Abstract
The difference between male and female behavior and male and female susceptibility to a number of neuropsychiatric conditions is not controversial. From a biological perspective, one might expect to see at least some of these differences underpinned by identifiable physical differences in the brain. This Mini‐Review focuses on evidence that plasticity mechanisms differ between males and females and ask at what scale of organization the differences might exist, at the systems level, the circuits level, or the synaptic level. Emerging evidence suggests that plasticity differences may extend to the scale of synaptic mechanisms. In particular, the CaMKK, NOS1 and estrogen receptor pathways show sexual dimorphisms with implications for plasticity in the hippocampus and cerebral cortex. © 2016 The Authors. Journal of Neuroscience Research Published by Wiley Periodicals, Inc.
Keywords: neuropsychiatric conditions, CaMKK, NOS1, estrogen receptor, hippocampus, cerebral cortex
The difference between male and female animals is unmistakable on the outside, and from a biological perspective one might expect to find many differences on the inside too. It is not controversial that male and female behavior is different both in humans and in less sentient animals and highly likely that many of those behavioral differences can be attributed to differences in brain structure. Over what spatial scale might such differences occur? At the systems level, male and female brains differ in size (Goldstein et al., 2001; Gur et al., 2012) and connectivity (Ingalhalikar et al., 2014); the hypothalamic structures and circuits are different because of their roles in reproduction and hormone regulation (Simerly, 2002; Scott et al., 2015), and sex hormones are known to have effects on the function and development of neuronal circuits (Dohler et al., 1986; Scharfman and MacLusky, 2006). However, is it also possible that differences between the sexes exist on a finer scale, perhaps down to the level of individual synapses and the molecular mechanisms that are involved in synaptic plasticity.
To date, sex differences remain relatively underexplored in neuroscience. The relative lack of exploration may rest partially on practical grounds, such as the belief that biological results are more variable in females than in males, which leads to experimental designs employing only male animals. In a similar vein, because at least twice as many animals would be required to test for a difference in the role of a particular variable between male and female mice, the financial cost of doing so is twice as high and requires twice the time, and the subsequent cost may discourage the practice. In addition to errors of commission there may also be errors of omission. Pogun, noted as recently as 2001 that
Although males and females are unmistakably different, the recognition of sex as a key variable in science and medicine is considered a revolution in some circles. Sex differences transcend reproductive functions, are evident in the structural and functional organization of the brain, and are reflected in group differences in cognitive abilities and behavior. (Pogun, 2001).
Indeed, given the findings in mouse studies that genetic background can have a large effect on learning, plasticity, and behavior (Nguyen et al., 2000; Ranson et al., 2013), it seems almost inevitable that a far less subtle genetic difference between animals, such as an entire chromosome difference, would have some effect. This Mini‐Review explores some of the evidence for differences in plasticity at the cellular level and how those differences might impact learning and memory at the behavioral level. Particular attention is given to the role of estrogen in structural plasticity of dendritic spines and the differing degrees to which nitric oxide synthase (NOS) plays a role in synaptic potentiation in males vs. females. Given recent findings showing that synaptic proteins are major factors in mental health conditions (Hall et al., 2015; Reichelt et al., 2012), we further explore the evidence for sex differences in schizophrenia and autism‐spectrum disorders (ASD) as a possible cause. We begin by summarizing some of the molecular differences reported to date between synapses in males and females.
MOLECULAR ORGANIZATION OF THE SYNAPSE IN MALES AND FEMALES
Calcium/calmodulin kinase kinase (CaMKK) signaling has been found to differ in male and female mice, in both behavioral and plasticity studies (Fig. 1). The two isoforms of CaMKK, CaMKKα and CaMKKβ, act by phosphorylating CaMKI and CaMKIV, which in turn modulate the activity of the transcription factor, cAMP‐responsive element binding protein (CREB; Bito et al., 1997), most likely by calcium entry into the cytoplasm through NMDA receptors and L‐type calcium channel (Deisseroth et al., 1998). Mice lacking either CaMKKα or CaMKKβ reveal striking sex differences in tests of behavior and hippocampal synaptic plasticity (for review see Mizuno and Giese, 2010). Male mice lacking CaMKKα have deficits in contextual fear conditioning, which may relate to the lack of upregulation of brain‐derived neurotrophic factor (BDNF) by CaMKKα in males that would normally accompany this task in females (Mizuno et al., 2006). Male CaMKKβ‐deficient mice are impaired in spatial learning and lack hippocampal CREB activation compared with female null mice and also lack late‐phase hippocampal long‐term potentiation (LTP; Mizuno et al., 2007). Baseline sex differences in CREB signaling also exist, with male neonatal rodents having greater phosphorylated CREB expression in hippocampal CA1 (Auger et al., 2001). It is known that CREB is involved in neocortical experience‐dependent plasticity (Glazewski et al., 1999; Barth et al., 2000), but it is not known whether the hippocampal differences in CaMKK and CREB signaling extend to neocortical circuits. These studies provide clear evidence for the basis of sex differences in synaptic plasticity, but further work is required to 1) determine whether CaMKK differences generalize to other brain regions, including the cortex, and 2) identify further sex‐related protein candidates for differences in plasticity.
Figure 1.
