Abstract
Background and aim
The aim of the study was to evaluate vitamin E effect upon oxidative stress associated with toluene −2, 4-diisocyanate (TDI)-induced asthma in rats.
Methods
The five study groups were: control, vehicle, TDI, vehicle+E, TDI+E. TDI animals were sensitized by nasal administration of TDI 10% (5μl/nostril) between days 1–7 and 15–21. Between days 22–28 groups TDI+E and vehicle+E rats received vitamin E (50 mg/kg, i. v.), and control, vehicle and TDI groups received saline solution. On day 29 the rats were challenged by intranasal application of 5% TDI (5 μl/nostril). On day 30 blood, BALF and lung biopsy were harvested. Oxidative stress tests were malondialdehyde (MDA), protein carbonyls (PC), total thiols (tSH), 1,1-diphenyl-2-picryl hydrazyl (DPPH) and reduced glutathione (GSH).
Results
TDI sensitization increased oxidative stress systemically, but also locally in the respiratory airways and lung tissue. There was an increase of MDA and PC formation associated with a deficiency of the antioxidant defense reflected by DPPH decreases. There were no differences between systemic and local lung concentrations of oxidized molecules.
After vitamin E treatment oxidative stress was reduced mostly due to serum, BALF and lung tissue GSH and DPPH increase.
Conclusion
The study showed that in rat TDI-induced asthma there was oxidative stress caused by increased ROS production and antioxidants deficiency, and vitamin E reduced ROS production and improved antioxidant defense.
Keywords: oxidative stress, asthma, vitamin E, isocyanates
Background and aims
Isocyanates is a group of reactive low-molecular weight chemicals commonly utilized in the manufacturing of polyurethane foams, paints, coatings, elastomers, adhesives, and many other products [1,2,3,4]. In the last 50 years, the production and use of isocyanates have rapidly increased. The three major diisocyanates present in the workplace include toluene diisocyanate (TDI), diphenyl-methane diisocyanate and hexamethylene diisocyanate [1]. Adverse health effects due to exposure to isocyanates have been known for a long time [5]. Humans may be exposed to TDI by inhalation, ingestion, dermal or eye contact. It is a powerful irritant to the mucosal membranes and can cause severe allergy and asthma, but patients may also present ophthalmic, dermatological, and gastrointestinal manifestations [6]. Occupational asthma is the most common work-related lung disease. Studies on the incidence and causative factors of occupational asthma identify isocyanates as one of the leading causes of occupational asthma worldwide. The increased use of isocyanate-containing products has raised the awareness of health problems related to non-occupational exposure as well [5].
Experimental animal models of diisocyanate occupational asthma have demonstrated an immunological basis for the disease. Animals can be sensitized by dermal or respiratory exposure, suggesting that either the exposure route may be important in the workplace. It was also found that isocyanate-induced lung disease is an oxidant stress-dependent pulmonary inflammation. Diisocyanates can react with -OH, -SH, and -NH2 groups of endogenous proteins [7] and are able to bind to glutathione (GSH) in the skin or mucosal surfaces [8,9,10]. GSH, one of the major anti-oxidants of the lung, has been linked to the response to isocyanate exposure. Experimental data suggest that airway GSH may help prevent the development of allergic sensitization and asthma [11,12].
To address the oxidative stress involved in isocyanates-induced asthma, in the present study attention was focused on vitamin E effect in rat experimental isocyanate-induced asthma.
Methods
Animals
Wistar rats aged 8–10 weeks (200–220 g) were purchased from the Iuliu Hatieganu University of Medicine and Pharmacy, Cluj Napoca, Romania. The animals were housed in a conventional animal facility at a constant temperature of 25±2°C, relative humidity of 50–70%, with 12-h dark/light cycles, and received water and pelleted food ad libitum. After one week of acclimation in our laboratory, the rats were randomized into five groups (n=10). Rats were housed at a maximum of five per cage. All experimental procedures were approved by the local Ethical Committee for Animal Experiments, and all studies were conducted in accordance with the regulations on the use and care of animals.
