Abstract
In a cross-sectional study we investigated the relationship between muscle and bone parameters in mid-thigh in older people using data from a single axial computed tomography (CT) section through the mid-thigh. Additionally we studied the association of these variables with incident low trauma lower limb fractures. A total of 3762 older individuals (1838 men and 1924 women) age 66-96 years, participants in the AGES-Reykjavík Study, were studied. The total cross-sectional muscular area and knee extensor strength declined with age similarly in both sexes. Muscle parameters correlated most strongly with cortical area and total shaft area (adjusted for age, height and weight) but explained less than 10% of variability in those bone parameters. The increment in medullary area and buckling ratio with age was almost fourfold greater in women than men. The association between medullary area and muscle parameters was non-significant. One hundred-thirteen women and 66 men sustained incident lower limb fractures during median follow-up of 5.3 years. Small muscular area, low knee extensor strength, large medullary area, low cortical thickness and high buckling ratio were significantly associated with fractures in both sexes. Our results show that bone and muscle loss proceeds at different rates and with different gender patterns.
Keywords: mid-thigh, muscle-bone relationship, aging, fracture risk, CT
Introduction
Harold Frost's mechanostat hypothesis [1] suggests that physical activity and muscle dominate bone remodelling and that an age related reduction in physical activity and muscle mass alone may be enough to cause age-related bone loss and lead to osteoporosis [1, 2]. Animal studies of mechanical loading support this theory [3, 4]. Muscle strength and bone strength are in close relationship to each other across the paediatric age range and children's bones become adapted to the increasing muscle forces as they grow [5]. As recently reviewed by Bonnet and Ferrari [6], studies have indicated that physical activity with sufficient loading intensity and frequency can effectively improve bone mass, structure and strength during adolescence, especially during the pre-pubertal years [7-9]. Whether this close relationship exists between bone and muscle in adults and older persons is controversial. Experimental data show conflicting results, but this may be due to difference in the age groups included in the studies as well as by differences in adjustment for body size.
Studies using dual energy X-ray absorptiometry (DXA) have found a correlation between bone parameters and muscle mass supporting the mechanostat theory [10-13]. Those studies were, however, based on 2D measurements with DXA which does not allow a direct estimate of bone size. In a population-based study of women and men (age, 21-97 years) using central and peripheral QCT, Melton III et al [14] did not find a close relationship between change in habitual load and change in bone strength or any consistent pattern. In older populations there is a limited evidence supporting that physical activity has postitive effects on bone [6]. However, a recent pQCT study in men by Cousins et al [15] found an association between bone strength and physical activity as well as bone strength and muscle power indices that were primarily related to greater total bone area but not bone volumetric density. Several studies have shown reduction of fracture rate in older people who exercise [16]. Lanyon and Skerry [17] have proposed that osteoporosis is the result of a maladaptation to mechanical loading in which changes of the hormonal milieu, such as estrogen deficiency, alter the setpoint of the mechanostat, impairing the response of bone remodelling to mechanical strain.
Ferretti et al [2] and Schoenau et al [5] introduced an extension of the mechanostat theory. They recommended that the diagnostic evaluation of skeletal disease should include an assessment of the musculature. They introduced an approach to measure the ratio of bone mass/strength to muscle mass/strength to distinguish between disuse in muscle and bone and primary or secondary osteoporosis.
The question whether decline in muscle mass with age may explain age-related bone loss and osteoporotic fractures remains a crucial one. In order to better understand possible interaction between those parameters as predictors of future fractures in old age we have used data from a CT section through mid-thigh to investigate the relationship between muscle and bone parameters in older people and how these parameters are associated with incident low trauma lower limb fractures during median 5.3 years of follow up. All forces on femur exerted by gravity and muscle action, may it be proximal or distal, must be projected through the mid thigh area. Our work is to fill in the mid femoral knowledge gap. Although mid-femur is not a usual osteoporotic fracture site, this site provides an opportunity to evaluate bone parameters such as cortical thickness which is difficult at other sites.
Methods
Study participants
The study cohort consisted of Icelandic men and women, aged 66-96 years, who participated in the Age Gene/Environment Susceptibility-Reykjavik Study (AGES-REYKJAVIK), a single-center prospective ongoing population study. Design and recruitment have been described in detail [18]. All participants provided written informed consent, and the study was approved (VSN 00-063) by the National Bioethics Committee in Iceland as well as the Institutional Review Board of the Intramural Research Program of the National Institute of Aging.
At the baseline visit, height and weight were measured and participants were asked to bring all medications to the clinic that they had used in the previous 2 weeks. Height and weight were measured using a Seca stadiometer and a digital scale (Marel, Iceland) and body mass index (BMI) calculated as kg/m2. Medical records were checked biannually from all hospitals receiving fractures in Iceland. From these records, we identified all incident low trauma lower limb fractures, defined as a fracture resulting from a fall from a standing position or lower. The fractures were categorized as a fracture at hip (S72.0-S72.2), at femoral shaft (S72.3), at lower leg (S82.1-S82.4) and at ankle (S82.5-S82.9).
Of the 5248 participants who completed the thigh CT-scan, we excluded those who were on medications known to affect bone density, including estrogen replacement therapy, tibolone, antiepileptics, systemic glucocorticosteroids and agents for the treatment of osteoporosis (raloxifene, calcitonin or bisphosphonates) (n=754), those who were physically unable to complete the knee extensor test (n=392) and those who had a history of lower limb fracture (n=440). A total of 3762 participants (72% of the original cohort, 1838 men and 1924 women) were available for analysis. Each participant was followed for up to 6 years until fracture or death or was censored at the end of follow-up. The median follow-up time was 5.3 years. Of those who were available for analysis, 113 women and 66 men sustained incident low trauma lower limb fracture during follow-up.
Computed tomography (CT) scanning
Computed tomography (CT) measurements were performed in the mid-thigh using a 4-row detector CT system (Sensation, Siemens Medical Systems, Erlangen, Germany) as previously described [18]. A single axial section through the mid-thigh (120kVp, 10 mm slice thickness) was used to quantify the geometry of mid-thigh. The axial image of the thigh at mid-femur was obtained after measuring the maximum length of femur to find the center of the long axis of the femur. We reported values from the right thigh.
CT-derived measures
Images were processed to extract measures of bone and muscle variables in the mid-thigh. We estimated total shaft area (cm2), medullary area (cm2), cortical area (cm2), cortical thickness (cm), buckling ratio and muscular area (cm2). A computer program was used to track the periosteal and medullary boundaries of the femoral shaft using a region-growing algorithm operated at a threshold of 176 Hounsfield Units (HU), a value which was found to provide accurate periosteal and endosteal cortical contours. From the mid-shaft scan we computed the total cross-sectional periosteal area (shaft CSA) and the medullary area (MA). The cortical area was defined by subtracting MA from shaft CSA. From the total shaft and medullary areas, a cortical thickness index (iCThi) was calculated as:
The buckling ratio (BR) was computed as the ratio of bone radius to cortical thickness:
The BR is an index of cortical instability based on the principle that thin-walled tubes become locally unstable in bending when the ratio of the outer diameter to wall thickness exceeds some maximum value [19]. It should be noted that these calculations approximate that the inner and outer cortical boundaries as two concentric circles, that is physically reasonable but which is an assumption nonetheless.
