ABSTRACT
Neuropsychiatric disease is the leading cause of disability in the United States, and fourth worldwide.1,2 Not surprisingly, human genetic studies have revealed a common genetic predisposition for many forms of neuropsychiatric disease, potentially explaining why overlapping symptoms are commonly observed across multiple diagnostic categories. For example, the CACNA1C gene was recently identified in the largest human genome-wide association study to date as a risk loci held in common across 5 major forms of neuropsychiatric disease: bipolar disorder, schizophrenia, major depressive disorder (MDD), autism spectrum disorder and attention deficit-hyperactivity disorder.3 This gene encodes for the Cav1.2 subunit of the L-type voltage-gated calcium channel (LTCC), accounting for 85% of LTCCs in the brain, while the Cav1.3 subunit comprises the remainder.4 In neurons, LTCCs mediate calcium influx in response to membrane depolarization,5 thereby regulating neurotransmission and gene expression. Here, we describe our recent finding that Cav1.2 also controls survival of young hippocampal neurons in the adult brain, which has been linked to the etiology and treatment of neuropsychiatric disease. We also describe the effective restoration of young hippocampal neuron survival in adult Cav1.2 forebrain-specific conditional knockout mice using the neuroprotective compound P7C3-A20.
KEYWORDS: anxiety, BDNF, Cav, neurogenesis, neuroprotection; P7C3, P7C3A20
In order to investigate the function of Cav1.2, genetic mouse models have been applied. Because global knockdown of cacna1c (KO) is embryonic-lethal,6 mice harboring forebrain-specific conditional knockout of cacna1c (forebrain- Cav1.2 cKO) were generated to specifically study the role of Cav1.2 in neuropsychiatric disease. These animals show reductions in spatial memory,7,8 as well as high anxiety-like behavior,9 one of the core symptoms of all forms of neuropsychiatric disease in which CACNA1C has been implicated. Previous in vitro studies have shown that LTCCs mediate survival of cultured hippocampal neural precursor cells,10 and other investigators have shown that postnatal hippocampal neurogenesis is involved in memory, processing of fearful experiences, and anxiety.11-16 We therefore wondered whether forebrain Cav1.2 cKO mice might display an altered pattern of hippocampal neurogenesis, which might contribute to the role of CACNA1C in neuropsychiatric disease.17
To assess the magnitude of hippocampal neurogenesis in forebrain- Cav1.2 cKO mice, we began with a standard week-long assay of daily injection of bromodeoxyuridine (BrdU),17 a thymidine analog that is incorporated into replicating DNA, and observed a 50% reduction in labeled cells in the subgranular zone (SGZ) of the dentate gyrus, compared to age/sex-matched wild type (WT) littermates (Fig. 1). This was accompanied by a similar reduction in expression of the immature neuronal marker doublecortin (DCX) in the SGZ. To differentiate between developmental versus adult roles of Cav1.2, we next used viral delivery of Cre-recombinase into the hippocampus of floxed-cacna1c adult mice. Here, we again observed a 50% decrease in the magnitude of hippocampal neurogenesis using the same assay. This finding supports a postnatal role for Cav1.2 in hippocampal neurogenesis.
Figure 1.
