Abstract
The systemic delivery of therapeutic viruses, such as oncolytic viruses or vaccines, is limited by the generation of neutralizing antibodies. While pseudotyping of rhabdoviruses with the lymphocytic choriomeningitis virus glycoprotein has previously allowed for multiple rounds of delivery in mice, this strategy has not translated to other animal models. For the first time, we provide experimental evidence that antibodies generated against the lymphocytic choriomeningitis virus glycoprotein mediate robust complement-dependent viral neutralization via activation of the classical pathway. We show that this phenotype can be capitalized upon to deliver maraba virus pseudotyped with the lymphocytic choriomeningitis virus glycoprotein in a Fischer rat model in the face of neutralizing antibody through the use of complement modulators. This finding changes the understanding of the humoral immune response to arenaviruses, and also describes methodology to deliver viral vectors to their therapeutic sites of action without the interference of neutralizing antibody.
Introduction
Rhabdoviruses such as vesicular stomatitis virus (VSV) and maraba virus (MRB) have been validated preclinically as promising oncolytic1,2 and vaccine vectors3,4 and their clinical evaluation is underway.5 However, within the first week following administration, neutralizing antibodies which limit multiple rounds of dosing are generated against these highly cytolytic viruses.6 In contrast, lymphocytic choriomeningitis virus (LCMV) is known for its inability to generate early neutralizing antibodies.7 This property has been conferred to rhabdoviruses via pseudotyping,8 and has been used to deliver multiple therapeutic doses in mice.9,10
The complement system is a first line of defense of innate immunity with diverse contributions in both homeostasis and pathological states.11 The classical pathway is activated through the binding of C1q to antibody, and leads to the destruction of pathogens via the membrane attack complex. The neutralizing effect of antibodies against epitopes on viruses such as vaccinia virus is enhanced by complement,12,13 and complement inhibitors improve the delivery of vaccinia virus to tumors in preimmune hosts.14
Mouse complement inadequately recapitulates human complement. Low hemolytic activity is observed,15 in part resulting from a C4 polymorphism16 as well as an unspecified classical pathway inhibitor.17 Rat complement however has higher hemolytic activity15 and provides a better model to understand the systemic delivery of therapeutic viruses. Using a Balb/c mouse model, a Fischer rat model, and a macaque model, we have identified that the LCMV glycoprotein (GP) elicits early antibodies that mediate neutralization in a complement-dependent manner. We show that an LCMV GP pseudotyped MRB vector (MRB LCMV GP), in combination with complement depletion, evades neutralization, thereby increasing the effective dose delivered.
Results
Anti-LCMV GP antibodies neutralize pseudotyped virus in a complement-dependent manner
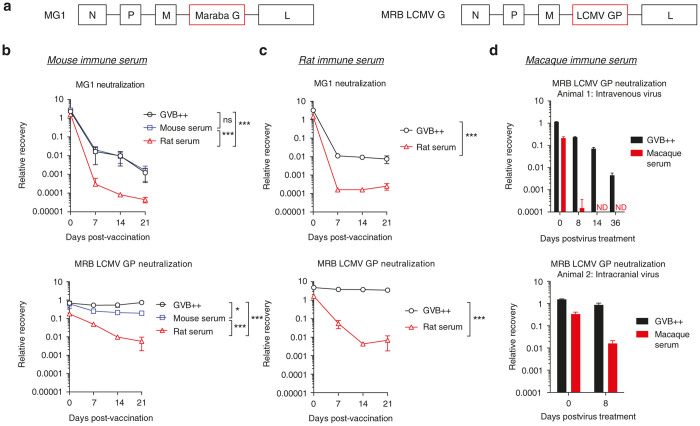
We engineered a MRB virus pseudotyped with the LCMV GP (Figure 1a). F344 Fischer rats and Balb/c mice were vaccinated with MRB LCMV GP or the MRB derivative MG1.2 The kinetics of anti-MG1 and MRB LCMV GP antibody production in mice and rats was assessed ex vivo using heat inactivated (HI) immune serum collected on days 7, 14, and 21 post-vaccination. Strongly neutralizing antibodies to MG1 were generated early in both mice and rats, and their neutralizing effect was enhanced by rat complement but not mouse complement. As previously shown,10 HI MRB LCMV GP mouse immune serum did not yield detectable neutralization in the absence of complement, or when mouse complement was reconstituted. Remarkably, in the presence of rat complement, antibodies to LCMV GP resulted in significant neutralization (average 103-fold neutralization with day 14 immune serum; Figure 1b). Similarly, rat anti-MRB LCMV GP antibodies did not induce detectable viral neutralization in the absence of complement, but in the presence of reconstituted rat complement led to an average 229-fold neutralization (day 14 immune serum; Figure 1c). The complement-dependent phenotype of the anti-LCMV GP antibodies in rats was stable for several weeks (Supplementary Figure S1a). The same complement-dependent neutralization was observed with MRB LCMV GP in ex vivo whole rat blood using the anticoagulant Relfudan18 (Supplementary Figure S1b,c). Moreover, the phenotype of the antibody was independent of the backbone and the mutation in the G protein of MG1 (Supplementary Figure S1e,f).
