Abstract
To develop objective assessments of work fatigue, we investigated the patterns of changes in salivary cortisol levels in emergency care providers working extended work shifts. Fourteen subjects, comprising seven physicians and seven physician assistants, provided unstimulated saliva samples at regular intervals over the course of a 24-h work shift and over their subsequent free day. There was a significant time effect, with early morning cortisol levels being significantly attenuated following the work shift. Native diurnal variations varied by gender, with the female subjects manifesting greater cortisol levels. Physicians also had higher cortisol profiles even though their wake–rest cycles were similar to those of the physician assistants. Our results suggest that temporal changes, as well as diurnal similarities, in the salivary cortisol patterns can reflect work-related stress and recovery. In particular, early morning cortisol levels may manifest individual reactivity to work stressors as well as sleep deprivation.
Keywords: Cortisol, Saliva, Diurnal variation, Similarity, Occupational stress
Introduction
The rapidly changing landscape of health care continues to exert its greatest influence on healthcare professionals entrusted with the responsibility of providing high-quality patient care with ever diminishing resources. Emergency personnel, in particular, often work long hours and are required to be “on call” (working through the night in the hospital) very frequently. Concerns about the negative effects of these long working hours on patient care and doctors’ well-being have led to regulations that limit working hours for USA-based doctors-in-training to 80 h per week [1]. Nevertheless, individual work shifts can still last up to 30 h, with a 24-h limit on continuous duty time and an additional period up to 6 h for continuity of care activities. The pressures of changing work patterns and lengthy shifts, coupled with the increased acuity of patients and complexity of care, set the stage for emotional, physical, and mental exhaustion in healthcare providers working in acute care settings [2, 3].
A growing body of research shows that work stress and possible burnout are very prevalent issues for emergency care professionals [4–6]. Beyond attrition from the profession, the combined effects of mental and physical exhaustion along with sleep deprivation have been related to higher rates of attentional failures [7, 8] as well as serious medical errors [9]. Furthermore, the emotional exhaustion and depersonalization linked to professional burnout has been linked to decreased empathy [10] and increased sickness absence [11]. Although regulating work hours is an important initial step, early recognition of those healthcare practitioners’ troubled by excessive and prolonged stress provides useful opportunities for early interventions. The commonly used approach of screening for the early behavioral signs and symptoms of burnout is challenging in the fast-paced, acute-care setting and burdened by the primary reliance on subjective self-reports for screening and gauging physical and mental exhaustion.
To address this clinical challenge, our research group has focused on developing technologies that utilize components of the human salivary proteome as non-invasive, qualitative indices of an individual’s response to stressors, either physical or emotional [12, 13]. Of the various putative stress indicators manifesting in saliva, most of the attention has focused on salivary cortisol as an expression of activation of the hypothalamic–pituitary–adrenal (HPA) axis [14–16]. Activation of the HPA axis by a stressor results in the release of neurohormones, such as the corticotropin-releasing hormone (CRH), which then stimulate the adrenal cortex to synthesize cortisol, the main glucocorticoid hormone of the human body. About 5–15% of the secreted cortisol circulates unbound in blood, and it is this “free” cortisol fraction that exerts its biological activity [17]. The biochemical properties of cortisol (low molecular weight, high lipid solubility) allow the unbound steroid to transfer from blood to saliva by passive diffusion [18] and results in a close correspondence between the saliva and the free serum cortisol fraction [19, 20]. As a measure of the bioactive cortisol in the body, salivary cortisol has several compelling attributes as a bioindicator of the individual stress response: (1) it has a simple relationship with CRH, one of the most important stress response mediators [21]; (2) the concentration of salivary cortisol is independent of saliva flow rate, thus rendering it less susceptible to conditions of reduced saliva flow or stimulation of saliva glands; (3) unlike other biofluids such as blood and urine, the collection of saliva is noninvasive and easily accomplished with minimal stress or embarrassment; (4) salivary cortisol is a rather stable molecule, and saliva samples can be kept at room temperature for at least 4 weeks without a significant drop in cortisol levels [19].
