Abstract
We report a case of dramatic outcome of severe hemolytic disease in a newborn due to RH1 incompatibility. A newborn with A RH1 blood group was admitted in the Mohammed V Military Teaching Hospital for the problem of hydrops fetalis associated with RH1 incompatibility. The blood group of his mother, aged 31, was AB RH1-negative and that of his 37 year old father was A RH1. The mother had a history of 4 term deliveries, 3 abortions, and 1 living child. There was no prevention by anti-D immunoglobulin postpartum. The mother’s irregular agglutinin test was positive and the pregnancy was poorly monitored. The laboratory tests of the newborn showed a high total serum bilirubin level (30 mg/L) and macrocytic regenerative anemia (Hemoglobin=4 g/dL, mean corpuscular volume = 183 fL, reticulocytes count =176600/m3). The blood smear showed 1256 erythroblasts per 100 leukocytes, Howell–Jolly bodies and many macrocytes. The direct antiglobulin test was positive. He was transfused with red blood cell concentrates and treated with conventional phototherapy. The evolution was unfavourable; he died three days after the death of his mother. The monitoring of these high-risk pregnancies requires specialized centers and a close collaboration between the gynaecologist and the blood transfusion specialist to strengthen the prevention, as well as clinico-biological monitoring in patients with a history of RH1 fetomaternal alloimunization.
Keywords: Hemolytic disease of the newborn, alloimmunization, prevention, RH1
Introduction
The RH1 alloimmunization responsible for the hemolytic disease of the newborn occurs when the RH1-negative mother’s blood comes into contact with the foetus’s RH1 positive red blood cells. After the passing of foetal red blood cells into the maternal circulation, the RH1 antigens on foetal red blood cells, which are foreign antigens to the maternal immune system, trigger the immunological processes producing anti-RH1 allo-antibodies of the immunoglobulin class IgG. These antibodies cross the placenta, attack foetal red blood cells and lead to a foetal hemolytic anemia [1].
The immunoprophylaxis by anti-RH1 immunoglobulins has been established since the 1970s, but this disease remains the leading cause of fetal anemia. The severe forms of hemolytic disease are observed in 10% of fetuses or newborn affected by this disease [2,3]. It exposes to fetal complications such as hydrops, hypoxic brain damage and fetal death [2,3].
We report a case of dramatic outcome of an observation of severe hemolytic anaemia in a newborn due to RH1 incompatibility, which led to death.
Case report
A male newborn presenting the antecedents of consanguinity was admitted 30 minutes of life to the pediatric department of Mohammed V Military Teaching Hospital for the issue of hydrops fetalis on Rhesus incompatibility; the birth weight was 1800g and his blood group was A RH1. After birth, the baby was intubated and placed on mechanical ventilation due to respiratory distress and hypoxia. The blood group of his mother, aged 31, was AB RH1-negative and that of his father aged 37 was A RH1.
The mother had a history of 4 term deliveries, 3 abortions, and 1 living child. There was no prevention by anti-D immunoglobulin postpartum. For the fourth pregnancy, she was sent to gynecology department of Mohammed V Military Teaching Hospital after the discovery of fetal ascites. Preterm birth was induced at 30 weeks of gestation by cesarean section under spinal anaesthesia and she was transferred to the medical intensive care unit and the newborn was transferred to the paediatric department. Mother‘s irregular agglutinins test was positive and the pregnancy was poorly monitored.
