Abstract
Many studies have examined the hypothesis that greater participation in physical activity (PA) is associated with less brain atrophy. Here we examine, in a sub-sample (n = 352, mean age 79.1 years) of the Age, Gene/Environment Susceptibility-Reykjavik Study cohort, the association of the baseline and 5-year change in magnetic resonance imaging (MRI)-derived volumes of gray matter (GM) and white matter (WM) to active and sedentary behavior (SB) measured at the end of the 5-year period by a hip-worn accelerometer for seven consecutive days. More GM (β = 0.11; p = 0.044) and WM (β = 0.11; p = 0.030) at baseline was associated with more total physical activity (TPA). Also, when adjusting for baseline values, the 5-year change in GM (β = 0.14; p = 0.0037) and WM (β = 0.11; p = 0.030) was associated with TPA. The 5-year change in WM was associated with SB (β= −0.11; p = 0.0007). These data suggest that objectively measured PA and SB late in life are associated with current and prior cross-sectional measures of brain atrophy, and that change over time is associated with PA and SB in expected directions.
Keywords: Physical activity, Sedentary behavior, Brain atrophy, Elderly, MRI
1. Introduction
It is hypothesized that physical activity (PA) helps to preserve and maintain cognitive function and decrease the risk of dementia and Alzheimer disease [1–4]. Change in cognitive ability has been associated with brain atrophy [5–17]. It has been shown that the brain atrophies with age due to volume loss in both white (WM) and gray matter (GM) and increase in white matter lesions [18]. GM has been shown to linearly decline with increasing age starting at early adulthood, while WM deterioration shows nonlinear changes [14,19,20]. WM has been shown to increase throughout adulthood, peaking at around the age of 40–60 years, followed by an accelerated decline starting around age 60 [14,19]. PA is also known to be negatively associated with age [21,22] and sedentary behavior (SB) is known to be positively associated with age [23]. This trend has been shown to start in the forties [22].
Cross-sectional studies have shown a positive relationship between GM and WM volumes in the older adult brain and physical fitness [24,25]. Furthermore, six months aerobic training was shown to increase both GM and WM volumes in older subjects [26]. A cross-sectional study, using questionnaire, showed PA levels to positively correlate with brain volumes [27]. Longitudinal studies, using questionnaires have shown that higher level of PA at baseline predicts larger GM volume [28–30], larger WM volume [29] and more total brain volume [29,30] in late life. Studies using objectively measured PA are needed to confirm these results.
Previous studies have shown that lower PA levels predict lower brain volumes and atrophy [28–30], indicating that PA affects brain volumes. Currently, there are no published studies on whether brain volumes or changes in brain volume, is associated with PA later in life. It might be expected that those with greater PA would have a history of greater brain volumes both in the past and in the present and show the best maintenance of brain volumes over time. The aims of this study are to quantify the prospective changes in magnetic resonance imaging (MRI)-derived brain atrophy measurements in a 5-year period and explore their association with objectively measured PA and SB in an older population. This study is the first to assess brain atrophy in a longitudinal study design in relation to objectively measured behavior outcomes. Furthermore, we will test the hypothesis that the association between brain volumes and the important behavioral variables, PA and SB, are independent of self-reported PA at baseline (SPA).
2. Methods
2.1. Study population and design
The Age, Gene/Environment Susceptibility Reykjavik Study (AGES-Reykjavik study) was a prospective cohort study designed to examine risk factors in relation to disease and disability in old age. The aim was to investigate the contributions of environmental factors, genetic susceptibility, and gene-environment interactions to aging of the neurocognitive, cardiovascular, musculoskeletal, body composition, and metabolic systems. The AGES-Reykjavik study is a continuation of the Reykjavik Study, which was initiated in 1967 by the Icelandic Heart Association and included men and women born in 1907–1935 and living in the Reykjavik area. From 2002 to 2006, new data were collected for the AGES-Reykjavik study, and details on the study design have been described elsewhere [31]. Data from this data collection was used as baseline measurements for the current study. The current study was a part of the AGESII-Reykjavik study which is a follow up of the AGES-Reykjavik study, with the time interval of approximately five years. Between April 2009 and June 2010, objective PA measurement by accelerometers was added to the AGESII-Reykjavik study test protocol [21]. During the PA sub-study measurement period, 1194 subjects participated in the AGESII-Reykjavik study and were eligible to be invited to participate in the sub-study. Of these, 150 participants were excluded for different reasons (e.g., blindness and other physical- and mental impairments), 84 refused and 294 did not participate because of scheduling conflicts. Five subjects lost the accelerometers. The remaining 671 (56.2%) participants received an accelerometer to measure their daily activity. Of these, 585 participants had four or more valid days (≥10h of wear time) of useable accelerometry data. After excluding those with mild cognitive impairment (MCI), dementia or scored 24 or less on MMSE, 18 or less on the DSST test and did not have both brain measurements in AGES-Reykjavik study and AGESII-Reykjavik study, the final number of subjects was 352. The study was approved by the Icelandic National Bioethics Committee (VSN: 00-063), the Icelandic Data Protection Authority, and the institutional review board of the US National Institute on Aging, National Institutes of Health. Signed informed consent was given by all participants.