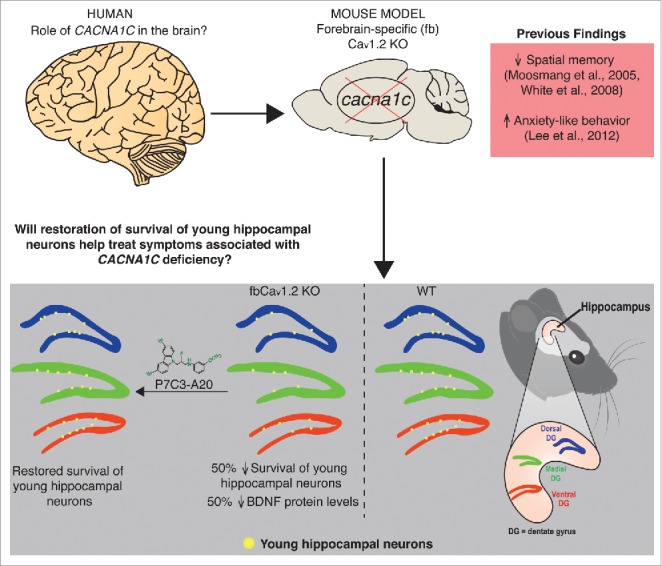
Role of Cav1.2 in the mouse forebrain. Forebrain-specific Cav1.2 deletion (fbCav1.2 KO) in mice results in anxiety-like behaviors and deficits in spatial memory. Heterozygous Cav1.2 mouse models also show deficits in acquisition of conditioned fear,44 as well as depression- and anxiety-like behaviors.45 Our recent findings show that fbCav1.2 KO mice exhibit 50% decrease in brain levels of BDNF, associated with a 50% decrease in survival of young hippocampal neurons in the adult brain. This deficit in neuron survival was overcome by treatment of mice with the neuroprotective compound P7C3-A20, without affecting levels of BDNF in the brain. Our findings identify a new role of the neuropsychiatric-associated gene CACNA1C in the brain, and future work will determine restoration of survival of young hippocampal neurons will help treat behavioral deficits associated with CACNA1C-deficiency. Our ultimate goal is to develop new treatment approaches for patients suffering from forms of neuropsychiatric disease associated with aberration in CACNA1C.
In the week-long assay of daily BrdU labeling, the magnitude of BrdU signal is due to the combined effects of proliferation and survival of newborn neurons in the hippocampus. These aspects of hippocampal neurogenesis are distinct physiologic processes that can be distinguished by pulse-chase BrdU labeling.17 Quantification of signal 1 hour after BrdU labeling showed equal rates of neural precursor cell proliferation in forebrain Cav1.2 cKO mice and age / sex-matched WT littermates. Thirty days after labeling, however, forebrain Cav1.2 cKO mice showed significantly fewer surviving BrdU+ cells, indicating that Cav1.2 mediates survival, and not proliferation, of young hippocampal neurons in the adult brain. This suggests that survival of these young neurons may be important in warding off neuropsychiatric disease. Since increased cell death in some cases may result in decreased hippocampal volume,18 we next performed morphometric analysis of the dentate gyrus, CA1 and CA3 hippocampal regions. We observed no differences in size of these regions between forebrain Cav1.2 cKO mice and age / sex-matched WT littermates, further supporting a more prominent role of Cav1.2 in adulthood rather than development.
Because the survival component of postnatal hippocampal neurogenesis is regulated in part by the neurotrophic agent brain-derived neurotrophic factor (BDNF),19 and LTCC-signaling controls BDNF expression in in vitro systems,20,21 we measured BDNF protein levels in forebrain Cav1.2 cKO mice. Here, we observed a 50% reduction relative to age / sex-matched WT littermates (Fig. 1), providing the first in vivo evidence that BDNF expression in the adult brain is controlled by Cav1.2. We next wondered whether we could restore normal hippocampal neurogenesis in forebrain Cav1.2 cKO mice without intervening in the underlying BDNF-deficit, as there are no current drug therapies that specifically target BDNF levels in the brain. To address this problem, we turned to the neuroprotective aminopropyl carbazole compound P7C3-A20, a small molecule that blocks neuronal cell death,22-28 thereby increasing the magnitude of hippocampal neurogenesis.29-31 Treatment of forebrain Cav1.2 cKO mice with P7C3-A20 restored normal survival of young hippocampal neurons (Fig. 1), as measured by BrdU-labeling and DCX expression. Restoration of normal hippocampal neurogenesis was not accompanied by an increase in BDNF, however, demonstrating that deficits in hippocampal neurogenesis associated with BDNF deficiency in the adult brain can be corrected with therapeutic agents that do not restore BDNF.