Figure 1.
Early antibodies elicited against lymphocytic choriomeningitis virus glycoprotein (LCMV GP) mediate robust complement-dependent neutralization. (a) Schematic of the genome of maraba (MRB) pseudotyped with the LCMV GP. (b) Mice were vaccinated with 107 pfu of MG1 or MRB LCMV GP and serum taken at the indicated time points. Neutralization was assessed following incubation (1 hour; 37oC) with heat inactivated (HI) immune serum combined with dextrose gelatin veronal buffer (GVB++) or with mouse serum or rat serum as a source of complement. Infectious virus was quantified by plaque assay. n = 3 mice/group; data is expressed as group mean ± SD. (c) Rats were vaccinated with 108 pfu and bled at the indicated time points. Neutralization was assessed following incubation (1 hour; 37oC) with HI immune serum combined with GVB++ or with rat serum as a source of complement. n = 2 rats/group; data is expressed as group mean ± SD. Statistical comparison indicate minimum significance level on days 7, 14, and 21. (d) Two cynomolgus macaques received 1010 pfu intravenously (Animal 1) or 109 pfu intracranially (Animal 2). Neutralization was assessed following incubation (1 hour; 37oC) with HI immune serum combined with GVB++ or with cynomolgus macaque serum as a source of complement. Data is expressed as the technical replicates ± SD. ns P > 0.05; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001
Using a cynomolgus macaque model, we demonstrated that this effect was not specific to rodents (Figure 1b, iii). Two animals were given MRB LCMV GP either intravenously or intracranially and viral neutralization was assessed ex vivo using serum samples collected at various time points postexposure. As early as 8 days after treatment, antibody-mediated, complement-dependent neutralization led to a significant reduction in infectivity and this was maintained in one animal for 5 weeks (Figure 1c).
The classical and terminal pathways are required for neutralization
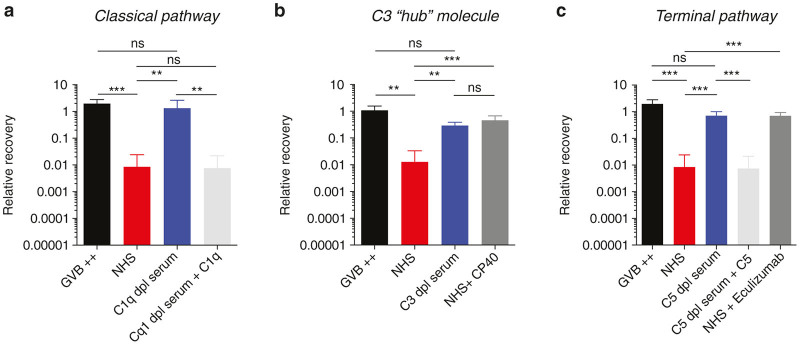
In order to determine which complement pathways were important for neutralization, MRB LCMV GP neutralization was assessed using HI MRB LCMV GP immune rat serum combined with normal human serum (NHS), NHS depleted of key complement components, or NHS treated with complement inhibitors (Figure 2). Anti-MRB LCMV GP antibodies did not induce viral neutralization in gelatin veronal buffer (GVB++) alone, however when combined with NHS, a 78-fold reduction in titer was observed. When the classical pathway molecule C1q was immunodepleted, neutralization was abrogated. Moreover, addition of C1q to physiologic concentration restored the neutralizing effect (Figure 2a). Immunodepletion of C3 precluded the loss of infectivity and was mirrored by the effect of the C3 inhibitory binding peptide, CP4019,20 (Figure 2b). To assess the terminal pathway, C5 immunodepleted serum and the C5 inhibitory monoclonal antibody, Eculizumab, were used. Both depletion and inhibition of C5 prevented viral neutralization, and this effect was reversed with the addition of C5 (Figure 2c). Inhibition of the classical pathway, the hub molecule C3, or the terminal pathway would therefore be of use to inhibit the functional activity of the anti-LCMV GP antibody. Finally, the disruption of viral particles by antibodies and complement was confirmed by electron microscopy (Supplementary Figure S1g).
