Abstract
Neuromodulators can alter the response properties of sensory neurons, including those in the auditory system. Dopamine, which plays a major role in reward and movement, has been shown to alter neural responses in the auditory brainstem and midbrain. Recently we identified the subparafascicular thalamic nucleus (SPF), part of the A11 dopaminergic cell group, as the source of dopamine to the inferior colliculus (IC). The superior olivary complex (SOC) is also a likely target of dopaminergic projections from the SPF because it receives projections from the SPF and contains fibers and terminals immunoreactive for tyrosine hydroxylase, the rate limiting enzyme in dopamine synthesis. However, it is unknown if the projections from the SPF to SOC are dopaminergic, and if single neurons in the SPF project to both the IC and SOC. Using anterograde tracing combined with fluorescent immunohistochemistry, we found that the SPF sends dopaminergic projections to the superior paraolivary nucleus and the medial nucleus of the trapezoid body, but not the lateral superior olive. We confirmed these projections using a retrograde tracer. By making dual retrograde deposits in the IC and SOC, we found that individual dopaminergic cells innervate both the IC and SOC. These results suggest dopaminergic innervation, likely released in a context dependent manner, occurs at multiple levels of the auditory pathway.
Keywords: superior olivary complex, dopamine, neuromodulation, mouse
1. Introduction
Neuromodulatory systems have the ability to significantly alter auditory processing based on social context and internal state (Hurley and Hall, 2011; Ikeda et al., 2015). A variety of studies have provided intriguing data suggesting that the neuromodulator dopamine plays an important role in modulating auditory responses. For example, Bender et al. (2010) showed that dopamine can modulate action potential initiation in the dorsal cochlear nucleus and Gittelman et al. (2013) showed that dopamine heterogeneously modulates response properties of neurons in the inferior colliculus (IC). Moreover, people with Parkinson’s disease and schizophrenia, both disorders characterized by a dysfunction in dopamine signaling, have speech processing problems (Gräber et al., 2002; Schröder et al., 2010; Kantrowitz et al., 2015) and people with schizophrenia may experience auditory hallucinations (Lennox et al., 2000). Thus, dopamine may be required for normal auditory processing.
The sources of dopamine in the auditory system, however, are not well understood. Recently, we discovered that the subparafascicular thalamic nucleus (SPF) is the source of the dopaminergic input to the IC (Nevue et al., 2016). The SPF is part of the A11 dopaminergic cell group and sends widespread dopaminergic projections to many structures in the brain (Takada et al. 1988; Takada, 1993). In addition to dopaminergic neurons, the SPF contains neurons that use GABA or various neuropeptides including substance P, enkephalin, and somatostatin (Moriizumi and Hattori, 1992; Sugimoto et al., 1984; Wamsley et al., 1980). The SPF receives projections from many auditory nuclei including the superior olivary complex, inferior colliculus, nuclei of the lateral lemniscus, and medial geniculate body, and therefore may act as a convergence center for auditory projections (LeDoux et al., 1985; Wang et al., 2006; Yasui et al., 1990). Studies in fish have also identified a dopaminergic cell group in the hypothalamus, likely a homolog to the SPF, which sends projections to auditory nuclei, the inner ear, and lateral line (Metcalfe et al., 1985; Bricaud et al., 2001; Forlano et al., 2014). Thus, it is likely that the SPF in mammals sends dopaminergic projections to other auditory nuclei besides the IC.
In particular, the superior olivary complex (SOC) is a likely target of dopaminergic projections from the SPF. The SOC is a group of brainstem nuclei that functions as a convergence center within the ascending and descending auditory pathway, and these different nuclei play important roles in both monaural and binaural processing (Thompson and Schofield, 2000; Oliver, 2000). While it is known that the SOC receives projections from the SPF (Yasui et al., 1992) and contains TH-positive fibers and terminals (Mulders and Robertson, 2001; 2005), it is not known whether the SPF projections are the dopaminergic source to the SOC. In addition, it is not known whether the same neurons in the SPF project to both the IC and SOC.
