Sickle cell anemia (SCA) and Plasmodium falciparum malaria are two major public health problems in the state of Odisha, India. The prevalence of sickle cell gene in the western part of Odisha is 13.1%,1P. falciparum contributes 23% of cases and 15% of malaria-related deaths in India. Various African studies have shown that, even though SCA protects from P. falciparum infection, the risk of severe illness and death due to malaria is higher.2,3 Though several factors are responsible for the disease severity in P.falciparum malaria in patients with SCA, it was recently found that fetal hemoglobin (HbF), a physiological hemoglobin usually found higher in patients with SCA had a negative epistatic interaction with HbS during protection against malaria.4 The role of HbF against P. falciparum malaria in cases with normal hemoglobin genotypes has been widely studied and found to be protective against severe disease manifestation. So it is necessary to investigate this association in the regions with high prevalence of sickle cell gene and high endemicity of P. falciparum malaria. This study aims to find out the effect of HbF level on the clinical manifestation of severe P.falciparum malaria in patients with SCA.
This prospective study was undertaken at the Sickle Cell Clinic and Molecular Biology Laboratory, Veer Surendra Sai Institute of Medical Sciences and Research, Burla, Odisha, India. Forty-six adult patients with SCA along with severe P. falciparum malaria admitted in the Department of Medicine of this institute were included in this study. The mean age of patients was 25.4±8.8 years (range, 17 to 60 years) with 58.7% (27/46) being males. The hemoglobin variants including HbF was estimated by Cation-Exchange high performance liquid chromatography (CE-HPLC) using Variant II −β-thalassemia short program (Bio-Rad laboratories, Hercules). The mean % HbF level was found to be 18. 2 ± 4.9 %; ranging from 6.0 to 29.0 %.
The severity of malaria was defined by WHO guideline in 2010.5 The severity due to the malarial infection was defined by the presence of single or multiple complications. Cerebral malaria, severe malarial anemia, jaundice, acute renal failure and/or hepatopathy were considered as the major clinical symptoms of the patients. Among the various clinical symptoms, the incidence of cerebral malaria was 37.0% (17/46) followed by severe malarial anemia (21.7%, 10/46). Episodes of vaso-occlusive crises were observed in 50.0% of cases. Death was recorded in 9 patients including six females. The demographic and clinical features of patients have been shown in Table 1. There were multiple complications responsible for mortality in these patients. The comparison of % HbF level in patients with a various number of complications they had revealed that the mean % HbF levels increased with the number of clinical complications in the patients. The average increase in the % HbF level was 15.7±4.0, 18.0±4.0, 18.9±6.1 and 20.8±1.2 respectively in patients with single, two, three and four complications. Further linear regression analysis between total hemoglobin level and % HbF level in the patients elucidated an inverse relationship (r, −0.356; p, 0.015), which indicates that patients with higher % HbF level had lower total hemoglobin level.
Table 1.
The demographic and clinical features of severe Plasmodium falciparum malaria parients with sickle cell anemia (N=46).
| Characteristics | Severe P. falciparum malaria patients with sickle cell anemia (N=46) Number (%) |
|---|---|
| Age in year | 25.4±8.8 (Range, 17–60 years) |
| Gender | |
| Male | 27 (58.7) |
| Female | 19 (41.3) |
| Hemoglobin (mg/dL) | 7.4±2.3 |
| HbF (%) | 18.2±4.9 |
| Severe Malaria Anemia | 10 (21.7) |
| Cerebral Malaria | 17 (37.0) |
| Hepatopathy | 10 (21.7) |
| Acute Renal Failure | 5 (10.9) |
| Jaundice | 20 (43.5) |
| Episodes of Vaso-Occlusive Crises | 23 (50.0) |
| Fatal Outcome | 9 (19.6) |
Note: Age, Hemoglobin (mg/dL) and HbF (%) were represented as mean ±SD.