Anxiety-like behavior, which is prominently displayed by forebrain-conditional Cav1.2 KO mice,9 can occur as a consequence of over-activation of the hypothalamic-pituitary-adrenal (HPA) axis, leading to elevated levels of circulating corticosterone.32 Moreover, environmental stress, either chronic or acute, is known to augment the development and progression of mental illness.33-37 Since forebrain Cav1.2 cKO mice show elevated anxiety-like behavior, we tested whether they also displayed altered levels of plasma corticosterone. We found no difference in either basal or acute stress-induced corticosterone levels in forebrain Cav1.2 cKO mice compared to age / sex-matched WT littermates. Thus, the anxiety-like behavior in this preclinical model of neuropsychiatric disease is independent of the HPA axis, further underscoring the importance of CACNA1C in precipitating common symptoms across multiple forms of neuropsychiatric disease.
In addition to the HPA axis, a substantial body of literature supports a role for hippocampal neurogenesis in depression and anxiety, including evidence that increasing postnatal hippocampal neurogenesis reduces anxiety and depression-like behaviors in mice.12,38-40 Furthermore, a recent small Phase 1B, randomized, double blind, placebo controlled, multiple-dose escalation study using a new drug that stimulates hippocampal neurogenesis, known as NSI-189 phosphate, has shown favorable outcome in treatment for MDD in humans, with significant improvement shown in the Symptoms of Depression Questionnaire and Cognitive and Physical Functioning Questionnaire.41 Future work in forebrain Cav1.2 cKO mice will determine whether restoration of normal hippocampal neurogenesis in adulthood will ameliorate anxiety-like behavior.
Taken together, our recent work demonstrates that Cav1.2 serves a critical role in protecting young hippocampal neurons from death in the adult brain. This is in contrast to the known role of the other predominant LTCC in the hippocampus, Cav1.3, which is involved in both proliferation and maturation of newborn hippocampal neurons.42 Mice harboring genetic deficiency in Cav1.3 also show smaller dentate gyrus volume, underscoring the likelihood of a developmental role for Cav1.3 as well.42 Future delineation of the distinct and overlapping physiologic roles of Cav1.2 and Cav1.3 in hippocampal neurogenesis will provide insight into new therapeutic strategies to augment neurogenesis in ways that may benefit patients.
Another critical future direction is to determine whether the survival deficit in young hippocampal neurons of forebrain Cav1.2 cKO mice is a cell autonomous or non-autonomous effect. Since BDNF is released from neurons and promotes neuronal survival,43 and we observe a 50% BDNF reduction in these mice, our results suggest a non-autonomous effect. However, this does not exclude the possibility that Cav1.2 may also play a role in neuronal cell death by regulating calcium signaling in a cell-autonomous manner.
If the latter possibility proved to be true in young hippocampal neurons, this would raise the possibility that Cav1.2 might also play a role in regulating death of mature nerve cells as well. If so, this could point to new therapeutic opportunities to mitigate neuronal cell death in a broad array of neuropsychiatric conditions, including neurodegenerative disease and normal aging, while also providing evidence for a role of neurodegenerative processes in progression of multiple forms of mental illness. Indeed, the progressive nature of many forms of neuropsychiatric disease, including those linked to CACNA1C, is consistent with neurodegenerative processes that would be amenable to treatment with neuroprotective agents.46 In this light, it is useful to note that P7C3 compounds have demonstrated protective efficacy in preclinical models of Parkinson disease,24,28-29 cognitive decline with aging,31 amyotrophic lateral sclerosis,23 traumatic brain injury,25-27 peripheral nerve injury,22 neurodegeneration-associated depression,46 and now cell death associated with genetic susceptibility to mental illness.17 It has recently been reported that P7C3 compounds enhance flux of the nicotinamide adenine dinucleotide (NAD) salvage pathway in normal mammalian cells, and also facilitate NAD recovery following doxorubicin exposure.36 NAD is important in regulating essential substrates for mitochondrial metabolism, and is also a major substrate for ATP production important to neuronal survival.37 Thus, P7C3 compounds, or other pharmacologic interventions with neuroprotective or NAD-augmenting capability, might be useful for mitigating disease progression in many forms of mental illness, for which only symptomatic treatment is currently available.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by funds from the University of Iowa Carver College of Medicine to A.A.P, funds from an anonymous donor to the Mary Alice Smith Fund for Neuropsychiatry Research to A.A.P, a National Science Foundation fellowship to H.DJ-C. Funding from The Hartwell Foundation to A.M.R and A.A.P., and Weill Cornell Autism Research Program funding to A.M.R. A.A.P. holds patents on the P7C3 family of neuroprotective compounds.