Figure 2.
Neutralization requires classical pathway and terminal pathway activation. Maraba virus (MRB) lymphocytic choriomeningitis virus glycoprotein (LCMV GP) neutralization was assessed following incubation (1 hour; 37oC) with heat inactivated (HI) rat immune serum collected 18 or 21 days post-vaccination combined with GVB ++ or normal human serum (NHS) or C1q immunodepleted NHS (a), C3 immunodepleted NHS (b), or C5 immunodepleted NHS (c) as a source of complement. Additionally, C1q or C5 was added back at a concentration of 70 or 75 µg/ml, respectively. Additionally, CP40 was added at a concentration of 25 µmol/l to inhibit human C3 or the C5 monoclonal antibody, eculizumab, was used to inhibit C5 at a concentration of 100 µg/ml. The immune serum used in the C3 studies was collected from animals treated with 35U cobra venom factor (CVF) the day prior to blood draw. Infectious virus was quantified by plaque assay. n = 3 or 4 rats/group. Data is expressed as group mean ± SD. ns P > 0.05; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001
Depletion of complement increased delivery of viral vectors pseudotyped with LCMV GP
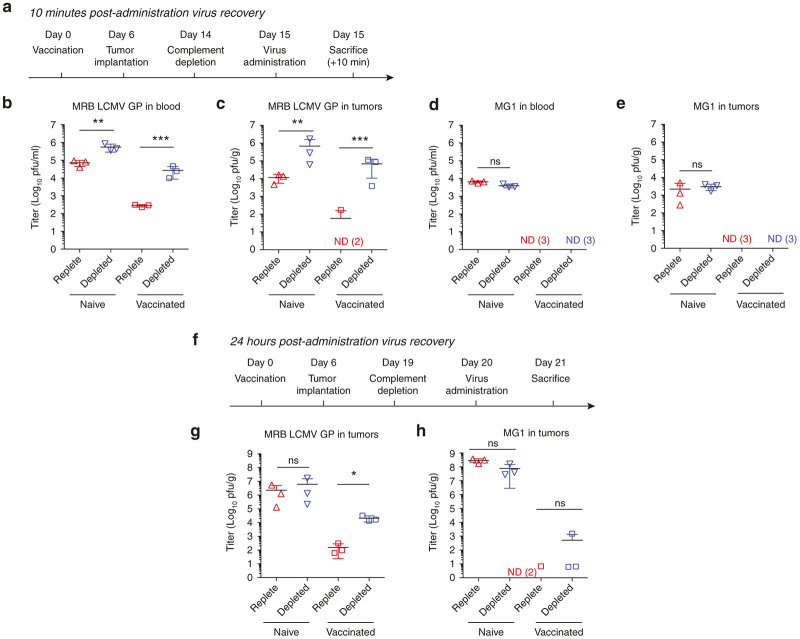
In a Fischer rat model, we assessed whether complement depletion would increase the stability of the pseudotyped MRB virus in the blood, and increase its delivery to tumors. Vaccinated or naive rats were given a complement depleting dose of cobra venom factor (CVF) or a sham injection of phosphate buffered saline (PBS) as shown in Figure 3a. Virus was administered intravenously and infectious virus in the blood and tumors 10 minutes postinjection was quantified. We observed an average 97-fold increase in infectious virus recovery from the blood of complement-depleted MRB LCMV GP immune animals 10 minutes postinjection (Figure 3b). We also observed a corresponding increase in delivery to the tumors of complement-depleted immune animals (Figure 3c). In contrast, we did not observe any benefit from complement depletion on the stability of MG1 in immune or naive animals (Figure 3d, e).