The stress-dependent activation of salivary cortisol has led to its common use as an indicator of fatigue and burnout [22, 23]. Several researchers [24–26] have reported low levels of cortisol in disorders, including post-traumatic stress disorder, chronic fatigue syndrome (CFS), and fibromyalgia, which feature fatigue as a symptom. Additionally, Padilha et al. [27] reported that fixed work shifts, such as the day shift, the night shift, and the early morning shift, resulted in markedly differences in the diurnal patterns of cortisol. Thus, salivary cortisol is very attractive as an objective index of physical and mental exhaustion in healthcare personnel working extended work shifts. We hypothesized that the temporal patterns of salivary cortisol secretions through work-related stress and recovery would clarify occupationally induced stress reactivity and fatigue in emergency care providers. The primary purpose of our study was to evaluate whether salivary cortisol secretion profiles reflect physical and emotional exhaustion in healthcare providers. To account for the circadian rhythm inherent to salivary cortisol levels [28], we utilized the same cohort of emergency care providers and conducted repeated sampling through extended work days as well as the subsequent recovery days.
Methods
Subjects
A cohort of 14 subjects was recruited from the medical staff [occupational breakdown: 7 physicians (P) and 7 physician assistants (PA)] of the emergency department of a large, metropolitan level 1 trauma center. Consistent with work conditions typical for the emergency care team, all study participants work regularly in extended 24-h shifts, providing medical care in a high-pressure, dynamic environment. As summarized in Table 1, the subjects included nine male and five female adults (age range 31–54 years, career duration range 6–33 years). All participants were healthy, non-obese, and non-smokers. Body weight, height, and body mass index of all subjects was 63.0 ± 12.0 kg, 169.1 ± 10.0 cm, and 21.9 ± 2.5, respectively. The recruitment and study protocol was approved by the Ethical Committee of Iwate University and the Institutional Review Board of Tokyo Metropolitan Hiroo Hospital.
Table 1.
Demographics of study participants
| Condition | All (n = 14) | Physician (n = 7) | Physician assistant (n = 7) | p valuea |
|---|---|---|---|---|
| Male:female | 9:5 | 5:2 | 4:3 | |
| Age (years) | 39.4 ± 5.9 | 37.6 ± 4.5 | 41.3 ± 6.8 | NS |
| Career duration (years) | 16.1 ± 7.0 | 12.6 ± 8.0 | 19.7 ± 7.2 | NS |
NS Not significant
Data are presented as the mean ± standard deviation (SD)
aWelch’s t test
Saliva collection
All participants were well rested and had not taken any medications prior to each test cycle. Each participants provided seven saliva samples over the course of their extended 24-h shift and over their subsequent day off. Saliva samples were collected at predetermined time points: A1, morning of work day (W); A2, before evening meal of work day (W); A3, around midnight of work day (W); A4, morning following work day (W), which was the morning of the day off (O); A5, before evening meal of day off (O); A6, bedtime of day off (O); A7, morning after the day off (O) (Fig. 1). Three series of saliva samples were collected (total = 3 cycles) over the course of 1 month. In the case of the female subjects, the saliva sampling occurred in the luteal phase of their menstrual cycles. Participants were instructed to record the time of sample collection in a logbook and to collect the saliva sample within ±60 min of the scheduled sampling time. Participants were instructed to sit still and upright for several minutes prior to saliva collection at the A2, A3, A4, A5, and time A6 points and to collect samples immediately after awakening at the A1 and A7 time points. The collecting procedure comprised collecting saliva pooled on the floor of the mouth for 3 min without any stimulation by passive drool in polypropylene tubes.
Fig. 1.