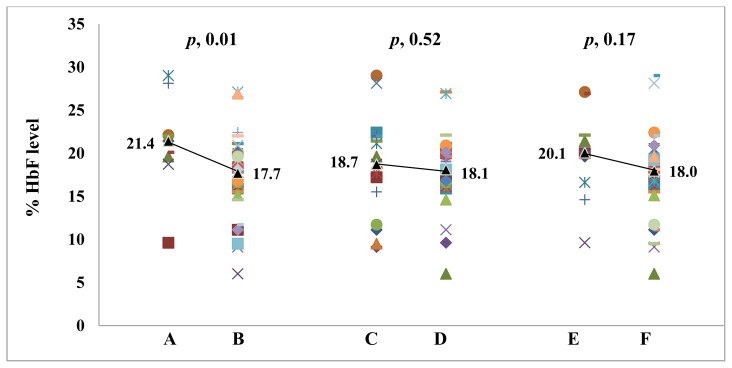
In another comparison, we found that there was a trend in increasing % HbF level in patients with severe malarial anemia compared to patients without it. A similar tendency was observed in patients with cerebral malaria. The average % HbF level also significantly increased averagely in patients who died compared to patients who survived (p, 0.01). The % HbF differences in the patients with severe malarial anemia, cerebral malaria, and fatality, has been illustrated in Figure 1.
Figure 1.
Comparison of % HbF level in patients with severe Plasmodium falciparum malaria with different clinical manifestation.
A. HbF level in death patients; B. HbF level in survived patients; C. HbF level in patients with cerebral malaria; D. HbF level in patients with no cerebral malaria; E. HbF level in patients with severe malarial anemia; F. HbF level in patients with no severe malarial anemia. The line joined the median value in each category.
Like HbS, alpha thalassemia has also been found to have a protective role against P. falciparum malaria. However, this protection afforded by alpha thalassemia becomes relatively sluggish when co-inherited with HbS. Because of a higher prevalence of alpha thalassemia in the study area,1 we have attempted to compare the hematological and clinical parameters in patients with heterozygous and homozygous alpha thalassemia separately against patients with normal alpha globin genotype. There were no statistically significant differences between the groups in hematological (Supplementary Table 1) as well as clinical parameters except for severe malarial anemia which was higher in patients with homozygous alpha thalassemia (Supplementary Table 2). Severe malarial anemia was associated with alpha thalassemia whereas alpha thalassemia has no impact on mortality, so we have analyzed the association of severe malarial anemia with mortality and was found insignificant (OR, 0.2; 95%CI [0.038–0.98]; p, 0.06).
From the three factors above, which are associated with severity and mortality due to P. falciparum malaria in patients with SCA, derived that HbF has a negative role in protection against severe disease manifestation. It has been found that HbF provides protection from P. falciparum malaria by diminishing the growth of the parasites inside the RBCs.6 Instead of giving protection, the high level of HbF in our patients is associated with a major severity of P. falciparum infection. This might be due to the peripheral selection and not for its increased synthesis of HbF in patients with SCA.
Though, HbF in SCA is found to be supportive in reducing episodes of vaso-occlusive crises and requirements of blood transfusion,7 the protection afforded by HbS against severe malaria reduced with increased % HbF level. This study agrees with the hypothesis of a negative epistatic interaction between HbS and HbF in reducing protection against severe malaria by Mmbando et al.,4 In this situation, use of hydroxyurea (a drug which usually increases HbF level) in patients with SCA in malaria endemic regions is debatable. In general, hydroxyurea must be started in severe patients with SCA because (1) increasing HbF level following hydroxyurea therapy is the principal but not the sole determinant of clinical responses in these patients; (2) some patients with minimal or no increased in HbF level following hydroxyurea therapy also showed significant clinical responses.8 In the present study, 26 patients were on hydroxyurea therapy, from which 15.4% (4/26) of patients died compared to 25.0% (5/20) of death in patients without hydroxyurea therapy. A large cohort study in a malaria endemic region is essential to give conclusive results on the association of HbF and use of hydroxyurea in patients with SCA.
Supplementary materials
Supplementary Table 1.