Experimental protocol
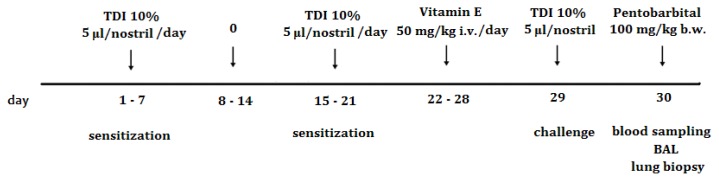
Toluene-2,4-diisocyanate (98%; Fluka, CAS 584-84-9) was obtained from Aldrich Chemical Co. (Taufkirchen, Germany). TDI was prepared at a concentration of 10% for sensitization and 5% for the challenge, using ethyl acetate as the vehicle. The five study groups were: CONTROL, group that received no treatment; VEHICLE, rats that received the vehicle on all occasions; TDI, rats that received sensitization and challenge with TDI; VEHICLE+E, rats that received vehicle and vitamin E; TDI+ E, rats that received TDI on all occasions and vitamin E treatment. The rats in groups TDI and TDI+E were sensitized by nasal administration of TDI 10% (5 μl/nostril) from day one to day 7, and from day 15 to 21, with a rest period from day 8 to 14. From day 22 to day 28 in groups TDI+E and VEHICLE+E rats received Vitamin E (50 mg/kg, i. v.) [13], and in groups CONTROL, VEHICLE and TDI rats received saline solution. In day 29 the rats were challenged by intranasal application of 5% TDI (5 μl/nostril) [14,15] (Figure 1).
Figure 1.
Schematic diagram of the experimental TDI-induced asthma protocol and vitamin E treatment.
Sample collections
At the end of the experiment period, all animals were sacrificed with pentobarbital overdose (100 mg/kg, i.p.) on day 30 [15]. Blood samples were taken from the retro-orbital sinus, rest for 1 h at room temperature, then centrifuged (3000×g, 20 min) and supernatants were harvested and stored at −80°C until further analysis.
To perform the bronchoalveolar lavage (BAL), a cannula was placed in the trachea and the lungs were lavaged in situ, three times with 0.8 mL sterile saline (0.9% NaCl), and the recovered fluid was pooled. The total BAL fluid (BALF) volume was measured, and the BALF was then centrifuged at 1200 rpm for 10 min. The resultant supernatant was collected and stored at −80 °C until further analysis [1,4,15].
Lung biopsy was also performed. Briefly, after harvesting the lungs, tissue fragments were homogenized with a Polytron homogenizer (Brinkmann Kinematica) for 3 minutes on ice in phosphate buffered saline (pH, 7.4), added in a ratio of 1:4 (w/v). The suspension was centrifuged for 5 minutes at 3000 g at 4°C to prepare the cytosolic fractions. The proteins levels in homogenates were measured by using the Bradford method [16,17]. The homogenate were maintained at −80°C until further analysis.
Chemicals
1,1-diphenyl-2-picryl hydrazyl (DPPH), dimethylsulfoxide (DMSO), 2,4-dinitrophenylhydrazine (DNPH) were purchased from Sigma Aldrich, St. Louis, MO. All other chemicals used in the experiments were of analytical grade.
Determination of MDA
The malondialdehyde (MDA) content was measured by assessing the concentration of thiobarbituric acid reactive substances (TBARS) [18]. A solution of 10 mM 2- thiobarbituric acid in 75 mM K2HPO4 solution (pH 3.0) was added to the samples and then heated in a water bath for one hour. After cooling, the reacting product was extracted in n-butanol. MDA was measured spectrofluorimetrically using a synchronous technique, with excitation at 534 nm and emission at 548 nm. MDA values were conveyed in nmol/mLin serum and BALF and nmol/mg protein in lung homogenate supernatant.