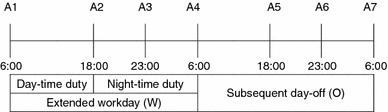
Summary of repeated saliva sampling time points. A1 Morning of work day, A2 before evening meal of work day, A3 around midnight of work day, A4 morning following work day (morning of free day), A5 before evening meal of day off, A6 bedtime of day off, A7 morning following day off
Salivary cortisol analysis
The saliva samples were centrifuged at 1,200 rpm for 30 min, and the resulting supernatant was stored at −20°C for 1 month for subsequent batch analysis. Salivary cortisol levels were measured using commercial-linked immunosorbent assay kits (1-3002; Salimetrics LLC, State College, PA) and a plate reader (450 nm measurement wavelength; ARVO MX; Perkin Elmer Life Science, Boston, MA). The intra- and inter-assay variations were 3.35–3.36 and 3.75–6.41%, respectively. All samples were assayed both singly and pooled. Cortisol concentration was expressed as nanomoles per liter.
Activity recording
A portable metabolic rate monitor ( 40 g, dimensions W 62.5 × L 46.5 × H = 26 mm3; Suzuken Co, Nagoya, Japan) was attached to the lumbar region of the subjects to enable measurement of their metabolic rate (calorie, cal) and activities every 2 min over the 48-h period of observation. The subjects also kept activity records containing information on the timing of their meals, rests, and sleep patterns, including ambient light conditions. The number of resting hours on the work day and sleeping hours on the day off were corroborated using this monitor and the activity records.
Dynamic programming method
Because the stress response varies by personality and individual differences, an objective parameter was needed to evaluate the diurnal variation of salivary cortisol. We used similarity as the parameter for comparing the phase and shape between two diurnal curves and calculated this parameter using the dynamic programming method [29], which compares the phase and shape characteristics of two curves. This method can compare the profile itself between two standardized curves without considering the range of salivary cortisol levels. Each curve was defined by four points, on work day as A1–A4 and on the day off as A4–A7. When two curves were asymmetrical, the similarity was −1; when they overlapped, the similarity was 1.
Statistical analysis
Data analysis was performed with proprietary statistical software [JMP ®) 8.0.2; SAS Institute, Cary, NC), and data were presented as mean values and standard deviation (SD). Welch’s t test was used for comparisons between physicians and physician assistants. Cortisol data were analyzed using two-way analysis of variance (ANOVA) with repeated measurement [time (repeated factor 7) by gender or occupation (two groups)]. We verified repeated-measures results using Greenhouse–Geisser correlations where appropriate (heterogeneity of error covariances in the Mauchly test of sphericity). Significant differences of post hoc comparisons among groups were calculated with the Bonferroni test (m = 9). All analyses were two-tailed, with the level of significance set at p < 0.05.
Results
Cortisol data were discarded from five collections where the metabolic rate monitor indicated unusual movement or activity immediately prior to the sample collection. In all, cortisol data from 289 samples were available for analysis. Average recording time for each saliva sampling time point was: A1, 06:34 ± 41 min; A2, 17:39 ± 45 min; A3, 01:22 ± 108 min; A4, 06:35 ± 84 min; A5, 18:23 ± 52 min; A6, 23:03 ± 81 min; A7, 06:54 ± 59 min. There were no significant differences among three sampling time points A1, A4, and A7. The duration of rest times was 192 ± 77 min on the work day, and duration of sleeping time was 433 ± 73 min on the day off. There were significant differences in the rest times between the work day and the day off (p < 0.01). In the subgroup analysis, average rest times for the physicians and physician assistants on work days was 174 ± 170 and 200 ± 54 min, respectively. The average sleeping time on the day off for the physicians and physician assistants was 489 ± 50 and 435 ± 69 min, respectively. There were no significant differences between the two groups in terms of sleeping time on the day off.
None of the salivary cortisol levels at each data point exhibited normal distributions (Fig. 2). Furthermore, no significant correlation coefficient was observed among the data points, with no correlation between them (−0.181 to 0.510).
Fig. 2.