References
- [1].Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, et al.. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2197-223; PMID:23245608; http://dx.doi.org/ 10.1016/S0140-6736(12)61689-4 [DOI] [PubMed] [Google Scholar]
- [2].Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, et al.. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 2013; 382:1575-86; PMID:23993280; http://dx.doi.org/ 10.1016/S0140-6736(13)61611-6 [DOI] [PubMed] [Google Scholar]
- [3].Cross-Disorder Group of the Psychiatric Genomics C . Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet 2013; 381:1371-9; PMID:23453885; http://dx.doi.org/ 10.1016/S0140-6736(12)62129-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sinnegger-Brauns MJ, Huber IG, Koschak A, Wild C, Obermair GJ, Einzinger U, Hoda JC, Sartori SB, Striessnig J. Expression and 1,4-dihydropyridine-binding properties of brain L-type calcium channel isoforms. Mol Pharmacol 2009; 75:407-14; PMID:19029287; http://dx.doi.org/ 10.1124/mol.108.049981 [DOI] [PubMed] [Google Scholar]
- [5].Hofmann F, Flockerzi V, Kahl S, Wegener JW. L-type CaV1.2 calcium channels: from in vitro findings to in vivo function. Physiol Rev 2014; 94:303-26; PMID:24382889; http://dx.doi.org/ 10.1152/physrev.00016.2013 [DOI] [PubMed] [Google Scholar]
- [6].Seisenberger C, Specht V, Welling A, Platzer J, Pfeifer A, Kuhbandner S, Striessnig J, Klugbauer N, Feil R, Hofmann F. Functional embryonic cardiomyocytes after disruption of the L-type alpha1C (Cav1.2) calcium channel gene in the mouse. J Biol Chem 2000; 275:39193-9; PMID:10973973; http://dx.doi.org/ 10.1074/jbc.M006467200 [DOI] [PubMed] [Google Scholar]
- [7].Moosmang S, Haider N, Klugbauer N, Adelsberger H, Langwieser N, Muller J, Stiess M, Marais E, Schulla V, Lacinova L, et al.. Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J Neurosci: Official J Society Neurosci 2005; 25:9883-92; PMID:16251435; http://dx.doi.org/ 10.1523/JNEUROSCI.1531-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].White JA, McKinney BC, John MC, Powers PA, Kamp TJ, Murphy GG. Conditional forebrain deletion of the L-type calcium channel Ca V 1.2 disrupts remote spatial memories in mice. Learning Memory 2008; 15:1-5; PMID:18174367; http://dx.doi.org/ 10.1101/lm.773208 [DOI] [PubMed] [Google Scholar]
- [9].Lee AS, Ra S, Rajadhyaksha AM, Britt JK, De Jesus-Cortes H, Gonzales KL, Lee A, Moosmang S, Hofmann F, Pieper AA, et al.. Forebrain elimination of cacna1c mediates anxiety-like behavior in mice. Mol Psychiatry 2012; 17:1054-5; PMID:22665262; http://dx.doi.org/ 10.1038/mp.2012.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron 2004; 42:535-52; PMID:15157417; http://dx.doi.org/ 10.1016/S0896-6273(04)00266-1 [DOI] [PubMed] [Google Scholar]
- [11].Besnard A, Sahay A. Adult Hippocampal Neurogenesis, Fear Generalization, and Stress. Neuropsychopharmacol: Official Publ Am College Neuropsychopharmacol 2016; 41:24-44; PMID:26068726; http://dx.doi.org/ 10.1038/npp.2015.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hill AS, Sahay A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to reduce anxiety and depression-like behaviors. Neuropsychopharmacol: Official Publ Am College Neuropsychopharmacol 2015; 40:2368-78; PMID:25833129; http://dx.doi.org/ 10.1038/npp.2015.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kheirbek MA, Klemenhagen KC, Sahay A, Hen R. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat Neurosci 2012; 15:1613-20; PMID:23187693; http://dx.doi.org/ 10.1038/nn.3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 2011; 472:466-70; PMID:21460835; http://dx.doi.org/ 10.1038/nature09817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sahay A, Wilson DA, Hen R. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron 2011; 70:582-8; PMID:21609817; http://dx.doi.org/ 10.1016/j.neuron.2011.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wu MV, Sahay A, Duman RS, Hen R. Functional differentiation of adult-born neurons along the septotemporal axis of the dentate gyrus. Cold Spring Harbor Perspectives Biol 2015; 7:a018978; PMID:26238355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lee AS, De Jesus-Cortes H, Kabir ZD, Knobbe W, Orr M, Burgdorf C, Huntington P, McDaniel L, Britt JK, Hoffmann F, et al.. The neuropsychiatric disease-associated gene cacna1c mediates survival of young hippocampal neurons. Eneuro 2016; 3(2); PMID:27066530; http://dx.doi.org/ 10.1523/ENEURO.0006-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pieper AA, Wu X, Han TW, Estill SJ, Dang Q, Wu LC, Reece-Fincanon S, Dudley CA, Richardson JA, Brat DJ, et al.. The neuronal PAS domain protein 3 transcription factor controls FGF-mediated adult hippocampal neurogenesis in mice. Proc Natl Acad Sci U S A 2005; 102:14052-7; PMID:16172381; http://dx.doi.org/ 10.1073/pnas.0506713102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Choi SH, Li Y, Parada LF, Sisodia SS. Regulation of hippocampal progenitor cell survival, proliferation and dendritic development by BDNF. Mol Neurodegeneration 2009; 4:52; PMID:20025751; http://dx.doi.org/ 10.1186/1750-1326-4-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ghosh A, Carnahan J, Greenberg ME. Requirement for BDNF in activity-dependent survival of cortical neurons. Science 1994; 263:1618-23; PMID:7907431; http://dx.doi.org/ 10.1126/science.7907431 [DOI] [PubMed] [Google Scholar]
- [21].Tabuchi A, Nakaoka R, Amano K, Yukimine M, Andoh T, Kuraishi Y, Tsuda M. Differential activation of brain-derived neurotrophic factor gene promoters I and III by Ca2+ signals evoked via L-type voltage-dependent and N-methyl-D-aspartate receptor Ca2+ channels. J Biol Chem 2000; 275:17269-75; PMID:10748141; http://dx.doi.org/ 10.1074/jbc.M909538199 [DOI] [PubMed] [Google Scholar]
- [22].Kemp SW, Szynkaruk M, Stanoulis KN, Wood MD, Liu EH, Willand MP, Morlock L, Naidoo J, Williams NS, Ready JM, et al.. Pharmacologic rescue of motor and sensory function by the neuroprotective compound P7C3 following neonatal nerve injury. Neuroscience 2015; 284:202-16; PMID:25313000; http://dx.doi.org/ 10.1016/j.neuroscience.2014.10.005 [DOI] [PubMed] [Google Scholar]
- [23].Tesla R, Wolf HP, Xu P, Drawbridge J, Estill SJ, Huntington P, McDaniel L, Knobbe W, Burket A, Tran S, et al.. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A 2012; 109:17016-21; PMID:23027932; http://dx.doi.org/ 10.1073/pnas.1213960109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].De Jesus-Cortes H, Xu P, Drawbridge J, Estill SJ, Huntington P, Tran S, Britt J, Tesla R, Morlock L, Naidoo J, et al.. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of Parkinson disease. Proc Natl Acad Sci U S A 2012; 109:17010-5; PMID:23027934; http://dx.doi.org/ 10.1073/pnas.1213956109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Blaya MO, Bramlett HM, Naidoo J, Pieper AA, Dietrich WD. Neuroprotective efficacy of a proneurogenic compound after traumatic brain injury. J Neurotrauma 2014; 31:476-86; PMID:24070637; http://dx.doi.org/ 10.1089/neu.2013.3135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yin TC, Britt JK, De Jesus-Cortes H, Lu Y, Genova RM, Khan MZ, Voorhees JR, Shao J, Katzman AC, Huntington PJ, et al.. P7C3 neuroprotective chemicals block axonal degeneration and preserve function after traumatic brain injury. Cell Reports 2014; 8:1731-40; PMID:25220467; http://dx.doi.org/ 10.1016/j.celrep.2014.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dutca LM, Stasheff SF, Hedberg-Buenz A, Rudd DS, Batra N, Blodi FR, Yorek MS, Yin T, Shankar M, Herlein JA, et al.. Early detection of subclinical visual damage after blast-mediated TBI enables prevention of chronic visual deficit by treatment with P7C3-S243. Invest Ophthalmol Visual Sci 2014; 55:8330-41; PMID:25468886; http://dx.doi.org/ 10.1167/iovs.14-15468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].De Jesús-Cortés H, Miller AD, Britt JK, DeMarco AJ, De Jesús-Cortés M, Stuebing E, Naidoo J, Vázquez-Rosa E, Morlock L, Williams NS, et al.. Protective efficacy of P7C3-S243 in the 6-hydroxydopamine model of Parkinson disease. NPJ Parkinson Dis 2015; 1; PMID:27158662; http://dx.doi.org/ 10.1038/npjparkd.2015.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Naidoo J, De Jesus-Cortes H, Huntington P, Estill S, Morlock LK, Starwalt R, Mangano TJ, Williams NS, Pieper AA, Ready JM. Discovery of a neuroprotective chemical, (S)-N-(3-(3,6-dibromo-9H-carbazol-9-yl)-2-fluoropropyl)-6-methoxypyridin-2-amine [(−)-P7C3-S243], with improved druglike properties. J Med Chem 2014; 57:3746-54; PMID:24697290; http://dx.doi.org/ 10.1021/jm401919s [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pieper AA, McKnight SL, Ready JM. P7C3 and an unbiased approach to drug discovery for neurodegenerative diseases. Chem Society Rev 2014; 43:6716-26; PMID:24514864; http://dx.doi.org/ 10.1039/C3CS60448A [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pieper AA, Xie S, Capota E, Estill SJ, Zhong J, Long JM, Becker GL, Huntington P, Goldman SE, Shen CH, et al.. Discovery of a proneurogenic, neuroprotective chemical. Cell 2010; 142:39-51; PMID:20603013; http://dx.doi.org/ 10.1016/j.cell.2010.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry 1999; 46:1167-80; PMID:10560023; http://dx.doi.org/ 10.1016/S0006-3223(99)00164-X [DOI] [PubMed] [Google Scholar]
- [33].Benjet C, Borges G, Medina-Mora ME. Chronic childhood adversity and onset of psychopathology during three life stages: childhood, adolescence and adulthood. J Psychiatric Res 2010; 44:732-40; PMID:20144464; http://dx.doi.org/ 10.1016/j.jpsychires.2010.01.004 [DOI] [PubMed] [Google Scholar]
- [34].Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological Psychiatry 2001; 49:1023-39; PMID:11430844; http://dx.doi.org/ 10.1016/S0006-3223(01)01157-X [DOI] [PubMed] [Google Scholar]