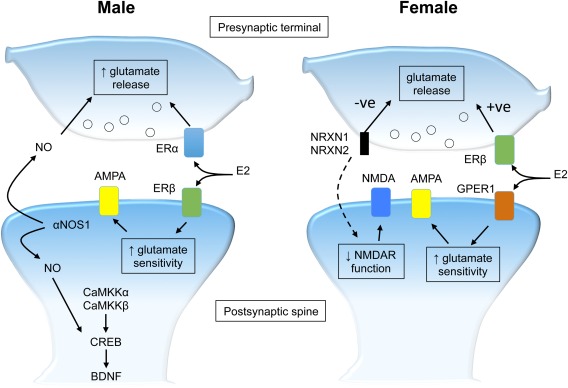
Summary of synaptic plasticity and molecular pathways that differ between the sexes. Neocortical αNOS1 in males has been shown to be involved in in vitro and in vivo synaptic plasticity, with NO acting both pre‐ and postsynaptically. NO and αNOS1 are also more abundant in the male than in the female hippocampus. CaMKKα and CaMKKβ are also more crucial in the male brain for memory tasks, LTP, and CREB transcription. E2 acts via different pre‐ and postsynaptic disposition of oestrogen receptors between the sexes to facilitate increased presynaptic glutamate release and greater postsynaptic glutamate sensitivity; males require presynaptic ERα and postsynaptic ERβ, whereas females employ presynaptic ERβ and postsynaptic GPER1. Deletions of αNrxn1 and αNrxn2 have been shown to impair female behaviors, including anxiety, sociability, and memory, and a loss of presynaptic NRXN2 impairs glutamate release and postsynaptic NMDA receptor function in the neocortex. Dashed line represents the effect of mutations in the NRXN genes that would impair synaptic function. NB: the molecules depicted are expressed in males and females but have specific actions or pre‐/postsynaptic locations only in one or the other as shown.
SEX DIFFERENTIATION OF THE ROLE OF α‐NITRIC OXIDE SYNTHASE‐1 IN CORTICAL PLASTICITY
Studies in the barrel cortex have shown that both pre‐ and postsynaptic mechanism are activated during expression of cortical synaptic potentiation (Hardingham and Fox, 2006). The postsynaptic aspect of potentiation is dependent on αCaMKII autophosphorylation and GluA1 (GluR1), whereas the presynaptic component depends on postsynaptically located αNOS1 (Hardingham et al., 2003; Hardingham and Fox, 2006). Studies from hippocampus, cerebral cortex, and several other brain structures all suggest that nitric oxide is involved in increasing transmitter release, most likely through a coordinated and synergistic action on several components of the presynaptic release machinery (Hardingham et al., 2013). Theoretically, both activity‐dependent isoforms of nitric oxide synthase (NOS1 and NOS3) could be the source of the nitric oxide signal, and indeed there is evidence that NOS3 and αNOS1 are involved in providing a tonic and phasic release of nitric oxide, respectively (Hopper and Garthwaite, 2006).
The phasic component of nitric oxide release is calmodulin‐ and αNOS1‐dependent and can be triggered by NMDA receptor activation. Early indications that the function of αNOS1 might be different between males and females came from studies showing that knocking out αNOS1 confers some neuroprotection from ischemic damage produced by stroke in the male brain but has no protective effect on the female brain (Huang et al., 1994; McCullough et al., 2005). Subsequently, it was found that αNOS1 is also necessary for LTP in male but not female mice (Dachtler et al., 2012). The residual LTP in female mice is not susceptible to a general NOS antagonist, suggesting that the main component of LTP does not rely on nitric oxide signaling at all in female animals (Fig. 2). The dependence of male plasticity on NO may relate to baseline differences between the sexes. Females have, within the hippocampus, less abundant NO and reduced NOS1 expression compared with males, although the application of estradiol increases hippocampal NOS1 expression (Hu et al., 2012). Therefore, a possible explanation for this sex difference is that females lack available NO for the induction of plasticity and thus rely on other molecular pathways instead. It is conceivable that the sex differences in plasticity and susceptibility to stroke damage are related. If nitric oxide is released during ischemic damage, it would tend to potentiate excitatory transmission in the male brain, leading to greater NMDA receptor activation, greater calcium entry, and greater excitotoxic damage.
Figure 2.
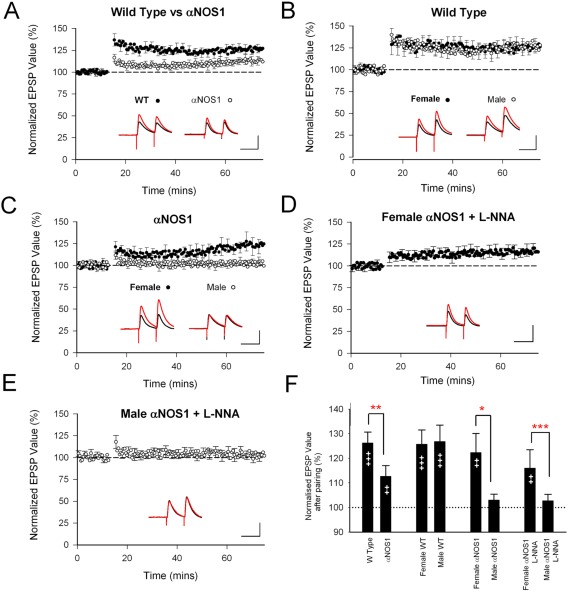
LTP is reduced in αNOS1 knockouts in a sex‐specific manner. A: LTP is reduced in αNOS1 knockouts compared with wild types (WTs; sexes combined). B: Male and female WTs showed similar magnitudes of LTP. C: Male αNOS1s show no significant LTP, whereas female αNOS1 knockouts do show LTP. L‐NNA has no effect on LTP in female αNOS1 knockouts (D) or male αNOS1 knockouts (E), which already lack LTP. F: Average level of potentiation observed at 60 min, showing within‐group significance (††P < 0.01, †††P < 0.001, paired t‐test) and comparisons between genotypes or sexes (*P < 0.05, **P < 0.01, ***P < 0.005). Scale bar = 100 msec/5 mV for example paired pulse EPSPs. Reproduced from the Journal of Neuroscience Dachtler et al. (2012) with permission.