Determination of protein carbonyls
Protein oxidation was estimated by protein carbonyls (PC) content [19]. From 0.5 ml of sample, proteins were precipitated using 0.25 ml of trichloroacetic acid (TCA) 10%. 0.75 ml of 10 mM DNPH in 2 M HCl was added to the precipitate and incubated at room temp for 30 min. The reaction mixture was recentrifuged using the same amount of TCA as before. The precipitate, thus obtained, was washed twice with 1 ml ethanol/ethyl acetate (1:1 v/v) mixture. The precipitate was dissolved in 0.75 ml protein dissolving solution (2 g sodium dodecylsulphate and 50 mg EDTA in 100 ml 80 mM phosphate buffer, pH 8.0), incubated for 10 min at room temp. The color intensity was measured at 370 nm against 2 M HCl. PC content was calculated using the molar extinction coefficient 22,000 M-1cm-1. PC concentration was expressed as nmol/ml for serum and nmol/mg protein for lung homogenates.
Determination of total thiols
Total thiols (tSH) were estimated using Ellman’s reagent [20,21]. In final volume of 4.0 ml, was added 0.2 ml sample and 0.6 ml of 20 mM tris-HCl buffer pH 8.2, followed by addition of 0.04 ml of 10 mM DTNB in absolute methanol and 3.16 ml of absolute methanol. The tubes were capped and color was developed for 15 min at room temperature. The tubes were then centrifuged at 3,000 g for 20 min. Supernatant was collected and absorbance was measured at 412 nm. tSH concentration was expressed as mmol/ml.
DPPH Radical Scavenging Assay
The hydrogen donating or radical scavenging ability was measured using the stable DPPH radical method [22]. Samples (200 ml) of 0.025–1 mg/ml in DMSO were mixed with 1.5 ml of 0.1 mM DPPH in methanol, and the final volume was adjusted to 3.5 ml with DMSO. The mixture was then shaken vigorously and allowed to stand for 30 min in the dark at room temperature. The absorbance was determined at 517 nm. Trolox was used as a reference compound. Absorbance of DPPH in DMSO was used as a control. The percent inhibition of DPPH free radical by the samples was calculated according to the following formula: DPPH scavenging ability = (Acontrol − A sample/Acontrol) × 100, where Acontrol is the absorbance of DPPH radical + methanol (containing all reagents except the sample) and Asample is the absorbance of DPPH radical + sample. The IC50 value represented the concentration that caused 50% inhibition. So, IC50 ≤ 50 μg/mL value means a high antioxidant capacity; 50 μg/mL < IC50 ≤ 100 μg·mL−1 value means a moderate antioxidant capacity and IC50 > 200 μg·mL−1 value means no relevant antioxidant capacity [23].
Determination of GSH
We measured reduced glutathione (GSH) fluorimetrically by using o-phtalaldehyde [20]. Samples were treated with TCA (10%) and centrifuged. A solution of o-phtalaldehyde (1 mg/mL in methanol) was added to supernatants diluted with sodium phosphate buffer 0.1 M and EDTA 5 mM (pH, 8.0). After 15 minutes, the fluorescence was recorded (350-nm excitation and 420-nm emission). GSH concentration was determined by using a standard curve and was expressed as nmol/mL and nmol/mg protein respectively.
Statistical analysis
Values are the mean and standard deviation (SD). Otherwise, the median and quartiles are reported (Q1 = first quartile; Q3 = third quartile). For multiple group comparisons, one-way ANOVA was used, as appropriate. If significant differences were determined with ANOVA, post hoc analyses were conducted using the Tukey test to determine differences between individual groups. The Mann–Whitney test was used for non-parametric data. Pearson’s and Spearman’s correlation analyses were used to calculate relationships between parameters. P<0.05 was considered significant. Analyses were conducted using SPSS v20.0 (SPSS, Chicago, Il, USA).
Results
Serum oxidative stress tests
After TDI challenge, compared to CONTROL there was a significant increase of serum MDA in all the groups (p<0.001). No significant differences were found in serum MDA concentration between groups VEHICLE, TDI, VEHICLE+E and TDI+E (p>0.05) (Table I).
Table I.