An operator used a manual contouring program to draw the contours of the hamstring, sartorius and quadriceps muscles of the thigh and total muscular cross-sectional area was calculated.
Reproducibility of the CT-measurements; twenty-six randomly selected participants of the study underwent a repeated CT scan after repositioning. Calculated coefficient of variation (CV %) was 1.1% for total shaft bone area, 2.4% for cortical area, 3.5% for medullary area, 2.6% for cortical thickness, 2.3% for buckling ratio, and 3.5% for muscular area. There was no significant difference between the repeated mesurements.
Maximal isometric knee extension strength
Muscle strength was assessed as a maximal isometric strength of the right leg while the individual was sitting in an adjustable dynamometer chair (Good Strength, Metitur Ltd., Palokka, Finaland). The seat belt was fastened in the pelvis area to prevent movement of the body during the test and the ankle fastened by a belt to a strain-gauge transducer. The knee extension strength was measured at a knee angle of 60 degrees from full extension toward flexion. The examiner ensured that the individuals understood the instruction by a trial check before the measurement performed. Three maximal efforts, separated by 30-seconds rest, were conducted. During the measurements, the individuals were encouraged verbally to produce at their maximum capability and the highest value was used.
Statistical analysis
General linear models were used to estimate the association with age and mean values of variables adjusted to age 75 years. Current height and weight was used to correct for body size. The correlation between height and weight was r=0.31 and r=0.48 in women and men respectively; both p<0.001. The estimates were obtained by using linear combinations of regression parameters with an intercept, age set at 75 years, height at 175 cm for men and 161 cm for women, weight at 83 kg for men and 71 kg for women. To estimate percent variances per 10-years in age interval the outcomes were analyzed on a natural log scale. Then the regression parameter (slope) for age has the interpretation of percent change for 1-year difference. This effect was then scaled to represent effect of 10 years. One-way analysis of variance (ANOVA) was used to compare men and women. Associations between variables were estimated by Pearson's correlations and partial correlations were estimated from linear regression. In Fig. 2 the adjusted values for cortical area and muscular area were estimated from a regressison model. In a regression model the cortical area and muscular area were the outcomes and age, height and weight were the parameters (subtracted from the mean in each parameter). The adjusted values were estimated as the residuals from the regression model and scaled with the intercept. ANOVA was used to compare fracture cases and controls. The Cox proportional hazards regression model was used to estimate risk of fracture, using time from visit to the AGES-Study as the time scale with adjustment for age, height and weight at entry. The analyses were performed for men and women separately.
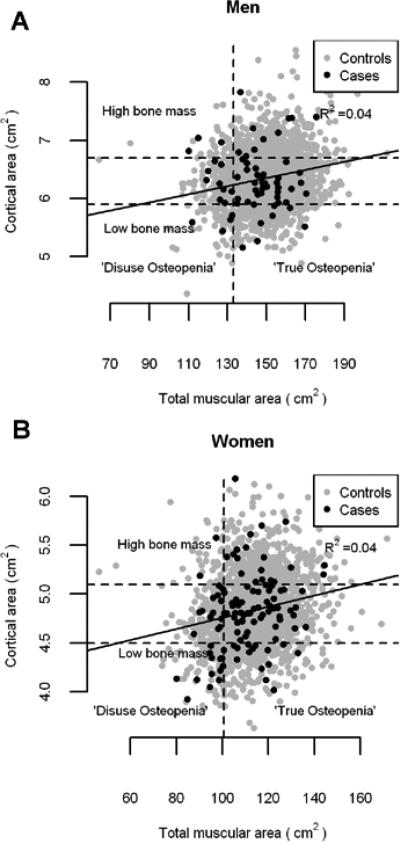
Figure 2.
Anthropometric relationship between cortical area and muscular area at mid-thigh in men (a) and women (b) fracture cases and controls. Both variables adjusted for age, height and weight. Slopes: p<0.0001, Intercepts: p<0.0001 and R2=0.04. The relationship was not significantly different between controls and fracture cases in men or women (p=0.16 and p=0.39, respectively). The individuals below the lower horizontal line are within the lowest quartile of cortical area. Those who are above the upper horizontal line are within the highest quartile of cortical area. The individuals who are left to the vertical line are within the lowest quartile of muscular area.
Results
Descriptive characteristics
A total of 3762 participants (1838 men and 1924 women), from the AGES-Reykjavik study were included in the study. The mean age at entry for men was 76.5±5.3 years and mean age at entry for women was 76.4±5.6 years. The age distribution is shown in table 1 as well as height and weight, showing a difference in mean height of 4.9 cm (2.8%) higher among men and 6.1 cm (3.9%) higher among women during the 30 years span in cohort birth year. The cohort difference in mean weight was 13.9 kg (18%) higher among men and 10.4 kg (16%) higher among women. Baseline characteristics of participants are presented in table 2.
Table 1.
Anthropometric variables for the whole study group (n=3762)
| Men (n=1838) | Women (n=1924) | |
|---|---|---|
| Mean (SD) |
Mean (SD) |
|
| Age (years) | 76.5 (5.3) | 76.4 (5.6) |
| Height (cm) | 175.5 (6.1) | 160.8 (5.6) |
| Weight (kg) | 82.9 (13.2) | 70.7 (12.9) |
| BMI (kg/cm2) | 26.9 (3.7) | 27.3 (4.7) |
| Age group/years | n | Height (cm) | Weight (kg) | n | Height (cm) | Weight (kg) |
|---|---|---|---|---|---|---|
| −69 | 162 | 177.1 (5.6) | 90.0 (12.2) | 208 | 162.9 (5.2) | 73.9 (14.1) |
| 70-74 | 565 | 177.3 (5.8) | 86.6 (13.8) | 594 | 162.7 (5.3) | 73.5 (12.6) |
| 75-79 | 554 | 175.6 (6.0) | 82.7 (12.5) | 538 | 160.9 (5.1) | 70.7 (12.3) |
| 80-84 | 410 | 173.3 (5.8) | 78.9 (12.0) | 420 | 158.8 (5.1) | 67.7 (12.2) |
| 85+ | 147 | 172.2 (5.5) | 76.1 (11.0) | 164 | 156.8 (5.4) | 63.5 (10.9) |
Table 2.