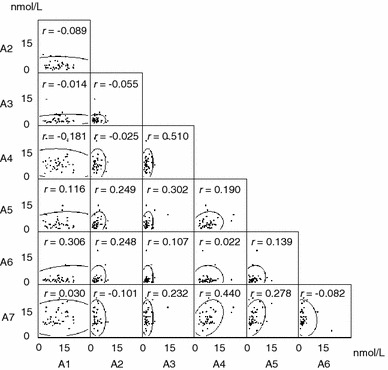
Scatter plot matrix from 289 samples with 95% equal probability ellipse and correlation coefficient between the variables. A1–A7 Sampling time points (see Fig. 1) over the 48-h study cycle, r correlation coefficient
The results obtained by the two-way ANOVA with repeated measures revealed significant gender differences in salivary cortisol levels [F(1, 34) = 17.3, p = 0.0002], with female participants manifesting higher cortisol levels. In contrast, there was no significant gender by time interaction effect [Wilks’ lambda = 0.63; F(2.33, 79.3) = 2.25, p = 0.10].
There was a significant main effect of time on salivary cortisol levels using two-way ANOVA with repeated measures [F(2.33, 79.3) = 40.1, p < 0.0001]. During the extended work days, salivary cortisol levels were lowest (2.5 ± 2.3 nmol/L) around midnight of the work day (A3) and highest (11.9 ± 8.1 nmol/L) in the morning (A1). Salivary cortisol levels on the subsequent day off were lowest (2.2 ± 3.0 nmol/L) just before sleeping (A6) and highest (10.2 ± 4.3 nmol/L) in the morning (A7) (Fig. 3). Post hoc analyses (Bonferroni test) revealed significant differences, as summarized by Fig. 3. Cortisol levels on the morning of work days (A1) were higher than those measured before the evening meal (A2: 3.2 ± 2.0 nmol/L) and before sleeping (A3) on work days and on the next morning of the extended work day (A4: 7.4 ± 4.1 nmol/L, p < 0.01). Salivary cortisol levels on the morning following the day off (A7) were higher than those measured before the evening meal (A5: 3.6 ± 2.7 nmol/L) or at bedtime (A6) on the day off as well as on the morning following the 24-h work shift (A4, p < 0.01).
Fig. 3.
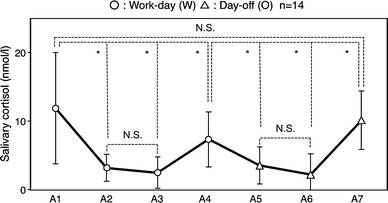
Mean diurnal variations in salivary cortisol on extended work days and day offs. *p < 0.01. NS Not significant
Results obtained by using two-way ANOVA with repeated measures indicated a significant main effect of occupation [F(1, 34) = 11.6, p = 0.0018], while there was no significant occupation by time interaction effect [Wilks’ lambda = 0.63, F(2.33, 79.3) = 2.48, p = 0.08]. In the analysis of effect of two different conditions between the work day and day off, there was a significant difference in the salivary cortisol levels between occupational groups on the work day (A1–A4) [F(1, 36) = 11.8, p = 0.0015], with cortisol levels being higher in physicians than in physician assistants (Fig. 4), whereas this difference was not manifest between these occupational groups on their day off (A4–A7) [F(1, 39) = 3.47, p = 0.071] (Fig. 4).
Fig. 4.
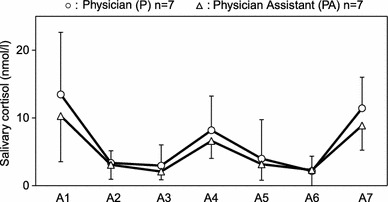
Mean diurnal variations of salivary cortisol on work days (A1–A4) and on days off (A4–A7) in physicians (P; n = 7) and physician assistants (PA; n = 7). *p < 0.01. NS not significant
In order to evaluate differences among the cortisol profile of each individual, we calculated the similarity between the mean curve of all subjects and an individual’s curve. Because of the significant gender differences in cortisol levels, the mean curve was used for each gender was used for the calculation. The results revealed a high similarity between two conditions with similarities during a work day (0.80 ± 0.29) comparable to those on the day off (0.83 ± 0.30 (Fig. 5). Five curves from a work day (12.8%) and two curves from a day off (5.1%) exceeded 3 SD. No significant differences were observed between the two conditions.