2.2. Assessment of PA
Participants were asked to wear the ActiGraph GT3X accelerometer (Actigraph Inc., Pensacola FL) monitor at the right hip for one complete week and to remove the monitor only before going to bed and during showers, bathing, or other water activities. Non-wear was defined as a period of at least 60 consecutive minutes during which the activity monitor recorded zero counts in all axes, allowing 1–2 min of vertical-axis counts between 0 and 100. A day of accelerometer wear was considered valid if the wear time was ≥10 h. Participants with fewer than four valid days over the week of measurement were excluded. Activity variables were derived from vertical-axis count values, and included: Total PA (TPA) defined as total counts during an average day (counts × day−1) and SB as hours × day−1 of activity <100 count × min−1 during wear time. Lifestyle PA was defined as ≥760 counts × min−1 [21,32,33].
2.3. MRI image acquisition
MRI including T1-, proton density-, and T2-weighted and fluid-attenuated inversion recovery (FLAIR) images were acquired on a 1.5-Tesla Signa Twinspeed EXCITE system (General Electric Medical Systems, Waukesha, WI) in the AGES-Reykjavik study. Brain tissue volumes, including GM, WM, cerebral spinal fluid (CSF), and white matter hyperintensities (WMH), were generated separately, using the multispectral MR images and a high-throughput automatic image analysis pipeline, which is based on the Montreal Neurological Institute (MNI) pipeline and optimized for use in the AGES-Reykjavik study (AGES-RS/MNI pipeline) [18]. The key processing stages were as follows: stereotaxic registration was achieved after signal non-uniformity correction by an affine transformation of the T1-weighted images to the ICBM152 template. Intersequence registration was performed by registering images from the individual (T2/proton density, fluid-attenuated inversion recovery) sequences to the T1-weighted images in order to accurately align all image volumes acquired during an acquisition session. Linear signal intensity normalization was then applied to correct for signal intensity variations across images in the different sequences. Finally, tissue classification was achieved with an artificial neural network classifier. The absolute volumes of the four tissue types were subsequently calculated and converted to native space volumes using the scale factor obtained from the stereotaxic registration transformation. Intra-cranial volume (ICV) was calculated by adding the volumes of GM, normal WM, WMH and CSF. All tissue volumes are presented as percent of the total ICV. The acquisition and post-processing of the MRI have been described in detail elsewhere [18]. The methods used in the follow-up MRI were the same as used in the baseline measurements. The 5-year change (Δ) in GM and WM volumes was calculated as the difference between the relative volume at follow-up and baseline.
2.4. Covariates
Covariates measured at baseline included age, sex and education [34]. Weight and body mass index (BMI) [35] was measured at follow-up. BMI was calculated as weight [kg] divided by squared height [m2]. Education was categorized as primary, secondary, college and university degree. SPA gathered from questionnaires from the AGES-Reykjavik study at baseline. Participants answered questions about how often they had participated in moderate or vigorous PA in the past 12 months (six categories to answer; (1) never, (2) rarely, (3) weekly but less than 1 h/week, (4) 1–3 h/week, (5) 4–7 h/week or (6) more than 7 h/week). The questions regarding PA were answered on a take-home questionnaire, that was reviewed by a trained interviewer when the participant came to a second visit to the clinic and returned the questionnaire. The following health factors measured at baseline were also used for adjustments: number of brain infarcts [18] depression [36], type 2 diabetes [37,38], mean arterial pressure (MAP) [39] and smoking status [30,38]. The presence of depression was assessed using the MINI International Neuropsychiatric Interview [40]. Participants were eligible for the MINI Interview if they had a score ≥6 on the 15-item Geriatric Depression Scale [41], had a history of anxiety or depression or were taking anti-depressant medication. Type 2 diabetes was defined as a fasting blood glucose level ≥7.0 mmol/L, the use of diabetic medication, or self-report of physician’s diagnosis of diabetes. MAP was calculated from the participants diastolic- (DP) and systolic (SP) pressure (MAP = DP + [1/3] × [SP–DP]). Smoking status was assessed by questionnaire and assigned as current/former smoker versus never smoked. SB was adjusted for lifestyle PA and wear time in all statistical models.
Analyses were performed using IBM SPSS 20.0 (SPSS Inc., Chicago, IL). The association between the accelerometer variables and brain volume measurements was analyzed using linear regression models. The PA variables were log transformed to correct for skewness. For Tables 2 and 3, linear regressions were performed and several models were formed. First, Model 1 was adjusted for age and sex and coefficients reflect association for individual brain volume measurements variables in separate models. In Model 2 each brain volume variable was adjusted for age, sex, brain infarcts, days between baseline and follow-up measurements, SPA, BMI, depression, MAP, type 2 diabetes, smoking status and education. Further, in Model 3, all baseline measurement variables and 5-year change variables were entered in the same model adjusted for same covariates as in Model 2.