Experience‐dependent potentiation in the somatosensory cortex depends on many of the same factors as LTP. For example, both αNOS1 and GluA1 are required for pre‐ and postsynaptic components of LTP, respectively, and both are important for experience‐dependent plasticity in the barrel cortex (Dachtler et al., 2011). Knocking out both GluA1 and αNOS1 abolishes experience‐dependent potentiation in the cortex, whereas knocking out either gene individually produces a reduction in but not a complete elimination of plasticity (Dachtler et al. 2011). Subsequent, experiments showed that the αNOS1 component of experience‐dependent plasticity was also sex dependent, as with LTP (Dachtler et al., 2012). Depriving mice of all but one whisker for a period of 3–4 weeks normally results in expansion of the spared whisker domain within the barrel cortex, such that neurons located in barrels surrounding the spared whisker's home barrel are far more sensitive to spared whisker stimulation than in a normal animal (Glazewski et al., 1996). This process is dependent on intracortical circuits and, crucially, on excitatory connections between cortical columns (Fox, 1994; Glazewski et al., 2000). Studies comparing experience‐dependent plasticity in male and female αNOS1 knockout mice showed that experience‐dependent potentiation was almost completely absent in αNOS1–/– males and intact in αNOS1–/– females (Dachtler et al., 2012). Taken together with the lack of LTP in the male αNOS1 knockouts, these findings suggest either that potentiation is αNOS1 dependent only in male mice or that females have an alternate (or compensating) mechanism that comes into play when αNOS1 is knocked out and this compensation system is absent in males. In either case, these studies provide evidence for the theory that cortical synaptic plasticity is differentiated at the level of the synapse between the two sexes.
ROLE OF ESTROGEN IN SYNAPTIC PLASTICITY
CaMKK and αNOS1 are examples of distinct, sexually dimorphic molecular pathways underpinning synaptic plasticity. However, the most obvious difference between the sexes is the presence of circulating estrogen (predominantly as 17β‐estradiol [E2]), a molecule known to affect synaptic plasticity directly (Cordoba Montoya and Carrer, 1997). High levels of estrogen are present in the female brain from circulating hormone, and noncirculating estrogen is also present in the male brain, albeit at far lower concentrations, where testosterone acts as a precursor to estrogen by aromatase catalysis (Gillies and McArthur, 2010).
Does Estrogen Have a Direct Role in Synaptic Plasticity?
Warren et al. (1995) performed a simple assay to examine whether estrogen has a role in LTP by testing females at different points in their estrous cycle. They found that, when females were at proestrus, the magnitude of hippocampal LTP was greater than that at either diestrus or estrus (Warren et al., 1995). Prior to this study, others had noted that the abundance of E2 was correlated with dendritic spine plasticity in the hippocampus; spine density was 32% lower at estrus compared with the proestrus phase of the cycle (Woolley and McEwen, 1992). The E2‐dependent increase in spine density at proestrus could be stabilized by NMDA receptors; it was prevented by NMDA receptor antagonists, whereas AMPA or muscarinic receptor antagonists had no effect (Woolley and McEwen, 1994). E2 acted to increase the sensitivity of synapses to NMDA receptor‐mediated input (Woolley et al., 1997); LTP increased only when both spine density increased and there was an enhancement of NMDA receptor transmission relative to AMPA receptor transmission (Smith and McMahon, 2005). Indeed, although E2 was found to cause spinogenesis, synapses remained “silent” unless NMDA receptor activation occurred, allowing the spine to stabilize (Srivastava et al., 2008). Specifically, E2‐dependent facilitation of LTP appears to act through GluN2B‐containing (also known as NR2B) NMDA receptors (Smith and McMahon, 2006). Overall, acute treatment of E2 causes increases in spine density and facilitation of LTP through GluN2B receptors, which could be linked to the estrus cycle in females.
Sex Difference In Estrogen and Plasticity
E2 treatment in slices of hippocampal CA1 acutely potentiates glutamatergic synapses of both sexes. Although males and females arrive at the same plasticity outcome of E2 treatment, the mechanisms by which they do it vary. The compound WAY20070 is an agonist of the β form of the estrogen receptor (ERβ) and causes an increase in the frequency of miniature excitatory postsynaptic currents (mEPSCs) in females but not males and, conversely, increases the amplitude of mEPSCs in males but not females. An ERα agonist (PPT) increases mEPSC frequency only in males (Oberlander, 2016). Hence, E2 acts via distinct estrogen receptors at pre‐ and postsynaptic locations. Furthermore, an agonist to the G‐protein‐coupled estrogen receptor‐1 (GPER1) causes increases in mEPSC amplitude in females but not in males (Oberlander, 2016). Therefore, at glutamateric synapses, in females, E2 facilitates potentiation by postsynaptic GPER1 and presynaptic ERβ, whereas, in males, E2 acts through postsynaptic ERβ and presynaptic ERα (Fig. 1). Currently it is unclear what advantage this sex difference would convey, and whether the recruitment of other downstream elements of the pre‐ and postsynaptic machinery (such as NOS) are consequently differentially modulated.