Fig. 5.
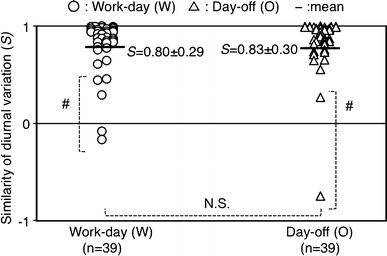
Similarities in diurnal variations between mean diurnal curve and individual diurnal curves on the work day (n = 39) versus day off (n = 39). Horizontal black lines Mean value in each group, S similarity of diurnal variation, W work day, O day off, NS not significant. #Difference exceeded 3 standard deviations
Discussion
The results of our naturalistic study of emergency care providers indicate that temporal changes in salivary cortisol levels, even after accounting for native diurnal variation and gender differences, can indicate work-related stress and recovery. Overall, our female subjects had higher salivary cortisol levels that their male colleagues, reinforcing the recommendation that gender should be considered in any comparison of salivary cortisol levels [30]. Cortisol levels in our subjects were highest in the morning and lowest at midnight. This diurnal pattern substantiates the findings of other researchers, including Vining et al. [18]. We found that the cortisol levels at each collection point exhibited a non-normal distribution, with a large excess and small skewness, and that this distributional tendency was most pronounced in the salivary samples collected at midnight. The large variation in cortisol levels measured in the morning of both the work day and the day off suggests that morning cortisol levels are highly dependent on the individual. Hennig et al. [31] reported this same high variability in early morning cortisol values in their research on implications for tolerance to shiftwork by nurses.
Morning cortisol levels were significantly attenuated following the work shift (A4) but rose again following a recovery day (day off). The lack of significant differences between salivary cortisol levels measured before evening meals (A2, A5) and before sleeping (A3, A6) on both the work day and the day off suggest that cortisol values cannot be estimated from point measurements. Several researchers [31–34] have suggested that these attenuated morning cortisol levels are influenced by night shifts. Simply put, the levels of salivary cortisol vary during the day, and the temporal changes from individual baseline levels may well be more useful in gauging stress reactivity.
The residual difference in cortisol patterns between the 2 days may be explained by the fatigue and sleep deprivation caused by the significantly shorter rest periods during the extended 24-h work shift in comparison to the day off. Because the recorded sampling times for the three morning collections (A1, A4, and A7) were comparable, it is unlikely that any of the lower levels of morning salivary cortisol recorded could be attributed to a shift in the cortisol awakening response. It is likely that the continual work stressors and lack of sleep possibly altered the production of cortisol, resulting in lower levels on the morning following the end of the extended work shift. A report by Leese et al. [34] about disrupted HPA axis responses to CRH after night shifts tends to support our hypothesis. As Chida and Steptoe [35] summarize in a recent systematic review, the magnitude of the cortisol-awakening response is influenced by mediators such as fatigue as well as sleep deprivation and quality. By the end of the recovery period, when the subjects were in a more fully rested state, the salivary cortisol rebounded to baseline morning levels. Additionally, the finding of higher cortisol levels on work days in physicians than physician assistants suggests a differential experience of work-related stress. Because the rest times between the physicians and physician assistants did not differ significantly, it is likely that the physicians, who have the primary responsibility for patient care, experience a higher level of work stress, resulting in a stronger stimulation of the HPA axis.