Baseline characteristics of participants, fracture cases and controls.
| Men | Women | |||
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| Controls | Cases | Controls | Cases | |
| N | 1772 | 66 | 1811 | 113 |
| Age (years) | 76.4 (5.3) | 79.4 (6.1) | 76.2 (5.6) | 78.4 (5.8) |
| Height (cm) | 175.5 (6.1) | 176.0 (6.3) | 160.9 (5.6) | 160.3 (5.7) |
| Weight (kg) | 83.0 (13.2) | 80.9 (13.2) | 70.9 (12.8) | 67.3 (13.7) |
| BMI (kg/cm2) | 26.9 (3.7) | 26.1 (4.0) | 27.4 (4.7) | 26.1 (4.8) |
| Total shaft area (cm2) | 7.75 (0.73) | 7.89 (0.91) | 6.11 (0.58) | 6.14 (0.59) |
| Medullary area (cm2) | 1.40 (0.36) | 1.56 (0.37) | 1.27 (0.36) | 1.40 (0.40) |
| Cortical area (cm2) | 6.35 (0.60) | 6.33 (0.69) | 4.84 (0.47) | 4.74 (0.47) |
| Cortical thickness (cm) | 0.91 (0.07) | 0.88 (0.07) | 0.76 (0.08) | 0.74 (0.08) |
| Buckling ratio | 1.74 (0.14) | 1.80 (0.13) | 1.84 (0.19) | 1.92 (0.21) |
| Total muscular area (cm2) | 150 (24) | 139 (24) | 114 (19) | 106 (17) |
| Quadriceps area (cm2) | 65.8 (10.6) | 61.1 (10.2) | 47.2 (7.6) | 43.7 (7.2) |
| Knee extensor strength (N) | 406 (107) | 351 (90) | 259 (75) | 229 (71) |
| Bone area/muscular area ratio | 0.043 (0.007) | 0.047 (0.009) | 0.043 (0.007) | 0.045 (0.007) |
Total female fracture cases were 113, the fractures were categorized as 81 at hip (S72.0-S72.2), 6 at femoral shaft (S72.3), 8 at lower leg (S82.1-S82.4) and 18 at ankle (S82.5-S82.9). Total male fracture cases were 66, the fractures were categorized as 50 at hip, 2 at femoral shaft, 6 at lower leg and 8 at ankle.
Cross-sectional variations and gender differences in muscle and bone parameters by age
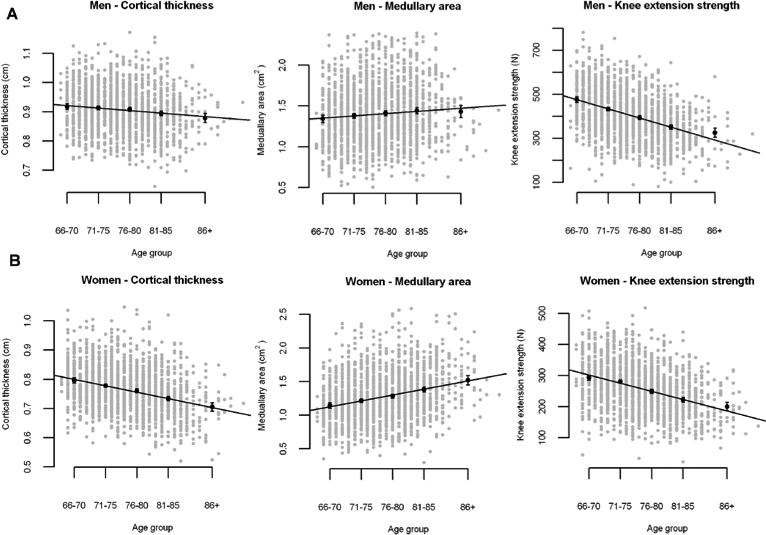
Table 3 shows the mean values at age 75 years corrected for body size and the variations with age in bone and muscle variables in this cross-sectional part of the study. There was no change in total shaft area either in men nor women based on the very low variance. On the other hand the medullary area increased in both sexes with age. The increment in medullary area was almost fourfold higher in women than in men or 15.0 % per decade (95%CI: 12.2 %, 17.7 %). Buckling ratio, reflecting the ratio between radius of the bone and cortical thickness, increased significantly by age and almost fourfold more in women. The quadriceps area declined by −11.5 (95%CI: −12.8 %, −10.4 %) in men and −10.5 % (95%CI: −11.6 %, −9.4 %) in women per decade. Both total muscular area and knee extensor strength did decline similarly in both sexes. The total muscular area declined by −10.3 % (95%CI: −11.5 %, −9.1 %) in men and −9.0 % (95%CI: −10.2 %, −7.8 %) in women per decade. The muscular strength declined about twofold more than the muscular area or −19.6 % (95%CI: −21.4 %, −17.7 %) in men and −19.4 % (95%CI: −21.4 %, −17.4 %) in women per decade. In Fig 1. the least square means per age group is displayed for cortical thickness, medullary area and knee exenstion strength, (a) men, b) women, with the data superimposed.
Table 3.
The means in bone and muscle variables through mid-thigh among controls corrected for height and weight and cross-sectional variations with age in these variables.
| Men | Women | Men | Women | ||
|---|---|---|---|---|---|
| Variable | Mean adjusted to age 75 years (SE) | Variation (%) per 10 years in age (CI) | P –value on gender difference | ||
| Total shaft area (cm2) | 7.74 (0.01) | 6.09 (0.01) | 0.0 (−1.3, 0.3) | 0.0 (−0.8, 0.8) | 0.37 |
| Medullary area (cm2) | 1.39 (0.01) | 1.24 (0.01) | 4.0 (1.6, 6.4) | 15.0 (12.2, 17.7) | <0.0001 |
| Cortical area (cm2) | 6.35 (0.01) | 4.84 (0.01) | −1.5 (−2.3, −0.7) | −3.7 (−4.4, −2.9) | 0.0002 |
| Cortical thickness (cm) | 0.908 (0.002) | 0.768 (0.002) | −1.9 (−2.6, −1.2) | −5.7 (−6.5, −5.0) | <0.0001 |
| Buckling ratio | 1.736 (0.003) | 1.827 (0.004) | 1.7 (1.0, 2.4) | 6.1 (5.2, 7.0) | <0.0001 |
| Total muscular area (cm2) | 150.66 (0.36) | 114.87 (0.32) | −10.3 (−11.5, −9.1) | −9.0 (−10.2, −7.8) | 0.13 |
| Quadriceps area (cm2) | 94.78 (0.19) | 47.65 (0.14) | −11.5 (−12.8, −10.4) | −10.5 (−11.6, −9.4) | 0.20 |
| Knee extensor strength (N) | 416.4 (2.3) | 265.2(1.7) | −19.6 (−21.4, −17.7) | −19.4 (−21.4, −17.4) | 0.91 |
| Bone area/muscular area ratio | 0.0430 (0.0001) | 0.0431 (0.0001) | 9.8 (8.5, 11.2) | 5.9 (4.6, 7.2) | <0.0001 |
Figure 1.