Figure 3.
Complement inhibition in vivo increases the effective dose of lymphocytic choriomeningitis virus glycoprotein (LCMV GP) pseudotyped virus that is delivered to tumors. (a) Maraba virus (MRB) LCMV GP (4 × 108 pfu) or MG1 (4 × 107 pfu) was delivered intravenously to Fischer rats bearing bilateral 13762 MATBIII tumors, according the schedule. As per the treatment groups, rats were vaccinated intravenously with 1 × 107 pfu and or depleted of complement with 35 U of cobra venom factor (CVF). Infectious virus in the blood (b,d) and in subcutaneous tumors (c,e) 10 minutes postvirus-administration was quantified by plaque assay. (f). As per the treatment groups, rats were vaccinated intravenously with 1 × 107 pfu and or depleted of complement with 35 U of CVF. 1 × 107 pfu of MRB LCMV GP (g) or MG1 (h) was delivered intratumorally to Fischer rats bearing bilateral 13762 MATBIII tumors. Subcutaneous tumor titers are shown for animals sacrificed 24 hours postvirus-administration. (n =3–4 per group) All tumor titers represent the average of both bilateral tumors. Data are represented as group mean ± SD. Each dot represents a rat. ND, not detected (*P < 0.05, ns P > 0.05).
Complement plays an important role both in the blood stream and in the tumor microenvironment to limit viral infection.14 Naive and vaccinated rats were treated with CVF or sham and subsequently given an intratumoral dose of MG1 or MRB LCMV GP virus (Figure 3f). Complement depletion increased the titer of MRB LCMV GP that was recovered from tumors of immune rats 24 hours after virus administration (mean 135-fold increase), but not naive rats (Figure 3g). MG1 replicates more rapidly than MRB LCMV GP in vitro (Supplementary Figure S2) and this is reflected by the titers recovered 24 hours postintratumoral injection in naive rats. However, very little infectious MG1 was recovered from the tumors of vaccinated rats and complement depletion did not increase recovery from naive or immune animals (Figure 3h).
Discussion
We show here for the first time that the antibodies generated against the LCMV GP in three different species induce robust neutralization in a complement-dependent manner (Figure 1). Specifically, antibody binding to virus pseudotyped with LCMV GP mediates C1q binding and neutralization via the membrane attack complex (Figure 2). We have demonstrated that we can capitalize on this phenotype of the LCMV GP by inhibiting complement in previously vaccinated rats to increase the proportion of the infectious dose that reaches the tumor (Figure 3). Antibody mediated complement dependent neutralization was important in both the blood and in the tumor. We also observed reduced neutralization of MRB LCMV GP in naive complement depleted animals, and we would speculate that IgM mediates a significant degree of complement dependent neutralization.
The complement system of mice inadequately recapitulates that of humans, and has masked an important phenotype of humoral immunity to arenaviruses. This study therefore, underscores the importance of critical interpretation of translational studies performed solely in mouse models and offers novel insight into the biology of arenaviruses; a clade of viruses with vast global health impact. A combination pseudotyping and complement inhibition strategy enables the delivery of infectious virus to tumors, despite the presence of antiviral antibody. Importantly, this type of strategy which increases the effective infectious viral dose could enable the repeated expression of virally encoded therapeutic transgenes, cytokines, or tumor antigens at the site of the tumor. Further investigation of LCMV GP pseudotyped vectors in combination with complement inhibition will elucidate the full breadth of the utility of unrestricted repeated vector delivery.
Materials and Methods
Viruses and cells
Vero and 13762 MAT B III cells were purchased from the American Type Culture Collection (Manassas, VA). 13762 MAT B III cells were maintained in McCoy’s 5A (ATCC) supplemented with 10% fetal bovine serum (FBS) (HyClone, Logan, UT). Vero cells were maintained in Dulbecco’s Modified Eagle’s medium (DMEM) (HyClone) supplemented with 10% FBS (HyClone).
Maraba and MG1 were used as previously described.2 MRB LCMV GP was constructed as previously described with VSV.10 VSVΔ51 and VSV LCMV GP were used as previously described.1,10
In vitro neutralization experiments with serum
Female F344 Fischer rats weighing 100–150 g and female Balb/c mice aged 6–8 weeks were purchased from Charles River (Wilmington, MA). All animals were housed in pathogen-free conditions and all studies conducted were in accordance with the guidelines of the Animal Care Veterinary Service facility of the University of Ottawa.