- [35].Kessler RC, McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, Aguilar-Gaxiola S, Alhamzawi AO, Alonso J, Angermeyer M, et al.. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br J Psychiatry: J Mental Sci 2010; 197:378-85; PMID:21037215; http://dx.doi.org/ 10.1192/bjp.bp.110.080499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Scott KM, McLaughlin KA, Smith DA, Ellis PM. Childhood maltreatment and DSM-IV adult mental disorders: comparison of prospective and retrospective findings. Br J Psychiatry: J Mental Sci 2012; 200:469-75; PMID:22661679; http://dx.doi.org/ 10.1192/bjp.bp.111.103267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Varese F, Smeets F, Drukker M, Lieverse R, Lataster T, Viechtbauer W, Read J, van Os J, Bentall RP. Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophrenia Bulletin 2012; 38:661-71; PMID: 22461484; http://dx.doi.org/ 10.1093/schbul/sbs050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, et al.. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003; 301:805-9; PMID:12907793; http://dx.doi.org/ 10.1126/science.1083328 [DOI] [PubMed] [Google Scholar]
- [39].Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci 2007; 10:1110-5; PMID: 17726477; http://dx.doi.org/ 10.1038/nn1969 [DOI] [PubMed] [Google Scholar]
- [40].Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, Hen R, Belzung C. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry 2008; 64:293-301; PMID:18406399; http://dx.doi.org/ 10.1016/j.biopsych.2008.02.022 [DOI] [PubMed] [Google Scholar]
- [41].Fava M, Johe K, Ereshefsky L, Gertsik LG, English BA, Bilello JA, Thurmond LM, Johnstone J, Dickerson BC, Makris N, et al.. A Phase 1B, randomized, double blind, placebo controlled, multiple-dose escalation study of NSI-189 phosphate, a neurogenic compound, in depressed patients. Mol Psychiatry 2015; PMID:26643541; http://dx.doi.org/ 10.1038/mp.2015.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Marschallinger J, Sah A, Schmuckermair C, Unger M, Rotheneichner P, Kharitonova M, Waclawiczek A, Gerner P, Jaksch-Bogensperger H, Berger S, et al.. The L-type calcium channel Cav1.3 is required for proper hippocampal neurogenesis and cognitive functions. Cell Calcium 2015; 58:606-16; PMID:26459417; http://dx.doi.org/ 10.1016/j.ceca.2015.09.007 [DOI] [PubMed] [Google Scholar]
- [43].Lipsky RH, Marini AM. Brain-derived neurotrophic factor in neuronal survival and behavior-related plasticity. Annals N York Acad Sci 2007; 1122:130-43; PMID:18077569; http://dx.doi.org/ 10.1196/annals.1403.009 [DOI] [PubMed] [Google Scholar]
- [44].Langweiser N, Christel CJ, Kleppisch T, Hofmann F, Wotjak CT, Moosmang S. Homeostatis switch in hebbian plasticity and fear learning after sustained loss of Cav1.2 calcium channels. J Neuroscience 2010; 30:8367-75; PMID:20573883; http://dx.doi.org/ 10.1523/JNEUROSCI.4164-08.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dao DT, Mahon PB, Cai X, Kovacsics CE, Blackwell RA, Arad M, Shi J, Zandi PP, O'Donnell P, Bipolar Genome Study (BiGS) Consortium, Knowles JA, et al.. Mood disorder susceptibility gene CACNA1C modifies mood-related behaviors in mice and interacts with sex to influence behavior in mice and diagnosis in humans. Biol Psychiatry 2010; 68:801-10; PMID:20723887; http://dx.doi.org/ 10.1016/j.biopsych.2010.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Dodd S, Maes M, Anderson G, Dean OM, Moylan S, Berk M. Putative neuroprotective agents in neuropsychiatric disorders. Prog Neuro-Psychopharmacol Biol Psych 2013; 42:135-45; http://dx.doi.org/ 10.1016/j.pnpbp.2012.11.007 [DOI] [PubMed] [Google Scholar]