Values and regression lines with age for cortical thickness, medullary area and knee extension strength (least square means and 95% confidence limits) per age group (66-70; 71-75; 76-80, 81-85, 86+) a) Men b) Women.
The relationship between muscle and bone parameters
Table 4 shows the simple and partial correlation between bone and muscle variables when corrected for age, height and weight. Using simple correlation coefficients, total shaft area, cortical area as well as cortical thickness correlated positively with total muscular area (r = 0.32−0.47, p<0.0001) and knee extensor strength (r = 0.12−0.24, p<0.0001) for both men and women. The muscular area and knee extensor strength correlated strongest with cortical area and total shaft area of the bone variables after adjustment. The association between medullary area and muscle parameters was non-significant except the simple correlation among women. The association between buckling ratio and muscle parameters was significant but the association was minimal (table 4). The association between the muscular strength and the bone variables were less than between muscular area and bone variables. The results obtained using quadriceps area gave similar results as total muscular area.
Table 4.
Correlation of various measures in controls (n=3583). All partial correlation coefficients are adjusted for age, height and weight. In partial correlations, the bone variable is the response and the muscle variable an explanatory variable.
| Men | Women | |||
|---|---|---|---|---|
| Relationship | Simple correlation coefficient | Partial correlation coefficient* | Simple correlation coefficient | Partial correlation coefficient* |
| Muscular area vs. | ||||
| Total shaft area | 0.36 (p<0.0001) | 0.22 (p<0.0001) | 0.32 (p<0.0001) | 0.20 (p<0.0001) |
| Medullary area | 0.02 (p=0.50) | −0.03 (p=0.42) | −0.11 (p<0.0001) | 0.03 (p=0.39) |
| Cortical area | 0.43 (p<0.0001) | 0.28 (p<0.0001) | 0.47 (p<0.0001) | 0.23 (p<0.0001) |
| Cortical thickness | 0.34 (p<0.0001) | 0.25 (p<0.0001) | 0.39 (p<0.0001) | 0.13 (p<0.0001) |
| Buckling ratio | −0.14 (p<0.0001) | −0.13 (p=0.0006) | −0.25 (p<0.0001) | −0.06 (p=0.05) |
| Height | 0.28 (p<0.0001) | −0.15a (p<0.0001) | 0.20 (p<0.0001) | −0.07a (p=0.0002) |
| Weight | 0.76 (p<0.0001) | 0.78b (p<0.0001) | 0.70 (p<0.0001) | 0.68b (p<0.0001) |
|
Knee extensor strength vs. | ||||
| Total shaft area | 0.19 (p<0.0001) | 0.11 p<0.0001) | 0.12 (p<0.0001) | 0.07 (p=0.0009) |
| Medullary area | −0.01 (p=0.56) | −0.02 (p=0.52) | −0.12 (p<0.0001) | −0.03 (p=0.24) |
| Cortical area | 0.25 (p<0.0001) | 0.14 (p<0.0001) | 0.24 (p<0.0001) | 0.11 (p<0.0001) |
| Cortical thickness | 0.21 (p<0.0001) | 0.12 (p<0.0001) | 0.24 (p<0.0001) | 0.09 (p<0.0001) |
| Buckling ratio | −0.10 (p=0.0001) | −0.07 (p=0.01) | −0.19 (p<0.0001) | −0.06 (p=0.007) |
| Muscular area | 0.48 (p<0.0001) | 0.54 (p<0.0001) | 0.37 (p<0.0001) | 0.38 (p<0.0001) |
| Height | 0.24 (p<0.0001) | 0.04a (p=0.09) | 0.21 p<0.0001) | 0.06a (p=0.007) |
| Weight | 0.30 (p<0.0001) | 0.18b (p<0.0001) | 0.20 p<0.0001) | 0.09b (p<0.0001) |
|
Cortical area vs. | ||||
| Height | 0.50 (p<0.0001) | 0.37a (p<0.0001) | 0.46 (p<0.0001) | 0.33a (p<0.0001) |
| Weight | 0.48 (p<0.0001) | 0.34b (p<0.0001) | 0.52 (p<0.0001) | 0.42b (p<0.0001) |
adjusted for age and weight
adjusted for age and height
In Fig. 2 the anthropometric relationship between cortical area and muscular area (both variables were adjusted for age, height and weight) is presented. The individuals within the lowest quartile of cortical area were classified as having low bone mass and the highest quartile of cortical area as having high bone mass. The individuals were further classified as those within the lowest quartile of muscular area into low muscle mass. Those with low bone and muscle mass were considered as “disuse osteopenic”, caused by a lack of mechanical stimulation, and those with low bone but normal muscle mass as “true osteopenic”, caused by primary disturbances of bone cells, as suggested by Ferretti et al [2]. The Fig. 2 shows diffuse scattering without any clear cut separation between cases and controls in the relationship between cortical and muscular area. The correlation between cortical area and muscular area was not significantly different between fracture cases and controls in men or women (p=0.16 and p=0.39, repectively).
The association of muscle and bone parameters with incident low trauma lower limb fractures
Table 5 shows age, height and weight adjusted hazard ratios for incident low trauma lower limb fracture associated with 1 standard deviation (SD) change in each bone and muscle parameters. In most cases the hazard ratios and significant associations were similar for both sexes. The bone parameters, cortical thickness, medullary area and buckling ratio as well as muscular area and knee extensor strength were significantly associated with incident lower limb fracture in both men and women. In both men and women muscular area and knee extensor strength were significant parameters when included in the model with either medullary area or buckling ratio (table 6). The hazard ratios were similar for both sexes, 0.5-0.7 for the muscle parameters and 1.2-1.4 for the bone variables when included in the same model. Medullary area and buckling ratio were highly correlated. Similar results were achieved when bone variables were combined with the muscular area instead of knee extensor strength but the results were more significant using knee extensor strength in women (table 6). To put in context the age variation of the measured parameters with possible risk of fracture we can calculate the following; Among women 1 SD in these two bone parameters and muscular area equals roughly half of 10 years variation in those parameters but 10 years variation in knee extension strength is approximately 70% of 1 SD in that parameter. In men, the 10 years variation in muscle parameters equals roughly to 60-70% of 1 SD in these parameters.
Table 5.