Serum was collected from mice or rats at various time points relative to vaccination (pre 7, 14, or 21 days post-vaccination) by cardiac puncture. Blood was allowed to clot at room temperature and serum was collected following an 800g centrifugation. Mice were vaccinated with 107 pfu of MRB LCMV GP or MG1 intravenously. Rats were vaccinated with 107 or108 pfu or MRB LCMV GP, MG1, MRB virus, VSVΔ51 or VSV LCMV GP intravenously. Serum (25 µl) was HI (56oC for 30 minutes) and used as a source of antibody. Complement was supplemented with an equal volume (25 µl) of rat serum (CompTech, Tyler, TX) or Balb/c mouse complement serum (Innovative Research, MI). Alternatively, 25 µl of dextrose gelatin veronal buffer (GVB++; Lonza, Allendale, NJ) was used. Serum was diluted into GVB++ and neutralization was assessed following incubation with virus at a concentration of 5 × 105 pfu per reaction for 1 hour at 37oC. Remaining infectious virus was titered on Vero cells. Neutralization was also assessed using rat serum pretreated with 10 U/ml (CVF; Quidel, San Diego, CA) for 1 hour at 37oC.
Two cynomolgus macaques received 1010 pfu of MRB LCMV GP intravenously (Animal 1) or 109 pfu intracranially (Animal 2) under a protocol approved by the Animal Resource Center, University Health Network, Toronto, ON, Canada. Serum was collected at various time points (pre 8, 14, or 36 days postadministration). As described with rat and mouse immune serum, neutralization was assessed following incubation of HI immune serum (1 hour; 37°C) GVB++ or with cynomolgus macaque serum (Innovative Research, Novi, MI).
Neutralization of MRB LCMV GP was assessed with rat immune serum supplemented with human serum (NHS) or serum immunodepleted of key complement components. C1q depleted, C3 depleted, and C5 depleted serum as well as NHS (CompTech) or NHS preincubated (15 minutes at 37°C) with the Compstatin analog, CP40 (25 μmol/l) or Eculizumab (100 µg/ml) was combined with 25 µl of HI rat immune serum and 5 × 105 pfu for 1 hour at 37°C. Immune rat serum that was combined with human C3 immuno-depleted serum originated from animals treated with CVF 2 days prior to blood draw.
In vitro neutralization with whole blood
Rats were vaccinated with 107 pfu of MG1 or MRB LCMV GP intravenously 2 weeks prior to the terminal bleed and select rats depleted of complement with 35 U CVF the day prior to the bleed. Blood was collected from rats using serum collection vacutainer tubes and treated immediately with the anticoagulant Refludan (50 μg/ml). Blood was spun at 800g for 10 minutes to obtain plasma. Plasma aliquots were incubated for 30 minutes at 56°C to inactivate complement. 200 µl of blood or fractions thereof were incubated for 1 hour at 37°C with 2 × 106 pfu of MRB LCMV GP or MG1. Remaining infectious virus was titered on Vero cells.
In vivo animal studies
F344 Fischer rats were vaccinated with 107 pfu of MG1 or MRB LCMV GP intravenously 2 weeks prior to their virus treatment. Tumors were established by injecting 1 × 106 13762 MATBIII cells subcutaneously, unilaterally, or bilaterally in the left and right flanks. For the depletion of complement, 35 U of CVF was administered intraperitoneally 24 hours prior to virus. To examine the stability of the virus early after administration, animals were treated intravenously with MRB LCMV GP (4 × 108 pfu) or MG1 (4 × 107 pfu) and animals killed 10 minutes post-treatment. Blood was collected by cardiac puncture using ethylenediaminetetraacetate (EDTA) collection vacutainer tubes (BD Bioscience, San Jose, CA) and tumors resected. Blood was titered on Vero cells and tumors were flash frozen, homogenized, and then titered on Vero cells to quantify infectious virus.
Virus naive or vaccinated rats were also treated intratumorally with 107 pfu of MG1 or MRB LCMV GP. Tumors were collected 24 hours postvirus-treatment and immediately frozen. Infectious virus was quantified by plaque assay on Vero cells.