Recent studies have also demonstrated a role for E2 in inhibition and hve begun to reveal not only that is this sex specific but that E2 acts through distinct molecular pathways. Acute E2 application to hippocampal slices has revealed a rapid suppression of GABAergic inhibitory synaptic transmission at perisomatic inputs to pyramidal cells within the hippocampal CA1, specifically through a molecular cascade including the α form of the estrogen receptor (ERα), metabotrophic glutamate receptor 1 (mGluR1), and endocannabinoid receptor 1 (Huang and Woolley 2012). Remarkably, this inhibitory effect was evident only in females; E2 had no effect on inhibitory postsynaptic currents (IPSCs) in males (Huang and Woolley 2012). Further work revealed this was because E2 promotes an ERα–mGluR1 interaction only in females, which in turn stimulates production of phospholipase C and inositol triphosphate (IP3), leading to postsynaptic endocannabinoid release (Tabatadze, 2015).
Is Circulating Estrogen the Source of the Sex Difference?
Theoretically, the sex differences in ERs discussed above could account for different effects of estrogen from the common source of aromatase, present in males and females. However, several studies suggest that circulating estrogen is in practice the cause of the spinogenic differences in hippocampal plasticity. First, E2 levels are very much lower in males (below detection limits [0.07pM]) than in females. Second, in vivo, E2 increases spine density only in females. Third, spinogenesis still occurs if aromatase is blocked with tetrazole in cycling females, but this has no effect in males (Fester et al., 2012). Finally, E2 increases LTP sensitivity only in females (Vierk et al., 2015). Although these findings do not preclude aromatase‐generated E2 as a determinant of other sex differences, they do imply that the major difference in LTP between the sexes can be attributed to the action of circulating hormone.
SEX DIFFERENCES IN BEHAVIOR AND NEUROPSYCHIATRIC DISORDERS INVOLVING THE CORTEX
Behavior is the final output of all the upstream synaptic functions, so sex differences in behavior can reveal insights into how cortical activity differs between males and females. Likewise, aberrations in behavior caused by certain psychiatric conditions tend to be sex specific in their expression, which can provide insight into which cortical circuits and synaptic mechanisms differ between the sexes.
Sex Differences in Cortex‐Dependent Behaviors
Males and females show differences in cortical connectivity that develop early in adolescence (Ingalhalikar et al., 2014). In particular, prefrontal cortical areas appear to be more strongly linked across hemispheres in females and more strongly linked within hemispheres in males (Ingalhalikar et al., 2014). Prefrontal cortical function also differs between males and females. The Iowa gambling task (IGT) probes probabilistic learning; the subject is asked to win as much money as possible without initial knowledge of a winning strategy. A winning strategy is to select cards from a pack that yields smaller rewards but overall monetary gain rather than packs containing larger rewards but overall monetary loss. Studies show that the winning strategy is adopted before the subject is aware of it (Bechara et al., 1997). fMRI measurements show that the IGT engages the dorsolateral prefrontal cortex, the insula and posterior cingulate cortex, and the orbitofrontal and ventromedial prefrontal cortex (Li et al., 2010). Males consistently perform better than females by learning more quickly to avoid the card selections that cause the greater monetary punishment (van den Bos et al., 2013). fMRI studies suggest that male and females engage different parts of the cortex during the IGT, which might explain the sex difference. Males show activity in the left and right lateral orbitofrontal cortex and the right dorsolateral prefrontal cortex, whereas females show activation of the left dorsolateral prefrontal cortex, left frontal gyrus, and temporal lobe (Bolla et al., 2004). In a rat‐based version of the IGT, performance is modulated by both serotonin and dopamine signaling (Zeeb et al., 2009), and, similarly to humans, male rats perform better than females (van den Bos et al., 2012). Further work is required to understand why males and females engage different parts of their brains during certain cortical behaviors and whether each sex employs different neurotransmitters and different synaptic plasticity mechanisms. Nevertheless, these studies do emphasize that, in addition to the sex differences seen at the synaptic scale, sex differences may also manifest themselves at the systems level, and indeed the two may interact. The sex differences seen in prefrontal cortex may be a factor in sex differences seen in psychiatric diseases, as discussed below.
Sex Differences in Cortical Function in Neurobehavioral Disorders
Schizophrenia
Schizophrenia is a psychiatric disorder that has a clear association with cortical impairments. In particular, changes to the structure and function of the prefrontal cortex are particularly characteristic of schizophrenia, with studies noting cortical thinning (Kuperberg et al., 2003), alterations in neural density (Heckers, 1997), and reduced activity during prefrontal‐dependent tasks (Weinberger et al., 1986). The risk ratio of developing schizophrenia in males compared with females is approximately 1.4 (Aleman et al., 2003), with males tending to develop schizophrenia at an earlier age (Faraone et al., 1994), suggesting that there could be underlying sex differences in cortical abnormalities that affect the disease expression. Indeed, there is evidence for this hypothesis. Gross anatomical sex differences in the schizophrenic brain as detected by MRI remain controversial (in part because of the lack of testing sex‐balanced groups), but studies suggest female‐specific reductions and male‐specific enlargements in white matter volume within the occipitoparietal lobe (Highley et al., 2003) and reductions in temporal lobe (Bryant et al., 1999) and anterior cingulate cortex volume (Bryant et al., 1999) in male patients. Differences in molecular signaling may also be sex dependent. Altered expression of GABAergic genes in the anterior cingulate cortex of schizophrenic patients varies by sex; in males, GABA‐Aα5, GABA‐Aβ1, and GABA‐Aε had reduced expression, whereas, in females, GABA‐Aβ1 and GAD67 were upregulated (Bristow et al., 2015).