Univariate hazard ratios for lower limb fracture per 1 SD of bone and muscle parameters at mid-thigh adjusted for age, height and weight.
| Men | Women | |
|---|---|---|
| HR 95% CI, p | HR 95% CI, p | |
| Total shaft area (cm2) | 1.2 (0.9-1.6), 0.25 | 1.1 (0.9-1.4), 0.21 |
| Medullary area (cm2) | 1.4 (1.1-1.8), 0.004 | 1.3 (1.1-1.5), 0.01 |
| Cortical area (cm2) | 0.9 (0.7-1.2), 0.53 | 0.9 (0.7-1.2), 0.42 |
| Cortical thickness (cm) | 0.7 (0.6-0.9), 0.01 | 0.8 (0.6-1.0), 0.03 |
| Buckling ratio | 1.4 (1.1-1.7), 0.006 | 1.3 (1.0-1.5), 0.01 |
| Muscular area (cm2) | 0.5 (0.4-0.8), 0.001 | 0.7 (0.6-1.0), 0.027 |
| Knee extensor strength (N) | 0.6 (0.5-0.9) ,0.002 | 0.7 (0.6-0.9), 0.004 |
Table 6.
Hazard ratios for incident lower limb fracture in multivariate models including a bone variable (medullary area, cortical thickness or buckling ratio) and a muscle parameter (muscular area or knee extensor strength) at mid-thigh adjusted for age, height, weight (standardized for 1 SD). In models 1-3, a bone variable and muscular area are included and in models 4-6, a bone variable and knee extensor strengh are included.
| Men | |||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| HR 95% CI, p | HR 95% CI, p | HR 95% CI, p | |
| Medullary area (cm2) | 1.4 (1.1-1.8), 0.004 | ||
| Cortical thickness (cm) | 0.8 (0.6-1.0), 0.04 | ||
| Buckling ratio | 1.4 (1.1-1.7), 0.009 | ||
| Muscular area (cm2) | 0.5 (0.3-0.8), 0.0008 | 0.5 (0.3-0.8), 0.002 | 0.5 (0.3-0.8), 0.001 |
| Model 4 | Model 5 | Model 6 | |
|---|---|---|---|
| Medullary area (cm2) | 1.4 (1.1-1.8), 0.006 | ||
| Cortical thickness (cm) | 0.8 (0.6-1.0), 0.03 | ||
| Buckling ratio | 1.3 (1.1-1.7), 0.01 | ||
| Knee extensor strength (N) | 0.7 (0.5-0.9), 0.003 | 0.7 (0.5-0.9), 0.005 | 0.7 (0.5-0.9), 0.004 |
| Women | |||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| HR 95% CI, p | HR 95% CI, p | HR 95% CI, p | |
| Medullary area (cm2) | 1.3 (1.1-1.6), 0.005 | ||
| Cortical thickness (cm) | 0.8 (0.6-0.9), 0.01 | ||
| Buckling ratio | 1.3 (1.1-1.6), 0.004 | ||
| Muscular area (cm2) | 0.7 (0.5-1.0), 0.026 | 0.8 (0.6-1.02),0.06 | 0.7 (0.6-1.0), 0.04 |
| Model 4 | Model 5 | Model 6 | |
|---|---|---|---|
| Medullary area (cm2) | 1.2 (1.0-1.5), 0.03 | ||
| Cortical thickness (cm) | 0.8 (0.7-1.0), 0.08 | ||
| Buckling ratio | 1.2 (1.0-1.4). 0.04 | ||
| Knee extensor strength (N) | 0.7 (0.6-0.9), 0.001 | 0.7 (0.6-0.9), 0.002 | 0.7 (0.6-0.9), 0.002 |
Discussion
It is well known that women lose more bone than men into old age [18, 20] and subsequently sustain more fractures. The purpose of our cross-sectional study was to explore if the association between muscular and bone parameters in mid-thigh differ by age and sex in older people. Furthermore our aim was to study if those parameters were associated with incident low trauma fractures in the lower limbs. Our results showed that the size of medullary area and consequent thinning of cortex with higher buckling ratio was significantly associated with lower limb fractures in both sexes. Those bone parameters varied differently with age by gender, considerably more so in women than men. The bone parameters that strongest associated with fractures were, however, mostly independent of baseline muscular parameters. Variations in muscular parameters with age were parallel in both genders. On the other hand muscle parameters (cross-sectional area and knee extensor strength) were protective against incident lower limb fractures in both sexes, independent of the bone parameters measured in our study.
Our cross-sectional data indicate similar loss in muscle mass in both sexes with age but as reported by others [21] the loss of muscle strength is somewhat greater than loss of total muscle area and quadriceps area with aging, implying that the quality of the muscle may be reduced. The correlation between knee extensor strength and muscular area was moderate. In older persons, the muscle mass may be incomplete predictor of muscle strength because of deteriorated function of neuromuscular junction [13] and fatty infiltration of muscle increases with age and results in reduced muscle strength. In our study, the correlation between muscular area and knee extensor strength was greater among men than women, suggesting different muscle quality possibly due to less fatty infiltration. The variations with age in cortical thickness was threefold greater in women than men, almost fourfold greater for medullary area and buckling ratio with no difference with age in total shaft area. These variations with age reflect presumably greater endocortical resportion but minimal periosteal apposition. Our aim of study was to compare these variations in bone with muscle parameters.
There is discordance in the literature regarding the relationship between muscle and bone in older adults. In the present study, muscular area and knee extensor strength were postively associated with total shaft area and cortical area, similarly in both sexes. The correlations were almost twofold higher between muscular area and bone parameters than knee extensor strength and bone parameters. We did adjust the analyses for age, weight and height because those variables have biological influence on both bone parameters and muscle strength/area but possibly to a different degree. However, it is a limitation in our study that we did not separate body weight into lean and fat mass with possibly different confounding effects [22].
Our data do confirm a strong positive relationship between bone mass and body mass reported by others, as well as between bone mass and body height [23, 24] that justifies our use of the weight and height to adjust the correlations. Numerous studies have revealed that body weight was the strongest predictor of variance in BMD at weight-bearing sites [25-27]. In our study muscular area and knee extensor strength explained less than 10% of the variability in total shaft and cortical area when corrected for age, height and weight. On the other hand the association between medullary area and buckling ratio with the muscle parameters was negative and minimal if it was significant. Numerous studies have reported weak to moderate postitive correlations between muscle strength and bone mineral density in adults but in most cases the relationships were not adjusted for body size [11, 28, 29]. Other studies have reported non-significant relationship between muscle strength and BMD [24, 30]. Melton III et al [14] showed that there was no close relationship between changes in habitual load (estimated by body mass, appendicular muscle mass, and habitual physical activities) and changes in bone strength at the femoral neck, lumbar spine and radius measured by QCT in men and women, age range 21-97 years. Grip torques were not significant independent contributors to radius bone strength indexes when body size and gender were accounted for in men and postmenopausal women over the age of 50 [31]. In contrast, in a study of 796 men, 50-85 years of age, the relative skeletal muscle mass index correlated postitively with BMC, aBMD and bone geometric measurements estimated by DXA [13]. The discordance can be related to different age range studied and methodological differences including not mathematically accounting for the influence of body weight and height on both the bone and muscle variables.