Electron microscopy
6 × 107 pfu of MRB LCMV GP was combined with either 20 µl PBS (HyClone) or 20 µl of serum from a rat vaccinated with 107 pfu of MRB LCMV GP ~3 weeks prior to bleed for 0 or 15 minutes at 37°C. An equal volume of 2.5% gluteraldehyde was added to fix the virus. 10 µl of the virus suspension was plated on carbon-coated EM grids and stained with 2% phosphotungstic acid. The grids were screened using a Hitachi H7100 Transmission Electron Microscope.
Western blotting
CVF treated rat serum (1 µl) was resolved on 4–12% polyacrylamide gels (BioRad) and transferred to nitrocellulose membranes (Amersham GE Healthcare Lifesciences, Baie d’Urfe QC). Membranes were incubated for 1 hour at room temperature with the rabbit antirat C3 antibody (Cedarlane, Burlington, ON) at a dilution of 1:100. Membranes were incubated with goat antirabbit horseradish peroxidase (HRP)-conjugated IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 hour at room temperature. Proteins were detected using Supersignal West Pico chemiluminescent substrate (Pierce Biotechnology, Rockford, IL) followed by exposure to X-ray film (Fuji Photo Film, Tokyo, Japan).
In vitro infections and growth curves
13762 MAT B III cells were infected with MRB LCMV GP or MG1-gfp at multiplicity of infections (MOIs) ranging from 0.003 to 3. Fluorescence or bright field images were captured 48 hours postinfection.
Vero cells were infected with MRB LCMV GP or MG1 at an MOI of 0.01. Supernatants were collected at 12, 24, 36, and 48 hours postinfection and the titer was assessed by plaque assay on Vero cells.
Statistics
All statistical analyses were performed in GraphPad Prism. Statistical analyses were performed on log-transformed data. Two way analysis of variances (ANOVAs) with time as a viable repeated measure function was used for antibody kinetics. All other experiments were analyzed using one way ANOVAs with a Tukey’s post hoc test. Data is expressed as group mean ± SD. ns P > 0.05; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Author Contributions
J.C.B, L.E., L.S., D.F.S., R.A., C.S.I., V.A.J, J.A.M., and J.D.L, conceived of the study and contributed to the discussion of the data. L.S. and C.L. constructed the viral vectors. S.A.A., C.S., T.F., M.C.B.D., L.S., M.M., B.K., and L.E. collected and processed samples. L.E. and J.C.B. wrote the manuscript. All authors critically reviewed the manuscript.
Acknowledgments
The authors thank S. Avery for assisting with nonhuman primate housing and experiments, K. Yates and E. MacDonald for technical assistance with the rat experiments and J. McClintock for assistance with the electron microscopy experiments. J.C.B. is supported by the Terry Fox Research Foundation, the Ontario Institute for Cancer Research, and the Ottawa Regional Cancer Foundation. J.A.M. is supported by the Ontario Institute for Cancer Research. J.D.L. is supported by the National Institutes of Health grants AI068730 and the European Community’s Seventh Framework Programme under grant agreement no. 602699 (DIREKT). L.E. is the recipient of a Canadian Institute for Health Research Canada Graduate Scholarship.
J.C.B, L.E, L.S., and Turnstone Biologics are inventors of a patent application describing complement inhibition in conjunction with arenavirus glygoprotein pseudotyped vectors. J.D.L. is the inventor of the patent and/or patent applications that describe the use of complement inhibitors for therapeutic purposes and the founder of Amyndas Pharmaceuticals, which is developing complement inhibitors for clinical applications.