Mouse models of schizophrenia have revealed sex‐dependent effects. Female mice carrying a missense mutation within the C‐terminal of Disrupted‐in‐Schizophrenia‐1 (Disc1) had altered sociability, hyperlocomotion, and heightened anxiety (Dachtler et al., 2016), a profile similar to that of elderly females harboring single‐nucleotide polymorphisms within DISC1 (Harris et al., 2010). A separate mouse model expressing inducible truncated Disc1 also showed sex‐specific behaviors, with males having enhanced spontaneous locomotor activity and alterations in social interaction and females having deficient reference spatial memory in the Morris water maze (Pletnikov et al., 2008). Recent evidence has highlighted that, without the normal expression of DISC1 during development, adult in vivo and in vitro synaptic plasticity is impaired in both the somatosensory (Greenhill et al., 2015) and the visual cortex (Tropea et al., 2016). Hence, DISC1 may contribute to the genesis of sex differences in schizophrenia.
Autism‐spectrum disorders
Autism is a heterogeneous cluster of behavioral abnormalities, which correspondingly has a differing diagnosis depending on the severity of these symptoms. Males consistently have a substantially greater incidence of autism compared with females, with male:female ratios up to 15:1, although on average this is closer to 4:1 (Wing, 1981). A possible cause of this sex difference could lie in hormonal effects during development and has been discussed in terms of the “extreme male brain” theory (Baron‐Cohen et al., 2011), which itself is underpinned by the fetal testosterone theory and the X chromosome theory. Several recent reviews have been published on this topic (Werling and Geschwind, 2013; Schaafsma and Pfaff, 2014; Mottron et al., 2015), so here we focus instead on other possible factors that might contribute to sex differences in autism.
Research into the causative factors explaining the male bias in autism have pointed toward sex differences in the structure and function of the cortex. It is well established that brain enlargement occurs in autism, with enlargement of the cerebral cortex evident before the second year of life (Hazlett et al., 2011). Widespread differences in cortical gray matter have been observed across the frontal (Abell et al., 1999; McAlonan et al., 2002), parietal (McAlonan et al., 2002), and temporal (Boddaert et al., 2004; Hazlett et al., 2011) lobes. However, some of these differences vary by sex. In comparing autistic girls and boys, significant differences in gray matter were observed in the motor cortex, supplementary motor area, insular cortex, and amygdala (Supekar and Menon, 2015). In males but not females, a significant negative correlation has been observed between behavioral autism traits and default‐mode functional connectivity of the medial prefrontal cortex (Jung et al., 2015) along with reduced gyrification in the ventromedial prefrontal and orbitofrontal cortex (Nordahl et al., 2015). Diffusion tenor imaging‐derived fiber tracking has revealed subtle differences in the corpus callosum of preschool‐aged children with autism. Males have a smaller callosal projection region to the orbitofrontal cortex, although females had a smaller region projecting to the anterior frontal cortex (Nordahl et al., 2015).
Sex differences in cortical function in autism may pertain to altered gene expression at the synapse. Retinoic acid‐related orphan receptor alpha (RORA), a gene found to be downregulated in autistic patients (Nguyen et al., 2010), is upregulated by E2 but, if the protein is deficient, will cause an accumulation of testosterone through the lack of suppression of CYP19A1 activity (encoding aromatase; Sarachana et al., 2011). The regulation of RORA and its transcriptional targets, including CYP19A1, is tightly regulated in the male cortex but less so in females (Hu et al., 2015), suggesting that RORA dysregulation could have greater impact on E2 and testosterone regulation and aromatase activity in cortex of male autism patients. Dysregulation of synaptic genes in autism mouse models has revealed sex differences in autistic‐like phenotypes. Mutations within the neurexin genes (NRXN1–3) have been widely associated with autism (Chen et al., 2014), and female mice with deletions of αNrxn1 exhibit impairments in fear learning, although female αNrxn2 knockout mice have reduced sociability and increased repetitive behaviors (Born et al., 2015; Dachtler et al., 2015). Deficiency of Nrxn2 has been shown to impair NMDA receptor function, short‐term plasticity, and excitatory transmitter release in cortical layer V cells (Born et al., 2015), implying that some of the behavioral effects of NRXN deletion could be related to impaired cortical function.
Taken together, these studies show that differences at the synaptic level play a part in some of the behavioral impairments associated with autism between males and females, in addition to differences in cortical structure and connectivity.
CONCLUSIONS
We began this Mini‐Review by posing the question of whether sex differences might extend down to the synaptic scale and whether plasticity might differ between males and females as a result. The current literature yields a number of examples of such differences at the synaptic level, most notably the CaMKK pathway, the NOS1 pathway, and the differential effect of circulating estrogen on synaptogenisis, NOS1, and GABAergic transmission. The NOS1, CaMKK, and estrogen effects are all capable of influencing the exact nature of plasticity and hence of affecting learning, memory, and cognition. It remains to be determined the extent to which these are general cortical mechanistic differences, however, because some features such as the effects of estrogen on spinogenesis have thus far been documented only in the hippocampus.
Recent studies have shown that many of the risk factors for psychiatric diseases affect synaptic proteins. Furthermore, studies are now emerging showing that plasticity is altered or impaired in mouse models of psychiatric diseases. For example, a transient disruption of normal DISC1 activity during a critical period of early development affects cortical plasticity into adulthood. It is well known that schizophrenia and ASD show different prevalence across males and females. We therefore raise the possibility here that some of the differential susceptibility to neuropsychiatric disorders between males and females may arise from sex differences in the plasticity mechanisms that are perturbed in those conditions.
ROLE OF AUTHORS
JD and KF both wrote the review, JD conducted the original research on autism and NOS1 as cited, KF conceived and designed experiments for the NOS1 experiments as cited.