The muscle-bone hypothesis has been able to better account for the accrual of bone mass and strength during childhood and explain why certain types of exercise are able to prevent bone loss during immobilization [32, 33]. Bone loss is also a well-known result of skeletal unloading in long-duration spaceflight, with the most severe losses occurring in the load-bearing lower skeleton [34]. Schoenau et al [35] concluded that the interaction of muscle and bone system in forearm during puberty in boys and girls supported the ideas proposed by Frost [1], finding a strong correlation between muscle area and cortical area; in pubertal boys and girls r=0.88 and r=0.91, respectively. They did not adjust for body size. These correlations were approximately twofold higher than the simple correlations for the older individuals in our study. However, estrogen deficiency in our elderly women might have caused maladaptation to mechanical loading as suggested by Lanyon and Skerry [17] and further supported by studies in mice [36] showing an importance of estrogen receptor (ER-α) in this respect. As we did not measure sex steroids we can not exclude such an interaction. However we found closely similar relationship between bone and muscle in both sexes. Melton III et al [14] studying age 21-97 years did not find that sex steroids much influenced the strength-to-load ratio and suggested that the sex steroids are associated with skeletal muscle mass which was the primary estimate of habitual skeletal loading. Price et al [37] very recently suggested that there does not appear to be a unique mechanically sensitive pathway by which bone loading regulates bone mass and architecture to ensure adequate strength.
In our cross-sectional study we further examined the bone area/muscle area ratio and found a substantial inter-individual variation in this ratio in both sexes (Fig. 2). The bone area/muscle area ratio increased with age in both sexes and significantly more among men in disagreement with [2] where the ratio decreased after age of 50 in women and was rather stable in men. An extension to the mechanostat theory has been introduced by [2, 5] using the ratios of bone mass/strength to muscle mass/strength to distinguish between a primary and secondary bone disease. They proposed that since muscle and bone are closely functionally linked an assessment of both elements should be included in the pathogenetic evaluation of skeletal disease. Accordingly primary bone diseases are characterized by dysfunctional adaptation of bone to biomechanical load. Secondary bone diseases are characterized by decline in muscle force (sarcopenia) but with normal adaptation of bone to load. We tried such a separation of the individuals according to bone/muscle relationship into “disuse” and “true” osteopenia without any significant separation between controls and fracture cases. However, numerous factors other than the forces due to muscle contraction are known to influence bone, including hormones, genetic effect and potentially body composition which were not analyzed in our study.
Among the measured bone variables, our study indicates that medullary area and buckling ratio were associated with lower limb fractures. Buckling ratio, the ratio of bone radius to cortical thickness, and medullary area increase with age more in women than men. The importance of the buckling ratio in the femoral neck as a predictor of hip fracture has also been emphasized in the Rotterdam Study [38] and SOF Study [39] using DXA measurements, although it is important to take into account the limitations of indirectly determining bone geometry from projectional images. Increasing thigh muscle strength was, however, a significant independent protective factor against fractures in our multivariable hazards model. Muscle strength and area gave closely similar associative results. These factors were independent of the bone variables, which could suggest that muscle mass and strength, might act by preventing falls rather than acting directly on bones. It is however well documented that, whenever muscles are not properly actuated, bone is readily lost from the disused regions, for example for spinal cord injury [40] and stroke patients [41]. Our study refects bone and muscle loss with age in a relatively healthy population of older individuals because of exclusion criteria.
Our cross-sectional study has several important strengths. It included a large number of old individuals, both men and women and the CT scans were made at a single-center. All measurements were performed on data acquired before the occurrence of fractures, and cases and controls are a part of the same cohort, which ensures their comparability. Our detailed information on medication allows exclusion of persons on medications that may influence bone metabolism. This study has also some limitations. The cross-sectional nature of the data may underestimate the true rate of decline/increase with age as shown by some longitudinal studies [42, 43]. Thus, our findings could have been affected by secular changes in muscle mass or bone mass that occurred over the age span of our cohort or by confounding effect of fat [22]. Our data show similar secular changes in height for both sexes, 2.8% among men 3.9% among women during the 30 years span in cohort birth year. The secular changes in weight were much greater but similar by gender, 18% among men and 16% among women. Another limitation is that there were no measurements of sex hormones in the participants. The cross-sectional part is reflecting associations but not revealing causes and effects. All of our subjects were Caucasians and our results may therefore not be applicable to other ethnic groups. Isometric knee extensor strength and body weight are only surrogate estimates of the strains applied on the mid-thigh. The forces generated by the muscles in the cross-section of the mid-thigh are not applying the highest load on the mid femoral shaft. The highest load is expected at the proximal and distal ends of the femur. We can therefore not preclude that a stronger association might be between muscular and bone area if the bone parameters would have been estimated from regions where forces are higher. However, a large part of the quadriceps muscles does have origins at the femoral shaft. In our results, the quadriceps cross-sectional area and total muscular area correlated similarly with the bone parameters. Further, there was a relatively small number of incident fractures in our study, which reduced statistical power. The mid-femoral shaft is not a common location for osteoporotic fractures. However, this site allows a direct measurement of cortical bone that is the main part of the skeleton. Finally, we did not have an estimate of BMD or porosity in the femoral shaft cortex; previous studies of older individuals have reported no effect of exercise on cortical volumetric BMD [44, 45].
In conclusion, our cross-sectional results in older people show a weaker association between bone and muscle parameters (as measured in mid-thigh) than has been published in younger people [7, 9, 35]. Our results indicate that muscular area and strength are of importance for protection against incident lower limb fractures in bothsexes and this seems to be mostly independent of bone parameters. The study supports the idea that periosteal apposition is insufficient to protect bone strength against the medullary expansion due to endocortical resorption. This is consistent with a higher rate of fracture in women, as both genders show no change in total shaft area, but women have larger increment in medullary area. Thus, our results support a notion that endocortical resorption may be a key process for the development of bone fragility in lower limbs in old age. Better understanding of the determinants of endocortical resorption might thus be of importance in the prevention of low trauma lower limb fractures in older people. Our data were obtained on cross-section of mid-thigh and need to be confirmed at sites more commonly associated with osteoporotic fracture.
Acknowledgements
This study has been funded by NIH contract N01-AG-1-2100; the NIA Intramural Research Program; Hjartavernd (the Icelandic Heart Association); the Althingi (the Icelandic Parliament) and the memorial fund of Helga Jonsdottir and Sigurlidi Kristjansson. Dr Sigurdsson acknowledges support from the University of Iceland Research Fund. The study is approved by the Icelandic National Bioethics Committee, VSN: 00-063. The researchers are indebted to the participants for their willingness to participate in the study.