Serum oxidative stress tests.
| MDA | PC | GSH | tSH | DPPH | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CONTROL | 2.27 | ±0.59 | 1.31 | ±0.38 | 5.47 | ±1.57 | 0.25 | ±0.05 | 22.95 | ±10.6 |
| VEHICLE | 3.14 | ±0.46 | 2.01 | ±0.44 | 5.45 | ±2.27 | 0.22 | ±0.02 | 17.55 | ±9.94 |
| TDI | 3.57 | ±0.97 | 2.79 | ±0.71 | 5.45 | ±8.24 | 0.18 | ±0.06 | 17.71 | ±4.37 |
| VEHICLE+E | 3.22 | ±0.70 | 2.02 | ±0.67 | 16.32 | ±2.08 | 0.30 | ±0.02 | 19.00 | ±6.55 |
| TDI+E | 3.39 | ±0.47 | 2.32 | ±0.42 | 28.04 | ±5.44 | 0.21 | ±0.03 | 21.62 | ±5.69 |
Values are the mean ±SD. VEHICLE, ethyl acetate; TDI, toluene diisocyanate; E, vitamin E; MDA, manondialdehyde; PC, protein carbonyls; GSH, glutathione; tSH, total thiols; DPPH, 1,1-diphenyl-2-.
If compared to the CONTROL group, serum PC values of all the other groups increased significantly after TDI challenge (p<0.01), but there were no significant differences between the groups (p>0.05) (Table I).
In CONTROL, VEHICLE and TDI groups serum GSH had almost similar values (p>0.05) after TDI challenge. Vitamin E treatment increased GSH in VEHICLE+E and TDI+E (P<.0.001), and TDI+E had a significantly higher increase (p<0.001) (Table I).
Analyzing serum tSH values after TDI challenge it was found that TDI and vehicle alone or with vitamin E had no significant influence (p>0.05), and there were no important difference between TDI, VEHICLE and CONTROL groups (p>0.05) (Table I).
Compared to the CONTROL, after TDI challenge serum DPPH of vehicle or TDI animals decreased significantly (p<0.05). Vitamin E treatment increased DPPH significantly (p<0.01) in TDI+E animals (Table I).
BALF oxidative stress tests
From the BALF were determined just MDA, GSH and DPPH due to the low proteins concentration.
After TDI challenge BALF MDA did not changed significantly at VEHICLE and VEHICLE+E animal (p>0.05). TDI administration caused a significant increase of BALF MDA (p<0.001), and vitamin E treatment reduced BALF MDA to the control level (p<0.001) (Table II).
Table II.
BALF oxidative stress tests.
| MDA | GSH | DPPH | ||||
|---|---|---|---|---|---|---|
| CONTROL | 0.31 | ±0.03 | 12.08 | ±2.33 | 0.12 | ±0.02 |
| VEHICLE | 0.51 | ±0.09 | 16.95 | ±5.49 | 0.13 | ±0.04 |
| TDI | 1.23 | ±0.15 | 12.98 | ±4.48 | 0.11 | ±0.05 |
| VEHICLE+E | 0.15 | ±0.01 | 25.33 | ±7.62 | 0.11 | ±0.03 |
| TDI+E | 0.31 | ±0.03 | 14.67 | ±2.90 | 0.33 | ±0.02 |
Values are the mean ±SD. VEHICLE, ethyl acetate; TDI, toluene diisocyanate; E, vitamin E; MDA, manondialdehyde; GSH, glutathione; DPPH, 1,1-diphenyl-2-picryl hydrazyl;I, initial; f, final.
BALF GSH did not change after TDI challenge at VEHICLE animals (p>0.05), but vitamin E association caused a significant increase (p<0.001). TDI alone or associated with vitamin E did not affect BALF GSH (p>0.05) (Table II).
In BALF, after TDI challenge DPPH was not influenced by the VEHICLE alone or with vitamin E (p>0.05). TDI alone did not change BALF DPPH as well (p>0.05), but the association of vitamin E treatment increased DPPH (p<0.01) (Table II).
Lung oxidative stress tests
In lung tissue after TDI challenge MDA was not significantly influenced by vehicle alone or with vitamin E (p>0.05). TDI caused lung tissue MDA increase (p<0.01), and vitamin E association reduced those values significantly (p<0.01) (Table III).
Table III.