SIGNIFICANCE This Mini‐Review explores the question of whether male and female brains differ at the synaptic level. The significance of evaluating this question is twofold; first, because plasticity is important in memory disorders, psychiatric conditions such as autism and schizophrenia, and recovery from stroke, we have to know whether therapies for these conditions should stratified by biological sex. Second, reviewing the evidence will help those researchers studying plasticity and memory conditions to plan whether sex should be included as a variable in the experimental design.
The authors declare they have no conflicts of interest.
REFERENCES
- Abell F, Krams M, Ashburner J, Passingham R, Friston K, Frackowiak R, Happe F, Frith C, Frith U. 1999. The neuroanatomy of autism: a voxel‐based whole brain analysis of structural scans. Neuroreport 10:1647–1651. [DOI] [PubMed] [Google Scholar]
- Aleman A, Kahn RS, Selten JP. 2003. Sex differences in the risk of schizophrenia: evidence from meta‐analysis. Arch Gen Psychiatry 60:565–571. [DOI] [PubMed] [Google Scholar]
- Auger AP, Hexter DP, McCarthy MM. 2001. Sex difference in the phosphorylation of cAMP response element binding protein (CREB) in neonatal rat brain. Brain Res 890:110–117. [DOI] [PubMed] [Google Scholar]
- Baron‐Cohen S, Lombardo MV, Auyeung B, Ashwin E, Chakrabarti B, Knickmeyer R. 2011. Why are autism spectrum conditions more prevalent in males? PLoS Biol 9:e1001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth AL, McKenna M, Glazewski S, Hill P, Impey S, Storm D, Fox K. 2000. Upregulation of cAMP response element‐mediated gene expression during experience‐dependent plasticity in adult neocortex. J Neurosci 20:4206–4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. 1997. Deciding advantageously before knowing the advantageous strategy. Science 275:1293–1295. [DOI] [PubMed] [Google Scholar]
- Bito H, Deisseroth K, Tsien RW. 1997. Ca2+‐dependent regulation in neuronal gene expression. Curr Opin Neurobiol 7:419–429. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Chabane N, Gervais H, Good CD, Bourgeois M, Plumet MH, Barthelemy C, Mouren MC, Artiges E, Samson Y, et al. 2004. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel‐based morphometry MRI study. Neuroimage 23:364–369. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. 2004. Sex‐related differences in a gambling task and its neurological correlates. Cereb Cortex 14:1226–1232. [DOI] [PubMed] [Google Scholar]
- Born G, Grayton HM, Langhorst H, Dudanova I, Rohlmann A, Woodward BW, Collier DA, Fernandes C, Missler M. 2015. Genetic targeting of NRXN2 in mice unveils role in excitatory cortical synapse function and social behaviors. Front Synaptic Neurosci 7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow GC, Bostrom JA, Haroutunian V, Sodhi MS. 2015. Sex differences in GABAergic gene expression occur in the anterior cingulate cortex in schizophrenia. Schizophrenia Res 167:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant NL, Buchanan RW, Vladar K, Breier A, Rothman M. 1999. Gender differences in temporal lobe structures of patients with schizophrenia: a volumetric MRI study. Am J Psychiatry 156:603–609. [DOI] [PubMed] [Google Scholar]
- Chen J, Yu S, Fu Y, Li X. 2014. Synaptic proteins and receptors defects in autism spectrum disorders. Front Cell Neurosci 8:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordoba Montoya DA, Carrer HF. 1997. Estrogen facilitates induction of long term potentiation in the hippocampus of awake rats. Brain Res 778:430–438. [DOI] [PubMed] [Google Scholar]
- Dachtler J, Hardingham NR, Glazewski S, Wright NF, Blain EJ, Fox K. 2011. Experience‐dependent plasticity acts via GluR1 and a novel neuronal nitric oxide synthase‐dependent synaptic mechanism in adult cortex. J Neurosci 31:11220–11230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dachtler J, Hardingham NR, Fox K. 2012. The role of nitric oxide synthase in cortical plasticity is sex specific. J Neurosci 32:14994–14999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dachtler J, Ivorra JL, Rowland TE, Lever C, Rodgers RJ, Clapcote SJ. 2015. Heterozygous deletion of alpha‐neurexin I or alpha‐neurexin II results in behaviors relevant to autism and schizophrenia. Behav Neurosci 129:765–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dachtler J, Elliott C, Rodgers RJ, Baillie GS, Clapcote SJ. 2016. Missense mutation in DISC1 C‐terminal coiled‐coil has GSK3beta signaling and sex‐dependent behavioral effects in mice. Sci Rep 6:18748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, Heist EK, Tsien RW. 1998. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature 392:198–202. [DOI] [PubMed] [Google Scholar]
- Dohler KD, Coquelin A, Davis F, Hines M, Shryne JE, Sickmoller PM, Jarzab B, Gorski RA. 1986. Pre‐ and postnatal influence of an estrogen antagonist and an androgen antagonist on differentiation of the sexually dimorphic nucleus of the preoptic area in male and female rats. Neuroendocrinology 42:443–448. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Chen WJ, Goldstein JM, Tsuang MT. 1994. Gender differences in age at onset of schizophrenia. Br J Psychiatry 164:625–629. [DOI] [PubMed] [Google Scholar]
- Fester L, Prange‐Kiel J, Zhou L, Blittersdorf BV, Bohm J, Jarry H, Schumacher M, Rune GM. 2012. Estrogen‐regulated synaptogenesis in the hippocampus: sexual dimorphism in vivo but not in vitro. J Steroid Biochem Mol Biol 131:24–29. [DOI] [PubMed] [Google Scholar]