Table 2.
Association between brain atrophy measures and total objectively measured physical activity. Brain volume measurements are presented as a percent of intra-cranial volume.
| Variables | Total physical activity (counts × day −1)
|
P | |||
|---|---|---|---|---|---|
| Std. β | Lower 95% CL | Upper 95% CL | |||
| Model 1# | GMa | 0.16 | 0.047 | 0.27 | 0.0056 |
| WMa | 0.20 | 0.093 | 0.31 | 0.00030 | |
| GM-5yrb | 0.24 | 0.12 | 0.35 | <0.0001 | |
| WM-5yrb | 0.22 | 0.11 | 0.33 | <0.0001 | |
| Δ-GMc | 0.17 | 0.063 | 0.27 | 0.0016 | |
| Δ-WMc | 0.090 | −0.011 | 0.19 | 0.080 | |
| Model 2## | GMa | 0.12 | 0.012 | 0.23 | 0.029 |
| WMa | 0.13 | 0.031 | 0.23 | 0.010 | |
| GM-5yrb | 0.17 | 0.063 | 0.28 | 0.0021 | |
| WM-5yrb | 0.16 | 0.062 | 0.26 | 0.0016 | |
| Δ-GMc | 0.11 | 0.015 | 0.21 | 0.024 | |
| Δ-WMc | 0.11 | 0.0095 | 0.20 | 0.032 | |
| Model 3### | GMa | 0.11 | 0.0028 | 0.22 | 0.044 |
| WMa | 0.11 | 0.011 | 0.21 | 0.030 | |
| Δ-GMc | 0.14 | 0.047 | 0.24 | 0.0037 | |
| Δ-WMc | 0.11 | 0.010 | 0.21 | 0.030 | |
GM =gray matter, WM =white matter.
Model 1 =each variable entered separately and adjusted for age and sex.
Model 2 = Model 1 and additional adjustment for brain infarcts, days between baseline and follow-up measurements, education, SPA, BMI, depression, MAP, type 2 diabetes, smoking status and education.
Model 3 = baseline and the 5-year change brain measurement variables (Δ) included in the same model with same adjustments as in model 2.
Baseline measurement.
5-yr follow-up measurement.
5-year change (follow-up – baseline) (Δ).
Table 3.
Association between brain atrophy measures and objective sedentary behavior. Brain volume measurements are presented as a percent of intracranial volume.
| Variables | Sedentary behavior (hours × day−1)
|
p | |||
|---|---|---|---|---|---|
| Std. β | Lower 95% CL | Upper 95% CL | |||
| Model 1# | GMa | −0.011 | −0.075 | 0.054 | 0.74 |
| WMa | −0.061 | −0.12 | 0.00082 | 0.053 | |
| GM-5yrb | −0.023 | −0.091 | 0.044 | 0.49 | |
| WM-5yrb | −0.092 | −0.15 | −0.031 | 0.0032 | |
| Δ-GMc | −0.026 | −0.085 | 0.033 | 0.38 | |
| Δ-WMc | −0.080 | −0.14 | −0.024 | 0.0051 | |
| Model 2## | GMa | −0.0042 | −0.074 | 0.065 | 0.91 |
| WMa | −0.043 | −0.11 | 0.022 | 0.19 | |
| GM-5yrb | −0.011 | −0.083 | 0.061 | 0.76 | |
| WM-5yrb | −0.084 | −0.15 | −0.019 | 0.012 | |
| Δ-GMc | −0.015 | −0.077 | 0.047 | 0.64 | |
| Δ-WMc | −0.10 | −0.17 | −0.042 | 0.0010 | |
| Model 3### | GMa | 0.015 | −0.056 | 0.085 | 0.68 |
| WMa | −0.037 | −0.10 | 0.028 | 0.26 | |
| Δ-GMc | −0.034 | −0.10 | 0.029 | 0.28 | |
| Δ-WMc | −0.11 | −0.17 | −0.047 | 0.0007 | |
GM = gray matter, WM=white matter.
Model 1 = each variable entered separately and adjusted for age and sex, wear time and lifestyle PA.
Model 2 = Model 1 and additional adjustment for brain infarcts, days between baseline and follow-up measurements, education, SPA, BMI, depression, MAP, type 2 diabetes, smoking status and education.
Model 3 = baseline and the 5-year change brain measurement variables (Δ) included in the same model with same adjustments as in model 2.
Baseline measurement.
5-yr follow-up measurement.
5-year change (follow-up-baseline) (Δ).