The purposes of this study were to determine 1) if the SPF sends dopaminergic projections to the SOC, and 2) whether individual neurons in the SPF project to both the auditory midbrain and brainstem. We first placed an anterograde tracer in the SPF and examined whether anterogradely labeled fibers in the SOC nuclei were colocalized with tyrosine hydroxylase (TH). We then confirmed the SPF to SOC projections by placing retrograde tracer deposits in the individual SOC nuclei. We found that the superior paraolivary nucleus (SPN) and medial nucleus of the trapezoid body (MNTB), but not the lateral superior olive (LSO), receive dopaminergic projections from the SPF. By placing different retrograde tracers in the SOC nuclei and the IC in the same animals, we also found that individual dopaminergic cells in the SPF project to both the IC and SOC. These results suggest that the SPF has the ability to alter auditory processing in multiple nuclei, potentially facilitating plasticity or altering ongoing responses to salient stimuli.
2. Materials and Methods
2.1 Animals
We used normal hearing (CBA/CaJ or C57BL/6J) adult mice less than 3 months old (Zheng et al., 1999; Kane et al., 2012) (9 males, 8 females) obtained from Jackson Laboratory or bred in our colony. All animals had free access to food and water and were on a reversed 12-h light/12-h dark schedule. All care and procedures were in accordance with the guidelines of the National Institutes of Health and were approved by the Washington State University Institutional Animal Care and Use Committee.
2.2 Preparation of animals for tracer injections
We iontophoretically injected the tracers into the regions of interest in awake, restrained animals. To prepare the animals for the tracer injections, we mounted a headpost onto the skull of anesthetized mice using surgical techniques we have previously described (Muniak et al., 2012). Once the headpost was mounted, we made a craniotomy (about 1 mm × 1 mm) over the desired brain region based on stereotaxic coordinates (Paxinos and Franklin, 2001). We then covered the hole with petroleum jelly and/or bone wax to prevent the brain from dehydrating, applied lidocaine and Neosporin to the exposed muscle, and returned the mouse to its home cage to recover from the surgery for at least 1 day before a tracer deposit was made. On the experimental day, the animal was placed in a sound attenuating chamber with its headpost bolted into a custom stereotaxic apparatus (Muniak et al., 2012). Prior to the experiment, the animal was given acepromazine (<5 mg/kg, i.p.) to reduce any stress of putting the animal in the restraint.
2.3 Iontophoretic tracer injections
Methods were similar to Nevue et al. (2016). For anterograde tracing, we deposited 10% biotinylated dextran amine (BDA; 10,000 MW) (Life Technologies) made in 0.9% saline into the subparafascicular thalamic nucleus (SPF) in 4 mice. We located the SPF using stereotaxic coordinates (1.3 to 1.6 mm caudally from bregma and 0.1 to 0.5 mm laterally from midline; Paxinos and Franklin, 2001). The BDA was deposited by injecting 5μA of current for 10 minutes (7s on/7s off). The animal was then returned to its home cage for seven days survival time.
For retrograde tracing, we deposited 1% cholera toxin subunit B (CTB; List Biological Solutions) dissolved in distilled water into either the superior paraolivary nucleus (SPN) (n = 5), medial nucleus of the trapezoid body (MNTB) (n = 4), or lateral superior olive (LSO) (n = 3). One brain had tracer spread from the deposit to the SPN and MNTB, and was classified as such for quantification. In some animals (n = 6) we also deposited 2% Fluorogold (FG; Fluorochrome) in sodium acetate buffer into the inferior colliculus (IC), ipsilateral to the SOC deposit. We used glass micropipette electrodes (impedance 3 to 5 MΩ) filled with the tracer. The electrodes were advanced into the specific nucleus by a hydraulic micropositioner (David Kopf Instruments) driven from outside the sound attenuating chamber. We determined where to deposit the tracer using stereotaxic coordinates (Paxinos and Franklin, 2001) and electrophysiological response characteristics from extracellular recordings. For each penetration, the SPN was used as a reference point of location within the SOC because of our expertise in recording in this structure (Felix et al., 2012). The SPN has prominent spiking following the offset of the sound stimulus with little to no evoked spiking during stimulation (Felix et al., 2012) making it an easy target to identify. After locating the SPN, we found the MNTB by shifting the electrode medially and identifying primary-like spiking patterns that produced stronger responses to contralateral stimulation (Kopp-Scheinpflug et al., 2003). We found the LSO by shifting the electrode laterally from the SPN and identifying chopper-like patterns that produced stronger responses to ipsilateral stimulation (Adam et al., 1999; Karcz et al., 2011). The tracers were deposited using current (7s on/7s off) of 4 μA for 5–10 minutes. The animal was then returned to its home cage for seven days survival time.