Lung oxidative stress tests.
| MDA | PC | GSH | tSH | DPPH | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CONTROL | 0.20 | ±0.012 | 2.85 | ±0.49 | 0.56 | ±0.19 | 0.020 | ±0.003 | 39.88 | ±8.75 |
| VEHICLE | 0.27 | ±0.016 | 3.29 | ±0.62 | 0.69 | ±0.26 | 0.027 | ±0.005 | 50.98 | ±10.89 |
| TDI | 0.39 | ±0.04 | 3.53 | ±0.78 | 0.15 | ±0.04 | 0.023 | ±0.009 | 45.37 | ±11.37 |
| VEHICLE+E | 0.25 | ±0.012 | 1.76 | ±0.34 | 0.73 | ±0.25 | 0.031 | ±0.01 | 53.06 | ±4.85 |
| TDI+E | 0.29 | ±0.06 | 1.91 | ±0.27 | 0.65 | ±0.12 | 0.032 | ±0.001 | 52.17 | ±6.10 |
Values are the mean ±SD. VEHICLE, ethyl acetate; TDI, toluene diisocyanate; E, vitamin E; MDA, manondialdehyde; PC, protein carbonyls; GSH, glutathione; tSH, total thiols; DPPH, 1,1-diphenyl-2-picryl hydrazyl.
After TDI challenge vehicle caused a small increase of lung tissue PC (p<0.05), and vitamin E association reduced PC more than in the control group (p<0.001). TDI also increased PC in the lung (p<0.001), and vitamin E lowered it significantly (p<0.001) (Table III).
Vehicle alone or with vitamin E did not influenced significantly lung GSH (p>0.05) after TDI challenge. TDI reduced lung GSH (p<0.01), and TDI+E decreased it (p<0.01) almost like in control animals (p>0.05) (Table III).
Total thiols were not significantly changed after TDI challenge in none of the groups (p>0.05) (Table III).
In the lungs DPPH was increased by the vehicle (p<0.01), and association of vitamin E had almost the same effect (p>0.05) as vehicle. TDI did not influenced significantly DPPH in the lung (p>0.05), but TDI+E caused a significant increase (p<0.01) (Table III).
BALF MDA were significantly smaller than serum MDA (p<0.001). DPPH was also smaller in BALF (p<0.01) than in the serum. GSH had higher values in BALF (p<0.001) than in the serum only before vitamin E administration. After vitamin E GSH was higher in the serum of the VEHICLE+E group (p<0.001), and in BALF of the TDI+E group (p<0.001).
Discussion
The study showed that experimental rat TDI- induced asthma was associated with oxidative stress, and that vitamin E administration reduced oxidative stress.
Inhaled diisocyanates might cause various direct toxic effects on airways epithelial cells (AECs), but also indirect effects by initiating an immune response.
The direct toxic effects seem to be induced by the generation of reactive oxygen species (ROS) and by altering various antioxidant systems, such as thioredoxin, glutathione, ferritin, and heme oxygenase-1.
The imbalance between oxidant and antioxidant systems can initiate inflammatory responses in the airways through the activation of oxidative stress-sensitive transcription factors, like hypoxia-inducible factor HIF-1 and nuclear factor NF-kB, which in turn mediate the production of proinflammatory mediators, such as matrix metalloproteinase MMP-9, intercellular adhesion molecule ICAM-1, vascular cell adhesion molecule VCAM-1, TNF-α, interleukin IL-1, IL-8, and IL-6, from AECs. Various kinds of cytokines have an effect on the recruitment of cells that mediate the process of airway inflammation and remodeling [24,25].
For many years eosinophilic airway inflammation was recognized as the most important feature of patients with chronic, stable asthma. Today it has become apparent that occupational asthma is characterized by an influx of polymorphonuclear neutrophils (PMN) in the airways. PMN are key components of the first line of defense against microbial pathogens, being rapidly recruited to inflammatory sites in response to a variety of stimuli16. Recently, it has been demonstrated that PMN have an important role in TDI-induced asthma because they can release ROS having pathological effects on AECs of asthmatic individuals through the oxidative stress16. So, the nonimmune direct oxidative stress effects of diisocyanate may play an important role in the pathogenesis of TDI-induced asthma [26,27].