- Fox K. 1994. The cortical component of experience‐dependent synaptic plasticity in the rat barrel cortex. J Neurosci 14:7665–7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies GE, McArthur S. 2010. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex‐specific medicines. Pharmacol Rev 62:155–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazewski S, Chen CM, Silva A, Fox K. 1996. Requirement for alpha‐CaMKII in experience‐dependent plasticity of the barrel cortex. Science 272:421–423. [DOI] [PubMed] [Google Scholar]
- Glazewski S, Barth AL, Wallace H, McKenna M, Silva A, Fox K. 1999. Impaired experience‐dependent plasticity in barrel cortex of mice lacking the alpha and delta isoforms of CREB. Cereb Cortex 9:249–256. [DOI] [PubMed] [Google Scholar]
- Glazewski S, Giese KP, Silva A, Fox K. 2000. The role of alpha‐CaMKII autophosphorylation in neocortical experience‐dependent plasticity. Nat Neurosci 3:911–918. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS Jr, Faraone SV, Tsuang MT. 2001. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex 11:490–497. [DOI] [PubMed] [Google Scholar]
- Greenhill SD, Juczewski K, de Haan AM, Seaton G, Fox K, Hardingham NR. 2015. Neurodevelopment. Adult cortical plasticity depends on an early postnatal critical period. Science 349:424–427. [DOI] [PubMed] [Google Scholar]
- Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, Loughead J, Connolly JJ, Qiu H, Mentch FD, et al. 2012. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology 26:251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Trent S, Thomas KL, O'Donovan MC, Owen MJ. 2015. Genetic risk for schizophrenia: convergence on synaptic pathways involved in plasticity. Biol Psychiatry 77:52–58. [DOI] [PubMed] [Google Scholar]
- Hardingham N, Fox K. 2006. The role of nitric oxide and GluR1 in presynaptic and postsynaptic components of neocortical potentiation. J Neurosci 26:7395–7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham N, Glazewski S, Pakhotin P, Mizuno K, Chapman PF, Giese KP, Fox K. 2003. Neocortical long‐term potentiation and experience‐dependent synaptic plasticity require alpha‐calcium/calmodulin‐dependent protein kinase II autophosphorylation. J Neurosci 23:4428–4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham N, Dachtler J, Fox K. 2013. The role of nitric oxide in pre‐synaptic plasticity and homeostasis. Front Cell Neurosci 7:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SE, Hennah W, Thomson PA, Luciano M, Starr JM, Porteous DJ, Deary IJ. 2010. Variation in DISC1 is associated with anxiety, depression and emotional stability in elderly women. Mol Psychiatry 15:232–234. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe MD, Gerig G, Styner M, Chappell C, Smith RG, Vachet C, Piven J. 2011. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch Gen Psychiatry 68:467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S. 1997. Neuropathology of schizophrenia: cortex, thalamus, basal ganglia, and neurotransmitter‐specific projection systems. Schizophrenia Bull 23:403–421. [DOI] [PubMed] [Google Scholar]
- Highley JR, DeLisi LE, Roberts N, Webb JA, Relja M, Razi K, Crow TJ. 2003. Sex‐dependent effects of schizophrenia: an MRI study of gyral folding, and cortical and white matter volume. Psychiatry Res 124:11–23. [DOI] [PubMed] [Google Scholar]
- Hopper RA, Garthwaite J. 2006. Tonic and phasic nitric oxide signals in hippocampal long‐term potentiation. J Neurosci 26:11513–11521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu VW, Sarachana T, Sherrard RM, Kocher KM. 2015. Investigation of sex differences in the expression of RORA and its transcriptional targets in the brain as a potential contributor to the sex bias in autism. Mol Autism 6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Wu DL, Luo CX, Zhu LJ, Zhang J, Wu HY, Zhu DY. 2012. Hippocampal nitric oxide contributes to sex difference in affective behaviors. Proc Natl Acad Sci U S A 109:14224–14229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA. 1994. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science 265:1883–1885. [DOI] [PubMed] [Google Scholar]
- Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, Hakonarson H, Gur RE, Gur RC, Verma R. 2014. Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci U S A 111:823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M, Mody M, Saito DN, Tomoda A, Okazawa H, Wada Y, Kosaka H. 2015. Sex differences in the default mode network with regard to autism spectrum traits: a resting state fMRI study. PloS One 10:e0143126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SC, van der Kouwe AJ, et al. 2003. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry 60:878–888. [DOI] [PubMed] [Google Scholar]
- Li X, Lu ZL, D'Argembeau A, Ng M, Bechara A. 2010. The Iowa Gambling Task in fMRI images. Hum Brain Mapp 31:410–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan GM, Daly E, Kumari V, Critchley HD, van Amelsvoort T, Suckling J, Simmons A, Sigmundsson T, Greenwood K, Russell A, et al. 2002. Brain anatomy and sensorimotor gating in Asperger's syndrome. Brain 125:1594–1606. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. 2005. Ischemic nitric oxide and poly (ADP‐ribose) polymerase‐1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab 25:502–512. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Giese KP. 2010. Toward a molecular understanding of sex differences in memory formation. Trends Neurosci 33:285–291. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Ris L, Sanchez‐Capelo A, Godaux E, Giese KP. 2006. Ca2+/calmodulin kinase kinase alpha is dispensable for brain development but is required for distinct memories in male, though not in female, mice. Mol Cell Biol 26:9094–9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K, Antunes‐Martins A, Ris L, Peters M, Godaux E, Giese KP. 2007. Calcium/calmodulin kinase kinase beta has a male‐specific role in memory formation. Neuroscience 145:393–402. [DOI] [PubMed] [Google Scholar]