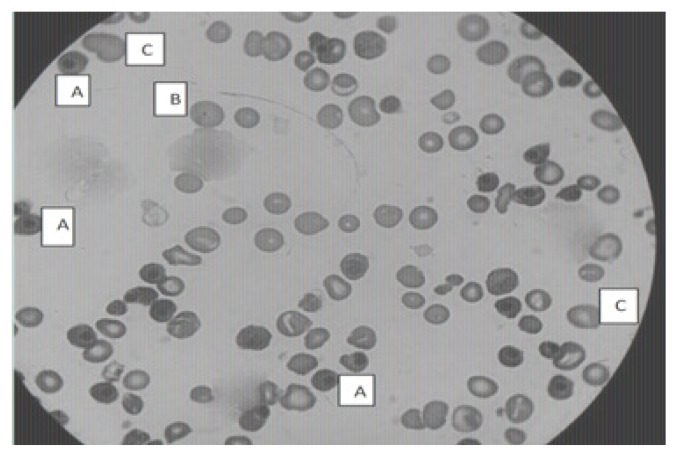
The laboratory tests of the newborn on the first day of life showed hyperbilirubinaemia (total serum bilirubin level =30 mg/L), hyperuremia (1.03 g/L), hyperglycemia (1.29 g/L), hyperkalaemia (7.2 mmol/L) and hyponatremia (134 mmol/L). The blood count showed bicytopenia with macrocytic regenerative anemia (Hemoglobin = 4g/dL, mean corpuscular volume = 183 fL, reticulocyte count = 176600/μL) associated with thrombocytopenia at 120 000/μL. The blood smear showed erythroblastosis (1256 erythroblasts per 100 leukocytes), Howell–Jolly bodies, anisocytosis and many macrocytes (Figure 1). The direct antiglobulin test was positive. After drainage of the ascites fluid, the newborn was transfused with red blood cell concentrates and was also treated with conventional phototherapy. The evolution was unfavorable with a steady increase of total serum bilirubin level (71 mg/L), hemoglobin (9.4 g/dL), reticulocytes (187–203/μL) and circulating erythroblasts (1386 erythroblasts per 100 leukocytes) and a decrease in platelet count (72 000/μL) on the second day (Table I) and died three days after the death of his mother, who died from pulmonary embolism in the intensive care unit.
Figure 1.
Image showing circulating erythroblasts (A), Howell–Jolly body (B) and many macrocytes (C) in the blood smear of the newborn (Staining: May-Grunwald-Giemsa. Magnification: × 1000).
Table I.
Summary table of the laboratory test of the newborn.
| Parameters | First day | Second day |
|---|---|---|
| White cells count (103/μL) | 202.7 | 79.8 |
| Corrected white cells count (103/μL) | 149.48 | 53.7 |
| Red cells count (106/μL) | 0.57 | 3.00 |
| Hemoglobin (g/dL) | 4.0 | 9.4 |
| Mean corpuscular volume (fL) | 183.1 | 90.0 |
| Reticulocyte count (/μl) | 176 600 | 187 203 |
| Platelets (103/μL) | 120 | 72 |
| Erythroblasts per 100 leukocytes | 1256 | 1386 |
| Morphological abnormalities of erythrocytes | Howell–Jolly bodies and many macrocytes | Anisocytosis, Howell–Jolly bodies and many macrocytes |
| Total serum bilirubin (mg/L) | 30 | 71 |
| Urea (g/L) | 1.03 | 1.08 |
| Sodium (mmol/L) | 134 | 131 |
| Potassium (mmol/L) | 7.20 | 6.4 |
| Glycaemia (g/L) | 1.29 | 0.68 |
Discussion
Our case report shows that there is RH1 incompatibility between the AB RH1-negative mother and the A RH1 newborn. The feto-maternal blood incompatibility constitutes the major cause of autoimmune hemolytic anaemia among newborns and must be evoked first before a neonatal anemia with early onset jaundice [1]. The allo-antibodies of the most common obstetrical interest are anti-RH1, anti-RH4 and anti-KEL1, representing respectively 35%, 37% and 13% of identified allo-antibodies; they are responsible for 88%, 8% and 2% respectively for severe fetomaternal incompatibilities [4].
Our patient presented anemia associated with erythroblastosis, Howell–Jolly bodies, many macrocytes and high reticulocytosis showing a very active erythropoiesis, to compensate for the hemolysis. Biological signs of autoimmune hemolytic anemia are regenerative anemia which can be macrocytic or normochromic normocytic anemia, a decrease in haptoglobin, an increase in lactate dehydrogenase related to the importance of hemolysis; a hemoglobinemia with hemoglobinuria in the case of intravascular hemolysis and sometimes an increase in unconjugated bilirubin and a decrease in the glycated hemoglobin and the direct antiglobulin test is positive in 95% of cases [5]. The direct antiglobulin test is based on the detection of erythrocytic autoantibodies either in serum, or when they are attached on red blood cells [6].