During the course of the present study we found that TDI sensitization increased oxidative stress systemically, but also locally in the respiratory airways and lung tissue. The mechanism was complex, because there was an increase of MDA and PC formation associated with a deficiency of the antioxidant defense reflected by DPPH decreases. These data are consistent with previously published works. The new finding was that there were no differences between systemic and local lung concentrations of oxidized molecules. After vitamin E treatment oxidative stress was reduced mostly due to serum, BALF and lung tissue GSH and DPPH increase.
The mechanisms that mediate diisocyanates immunogenicity are still unclear. It has been hypothesized that diisocyanates react with self-molecules, especially primary amine groups of proteins, in airway surfactant, epithelial fluid, or the extracellular skin compartment. In vivo, albumin appears to be the major protein target for diisocyanate reactivity, and diisocyanate-albumin is the only known “self” protein reaction product known to trigger innate and adaptive cellular immune responses, associated with airway inflammation and asthma. Animal experimental studies have suggested for albumin the possible existence of a “shuttle” mechanism through which diisocyanate is transported from the airways to the blood. The formation of antigenic diisocyanate-albumin conjugates mediates the pathogenic responses to occupational exposure, like the production of cytokine, specific IgE, oxidative stress, and innate immune proteins [28,29]. In the serum of exposed workers diisocyanate-albumin specific IgG is frequently found, and IgE isotypes are rarely observed [28,29].
In addition to albumin, diisocyanates are thought to react in vivo with GSH. GSH is the major free thiol of the lower airway fluid, where it is normally present at relatively high levels compared with peripheral blood [28,29]. Accordingly we found the higher GSH concentration in BALF.
There is also a theory that GSH serves also as a shuttle for systemic distribution of inhaled diisocyanate, because the reaction of isocyanate with GSH is reversible, and isocyanate groups are transferred from GSH to albumin or other peptides/proteins [28,29]. This mechanism may be the cause of the insignificant changes of serum and BALF GSH at TDI animals.
Moreover, the International Agency for Research on Cancer has classified TDI, but none of the other diisocyanates, as a possible human carcinogen. Considering the widespread use of isocyanates and their known health effects, a strategy in reducing diisocyanates pathological effects is needed. In the present study the exogenous antioxidant vitamin E proved to be efficient in reducing the oxidative stress systemically and locally, in BALF and lung tissue by a smooth decrease of ROS production and a significant increase of the antioxidant defense. We may consider that by using vitamin E TDI exposed persons may reduce carcinogenic risk as well.
In recent approaches isocyanate metabolites and isocyanate–protein conjugates have been measured in human biological samples as potential new exposure biomarkers. Immunologic responses such as isocyanate-specific IgG and IgE have also been measured as biomarkers in workers [30]. Common allergens have been shown to increase ROS production and through that to induce allergic airway inflammation. Although the exact mechanisms are not known, oxidase activity initiates immune activation through its ability to recruit inflammatory cells, including neutrophils. That was the reason for some studies to consider ROS as adjuvants of initiating allergic inflammation [25,31]. By reporting the results of this study, we may propose oxidative stress tests as diagnostic biomarkers for TDI induced asthma as well.
Conclusion
The study has showed that in rat TDI-induced asthma there is oxidative stress caused by increased ROS production and antioxidants deficiency. Due to that oxidative stress biomarkers may be useful diagnostic tests in TDI-induced asthma. Because vitamin E improved antioxidant defense and reduced ROS production, it may be recommended for TDI-asthma treatment and TDI-induced carcinogenesis prophylaxis.