References
- Stojdl, DF, Lichty, BD, tenOever, BR, Paterson, JM, Power, AT, Knowles, S et al. (2003). VSV strains with defects in their ability to shutdown innate immunity are potent systemic anticancer agents. Cancer Cell 4: 263–275. [DOI] [PubMed] [Google Scholar]
- Brun, J, McManus, D, Lefebvre, C, Hu, K, Falls, T, Atkins, H et al. (2010). Identification of genetically modified Maraba virus as an oncolytic rhabdovirus. Mol Ther 18: 1440–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottke, T, Errington, F, Pulido, J, Galivo, F, Thompson, J, Wongthida, P et al. (2011). Broad antigenic coverage induced by vaccination with virus-based cDNA libraries cures established tumors. Nat Med 17: 854–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol, JG, Zhang, L, Bridle, BW, Stephenson, KB, Rességuier, J, Hanson, S et al. (2014). Maraba virus as a potent oncolytic vaccine vector. Mol Ther 22: 420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miest, TS and Cattaneo, R (2014). New viruses for cancer therapy: meeting clinical needs. Nat Rev Microbiol 12: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, AT, Wang, J, Falls, TJ, Paterson, JM, Parato, KA, Lichty, BD et al. (2007). Carrier cell-based delivery of an oncolytic virus circumvents antiviral immunity. Mol Ther 15: 123–130. [DOI] [PubMed] [Google Scholar]
- Hangartner, L, Zinkernagel, RM and Hengartner, H (2006). Antiviral antibody responses: the two extremes of a wide spectrum. Nat Rev Immunol 6: 231–243. [DOI] [PubMed] [Google Scholar]
- Pinschewer, DD, Perez, M, Jeetendra, E, Bächi, T, Horvath, E, Hengartner, H et al. (2004). Kinetics of protective antibodies are determined by the viral surface antigen. J Clin Invest 114: 988–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tober, R, Banki, Z, Egerer, L, Muik, A, Behmüller, S, Kreppel, F et al. (2014). VSV-GP: a potent viral vaccine vector that boosts the immune response upon repeated applications. J Virol 88: 4897–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muik, A, Stubbert, LJ, Jahedi, RZ, Geiβ, Y, Kimpel, J, Dold, C et al. (2014). Re-engineering vesicular stomatitis virus to abrogate neurotoxicity, circumvent humoral immunity, and enhance oncolytic potency. Cancer Res 74: 3567–3578. [DOI] [PubMed] [Google Scholar]
- Ricklin, D, Hajishengallis, G, Yang, K and Lambris, JD (2010). Complement: a key system for immune surveillance and homeostasis. Nat Immunol 11: 785–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaever, T, Meng, X, Matho, MH, Schlossman, A, Li, S, Sela-Culang, I et al. (2014). Potent neutralization of vaccinia virus by divergent murine antibodies targeting a common site of vulnerability in L1 protein. J Virol 88: 11339–11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhnia, MR, McCausland, MM, Moyron, J, Laudenslager, J, Granger, S, Rickert, S et al. (2009). Vaccinia virus extracellular enveloped virion neutralization in vitro and protection in vivo depend on complement. J Virol 83: 1201–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evgin, L, Acuna, SA, Tanese de Souza, C, Marguerie, M, Lemay, CG, Ilkow, CS et al. (2015). Complement inhibition prevents oncolytic vaccinia virus neutralization in immune humans and cynomolgus macaques. Mol Ther 23: 1066–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong, GL and Mattes, MJ (1989). Mouse strains with typical mammalian levels of complement activity. J Immunol Methods 125: 147–158. [DOI] [PubMed] [Google Scholar]
- Ebanks, RO and Isenman, DE (1996). Mouse complement component C4 is devoid of classical pathway C5 convertase subunit activity. Mol Immunol 33: 297–309. [DOI] [PubMed] [Google Scholar]
- Ratelade, J and Verkman, AS (2014). Inhibitor(s) of the classical complement pathway in mouse serum limit the utility of mice as experimental models of neuromyelitis optica. Mol Immunol 62: 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bexborn, F, Engberg, AE, Sandholm, K, Mollnes, TE, Hong, J and Nilsson Ekdahl, K (2009). Hirudin versus heparin for use in whole blood in vitro biocompatibility models. J Biomed Mater Res A 89: 951–959. [DOI] [PubMed] [Google Scholar]
- Qu, H, Ricklin, D, Bai, H, Chen, H, Reis, ES, Maciejewski, M et al. (2013). New analogs of the clinical complement inhibitor compstatin with subnanomolar affinity and enhanced pharmacokinetic properties. Immunobiol 218: 496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risitano, AM, Ricklin, D, Huang, Y, Reis, ES, Chen, H, Ricci, P et al. (2014). Peptide inhibitors of C3 activation as a novel strategy of complement inhibition for the treatment of paroxysmal nocturnal hemoglobinuria. Blood 123: 2094–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.