2.4 Perfusion and tissue collection
The mice were deeply anesthetized in an induction chamber with isoflurane until breathing stopped and there was no nociceptive response. We then transcardially perfused the mouse using 60 mL of buffered 10% formalin or 4% paraformaldehyde in 0.1M phosphate buffer solution (PBS, pH 7.4). The brain was dissected and cryoprotected overnight in 20% sucrose solution in 0.1M PBS. We sectioned the brain coronally at a thickness of 40 μm using a Leica SM2000 R freezing microtome (Leica Biosystems) making sure to place a small fiducial mark on the right side of the brain to identify label that was ipsilateral or contralateral to the deposit site. Sections from the SOC, IC, and SPF were collected serially in 0.1M PBS and stored at 4°C until use. Nissl staining was performed on adjacent series to identify different nuclei within the SOC.
2.5 Immunohistochemistry
Sections were rinsed in PB and incubated overnight at room temperature in a primary antibody solution. The primary antibody solution consisted of polyclonal rabbit anti-TH (Millipore, 1:1500), 3% normal donkey solution (Millipore) and 0.4% Triton X-100 (Sigma-Aldrich) in 0.1M PB. For retrograde tracing we also used goat anti-CTB (List Biological Solutions, 1:10,000). After incubation in the primary antibody, sections were washed and then incubated in a secondary antibody for two hours at room temperature. We used donkey anti-rabbit Alexa Fluor 568, and donkey anti-goat 488 to visualize the primary antibodies (1:250, Life Technologies). For anterograde experiments we used Alexa Fluor 488 conjugated streptavidin (1:250, Life Technologies) to visualize BDA. The sections were then mounted on Superfrost Plus microscope slides (Fisher Scientific), dehydrated and cleared, and coverslipped with DPX (Electron Microscopy Sciences). Label was observed using a Leica TCS SP8 confocal microscope or Leica DMR fluorescent microscope (Leica Microsystems).
2.6 Data Analysis
For colocalization analysis, we examined every other 40μm section in the SPF. Approximately 4–5 sections were analyzed for each animal. Cells were counted with live images. In each section, we counted the number of cells that were retrogradely labeled with CTB, and if those cells were also labeled with TH or retrogradely labeled with FG. Because the data were not normally distributed, we used Mann-Whitney U tests to determine statistical significance between groups. Data are presented as mean +/− SD.