The observed similarities in the individual and mean diurnal variations on extended work days and on the day off and the lack of a significant difference between the two conditions suggest that nighttime duty did not affect the robustness of the natural diurnal variation of salivary cortisol. However, several of the subjects exhibited non-typical curves for work days and days off. In earlier studies, Smyth et al. [36] and Ice et al. [37] reported that about one-half of salivary cortisol diurnal cycles show a typical bath-tub curve. Furthermore, Stone et al. [38] reported that at least 10% of individuals may exhibit a flat diurnal profile. Our study results indicate that a dissimilarity with normal mean curves of diurnal variation could be used to study abnormalities in personal diurnal cortisol secretions and used as a basis for assessments and early interventions to prevent stress-related disorders.
Conclusion
Temporal patterns of salivary cortisol secretions reflect work-related stress and recovery and can be used to clarify occupationally induced stress reactivity and fatigue in emergency care providers. In particular, early morning salivary cortisol levels following an extended work shift may reflect HPA reactivity to work stressors as well as sleep deprivation. Beyond gender differences, the individual’s level of responsibility for critical clinical decisions also affects the variation in salivary cortisol profiles during the work day. Comparison of salivary cortisol secretion patterns and profiles could provide insights into how individuals react differentially to stressors and serve as the basis for assessing and preemptively tackling occupationally induced stress diseases in emergency care providers.
Acknowledgments
This research was supported by Grants No. 22500400 from the Japan Society for the Promotion of Science (P.I.-M. Yamaguchi), Japan, and 1U01DA023815 from the NIH/National Institute on Drug Abuse (P.I.-V. Shetty), USA.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Biller CK, Antonacci AC, Pelletier S, Homel P, Spann C, Cunningham MJ, Eavey RD. The 80-hour work guidelines and resident survey perceptions of quality. J Surg Res. 2006;135:275–281. doi: 10.1016/j.jss.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Kishi Y, Muraoka M, Kurosawa H, Koido Y, Yamamoto Y, Yazaki S. Burnout, depression and quality of life among Japanese emergency physicians. Jpn J Gen Hosp Psychiatry. 2000;12:135–143. [Google Scholar]
- 3.Okamoto H, Otaki N, Terasawa H, I S, Yamaguchi Y, Shimazaki S. A study of overwork among emergency physicians from 10 tertiary critical care emergency centers in Japan. Nihon Kyukyu Igakukai Zasshi (Japanese Association for Acute Medicine) 2009;20:191–200. doi: 10.3893/jjaam.20.191. [DOI] [Google Scholar]
- 4.DiGiacomo M, Adamson B. Coping with stress in the workplace: implications for new health professionals. J Allied Health. 2001;30:106–111. [PubMed] [Google Scholar]
- 5.Embriaco N, Papazian L, Kentish-Barnes N, Pochard F, Azoulay E. Burnout syndrome among critical care healthcare workers. Curr Opin Crit Care. 2007;13:482–488. doi: 10.1097/MCC.0b013e3282efd28a. [DOI] [PubMed] [Google Scholar]
- 6.Tokuda Y, Hayano K, Ozaki M, Bito S, Yanai H, Koizumi S. The interrelationships between working conditions, job satisfaction, burnout and mental health among hospital physicians in Japan: a path analysis. Ind Health. 2009;47:166–172. doi: 10.2486/indhealth.47.166. [DOI] [PubMed] [Google Scholar]