3. Results
3.1. Descriptive statistics
Descriptive characteristics for women and men are presented in Table 1. Participants were also subdivided into those with high (above median) and low (below median) baseline volumes of both GM and WM. TPA and SB for men and women, for low and high GM, and for low and high WM, are presented in Fig. 1 and Fig. 2, respectively. The mean age of the participants receiving an accelerometer at the AGESII-Reykjavik study was 79.1 (SD 4.4) years. Participants with lower GM averaged 106,000 counts × day−1 in TPA, but those with higher GM averaged 127,000 counts × day−1 in TPA. Participants with lower WM averaged 101,000 counts × day−1 in TPA, but those with higher WM averaged 132,000 in counts × day−1 in TPA. For SB, those with lower GM spent 10:20 h:min × day−1 sedentary, but those with higher GM spent 10:11 h:min × day−1 sedentary. Participants with lower WM spent 10:30 h:min × day−1, but those with higher WM spent 10:01 h:min × day−1 in SB.
Table 1.
Descriptive statistics for participants (n=352) shown separately for women and men, for those above (GM high) and below the median for GM at baseline (GM low), and for those above (WM high) and below (low WM) the median for WM at baseline. Data are presented as mean (±SD).
| Demographics | Men (n= 137) | Women (n = 215) | GM low(n=176)a | GM high(n = 176)a | WM low(n=176)a | WM high (n= 176)a |
|---|---|---|---|---|---|---|
| Agea | 79.2 (4.0) | 79.0 (4.6) | 80.3 (4.6) | 77.9 (3.7) | 80.6 (4.5) | 77.6 (3.7) |
| BMI (kg × m−2)a | 26.9 (3.8) | 26.9 (4.7) | 26.2(4.2) | 27.6 (4.4) | 27.1 (4.4) | 26.8 (4.3) |
| Weight (kg)a | 83.9 (13.6) | 70.9 (13.4) | 75.4(15.1) | 76.5 (14.7) | 76.2 (15.9) | 75.6 (13.9) |
| ICV(cm3)b | 1617 (122) | 1409 (96) | 1544 (145) | 1435 (129) | 1489 (144) | 1490 (151) |
| GM (%)b, c | 45.1 (2.7) | 47.3 (2.7) | 44.3 (1.9) | 48.7 (1.7) | 46.0 (2.8) | 47.0 (2.9) |
| WM (%)b, c | 26.1 (1.6) | 26.2 (1.7) | 26.0(1.7) | 26.4 (1.6) | 24.9 (1.1) | 27.5 (0.89) |
| GM-5yr(%)a, c | 43.9 (2.7) | 46.7 (2.8) | 43.4(2.1) | 47.9 (1.9) | 45.0 (3.0) | 46.2 (3.0) |
| WM-5yr(%)a, c | 24.7 (1.9) | 24.9 (1.9) | 24.5 (1.9) | 25.1 (1.8) | 23.4 (1.4) | 26.2 (1.1) |
| Δ-GM (%)d | −1.2 (1.1) | −0.65 (1.0) | −0.91 (1.2) | −0.85 (0.93) | −0.92(1.2) | −0.83 (1.0) |
| Δ-WM (%)d | −1.3 (0.83) | −1.4 (0.67) | −1.5 (0.82) | −1.2 (0.62) | −1.4 (0.81) | −1.3 (0.65) |
| Wear time (h:min × day−1)a | 13:59 (1:18) | 13:47 (1:13) | 13:45 (1:20) | 13:58 (1:10) | 13:46 (1:20) | 13:57 (1:10) |
| SB (h:min × day−1)a | 10:31 (1:27) | 10:06 (1:23) | 10:20 (1:21) | 10:11 (1:29) | 10:30 (1:27) | 10:01 (1:21) |
| TPA(1000 counts × day−1)a | 120 (68) | 114 (61) | 106 (56) | 127 (69) | 101 (57) | 132 (67) |
| Wear time PA (counts × min−1)a | 143 (81) | 136 (70) | 127 (65) | 151 (81) | 121 (67) | 157 (77) |
BMI = Body max index; ICV = intra-cranial volume; GM = gray matter, WM = white matter, PA = physical activity; SB = sedentary behavior.
Follow-up (5-yr) measurements.
Baseline measurements.
Brain volumes as percent of ICV.
5-year change (follow-up − baseline) (Δ).
Fig. 1.
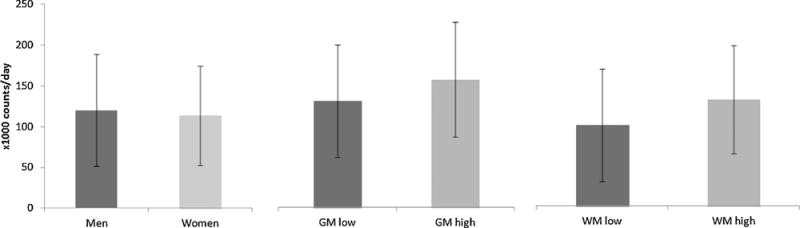
The mean (±SD) amount of total physical activity (TPA) for men and women, those with low gray matter (GM) and high GM, and those with low white matter (WM) and high WM.
Fig. 2.