Abbreviations
- QCT
quantitative computed tomography
- DXA
dual energy X-ray absorptiometry
- shaft CSA
total cross-sectional periosteal area
- MA
medullary area
- iCThi
cortical thickness index
- BR
buckling ratio
- BMI
body mass index
- CI
confidence interval
- SD
standard deviation
- BMC
bone mineral content
- aBMD
areal bone mineral density
- HR
hazard ratio
- ER-α
Estrogen receptor
Footnotes
DISCLOSURES: NONE
References
- 1.Frost HM. The mechanostat: a proposed pathogenic mechanism of osteoporoses and the bone mass effects of mechanical and nonmechanical agents. Bone Miner. 1987;2:73–85. [PubMed] [Google Scholar]
- 2.Ferretti JL, Cointry GR, Capozza RF, Frost HM. Bone mass, bone strength, muscle-bone interactions, osteopenias and osteoporoses. Mech Ageing Dev. 2003;124:269–79. doi: 10.1016/s0047-6374(02)00194-x. [DOI] [PubMed] [Google Scholar]
- 3.Hart KJ, Shaw JM, Vajda E, Hegsted M, Miller SC. Swim-trained rats have greater bone mass, density, strength, and dynamics. J Appl Physiol. 2001;91:1663–8. doi: 10.1152/jappl.2001.91.4.1663. [DOI] [PubMed] [Google Scholar]
- 4.Warner SE, Shea JE, Miller SC, Shaw JM. Adaptations in cortical and trabecular bone in response to mechanical loading with and without weight bearing. Calcif Tissue Int. 2006;79:395–403. doi: 10.1007/s00223-005-0293-3. [DOI] [PubMed] [Google Scholar]
- 5.Schoenau E, Fricke O. Interaction between Muscle and Bone. Horm Res. 2006;66:73–8. [Google Scholar]
- 6.Bonnet N, Ferrari S. Exercise and the skeleton: How it works and what It really does IBMS BoneKEy. 2010;7:235–48. [Google Scholar]
- 7.Bass SL. The prepubertal years: a uniquely opportune stage of growth when the skeleton is most responsive to exercise? Sports Med. 2000;30:73–8. doi: 10.2165/00007256-200030020-00001. [DOI] [PubMed] [Google Scholar]
- 8.MacKelvie KJ, Khan KM, Petit MA, Janssen PA, McKay HA. A School-Based Exercise Intervention Elicits Substantial Bone Health Benefits: A 2-Year Randomized Controlled Trial in Girls Pediatrics. 2003;112:e447–e52. doi: 10.1542/peds.112.6.e447. [DOI] [PubMed] [Google Scholar]
- 9.Matthews BL, Bennell KL, McKay HA, Khan KM, Baxter-Jones ADG, Mirwald RL, Wark JD. Dancing for bone health: a 3-year longitudinal study of bone mineral accrual across puberty in female non-elite dancers and controls. Osteoporos Int. 2006;17:1043–54. doi: 10.1007/s00198-006-0093-2. [DOI] [PubMed] [Google Scholar]
- 10.Capozza RF, Cointry GR, Cure-Ramírez P, Ferretti JL, Cure-Cure CA. DXA study of muscle-bone relationships in the whole body and limbs of 2512 normal men and pre- and post-menopausal women. Bone. 2004;35:283–95. doi: 10.1016/j.bone.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Ferretti JL, Capozza RF, Cointry GR, García SL, Plotkin H, Alvarez Filgueira ML, Zanchetta JR. Gender-related differences in the relationship between densitometric values of whole-body bone mineral content and lean body mass in humans between 2 and 87 years of age. Bone. 1998;22:683–90. doi: 10.1016/s8756-3282(98)00046-5. [DOI] [PubMed] [Google Scholar]
- 12.Rittweger J, Beller G, Ehrig J, Jung C, Koch U, Ramolla J, Schmidt F, Newitt D, Majumdar S, Schiessl H, Felsenberg D. Bone-muscle strength indices for the human lower leg. Bone. 2000;27:319–26. doi: 10.1016/s8756-3282(00)00327-6. [DOI] [PubMed] [Google Scholar]
- 13.Szulc P, Beck TJ, Marchand F, Delmas PD. Low skeletal muscle mass is associated with poor structural parameters of bone and impaired balance in elderly men--the MINOS study. J Bone Miner Res. 2005;20:721–9. doi: 10.1359/JBMR.041230. [DOI] [PubMed] [Google Scholar]
- 14.Melton LJ, 3rd, Riggs BL, Achenbach SJ, Amin S, Camp JJ, Rouleau PA, Robb RA, Oberg AL, Khosla S. Does reduced skeletal loading account for age-related bone loss? J Bone Miner Res. 2006;21:1847–55. doi: 10.1359/jbmr.060908. [DOI] [PubMed] [Google Scholar]
- 15.Cousins JM, Petit MA, Paudel ML, Taylor BC, Hughes JM, Cauley JA, Zmuda JM, Cawthon PM, Ensrud KE. Muscle power and physical activity are associated with bone strength in older men: The osteoporotic fractures in men study. Bone. 2010;47:205–11. doi: 10.1016/j.bone.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sherrington C, Whitney JC, Lord SR, Herbert RD, Cumming RG, Close JC. Effective exercise for the prevention of falls: a systematic review and meta-analysis. J Am Geriatr Soc. 2008;56:2234–43. doi: 10.1111/j.1532-5415.2008.02014.x. [DOI] [PubMed] [Google Scholar]
- 17.Lanyon L, Skerry T. Postmenopausal osteoporosis as a failure of bone's adaptation to functional loading: a hypothesis. J Bone Miner Res. 2001;16:1937–47. doi: 10.1359/jbmr.2001.16.11.1937. [DOI] [PubMed] [Google Scholar]
- 18.Sigurdsson G, Aspelund T, Chang M, Jonsdottir B, Sigurdsson S, Eiriksdottir G, Gudmundsson A, Harris TB, Gudnason V, Lang TF. Increasing sex difference in bone strength in old age: The Age, Gene/Environment Susceptibility-Reykjavik study (AGES-REYKJAVIK). Bone. 2006;39:644–51. doi: 10.1016/j.bone.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Kaptoge S, Dalzell N, Loveridge N, Beck TJ, Khaw KT, Reeve J. Effects of gender, anthropometric variables, and aging on the evolution of hip strength in men and women aged over 65. Bone. 2003;32:561–70. doi: 10.1016/s8756-3282(03)00055-3. [DOI] [PubMed] [Google Scholar]
- 20.Khosla S, Riggs BL. Pathophysiology of age-related bone loss and osteoporosis. Endocrinol Metab Clin North Am. 2005;34:1015–30. doi: 10.1016/j.ecl.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–64. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 22.Reid IR. Relationships between fat and bone. Osteoporos Int. 2008;19:595–606. doi: 10.1007/s00198-007-0492-z. [DOI] [PubMed] [Google Scholar]