3. Results
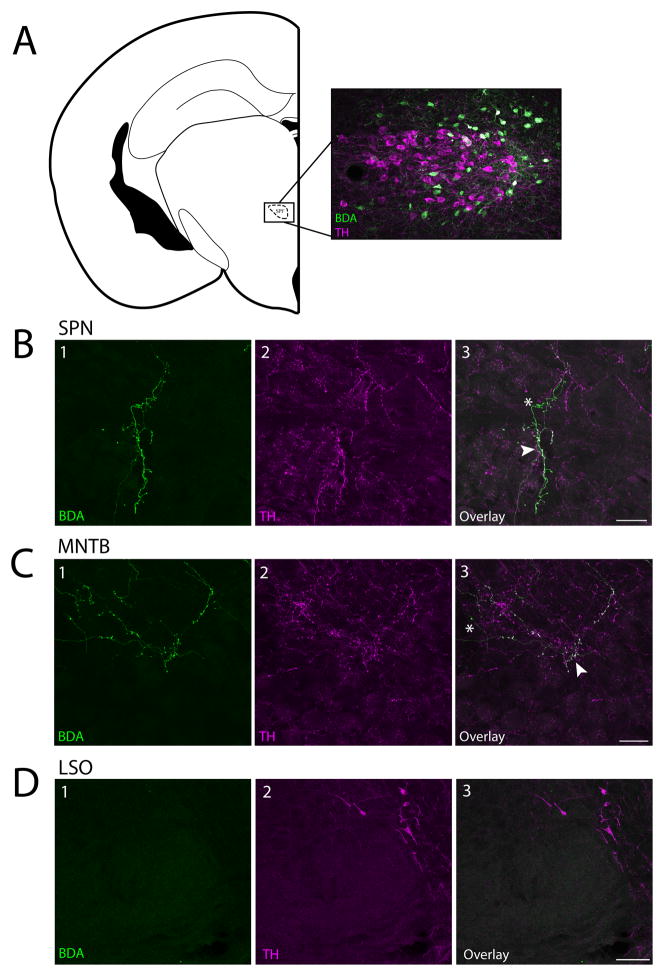
To determine if the SPF sends dopaminergic projections to the SOC, we iontophoretically injected an anterograde tracer (10K BDA) in the left SPF of 4 mice. The location of these deposits was confirmed after tissue processing (Figure 1A). Neurons in the SPF have been shown previously to be dopaminergic and not noradrenergic (Takada et al., 1988; Koblinger et al., 2014; Nevue et al., 2016). By staining the same brains for TH, we determined if there were anterogradely labeled fibers in the SOC, and if they were TH-positive. We found anterogradely labeled fibers colocalized with TH in the SPN and MNTB, but not LSO (Figure 1B–D). We counted the number of boutons in a 40μm × 40μm square area of each SPN (n =4) and MNTB (n =4) and found no statistical difference (p = 0.273) between bouton density in the SPN (8.50 ± 2.65) and the MNTB (11.5 ± 4.20).
Figure 1. Dopaminergic cells in the SPF project to the SPN and MNTB but not the LSO.
A: Coronal section showing a BDA deposit (green cell bodies) in the SPF. TH-positive cells are shown in magenta. B: Fibers in the SPN were anterogradely labeled and TH-positive. B1: BDA labeled fibers. B2: TH-positive fibers. B3: Overlay. C: Fibers in the MNTB were anterogradely labeled and TH-positive. C1: BDA labeled fibers. C2: TH-positive fibers. C3: Overlay. D. There were no anterogradely labeled fibers in the LSO. B–D: Arrows point to examples of anterogradely labeled fibers colocalized with TH, asterisks mark TH-negative anterogradely labeled fibers. Coronal section modified from Paxinos and Franklin (2001). Scale bar: A, D 100μm; B, C 25μm. Abbreviations: SPF, subparafascicular thalamic nucleus; SPN, superior paraolivary nucleus; MNTB, medial nucleus of the trapezoid body: LSO, lateral superior olive.