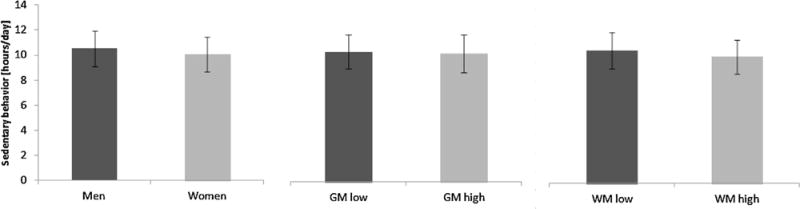
The mean (±SD) amount of sedentary behavior (SB) for men and women, those with low gray matter (GM) and high GM, and those with low white matter (WM) and high WM.
3.1.1. Regression analysis of physical activity and brain volume
Results from linear regression models for TPA are shown in Table 2. With adjustments for age and sex (Models 1), all brain measurement variables were separately and significantly positively associated with TPA (all p < 0.05), except the 5-year change in WM. Adding brain infarcts, days between baseline and follow-up measurements, SPA, BMI, depression, MAP, type 2 diabetes, smoking status and education as covariates (Model 2), did not change the significance or direction of the correlations, with the exception of the 5-year change in WM, which was found to have a significant, positive correlation with TPA (p< 0.05). When both baseline brain volume and the 5-year brain volume change were included in the same model (Model 3), which also adjusted for the above potential confounding variables, all brain volumes were significantly associated with TPA (all p < 0.05). Less brain volume at baseline and more 5-year loss, predict less PA.
3.1.2. Regression analysis of sedentary behavior and brain volume
Results from linear regression models for SB are shown in Table 3. For SB, only WM at follow-up (β= −0.092; p = 0.0032) and the 5-year change in WM (β= −0.080; p = 0.0051) were separately associated, negatively, with SB. Less WM at baseline and more 5-year decrease, predict more SB. When adjusting the models for the above covariates, lifestyle PA and wear time, the same brain parameters were significantly negatively associated with SB (WM at follow-up: β = −0.084; p = 0.012); (5-year change in WM: β = −0.10; p = 0.0010). These associations remained in Model 3.
4. Discussion
The main finding of this study is that more GM and WM at both baseline and follow-up are independently associated with more TPA, even when adjusted for self-reported PA questionnaire (SPA) and several other potential confounding variables. Furthermore, a 5-year change in both GM and WM was associated with less TPA. In addition, less WM at follow-up and the 5-year change in WM was independently associated with more SB, also after adjusting for lifestyle PA, wear time, SPA and other potential confounding variables. The results suggest that maintenance of brain volume is associated with PA in older adults and that WM atrophy is associated with SB, independent of lifestyle PA and SPA. Thus: (a) more GM and WM volumes, and less 5-year atrophy in these volumes, predicts more PA; and (b) more 5-year atrophy in WM, predicts more SB.
Previous longitudinal studies have shown higher PA and structured exercise to be associated with more global or regional brain volumes later in life, both GM and WM [28–30,42]. Most of those studies use self-reported questionnaires which are known to misestimate PA levels and SB in comparison to more objective measurements [43,44]. Interestingly, two longitudinal studies found no association between PA and brain volumes after adjusting for confounding factors [29,30]. However, in both studies the participants were slightly younger than in the present study. In the present study, we adjust for PA measured at baseline with questionnaire (SPA) when examining change in the brain measurements. Therefore, the observed association between brain volumes and brain volume changes on the one hand, and PA and SB at follow-up on the other hand, are independent of the SPA classification at baseline. Our results thus may suggest that the longitudinal relationship between brain volumes and PA could also be the other way around, i.e. brain atrophy associates with subsequent decline in PA and more SB. This bidirectional relationship, i.e. brain atrophy causes less PA and vice versa, forms a pattern that needs intervention. By breaking that bidirectional relationship, better physical- and brain health could be gained.
Only the 5-year decrease in WM independently predicted more SB after adjusting for lifestyle PA, wear time and potential confounding variables. In older people, SB is known to have the highest prevalence of all activity types compared to any other age group [23,45,46]. We have previously shown in this cohort that participants were on average sedentary for 10.1 h × day, or 74.5% of their non-sleeping time [21]. The increase in time spent in SB after the age of 60 may be due to positive factors such as increased leisure time following retirement or to negative factors such as worsening health conditions [23]. SB has been identified as a distinct risk factor for poor health [47] and mortality [48]. With increasing age, nerve fiber activity declines and affects brain function [49,50]. A recent study suggests that among older adults, the structural integrity of WM is not only dependent on levels of PA, but also on the amount of remaining time spent sedentary [51].
Future studies should also investigate whether atrophy of particular regions in the brain are more potent than other regions in terms of diminishing PA and increasing SB. Many studies have demonstrated the effects of both planned exercise and PA in changing the volume of most regions of the brain [26–28,30,35,42,52–55]. Blood flow in the brain has been shown to vary between types of exercise and intensities [56,57]. Furthermore, it has been well documented that increased blood flow in the brain during exercises promotes the development of new neurons [58–60] and thereby delays brain structural and functional decline [61]. Although, PA seems to affect some regions of the brain more than others, it cannot be assumed that the atrophy of the same regions are most potent in affecting the PA and SB.