In this pathology, it is necessary to exclude physiologic jaundice due to newborn’s immature liver. However, the physiologic jaundice of the newborn is never present at birth and appears from the 36th hour to reach a maximum on the 3rd-4th day and disappears before the 10th day. It is also necessary to eliminate ABO incompatibility which is exclusively found in newborns with A or B blood type and whose mothers are O blood type and neonatal jaundice associated with hyperhemolysis due to common congenital hemolytic anemia: red blood cell membrane disorders (hereditary spherocytosis, hereditary elliptocytosis, and hereditary pyropoikilocytosis), red blood cell enzyme defects (glucose 6 phosphate dehydrogenase deficiency, pyruvate kinase deficiency and other red blood cells enzymopathies) and neonatal hemolysis due to hemoglobinopathies(α-thalassaemia major and α-globin and γ-globin chain structural abnormalities) [7]. The cases of polycythemia vera and certain infectious syndromes can also be accompanied by jaundice. In case of prolonged jaundice, other etiologies should be sought including breast milk jaundice [4].
The irregular agglutinin test is an important test for pregnancy monitoring as part of the prevention of anti-RH1 alloimmunization and management of feto-maternal incompatibilities. It aims at detecting and identifying red cell alloantibodies directed against erythrocyte antigens other than A or B of unexpressed ABO system on the surface of its own red blood cells capable of inducing, by feto-maternal incompatibility, hemolytic disease in the fetus and/or newborn [8]. The irregular agglutinin test is done 2 times (1st and 6th or 7th prenatal examinations) in RH1 pregnant women without transfusion history and 4 times (1, 4, 6 and 7th prenatal examinations) in RH1 women with a history of transfusion or pregnancy and in the RH1 negative women. This test is also practiced at childbirth in RH1 negative women before the anti-D immunoglobulin injection [4].
Postnatal management of hemolytic disease of the newborn due to RH1 incompatibility aims at preventing postnatal death from anemia complications and neonatal kernicterus and may include: intensive phototherapy which is the most commonly used treatment and its effectiveness is evaluated by regular monitoring of the concentration of total serum bilirubin, exchange transfusion which is the last resort in the treatment of hyperbilirubinaemia and its adverse effects are numerous: hypocalcaemia and thrombocytopenia, convulsions, necrotizing enterocolitis, apnea, bradycardia, hyperkalemia and hypoglycemia. The treatment of hyperbilirubinaemia can also be done using intravenous immunoglobulin (IVIG) (0.5–1 g/kg). A severe complication of injection of IVIG is necrotizing enterocolitis. A few small randomized controlled trials showed that the use of IVIG reduced the need for exchange transfusion, the duration of intensive phototherapy and length of hospitalization [9], but a randomized controlled trial conducted in the Netherlands did not confirm these results [10]. Other drugs which have been proposed in the treatment of neonatal jaundice are: D-penicillamine and metalloporphyrins which inhibit hemeoxygenase and reduce the production of bilirubin, albumin which increases bilirubin transport capacity in the blood and reduces the blood concentration of unconjugated bilirubin, and phenobarbital which increases bilirubin uptake, conjugation and excretion [9,11]. Blood transfusions may also be needed to correct severe anemia [9]. Despite blood transfusion and treatment by intensive phototherapy, our patient died four days after his birth with hemolysis, kidney failure, jaundice and hypoxia.
The best treatment is to prevent causal anti-D immunization with intravenous (IV) anti-RH1 immunoglobulin in RH1 negative pregnant women to neutralize the foetal red blood cells in the maternal vascular compartment [12]. When the newborn is Rh1 negative, the rhesus is confirmed on the second sample. If negativity is confirmed, anti-D immunization in mothers is unnecessary. If the newborn is RH1, the prophylaxis of alloimmunization to RH1 antigen is based on the IV injection of anti-RH1immunoglobulins. It is necessary first of all to perform the double determination of ABO group and the phenotype of RH-Kell of the newborn, a direct antiglobulin test on the red blood cells of the newborn, the irregular agglutinin test on maternal serum at childbirth and Kleihauer test on maternal blood collected at least one hour after delivery. The IV injection of anti-RH1 Immunoglobulins is carried out within 72 hours at the latest following delivery [12].