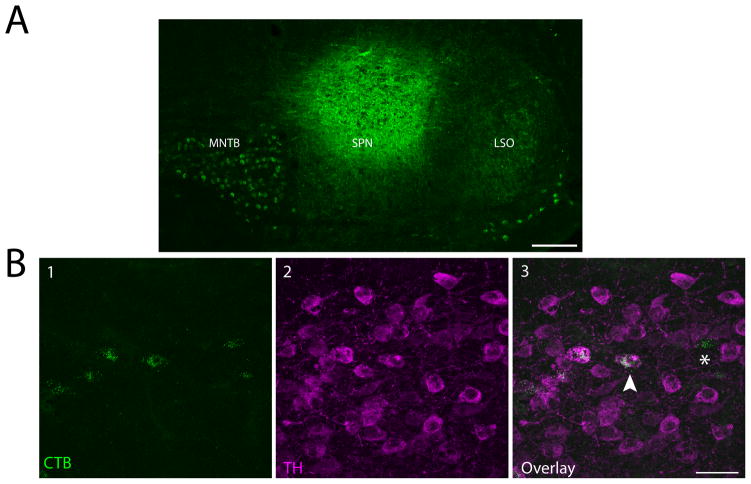
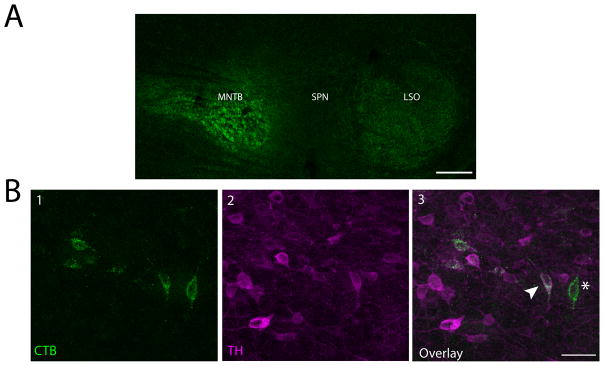
We confirmed our anterograde results using retrograde tracing. We injected 1% CTB in the SPN of 5 mice and the MNTB of 4 mice. One mouse had a CTB deposit that covered parts of both the SPN and MNTB, and is represented as such in the figures. Additionally, to confirm that the LSO was not receiving dopaminergic projections from the SPF, we injected 1% CTB in the LSO of 3 mice. We only analyzed data from deposit sites we considered to successfully hit the target nucleus. For the purposes of this paper and constraints of working in a mouse, we considered a deposit to be in the SPN even if there was small tracer spread into other periolivary nuclei, as long as the deposit was primarily in the SPN (Paxinos and Franklin, 2001). For MNTB and LSO deposits, we did not analyze data from experiments where the tracer spread to other subdivisions of the SOC. We were not concerned if there was minimal tracer spread outside of the SOC because there is no evidence that regions surrounding the SOC receive projections from the SPF. Representative deposits for the SPN and MNTB are shown in Figures 2A and 3A, respectively.
Figure 2. Retrograde tracing confirmed dopaminergic projections from the SPF to the SPN.
A: Cholera toxin subunit B (CTB) deposit in the SPN. SOC nuclei outlines based off of Paxinos and Franklin (2001). B1: Retrogradely labeled cells in the SPF. B2: TH-positive cells in the SPF. B3: Overlay showing colocalized cells. Arrow points to an example of a retrogradely labeled cell colocalized with TH, asterisk marks a retrogradely labeled, TH-negative cell. Scale bar: A 100μm; B 40μm.
Figure 3. Retrograde tracing confirmed dopaminergic projections from the SPF to the MNTB.
A: Cholera toxin subunit B (CTB) deposit in the MNTB. SOC nuclei outlines based off of Paxinos and Franklin (2001). B1: Retrogradely labeled cells in the SPF. B2: TH-positive cells in the SPF. B3: Overlay showing colocalized cells. Arrow points to an example of a retrogradely labeled cell colocalized with TH, asterisk marks a retrogradely labeled, TH-negative cell. Scale bar: A 100μm; B 40μm.
We found retrogradely labeled, TH-positive neurons in the SPF after deposits in the SPN (Figure 2B) and MNTB (Figure 3B), confirming the dopaminergic projections from the SPF to the SOC. Additionally, we observed retrogradely labeled cells that were not TH-positive in these same brains. No retrogradely labeled, TH-positive cells were seen in the SPF after retrograde deposits in the LSO, which is in agreement with anterograde tracing data (present study, Yasui et al., 1992).