The present study is based on the well-characterized, population based AGES-Reykjavik cohort of older men and women [31]. This cohort consists of healthy older adults of Caucasian descent. It is thus expected that these findings can be generalized for most western populations in this age range. The main strengths of this study include objectively measured PA at study follow-up. Also, we have a longitudinal design of brain measurements with five years interval. Earlier it has been shown that participants who wore the accelerometers had similar characteristics compared with those participants who did not receive an accelerometer [21]. Nonetheless, we acknowledge several limitations in the present study. PA at baseline was not measured by an objective method, as self-report questionnaires were used. Therefore, we did not have similar measurements of the PA and SB at baseline and at follow-up, and SB was not measured at baseline. It is possible that if objective measurement of PA at baseline would have been available and used to adjust the statistical models, the observed association would have become attenuated. A longitudinal study using objective measurements both at baseline and follow-up would be beneficial to further test our hypothesis. Also, even though objective measurements are considered to be more accurate than subjective measurements, it is known that hip-worn accelerometers fail to detect some movements, like upper body movements during activities such as weight lifting and heavy carrying. They also have limitations on detecting non-ambulatory activities like cycling [62], activity that is not common in this age group in Iceland [63]. However, a quarter of the participants in this cohort of older Icelanders, reported swimming as an exercise [21], which is not included in the presented TPA. Since we only have objective measurements at follow-up, it is unclear if the relationships observed are uni- or bi-directional. Other studies are necessary to identify the direction of these relationships.
5. Conclusions
Our study confirms that there is an association between brain atrophy and PA. We also show an association between brain atrophy and SB, independent of lifestyle PA. These relationships are robust to adjustment by a number of confounders. This study provides additional evidence of the positive association of PA and the brain. PA interventions aimed at alleviating this association could have important public health impact.
HIGHLIGHTS.
Accelerometer was used to measure physical activity and sedentary behavior at follow up.
Gray matter and white matter was measured both at baseline and follow up, with 5-year interval.
An association was found between brain atrophy and physical activity.
There was also an association between brain atrophy and sedentary behavior, independent of lifestyle physical activity.
Acknowledgments
This study has been funded by NIA contract N01-AG-1-2100, the NIA Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament). This work was also supported by the National Science Foundation Graduate Research Fellowship under Grant No. DGE-0940903 and by the National Institutes of Health Intramural Research Program, grant number: Z01 DK071013 and Z01 DK071014 to RJB and KYC. Thor Aspelund is acknowledged for statistical consultation. The researchers are indebted to the participants for their willingness to participate in the study.
Abbreviation
- AGES-Reykjavik study
Age, Gene/Environment Susceptibility Reykjavik Study
- AGESII-Reykjavik study
Age, Gene/Environment Susceptibility Reykjavik Study, second phase
- BMI
body max index
- CSF
cerebral spinal fluid
- DP
diastolic pressure
- GM
gray matter
- ICV
intra-cranial volume
- MAP
mean arterial pressure
- MRI
magnetic resonance imaging
- SB
sedentary behavior
- SP
systolic pressure
- SPA
self-reported PA questionnaire
- PA
physical activity
- WM
white matter
- WMH
white matter hyperintensities
Footnotes
Disclosure statement
The authors have no conflicts to disclose.