References
- 1.Świerczyńska-Machura D, Walusiak-Skorupa J, Nowakowska-Świrta E, Piasecka-Zelga J, Świercz R, Pałczyński C. Immunological determinants in a murine model of toluene diisocyanate-induced asthma. Int J Occup Med Environ Health. 2012;25(4):492–498. doi: 10.2478/s13382-012-0063-1. [DOI] [PubMed] [Google Scholar]
- 2.Lemons AR, Siegel PD, Mhike M, Law BF, Hettick JM, Bledsoe TA, et al. A murine monoclonal antibody with broad specificity for occupationally relevant diisocyanates. J Occup Environ Hyg. 2014;11(2):101–110. doi: 10.1080/15459624.2013.843783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang J, Zhao H, Yao L, Tang H, Dong H, Wu Y, et al. Phosphatidylinositol 3-kinases pathway mediates lung caspase-1 activation and high mobility group box 1 production in a toluene-diisocyanate induced murine asthma model. Toxicol Lett. 2015;236(1):25–33. doi: 10.1016/j.toxlet.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Swierczyńska-Machura D, Nowakowska-Świrta E, Walusiak-Skorupa J, Piasecka-Zelga J, Swiercz R, Nocuń M, et al. Effect of inhaled toluene diisocyanate on local immune response based on murine model for occupational asthma. J Immunotoxicol. 2014;11(2):166–171. doi: 10.3109/1547691X.2013.818745. [DOI] [PubMed] [Google Scholar]
- 5.Verschoor L, Verschoor AH. Nonoccupational and occupational exposure to isocyanates. Curr Opin Pulm Med. 2014;20(2):199–204. doi: 10.1097/MCP.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 6.Shadnia S, Ahmadimanesh M, Ghazi-Khansari M, Zamani N. Intestinal obstruction in acute inhalational toluene 2,4-diisocyanate gas toxicity. Int J Occup Environ Med. 2013;4(3):164–166. [PubMed] [Google Scholar]
- 7.Vandenplas O, Cartier A, Ghezzo H, Cloutier Y, Malo JL. Response to isocyanates: effect of concentration, duration of exposure, and dose. Am Rev Respir Dis. 1993;147(5):1287–1290. doi: 10.1164/ajrccm/147.5.1287. [DOI] [PubMed] [Google Scholar]
- 8.Vanoirbeek JA, De Vooght V, Synhaeve N, Nemery B, Hoet PH. Is toluene diamine a sensitizer and is there cross-reactivity between toluene diamine and toluene diisocyanate? Toxicol Sci. 2009;109(2):256–264. doi: 10.1093/toxsci/kfp065. [DOI] [PubMed] [Google Scholar]
- 9.Vanoirbeek JA, Tarkowski M, De Vooght V, Nemery B, Hoet PH. Immunological determinants in a mouse model of chemical-induced asthma after multiple exposures. Scand J Immunol. 2009;70(1):25–33. doi: 10.1111/j.1365-3083.2009.02263.x. [DOI] [PubMed] [Google Scholar]
- 10.Vanoirbeek JA, De Vooght V, Nemery B, Hoet PH. Multiple challenges in a mouse model of chemical-induced asthma lead to tolerance: ventilatory and inflammatory responses are blunted, immunologic humoral responses are not. Toxicology. 2009;257(3):144–152. doi: 10.1016/j.tox.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 11.Wisnewski AV. Developments in laboratory diagnostics for isocyanate asthma. Curr Opin Allergy Clin Immunol. 2007;7(2):138–145. doi: 10.1097/ACI.0b013e3280895d22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wisnewski AV, Liu Q, Liu J, Redlich CA. Glutathione protects human airway proteins and epithelial cells from isocyanates. Clin Exp Allergy. 2005;35(3):352–357. doi: 10.1111/j.1365-2222.2005.02185.x. [DOI] [PubMed] [Google Scholar]
- 13.Akindele OO, Kunle-Alabi OT, Adeyemi DH, Oghenetega BO, Raji Y. Effects of vitamin E and melatonin on serum testosterone level in sleep deprived Wistar rats. Afr J Med Med Sci. 2014;43(4):295–304. [PubMed] [Google Scholar]
- 14.Muti AD, Bolfa P, Muti LA, Mureşan A. Vitamin E Attenuates the Degree of Histopathological Lung Damage Following Toluene Diisocyanate Administration in Rats. Bulletin USAMV-Veterinary Medicine. 2014;71(1):165–173. [Google Scholar]