- 23.Blain H, Jaussent J, Thomas E, Micallef JP, Dupuy AM, Bernard PL, Mariano-Goulart D, Cristol JP, Sultan C, Rossi M, Picot MC. Appendicular skeletal muscle mass is the strongest independent factor associated with femoral neck bone mineral density in adult and older men. Experimental Gerontology. 2010;45 doi: 10.1016/j.exger.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Owings TM, Pavol MJ, Grabiner MD. Lower extremity muscle strength does not independently predict proximal femur bone mineral density in healthy older adults. Bone. 2002;30:515–20. doi: 10.1016/s8756-3282(01)00705-0. [DOI] [PubMed] [Google Scholar]
- 25.Andersen S, Boeskov E, Laurberg P. Ethnic differences in bone mineral density between inuit and Caucasians in north Greenland are caused by differences in body size. J Clin Densitom. 2005;8:409–14. doi: 10.1385/jcd:8:4:409. [DOI] [PubMed] [Google Scholar]
- 26.Edelstein SL, Barrett-Connor E. Relation between body size and bone mineral density in elderly men and women. Am J Epidemiol. 1993;138:160–9. doi: 10.1093/oxfordjournals.aje.a116842. [DOI] [PubMed] [Google Scholar]
- 27.Segal NA, Torner JC, Yang M, Curtis JR, Felson DT, Nevitt MC. Muscle Mass Is More Strongly Related To Hip Bone Mineral Density Than Is Quadriceps Strength Or Activity Level In Adults Over Age 50. J Clin Densitom. 2008;11:503–10. doi: 10.1016/j.jocd.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halle JS, Smidt GL, O‘Dwyer KD, Lin SY. Relationship between trunk muscle torque and bone mineral content of the lumbar spine and hip in healthy postmenopausal women. Phys Ther. 1990;70:690–9. doi: 10.1093/ptj/70.11.690. [DOI] [PubMed] [Google Scholar]
- 29.Taaffe DR, Pruitt L, Lewis B, Marcus R. Relationship between bone mineral density of the proximal femur and strength of the hip muscles in postmenopausal women. Am J Phys Med Rehabil. 1998;77:477–82. doi: 10.1097/00002060-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Orwoll ES, Bauer DC, Vogtthe TM. Study of Osteoporotic Fractures Research Group and K.M. Fox , Axial bone mass in older women. Ann Intern Med. 1996;124:187–96. doi: 10.7326/0003-4819-124-2-199601150-00001. [DOI] [PubMed] [Google Scholar]
- 31.Frank AW, Lorbergs AL, Chilibeck PD, Farthing JP, Kontulainen SA. Muscle cross sectional area and grip torque contraction types are similarly related to pQCT derived bone strength indices in the radii of older healthy adults. J Musculoskelet Neuronal Interact. 2010;10:136–41. [PubMed] [Google Scholar]
- 32.Binkley TL, Specker BL. Muscle-bone relationships in the lower leg of healthy pre-pubertal females and males. J Musculoskelet Neuronal Interact. 2008;8:239–43. [PubMed] [Google Scholar]
- 33.Rittweger J. Ten years muscle-bone hypothesis: What have we learned so far? . J Musculoskelet Neuronal Interact. 2008;8:174–8. [PubMed] [Google Scholar]
- 34.Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19:1006–12. doi: 10.1359/JBMR.040307. [DOI] [PubMed] [Google Scholar]
- 35.Schoenau E, Neu CM, Mokov E, Wassmer G, Manz F. Influence of puberty on muscle area and cortical bone area of the forearm in boys and girls. J Clin Endocrinol Metab. 2000;85:1095–8. doi: 10.1210/jcem.85.3.6451. [DOI] [PubMed] [Google Scholar]
- 36.Lee K, Jessop H, Suswillo R, Zaman G, Lanyon L. Bone adaptation requires oestrogen receptor-α. Nature. 2003;424:6947. doi: 10.1038/424389a. [DOI] [PubMed] [Google Scholar]
- 37.Price J, Sugiyama T, Galea GL, Meakin LB, Sunters A, Lanyon LE. Role of endocrine and paracrine factors in the adaptation of bone to mechancial loading. Curr Osteoporos Rep. 2011;9:76–82. doi: 10.1007/s11914-011-0050-7. [DOI] [PubMed] [Google Scholar]
- 38.Rivadeneira F, Zillikens MC, De Laet CE, Hofman A, Uitterlinden AG, Beck TJ, Pols HA. Femoral neck BMD is a strong predictor of hip fracture susceptibility in elderly men and women because it detects cortical bone instability: the Rotterdam Study. J Bone Miner Res. 2007;22:1781–90. doi: 10.1359/jbmr.070712. [DOI] [PubMed] [Google Scholar]
- 39.Kaptoge S, Beck TJ, Reeve J, Stone KL, Hillier TA, Cauley JA, Cumming SR. Prediction of incident hip fracture risk by femur geometry variables measured by hip structural analysis in the Study of Osteoporotic Fractures. J Bone Miner Res. 2008;23:1892–904. doi: 10.1359/JBMR.080802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giangregorio L, McCartney N. Bone loss and muscle atrophy in spinal cord injury: Epidemiology, fracture prediction, and rehabilitation strategies. J Spinal Cord Med. 2006;29:489–500. doi: 10.1080/10790268.2006.11753898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jørgensen L, Jacobsen BK, Wilsgaard T, Magnus JH. Walking after stroke: Does it matter? Changes in bone mineral density within the first 12 months after stroke. A longitudinal study. Osteoporos Int. 2000;11:381–7. doi: 10.1007/s001980070103. [DOI] [PubMed] [Google Scholar]
- 42.Lauretani F, Bandinelli S, Griswold ME, Maggio M, Semba R, Guralnik JM, Ferrucci L. Longitudinal changes in BMD and bone geometry in a population-based study. J Bone Miner Res. 2008;23:400–8. doi: 10.1359/JBMR.071103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riggs BL, Melton LJ, Robb RA, Camp JJ, Atkinson EJ, McDaniel L, Amin S, Rouleau PA, Khosla S. A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res. 2008;23:205–14. doi: 10.1359/JBMR.071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bailey CA, Kukuljan S, Daly RM. Effects of lifetime loading history on cortical bone density and its distribution in middle-aged and older men. Bone. 2010;47:673–80. doi: 10.1016/j.bone.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 45.Daly RM, Bass SL. Lifetime sport and leisure activity participation is associated with greater bone size, quality and strength in older men. Osteoporos Int. 2006;17:1258–67. doi: 10.1007/s00198-006-0114-1. [DOI] [PubMed] [Google Scholar]