References
- 1.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3:343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 2.Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161:1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- 3.Yaffe K, Fiocco AJ, Lindquist K, Vittinghoff E, Simonsick EM, Newman AB, et al. Predictors of maintaining cognitive function in older adults: the Health ABC study. Neurology. 2009;72:2029–2035. doi: 10.1212/WNL.0b013e3181a92c36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78:1323–1329. doi: 10.1212/WNL.0b013e3182535d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- 6.Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, et al. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- 7.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 8.Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, et al. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- 9.Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taki Y, Goto R, Evans A, Zijdenbos A, Neelin P, Lerch J, et al. Voxel-based morphometry of human brain with age and cerebrovascular risk factors. Neurobiol Aging. 2004;25:455–463. doi: 10.1016/j.neurobiolaging.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 11.DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, et al. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Enzinger C, Fazekas F, Matthews PM, Ropele S, Schmidt H, Smith S, et al. Risk factors for progression of brain atrophy in aging: six-year follow-up of normal subjects. Neurology. 2005;64:1704–1711. doi: 10.1212/01.WNL.0000161871.83614.BB. [DOI] [PubMed] [Google Scholar]
- 13.Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology. 2005;64:1032–1039. doi: 10.1212/01.WNL.0000154530.72969.11. [DOI] [PubMed] [Google Scholar]
- 14.Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, et al. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26:1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. discussion 75–8. [DOI] [PubMed] [Google Scholar]
- 15.Ikram MA, Vrooman HA, Vernooij MW, van der Lijn F, Hofman A, van der Lugt A, et al. Brain tissue volumes in the general elderly population. The rotterdam scan study. Neurobiol Aging. 2008;29:882–890. doi: 10.1016/j.neurobiolaging.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Visser PJ, Scheltens P, Verhey FR, Schmand B, Launer LJ, Jolles J, et al. Medial temporal lobe atrophy and memory dysfunction as predictors for dementia in subjects with mild cognitive impairment. J Neurol. 1999;246:477–485. doi: 10.1007/s004150050387. [DOI] [PubMed] [Google Scholar]
- 17.Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- 18.Sigurdsson S, Aspelund T, Forsberg L, Fredriksson J, Kjartansson O, Oskarsdottir B, et al. Brain tissue volumes in the general population of the elderly: the AGES-Reykjavik study. Neuroimage. 2012;59:3862–3870. doi: 10.1016/j.neuroimage.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol Aging. 2005;26:1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. discussion 79–82. [DOI] [PubMed] [Google Scholar]
- 20.Abe O, Yamasue H, Aoki S, Suga M, Yamada H, Kasai K, et al. Aging in the CNS: comparison of gray/white matter volume and diffusion tensor data. Neurobiol Aging. 2008;29:102–116. doi: 10.1016/j.neurobiolaging.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Arnardottir NY, Koster A, Van Domelen DR, Brychta RJ, Caserotti P, Eiriksdottir G, et al. Objective measurements of daily physical activity patterns and sedentary behaviour in older adults: Age, Gene/Environment Susceptibility-Reykjavik Study. Age Ageing. 2012 doi: 10.1093/ageing/afs160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 23.Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, et al. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167:875–881. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon BA, Rykhlevskaia EI, Brumback CR, Lee Y, Elavsky S, Konopack JF, et al. Neuroanatomical correlates of aging, cardiopulmonary fitness level, and education. Psychophysiology. 2008;45:825–838. doi: 10.1111/j.1469-8986.2008.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, et al. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A: Biol Sci Med Sci. 2003;58:176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- 26.Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A: Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 27.Benedict C, Brooks SJ, Kullberg J, Nordenskjold R, Burgos J, Le Greves M, et al. Association between physical activity and brain health in older adults. Neurobiol Aging. 2013;34:83–90. doi: 10.1016/j.neurobiolaging.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 28.Erickson KI, Raji CA, Lopez OL, Becker JT, Rosano C, Newman AB, et al. Physical activity predicts gray matter volume in late adulthood: the cardiovascular health study. Neurology. 2010;75:1415–1422. doi: 10.1212/WNL.0b013e3181f88359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gow AJ, Bastin ME, Munoz M, aniega S, Valdes H, ernandez MC, Morris Z, Murray C, et al. Neuroprotective lifestyles and the aging brain: activity, atrophy, and white matter integrity. Neurology. 2012;79:1802–1808. doi: 10.1212/WNL.0b013e3182703fd2. [DOI] [PubMed] [Google Scholar]
- 30.Rovio S, Spulber G, Nieminen LJ, Niskanen E, Winblad B, Tuomilehto J, et al. The effect of midlife physical activity on structural brain changes in the elderly. Neurobiol Aging. 2010;31:1927–1936. doi: 10.1016/j.neurobiolaging.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–1087. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gennuso KP, Gangnon RE, Matthews CE, Thraen-Borowski KM, Colbert LH. Sedentary behavior, physical activity, and markers of health in older adults. Med Sci Sports Exerc. 2013;45:1493–1500. doi: 10.1249/MSS.0b013e318288a1e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matthews CE. Calibration of accelerometer output for adults. Med Sci Sports Exerc. 2005;37:S512–22. doi: 10.1249/01.mss.0000185659.11982.3d. [DOI] [PubMed] [Google Scholar]