- 7.Taoda K, Nakamura K, Kitahara T, Nishiyama K. Sleeping and working hours of residents at a national university hospital in Japan. Ind Health. 2008;46:594–600. doi: 10.2486/indhealth.46.594. [DOI] [PubMed] [Google Scholar]
- 8.Lockley SW, Cronin JW, Evans EE, Cade BE, Lee CJ, Landrigan CP, Rothschild JM, Katz JT, Lilly CM, Stone PH, Aeschbach D, Czeisler CA. Effect of reducing interns’ weekly work hours on sleep and attentional failures. N Engl J Med. 2004;351:1829–1837. doi: 10.1056/NEJMoa041404. [DOI] [PubMed] [Google Scholar]
- 9.Landrigan CP, Rothschild JM, Cronin JW, Kaushal R, Burdick E, Katz JT, Lilly CM, Stone PH, Lockley SW, Bates DW, Czeisler CA. Effect of reducing interns’ work hours on serious medical errors in intensive care units. N Engl J Med. 2004;351:1838–1848. doi: 10.1056/NEJMoa041406. [DOI] [PubMed] [Google Scholar]
- 10.Shanafelt TD, Balch CM, Bechamps GJ, Russell T, Dyrbye L, Satele D, Collicott P, Novotny PJ, Sloan J, Freischlag JA. Burnout and career satisfaction among American surgeons. Ann Surg. 2009;250:463–471. doi: 10.1097/SLA.0b013e3181ac4dfd. [DOI] [PubMed] [Google Scholar]
- 11.Pai CW, Mullin J, Payne GM, Love J, O’Connell G, Edington DW. Factors associated with incidental sickness absence among employees in one health care system. Am J Health Promot. 2009;24:37–48. doi: 10.4278/ajhp.081117-QUAN-286. [DOI] [PubMed] [Google Scholar]
- 12.Yamaguchi M, Kanemori T, Kanemaru M, Takai N, Mizuno Y, Yoshida H. Performance evaluation of salivary amylase activity monitor. Biosens Bioelectron. 2004;20:491–497. doi: 10.1016/j.bios.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi M, Deguchi M, Wakasugi J, Takai N, Higashi T, Mizuno Y. Hand-held monitor of sympathetic nervous system using salivary amylase activity and driver fatigue assessment. Biosens Bioelectron. 2006;21:1007–1014. doi: 10.1016/j.bios.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Yehuda R. Neuroendocrine aspects of PTSD. Handb Exp Pharmacol. 2005;169:371–403. doi: 10.1007/3-540-28082-0_13. [DOI] [PubMed] [Google Scholar]
- 15.Yehuda R. Advances in understanding neuroendocrine alterations in PTSD and their therapeutic implications. Ann NY Acad Sci. 2006;1071:137–166. doi: 10.1196/annals.1364.012. [DOI] [PubMed] [Google Scholar]
- 16.Breslau N. Neurobiological research on sleep and stress hormones in epidemiological samples. Ann NY Acad Sci. 2006;1071:221–230. doi: 10.1196/annals.1364.017. [DOI] [PubMed] [Google Scholar]
- 17.Ekins R. Measurement of free hormones in blood. Endocr Rev. 1990;11:5–46. doi: 10.1210/edrv-11-1-5. [DOI] [PubMed] [Google Scholar]
- 18.Vining RF, McGinley RA, Maksvytis JJ, Ho KY. Salivary cortisol: a better measure of adrenal cortical function than serum cortisol. Ann Clin Biochem. 1983;20:329–335. doi: 10.1177/000456328302000601. [DOI] [PubMed] [Google Scholar]
- 19.Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: an overview. Neuropsychobiology. 1989;22:150–169. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- 20.Obminski Z, Stupnicki R. Comparison of the testosterone-to-cortisol ratio values obtained from hormonal assays in saliva and serum. J Sports Med Phys Fitness. 1997;37:50–55. [PubMed] [Google Scholar]