Comparison of hematological parameters on the basis of alpha globin genotype in severe Plasmodium falciparum patients with sickle cell anemia.
| Parameters | Normal alpha globin genotype (αα/αα) N=17 | Heterozygous Alpha thalassemia (−α/αα) N=20 | Homozygous Alpha thalassemia (−α/−α) N=9 | p value |
|---|---|---|---|---|
| Complete blood counts | ||||
| Hemoglobin (g/dL) | 7.7±1.8 | 7.1±2.6 | 7.1±2.6 | 0.741 |
| WBC (103 /μl) | 8.1±3.8 | 10.2±7.8 | 8.4±5.7 | 0.133 |
| RBC (106 /μl) | 2.9±0.5 | 3.0±1.2 | 3.0±0.6 | 0.717 |
| MCV (fL) | 87.3±12.0 | 78.6±13.0 | 71.0±7.8 | 0.009 |
| MCH (pg) | 28.9±3.9 | 25.7±3.8 | 25.0±3.7 | 0.017 |
| MCHC (g/dL) | 32.0±2.3 | 31.8±2.2 | 31.3±1.9 | 0.631 |
| Platelets (103 /μl) | 197.2±91.1 | 192.9±157.8 | 179.7±70.2 | 0.554 |
| Biochemical parameters | ||||
| Glucose (U/L) | 106.0±32.6 | 105.4±28.7 | 87.9±45.2 | 0.657 |
| Urea (mg/dL) | 34.2±22.3 | 36.3±25.6 | 46.2±41.7 | 0.094 |
| Creatinine (mg/dL) | 1.41±0.99 | 0.94±0.8 | 1.5±0.9 | 0.064 |
| SGOT (U/L) | 74.2±40.5 | 71.6±56.0 | 54.0±28.8 | 0.295 |
| SGPT (U/L) | 54.3±40.0 | 43.8±34.0 | 40.6±12.7 | 0.771 |
| Bil-T (mg/dL) | 3.1±1.7 | 4.2±2.7 | 3.8±1.8 | 0.301 |
| Bil-D (mg/dL) | 1.1±0.6 | 1.0±0.9 | 1.3±0.6 | 0.253 |
| Hb Variants by HPLC | ||||
| HbA2 (%) | 2.77±0.4 | 2.7±0.8 | 2.9±0.5 | 0.796 |
| HbF (%) | 19.7±3.9 | 18.3±5.2 | 17.4.1±3.7 | 0.635 |
| HbS (%) | 75.0±3.6 | 76.2±5.3 | 75.9±1.5 | 0.765 |
WBC, white blood corpuscle; RBC, Red blood corpuscle; MCV, mean corpuscular volume; MCH, mean corpuscular Hb; MCHC, mean corpuscular Hb concentration; HCT, hematocrit; SGOT, Aspartate transaminase; SGPT, alanine transaminase; Bil-T, bilirubin total; Bil-D, bilirubin direct; LDH, lactate dehydrogenase. Comparison between the groups were made by one way analysis of variance by using SPSS version 16.0 for window as statistical software.
Supplementary Table 2.
Comparison of clinical features on the basis of alpha globin genotype in severe Plasmodium falciparum patients with sickle cell anemia.