- 34.Foubert-Samier A, Catheline G, Amieva H, Dilharreguy B, Helmer C, Allard M, et al. Education, occupation, leisure activities, and brain reserve: a population-based study. Neurobiol Aging. 2012;33:423 4e15–25. doi: 10.1016/j.neurobiolaging.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 35.Ho AJ, Raji CA, Becker JT, Lopez OL, Kuller LH, Hua X, et al. The effects of physical activity, education, and body mass index on the aging brain. Hum Brain Mapp. 2011;32:1371–1382. doi: 10.1002/hbm.21113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28:1–8. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Bruehl H, Wolf OT, Sweat V, Tirsi A, Richardson S, Convit A. Modifiers of cognitive function and brain structure in middle-aged and elderly individuals with type 2 diabetes mellitus. Brain Res. 2009;1280:186–194. doi: 10.1016/j.brainres.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77:461–468. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muller M, van der Graaf Y, Visseren FL, Vlek AL, Mali WP, Geerlings MI. Blood pressure, cerebral blood flow, and brain volumes. The SMART-MR study. J Hypertens. 2010;28:1498–1505. doi: 10.1097/HJH.0b013e32833951ef. [DOI] [PubMed] [Google Scholar]
- 40.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 4–57. [PubMed] [Google Scholar]
- 41.Valdimarsdottir M, Jonsson JE, Einarsdottir S, Tomasson K. Validation of an Icelandic version of the geriatric depression scale (GDS) Laeknabladid. 2000;86:344–348. [PubMed] [Google Scholar]
- 42.Yuki A, Lee S, Kim H, Kozakai R, Ando F, Shimokata H. Relationship between physical activity and brain atrophy progression. Med Sci Sports Exerc. 2012;44:2362–2368. doi: 10.1249/MSS.0b013e3182667d1d. [DOI] [PubMed] [Google Scholar]
- 43.Chinapaw MJ, Slootmaker SM, Schuit AJ, van Zuidam M, van Mechelen W. Reliability and validity of the activity questionnaire for adults and adolescents (AQuAA) BMC Med Res Methodol. 2009;9:58. doi: 10.1186/1471-2288-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tudor-Locke CE, Myers AM. Challenges and opportunities for measuring physical activity in sedentary adults. Sports Med. 2001;31:91–100. doi: 10.2165/00007256-200131020-00002. [DOI] [PubMed] [Google Scholar]
- 45.Davis MG, Fox KR. Physical activity patterns assessed by accelerometry in older people. Eur J Appl Physiol. 2007;100:581–589. doi: 10.1007/s00421-006-0320-8. [DOI] [PubMed] [Google Scholar]
- 46.Hagstromer M, Troiano RP, Sjostrom M, Berrigan D. Levels and patterns of objectively assessed physical activity - a comparison between Sweden and the United States. Am J Epidemiol. 2010;171:1055–1064. doi: 10.1093/aje/kwq069. [DOI] [PubMed] [Google Scholar]
- 47.Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. 2010;38:105–113. doi: 10.1097/JES.0b013e3181e373a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koster A, Caserotti P, Patel KV, Matthews CE, Berrigan D, Van Domelen DR, et al. Association of sedentary time with mortality independent of moderate to vigorous physical activity. PLoS One. 2012;7:e37696. doi: 10.1371/journal.pone.0037696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen NK, Song AW. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim Biophys Acta. 2012;1822:386–400. doi: 10.1016/j.bbadis.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burzynska AZ, Chaddock-Heyman L, Voss MW, Wong CN, Gothe NP, Olson EA, et al. Physical activity and cardiorespiratory fitness are beneficial for white matter in low-fit older adults. PLoS One. 2014;9:e107413. doi: 10.1371/journal.pone.0107413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boyke J, Driemeyer J, Gaser C, Buchel C, May A. Training-induced brain structure changes in the elderly. J Neurosci. 2008;28:7031–7035. doi: 10.1523/JNEUROSCI.0742-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bugg JM, Head D. Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiol Aging. 2011;32:506–514. doi: 10.1016/j.neurobiolaging.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verstynen TD, Lynch B, Miller DL, Voss MW, Prakash RS, Chaddock L, et al. Caudate nucleus volume mediates the link between cardiorespiratory fitness and cognitive flexibility in older adults. J Aging Res. 2012;2012:939285. doi: 10.1155/2012/939285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nielsen HB, Boushel R, Madsen P, Secher NH. Cerebral desaturation during exercise reversed by O2 supplementation. Am J Physiol. 1999;277:H1045–H1052. doi: 10.1152/ajpheart.1999.277.3.H1045. [DOI] [PubMed] [Google Scholar]
- 57.Ide K, Horn A, Secher NH. Cerebral metabolic response to submaximal exercise. J Appl Physiol (1985) 1999;87:1604–1608. doi: 10.1152/jappl.1999.87.5.1604. [DOI] [PubMed] [Google Scholar]
- 58.Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, et al. Neurobiology of exercise. Obesity (Silver Spring) 2006;14:345–356. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- 60.Draganski B, May A. Training-induced structural changes in the adult human brain. Behav Brain Res. 2008;192:137–142. doi: 10.1016/j.bbr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 61.Rosano C, Venkatraman J, Guralnik VK, Newman AB, Glynn NW, Launer L, et al. Psychomotor speed and functional brain MRI 2 years after completing a physical activity treatment. J Gerontol A: Biol Sci Med Sci. 2010;65:639–647. doi: 10.1093/gerona/glq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen KY, Bassett DR., Jr The technology of accelerometry-based activity monitors: current and future. Med Sci Sports Exerc. 2005;37:S490–S500. doi: 10.1249/01.mss.0000185571.49104.82. [DOI] [PubMed] [Google Scholar]
- 63.Gudlaugsson J, Gudnason V, Aspelund T, Siggeirsdottir K, Olafsdottir AS, Jonsson PV, et al. Effects of a 6-month multimodal training intervention on retention of functional fitness in older adults: a randomized-controlled cross-over design, Int. J Behav Nutr Phys Act. 2012;9:107. doi: 10.1186/1479-5868-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]