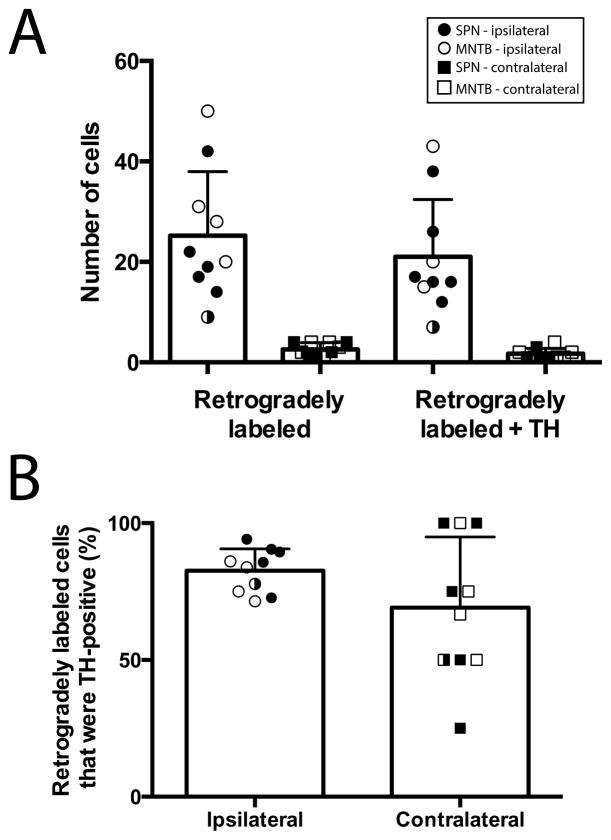
After deposits in either the SPN or MNTB, we found more retrogradely labeled cells in the ipsilateral SPF than in the contralateral SPF (ipsilateral, 25.2 ± 4.0; contralateral, 2.6 ± 0.4; p < 0.001; Figure 4A). However, there was no difference in the percentage of retrogradely labeled cells that were TH-positive between the ipsilateral and contralateral sides (82.6 ± 2.5% vs. 69.2 ± 8.2%; p = 0.106; Figure 4B).
Figure 4. The majority of retrogradely labeled cells in the SPF following deposits in either SPN or MNTB were TH-positive.
A: Average number of retrogradely labeled cells and retrogradely labeled cells that were TH-positive on each side (ipsilateral, circles; contralateral, squares) in the SPF following deposits in either the SPN (closed) or MNTB (open). Each symbol represents data from one deposit. One deposit spread to both SPN and MNTB, and is denoted by the half closed symbol. B: The average percentage of retrogradely labeled cells that were TH-positive in the ipsilateral and contralateral SPF was not significantly different. Each symbol represents data from one deposit.
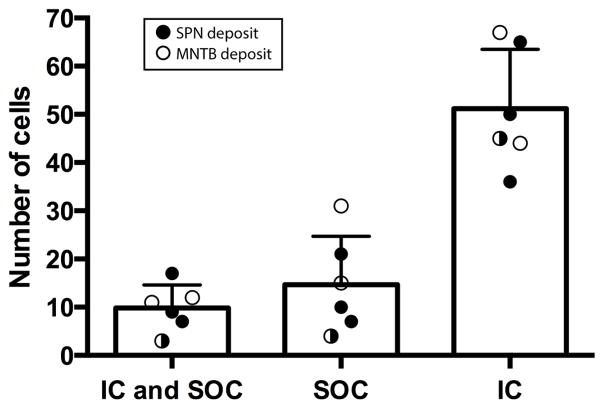
In six brains we made an additional retrograde deposit of FG in the IC. A representative deposit site is shown in Figure 5A. We found that some single cells in the SPF were retrogradely labeled with both CTB and FG (from the SOC and IC respectively), and were also TH-positive. These triple labeled cells were observed after both SPN and IC deposits, and MNTB and IC deposits (Figure 5B). There were more retrogradely labeled cells that were TH-positive from IC deposits (51.14 ± 12.35) than from the SOC (9.83 ± 4.75). Of the retrogradely labeled cells that project to the SOC, about 40% were TH-positive and also project to the IC (Figure 6).