- 21.Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- 22.Pruessner JC, Hellhammer DH, Kirschbaum C. Burnout, perceived stress, and cortisol responses to awakening. Psychosom Med. 1999;61:197–204. doi: 10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Kumari M, Badrick E, Chandola T, Adam EK, Stafford M, Marmot MG, Kirschbaum C, Kivimaki M. Cortisol secretion and fatigue: associations in a community based cohort. Psychoneuroendocrinology. 2009;34:1476–1485. doi: 10.1016/j.psyneuen.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Nater UM, Maloney E, Boneva RS, Gurbaxani BM, Lin JM, Jones JF, Reeves WC, Heim C. Attenuated morning salivary cortisol concentrations in a population-based study of persons with chronic fatigue syndrome and well controls. J Clin Endocrinol Metab. 2008;93:703–709. doi: 10.1210/jc.2007-1747. [DOI] [PubMed] [Google Scholar]
- 25.Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Van Den Eede F, Moorkens G, Van Houdenhove B, Cosyns P, Claes SJ. Hypothalamic–pituitary–adrenal axis function in chronic fatigue syndrome. Neuropsychobiology. 2007;55:112–120. doi: 10.1159/000104468. [DOI] [PubMed] [Google Scholar]
- 27.Padilha HG, Crispim CA, Zimberg IZ, Folkard S, Tufik S, de Mello MT. Metabolic responses on the early shift. Chronobiol Int. 2010;27:1080–1092. doi: 10.3109/07420528.2010.489883. [DOI] [PubMed] [Google Scholar]
- 28.Hellhammer DH, Wüst S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34:163–171. doi: 10.1016/j.psyneuen.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 29.Bellman R. On the theory of dynamic programming. Proc Natl Acad Sci USA. 1952;38:716–719. doi: 10.1073/pnas.38.8.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsson CA, Gullberg B, Rastam L, Lindbald U. Salivary cortisol differs with age and sex and shows inverse associations with WHR in Swedish women: a cross-sectional study. BMC Endocr Disord. 2009;9:16. doi: 10.1186/1472-6823-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hennig J, Kieferdorf P, Moritz C, Huwe S, Netter P. Changes in cortisol secretion during shiftwork: implications for tolerance to shiftwork? Ergonomics. 1998;41:610–621. doi: 10.1080/001401398186784. [DOI] [PubMed] [Google Scholar]
- 32.Costa G, Ghirlanda G, Tarondi G, Minors D, Waterhouse J. Evaluation of a rapidly rotating shift system for tolerance of nurses to nightwork. Int Arch Occup Environ Health. 1994;65:305–311. doi: 10.1007/BF00405694. [DOI] [PubMed] [Google Scholar]
- 33.Munakata M, Ichii S, Nunokawa T, Saito Y, Ito N, Fukudo S, Yoshinaga K. Influence of night shift work on psychologic state and cardiovascular and neuroendocrine responses in healthy nurses. Hypertens Res. 2001;24:25–31. doi: 10.1291/hypres.24.25. [DOI] [PubMed] [Google Scholar]
- 34.Leese G, Chattington P, Fraser W, Vora J, Edwards R, Williams G. Short-term night-shift working mimics the pituitary–adrenocortical dysfunction in chronic fatigue syndrome. J Clin Endocrinol Metab. 1996;81:1867–1870. doi: 10.1210/jc.81.5.1867. [DOI] [PubMed] [Google Scholar]
- 35.Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol Psychol. 2009;80:265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Smyth JM, Ockenfels MC, Gorin AA, Catley D, Porter LS, Kirschbaum C, Hellhammer DH, Stone AA. Individual differences in the diurnal cycle of cortisol. Psychoneuroendocrinology. 1997;22:89–105. doi: 10.1016/S0306-4530(96)00039-X. [DOI] [PubMed] [Google Scholar]
- 37.Ice GH, Katz-Stein A, Himes J, Kane RL. Diurnal cycles of salivary cortisol in older adults. Psychoneuroendocrinology. 2004;29:355–370. doi: 10.1016/S0306-4530(03)00034-9. [DOI] [PubMed] [Google Scholar]
- 38.Stone AA, Schwartz JE, Smyth J, Kirschbaum C, Cohen S, Hellhammer D, Grossman S. Individual differences in the diurnal cycle of salivary free cortisol: a replication of flattened cycles for some individuals. Psychoneuroendocrinology. 2001;26:295–306. doi: 10.1016/S0306-4530(00)00057-3. [DOI] [PubMed] [Google Scholar]