| Clinical signs and symptoms | Total (N=46) | αα/αα (N=17) | −α/αα (N=20) | Odd Ratio 95% CI | −α/−α (N=9) | Odd Ratio 95% CI |
|---|---|---|---|---|---|---|
| Severe Malarial Anemia | 10 (21.7) | 1 (5.9) | 5 (25.0) | 0.19 (0.02–1.8) | 4 (44.4) | 0.08* (0.007–0.87) |
| Cerebral Malaria | 17 (37.0) | 7 (41.2) | 8 (40.0) | 1.05 (0.28–3.93) | 2 (22.2) | 2.45 (0.38–15.5) |
| Hepatopathy | 10 (21.7) | 4 (23.5) | 6 (30.0) | 0.72 (0.16–3.13) | 0 | 6.33 (0.30–132.2) |
| Acute Renal Failure | 5 (10.9) | 3 (17.6) | 1 (5.0) | 4.07 (0.38–43.4) | 1 (11.1) | 1.71 (0.15–19.4) |
| Jaundice | 20 (43.5) | 6 (35.3) | 9 (45.0) | 0.67 (1.18–2.52) | 5 (55.6) | 0.43 (0.08–2.27) |
| Fatal Outcome | 9 (19.6) | 4 (23.5) | 3 (15.0) | 1.74 (0.33–9.19) | 2 (22.2) | 1.07 (0.16–7.42) |
The comparison has been analyzed separately for heterozygous (−α/αα) and homozygous (−α/−α) alpha thalassemia against normal alpha genotype. The data were presented as number (percentage).
p < 0.05
Acknowledgment
This study was supported by research funding from Indian Council of Medical Research (ICMR), New Delhi; Department of Science and Technology (DST), New Delhi; and National Health Mission, Govt. of Odisha, India.
Footnotes
Competing interests: The authors have declared that no competing interests exist.
References
- 1.Purohit P, Dehury S, Patel S, Patel DK. Prevalence of Deletional Alpha Thalassemia and Sickle Gene in a Tribal Dominated Malaria Endemic Area of Eastern India. ISRN Hematology. 2014;2014:e745245. doi: 10.1155/2014/745245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mmbando BP, Mgaya J, Cox SE, et al. Negative Epistasis between sickle and foetal haemoglobin suggests a reduction in protection against malaria. PLoS ONE. 2015;10(5):e0125929. doi: 10.1371/journal.pone.0125929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McAuley CF, Webb C, Makani J, et al. High mortality from Plasmodium falciparum malaria in children living with sickle cell anemia on the coast of Kenya. Blood. 2010;116:1663–1668. doi: 10.1182/blood-2010-01-265249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makani J, Komba AN, Cox SE, et al. Malaria in patients with sickle cell anemia: burden, risk factors, and outcome at the outpatient clinic and during hospitalization. Blood. 2010;115(2):215–220. doi: 10.1182/blood-2009-07-233528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Guidelines for the Treatment of Malaria. 2nd ed. Geneva: World Health Organization; 2010. http://www.who.int/malaria/publications/atoz/9789241547925/en/ [Google Scholar]
- 6.Pasvol G, Weatherall DJ, Wilson RJ. Effect of foetal haemoglobin on susceptibility of red cells to Plasmodium falciparum. Nature. 1977;270(5633):171–3. doi: 10.1038/270171a0. [DOI] [PubMed] [Google Scholar]
- 7.Mashon RS, Dash PM, Khalko J, et al. Higher fetal hemoglobin concentration in patients with sickle cell disease in eastern India reduces frequency of painful crisis. European Journal of Hematology. 2009;83(4):383–384. doi: 10.1111/j.1600-0609.2009.01290.x. [DOI] [PubMed] [Google Scholar]
- 8.Steinberg MH, Lu Z-H, Barton FB, et al. Fetal hemoglobin in sickle cell anemia: Determinants of response to hydroxyurea. Blood. 1997;89:1078–1088. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Comparison of hematological parameters on the basis of alpha globin genotype in severe Plasmodium falciparum patients with sickle cell anemia.