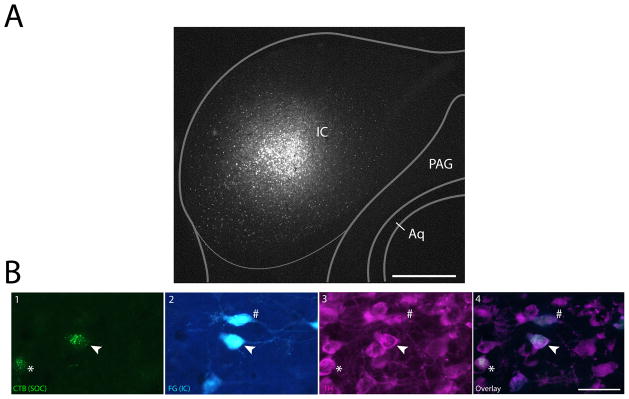
Figure 5. Single neurons in the SPF project to both the IC and SOC.
A: Fluorogold deposit in the IC, with an overlaid schematic coronal section based off of Paxinos and Franklin (2001). B1: Retrogradely labeled cells in the SPF after CTB deposit in the MNTB (deposit site shown in Fig. 3A). B2: Retrogradely labeled cells in the SPF after IC deposit. B3: TH-positive cells in the SPF. B4: Merged view showing colocalization of tracer and TH. Arrows show a TH-positive cell retrogradely labeled from IC and MNTB deposits. Asterisk marks a TH-positive cell that is retrogradely labeled from only the MNTB. Pound sign marks a TH-positive neuron that is retrogradely labeled only from the IC. Scale bar: A 250μm; B 40μm. Abbreviations: CNIC, central nucleus of inferior colliculus; ECIC, external cortex of inferior colliculus; DCIC, dorsal cortex of inferior colliculus; PAG, periaqueductal gray; Aq, aqueduct.
Figure 6. Some cells in the SPF project to both the SOC and the IC.
FG deposits were made in the IC and CTB deposits were made in the SPN (closed circles) or MNTB (open circles). First bar shows average number of TH-positive cells that were both FG and CTB positive indicating that individual cells in SPF project to both nuclei. Subsequent bars show TH positive cells that only project to SOC or IC. Each symbol represents data from one brain.
4. Discussion
Dopamine has widespread effects on neurons, including altering how auditory neurons respond to sound (Gittelman et al., 2013). Surprisingly, the source(s) of dopamine in auditory nuclei has not been well studied. Recently, we described dopaminergic projections from the subparafascicular thalamic nucleus (SPF) to the inferior colliculus (IC) (Nevue et al., 2016). The purpose of this study was to determine if the SPF also provides dopaminergic input to the superior olivary nuclei (SOC). The SOC contains TH-positive fibers and terminals (Mulders and Robertson, 2001; 2005) and receives projections from the SPF (Yasui et al., 1992), but, to our knowledge, this is the first study to examine whether the SPF to SOC projections are dopaminergic. In addition, we examined whether dopaminergic cells in the SPF project to both the IC and the SOC. We used tract tracing combined with immunofluorescence for TH and found that the SOC, specifically the SPN and MNTB, receives dopaminergic input from the SPF. Additionally, we found that TH-positive cells in the SPF project to both the IC and SOC. These results suggest that the dopaminergic neurons in the SPF are involved in modulating auditory responses at multiple levels throughout the auditory pathway.
Prior to this study, the IC was the only location within the central auditory pathway that was known to receive dopaminergic projections from the SPF (Nevue et al., 2016). Mulders and Robertson (2001, 2005) showed that the locus coeruleus sends noradrenergic projections to the SOC and made the claim that this was the only catecholaminergic innervation of the SOC. However, they did not examine any regions rostral to the superior colliculus, thus missing many major dopaminergic cell groups, including the dopaminergic cells in the SPF. Our more extensive examination clearly shows that the SPF has a large number of TH-positive cells that project to the SPN and MNTB. The finding that the SPF possesses markers of dopaminergic neurons and is DBH-negative (Koblinger et al., 2014; Nevue et al., 2016) provides strong evidence that the SPF cells that project to the SOC are dopaminergic rather than noradrenergic.