The physicians should be sensitized to be vigilant in preventing and monitoring RH1 alloimmunisation. The monitoring of these high-risk pregnancies requires specialized centres and collaboration between the gynecologist and the blood transfusion centre biologist.
Conclusion
Hemolytic disease of the newborn related to RH1 incompatibility is rare but serious. In order to avoid this drama, it is necessary to strengthen the prevention and clinico-biological monitoring in patients with a history of feto-maternal Rhesus alloimmunization by sensitizing and advising all RH1 negative unimmunized women that RH1 prophylaxis should be applied after all birthing of RH1 child and must also always be carried out after any miscarriage.
References
- 1.Tasseau A, Rigourd V. Anémie néonatale précoce: orientation diagnostique. Journal de pédiatrie et de puériculture. 2004;17:198–203. [Google Scholar]
- 2.Branger B, Winer N. Épidémiologie de l’allo-immunisation anti-D pendant la grossesse. J Gynecol Obstet Biol Reprod. 2006;35(1 Suppl):1S87–1S92. [PubMed] [Google Scholar]
- 3.CNGOF. Prévention de l’allo-immunisation Rhésus D foetomaternelle. Recommandations pour la pratique clinique. J Gynecol Obstet Biol Reprod. 2006;35:1S131–IS135. [Google Scholar]
- 4.Mannessier L. La surveillance immunohématologique de la femme enceinte et la nouvelle politique de prévention de l’allo-immunisation anti-RH1. Transfus Clin Biol. 2007;14(1):112–119. doi: 10.1016/j.tracli.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Rigal D, Meyer F. Anémies hémolytiques auto-immunes : diagnostic biologique et nouvelles approches thérapeutiques. Transfus Clin Biol. 2011;18:277–285. doi: 10.1016/j.tracli.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Philippe P. Diagnostic et prise en charge de l’anémie hémolytique auto-immune. Presse Med. 2007;36:1959–1969. doi: 10.1016/j.lpm.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 7.Murray NA, Roberts IA. Haemolytic disease of the newborn. Arch Dis Child Fetal Neonatal Ed. 2007;92(2):F83–F88. doi: 10.1136/adc.2005.076794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen-Bacrie S, Joubaud P, Krausé C, Morel P. La recherche d’anticorps anti-érythrocytaires (RAI): un examen au cœur de la réforme de la biologiemédicale. Rev Fr Lab. 2014;2014(467):37–44. [Google Scholar]
- 9.Smits-Wintjens VE, Walther FJ, Rath ME, Lindenburg IT, te Pas AB, Kramer CM, et al. Intravenous immunoglobulin in neonates with rhesus hemolytic disease: a randomized controlled trial. Pediatrics. 2011;127(4):680–686. doi: 10.1542/peds.2010-3242. [DOI] [PubMed] [Google Scholar]
- 10.Smits-Wintjens VE, Walther FJ, Lopriore E. Rhesus haemolytic disease of the newborn: Postnatal management, associated morbidity and long-term outcome. Semin Fetal Neonatal Med. 2008;13(4):265–271. doi: 10.1016/j.siny.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Dennery PA. Pharmacological interventions for the treatment of neonatal jaundice. Semin Neonatol. 2002;7(2):111–119. doi: 10.1053/siny.2002.0098. [DOI] [PubMed] [Google Scholar]
- 12.Miquel E, Cavelier B, Bonneau JC, Rouger P. Incompatibilités foetomaternelles érythrocytaires (IFME) : de la surveillance immunohématologique des femmes enceintes à la maladie hémolytique du nouveau-né (MHNN) Transfus Clin Biol. 2005;12:45–55. doi: 10.1016/j.tracli.2005.02.001. [DOI] [PubMed] [Google Scholar]