| Parameters | Normal alpha globin genotype (αα/αα) N=17 | Heterozygous Alpha thalassemia (−α/αα) N=20 | Homozygous Alpha thalassemia (−α/−α) N=9 | p value |
|---|---|---|---|---|
| Complete blood counts | ||||
| Hemoglobin (g/dL) | 7.7±1.8 | 7.1±2.6 | 7.1±2.6 | 0.741 |
| WBC (103 /μl) | 8.1±3.8 | 10.2±7.8 | 8.4±5.7 | 0.133 |
| RBC (106 /μl) | 2.9±0.5 | 3.0±1.2 | 3.0±0.6 | 0.717 |
| MCV (fL) | 87.3±12.0 | 78.6±13.0 | 71.0±7.8 | 0.009 |
| MCH (pg) | 28.9±3.9 | 25.7±3.8 | 25.0±3.7 | 0.017 |
| MCHC (g/dL) | 32.0±2.3 | 31.8±2.2 | 31.3±1.9 | 0.631 |
| Platelets (103 /μl) | 197.2±91.1 | 192.9±157.8 | 179.7±70.2 | 0.554 |
| Biochemical parameters | ||||
| Glucose (U/L) | 106.0±32.6 | 105.4±28.7 | 87.9±45.2 | 0.657 |
| Urea (mg/dL) | 34.2±22.3 | 36.3±25.6 | 46.2±41.7 | 0.094 |
| Creatinine (mg/dL) | 1.41±0.99 | 0.94±0.8 | 1.5±0.9 | 0.064 |
| SGOT (U/L) | 74.2±40.5 | 71.6±56.0 | 54.0±28.8 | 0.295 |
| SGPT (U/L) | 54.3±40.0 | 43.8±34.0 | 40.6±12.7 | 0.771 |
| Bil-T (mg/dL) | 3.1±1.7 | 4.2±2.7 | 3.8±1.8 | 0.301 |
| Bil-D (mg/dL) | 1.1±0.6 | 1.0±0.9 | 1.3±0.6 | 0.253 |
| Hb Variants by HPLC | ||||
| HbA2 (%) | 2.77±0.4 | 2.7±0.8 | 2.9±0.5 | 0.796 |
| HbF (%) | 19.7±3.9 | 18.3±5.2 | 17.4.1±3.7 | 0.635 |
| HbS (%) | 75.0±3.6 | 76.2±5.3 | 75.9±1.5 | 0.765 |
WBC, white blood corpuscle; RBC, Red blood corpuscle; MCV, mean corpuscular volume; MCH, mean corpuscular Hb; MCHC, mean corpuscular Hb concentration; HCT, hematocrit; SGOT, Aspartate transaminase; SGPT, alanine transaminase; Bil-T, bilirubin total; Bil-D, bilirubin direct; LDH, lactate dehydrogenase. Comparison between the groups were made by one way analysis of variance by using SPSS version 16.0 for window as statistical software.
Supplementary Table 2.
Comparison of clinical features on the basis of alpha globin genotype in severe Plasmodium falciparum patients with sickle cell anemia.
| Clinical signs and symptoms | Total (N=46) | αα/αα (N=17) | −α/αα (N=20) | Odd Ratio 95% CI | −α/−α (N=9) | Odd Ratio 95% CI |
|---|---|---|---|---|---|---|
| Severe Malarial Anemia | 10 (21.7) | 1 (5.9) | 5 (25.0) | 0.19 (0.02–1.8) | 4 (44.4) | 0.08* (0.007–0.87) |
| Cerebral Malaria | 17 (37.0) | 7 (41.2) | 8 (40.0) | 1.05 (0.28–3.93) | 2 (22.2) | 2.45 (0.38–15.5) |
| Hepatopathy | 10 (21.7) | 4 (23.5) | 6 (30.0) | 0.72 (0.16–3.13) | 0 | 6.33 (0.30–132.2) |
| Acute Renal Failure | 5 (10.9) | 3 (17.6) | 1 (5.0) | 4.07 (0.38–43.4) | 1 (11.1) | 1.71 (0.15–19.4) |
| Jaundice | 20 (43.5) | 6 (35.3) | 9 (45.0) | 0.67 (1.18–2.52) | 5 (55.6) | 0.43 (0.08–2.27) |
| Fatal Outcome | 9 (19.6) | 4 (23.5) | 3 (15.0) | 1.74 (0.33–9.19) | 2 (22.2) | 1.07 (0.16–7.42) |
The comparison has been analyzed separately for heterozygous (−α/αα) and homozygous (−α/−α) alpha thalassemia against normal alpha genotype. The data were presented as number (percentage).
p < 0.05