- 15.Tang H, Zhao H, Song J, Dong H, Yao L, Liang Z, et al. Ethyl pyruvate decreases airway neutrophil infiltration partly through a high mobility group box 1-dependent mechanism in a chemical-induced murine asthma model. Int Immunopharmacol. 2014;21(1):163–170. doi: 10.1016/j.intimp.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 16.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 17.Noble JE, Bailey MJ. Quantitation of protein. Methods Enzymol. 2009;463:73–95. doi: 10.1016/S0076-6879(09)63008-1. [DOI] [PubMed] [Google Scholar]
- 18.Conti M, Moran PC, Levillain P, Lemonnier A. Improved fluorometric determination of malonaldehyde. Clin Chem. 1991;37:1273–1275. [PubMed] [Google Scholar]
- 19.Reznick AZ, Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol. 1994;233:357–363. doi: 10.1016/s0076-6879(94)33041-7. [DOI] [PubMed] [Google Scholar]
- 20.Hu ML. Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol. 1994;233:380–385. doi: 10.1016/s0076-6879(94)33044-1. [DOI] [PubMed] [Google Scholar]
- 21.Tripathi P, Chandra M, Misra MK. Oral administration of L-arginine in patients with angina or following myocardial infarction may be protective by increasing plasma superoxide dismutase and total thiols with reduction in serum cholesterol and xanthine oxidase. Oxid Med Cell Longev. 2009;2(4):231–237. doi: 10.4161/oxim.2.4.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mossa AT, Nawwar GA. Free radical scavenging and antiacetylcholinesterase activities of Origanum majorana L. essential oil. Hum Exp Toxicol. 2011;30:1501–1513. doi: 10.1177/0960327110391686. [DOI] [PubMed] [Google Scholar]
- 23.Simirgiotis MJ. Antioxidant capacity and HPLC-DAD-MS profiling of Chilean peumo (Cryptocarya alba) fruits and comparison with German peumo (Crataegus monogyna) from southern Chile. Molecules. 2013;18:2061–2080. doi: 10.3390/molecules18022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park HS, Kim SR, Lee YC. Impact of oxidative stress on lung diseases. Respirology. 2009;14:27–38. doi: 10.1111/j.1440-1843.2008.01447.x. [DOI] [PubMed] [Google Scholar]
- 25.Shin YS, Kim MA, Pham LD, Park HS. Cells and mediators in diisocyanate-induced occupational asthma. Curr Opin Allergy Clin Immunol. 2013;13(2):125–131. doi: 10.1097/ACI.0b013e32835e0322. [DOI] [PubMed] [Google Scholar]
- 26.Ouyang B, Bernstein DI, Lummus ZL, Ying J, Boulet LP, Cartier A, et al. Interferon-γ promoter is hypermethylated in blood DNA from workers with confirmed diisocyanate asthma. Toxicol Sci. 2013;133(2):218–224. doi: 10.1093/toxsci/kft079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pham le D, Kim MA, Yoon MG, Lee SI, Shin YS, Park HS. Serum specific IgG response to toluene diisocyanate-tissue transglutaminase conjugate in toluene diisocyanate-induced occupational asthmatics. Ann Allergy Asthma Immunol. 2014;113(1):48–54. doi: 10.1016/j.anai.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 28.Wisnewski AV, Liu J, Redlich CA. Connecting glutathione with immune responses to occupational methylene diphenyl diisocyanate exposure. Chem Biol Interact. 2013;205(1):38–45. doi: 10.1016/j.cbi.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimber I, Dearman RJ, Basketter DA. Diisocyanates, occupational asthma and IgE antibody: implications for hazard characterization. J Appl Toxicol. 2014;34(10):1073–1077. doi: 10.1002/jat.3041. [DOI] [PubMed] [Google Scholar]
- 30.Lockey JE, Redlich CA, Streicher R, Pfahles-Hutchens A, Hakkinen PB, Ellison GL, et al. Isocyanates and human health: multistakeholder information needs and research priorities. J Occup Environ Med. 2015;57(1):44–51. doi: 10.1097/JOM.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med. 2011;208:417–420. doi: 10.1084/jem.20110367. [DOI] [PMC free article] [PubMed] [Google Scholar]