- Mottron L, Duret P, Mueller S, Moore RD, Forgeot d'Arc B, Jacquemont S, Xiong L. 2015. Sex differences in brain plasticity: a new hypothesis for sex ratio bias in autism. Mol Autism 6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen A, Rauch TA, Pfeifer GP, Hu VW. 2010. Global methylation profiling of lymphoblastoid cell lines reveals epigenetic contributions to autism spectrum disorders and a novel autism candidate gene, RORA, whose protein product is reduced in autistic brain. FASEB J 24:3036–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PV, Abel T, Kandel ER, Bourtchouladze R. 2000. Strain‐dependent differences in LTP and hippocampus‐dependent memory in inbred mice. Learn Mem 7:170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl CW, Iosif AM, Young GS, Perry LM, Dougherty R, Lee A, Li D, Buonocore MH, Simon T, Rogers S, et al. 2015. Sex differences in the corpus callosum in preschool‐aged children with autism spectrum disorder. Mol Autism 6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletnikov MV, Ayhan Y, Nikolskaia O, Xu Y, Ovanesov MV, Huang H, Mori S, Moran TH, Ross CA. 2008. Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol Psychiatry 13:173–186, 115. [DOI] [PubMed] [Google Scholar]
- Pogun S. 2001. Sex differences in brain and behavior: emphasis on nicotine, nitric oxide and place learning. Int J Psychophysiol 42:195–208. [DOI] [PubMed] [Google Scholar]
- Ranson A, Sengpiel F, Fox K. 2013. The role of GluA1 in ocular dominance plasticity in the mouse visual cortex. J Neurosci 33:15220–15225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt AC, Rodgers RJ, Clapcote SJ. 2012. The role of neurexins in schizophrenia and autistic spectrum disorder. Neuropharmacology 62:1519–1526. [DOI] [PubMed] [Google Scholar]
- Sarachana T, Xu M, Wu RC, Hu VW. 2011. Sex hormones in autism: androgens and estrogens differentially and reciprocally regulate RORA, a novel candidate gene for autism. PloS One 6:e17116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaafsma SM, Pfaff DW. 2014. Etiologies underlying sex differences in autism spectrum disorders. Front Neuroendocrinol 35:255–271. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. 2006. Estrogen and brain‐derived neurotrophic factor (BDNF) in hippocampus: complexity of steroid hormone‐growth factor interactions in the adult CNS. Front Neuroendocrinol 27:415–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott N, Prigge M, Yizhar O, Kimchi T. 2015. A sexually dimorphic hypothalamic circuit controls maternal care and oxytocin secretion. Nature 525:519–522. [DOI] [PubMed] [Google Scholar]
- Simerly RB. 2002. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci 25:507–536. [DOI] [PubMed] [Google Scholar]
- Smith CC, McMahon LL. 2006. Estradiol‐induced increase in the magnitude of long‐term potentiation is prevented by blocking NR2B‐containing receptors. J Neurosci 26:8517–8522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, Woolfrey KM, Jones KA, Shum CY, Lash LL, Swanson GT, Penzes P. 2008. Rapid enhancement of two‐step wiring plasticity by estrogen and NMDA receptor activity. Proc Natl Acad Sci U S A 105:14650–14655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Menon V. 2015. Sex differences in structural organization of motor systems and their dissociable links with repetitive/restricted behaviors in children with autism. Mol Autism 6:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropea D, Molinos I, Petit E, Bellini S, Nagakura I, O'Tuathaigh C, Schorova L, Mitchell KJ, Waddington J, Sur M, et al. 2016. Disrupted in schizophrenia 1 (DISC1) L100P mutants have impaired activity‐dependent plasticity in vivo and in vitro. Transl Psychiatry 6:e712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos R, Jolles J, van der Knaap L, Baars A, de Visser L. 2012. Male and female Wistar rats differ in decision‐making performance in a rodent version of the Iowa Gambling Task. Behav Brain Res 234:375–379. [DOI] [PubMed] [Google Scholar]
- van den Bos R, Homberg J, de Visser L. 2013. A critical review of sex differences in decision‐making tasks: focus on the Iowa Gambling Task. Behav Brain Res 238:95–108. [DOI] [PubMed] [Google Scholar]
- Vierk R, Bayer J, Freitag S, Muhia M, Kutsche K, Wolbers T, Kneussel M, Sommer T, Rune GM. 2015. Structure–function–behavior relationship in estrogen‐induced synaptic plasticity. Horm Behav 74:139–148. [DOI] [PubMed] [Google Scholar]
- Warren SG, Humphreys AG, Juraska JM, Greenough WT. 1995. LTP varies across the estrous cycle: enhanced synaptic plasticity in proestrus rats. Brain Res 703:26–30. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. 1986. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry 43:114–124. [DOI] [PubMed] [Google Scholar]
- Werling DM, Geschwind DH. 2013. Sex differences in autism spectrum disorders. Curr Opin Neurol 26:146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing L. 1981. Sex ratios in early childhood autism and related conditions. Psychiatry Res 5:129–137. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. 1994. Estradiol regulates hippocampal dendritic spine density via an N‐methyl‐D‐aspartate receptor‐dependent mechanism. J Neurosci 14:7680–7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. 1997. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor‐mediated synaptic input: correlation with dendritic spine density. J Neurosci 17:1848–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb FD, Robbins TW, Winstanley CA. 2009. Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacology 34:2329–2343. [DOI] [PubMed] [Google Scholar]