We also found TH-positive fibers in the SPN and MNTB that were not anterogradely labeled from SPF deposits. This could be because some neurons in the SPN that project to the SOC were not labeled by our injections, or more likely since our SPF deposits tended to be large, that the SPN and MNTB also receive noradrenergic inputs, most likely coming from the locus coeruleus. This is most certainly the case for the LSO, as we showed here that the LSO does not receive any input from the SPF and it is known to receive input from the locus coeruleus (Mulders and Robertson, 2001; 2005). It is highly likely that the LSO does not receive any dopaminergic input.
4.1 Functional implications of dopaminergic input to the SOC
Our findings here provide the first evidence that the SPF sends dopaminergic inputs to the nuclei of the superior olivary complex, but it is not clear what effect these inputs have on auditory processing. The LSO sends inhibitory inputs to the ipsilateral IC and excitatory inputs to the contralateral IC whereas the SPN sends predominantly inhibitory inputs to the ipsilateral IC (Loftus et al., 2004; Saldaña et al., 2009). While the LSO has been firmly implicated in the binaural processing of sound localization cues (Grothe, 2010; Tollin, 2003), less is known of SPN function. Neurons in the SPN possess both intrinsic (Felix et al., 2011) and synaptic (Kulesza et al., 2007) properties that make them well suited for encoding coarse temporal information important for vocalization processing (Eggermont, 2010). Thus, the LSO may be an early locus for the representation of “where” information of incoming sounds, while the SPN likely has a role in encoding “what” information necessary for sound identification. If the SPN has a role in the identification of salient sounds such as vocalizations at the level of the IC, both nuclei may be targets of SPF-derived dopaminergic inputs capable of modulating auditory processing based on the behavioral state of the animal. In contrast, if the primary function of the LSO is sound localization, modulation by dopamine that contributes to establishing social context may not be necessary. Unlike the LSO and SPN, the MNTB is not a major source of input to the IC (Adams, 1979; Kelly et al., 1998), but it does provide major projections to both the ipsilateral LSO and SPN (Banks and Smith, 1992; Sommer et al., 1993). Thus, the MNTB likely is a major part of both binaural “where” processing pathways that include the LSO and monaural “what” pathways of which the SPN is a part (Schofield, 2005). Future studies are necessary to determine whether other monaural areas implicated in auditory object identification such as the octopus cells of the cochlear nucleus (Felix et al., 2013) also receive dopaminergic inputs. Conversely, regions like the bushy cell area of the cochlear nucleus that give rise to binaural pathways (Cant and Benson, 2003) may be devoid of dopaminergic input. By establishing which nuclei share modulatory SPF connections, we can begin to explore the functional implications of dopamine modulation on binaural and monaural auditory processing.
Because we conducted this study in mice, we did not examine the medial superior olive (MSO). In mice, the MSO is small in number of neurons, and compared to the size of the SPN, MNTB, and LSO (Irving and Harrison, 1967; Ollo and Schwartz, 1979). It is even debatable if small animals with high frequency hearing even have a MSO (Ollo and Schwartz, 1979). Although they do not clearly identify the MSO, Yasui et al. (1992) did not find any anterograde labeling in the presumed MSO region after SPF deposits. In addition, Mulders and Robertson (2001, 2005) claimed there are no dopaminergic fibers in the MSO of the rat and guinea pig. Thus, it is likely that the MSO is not a target of dopaminergic modulation. Neuromodulation is still of interest in the MSO however, as it has recently been shown that serotonin modulates firing probability in MSO neurons (Ko et al., 2016).
4.2 The role of the SPF in auditory processing
The function of the SPF in auditory processing is not clear, but its afferent and efferent connections provide some indication of its potential role. The SPF receives input from a variety of auditory nuclei, including the auditory cortex, auditory thalamus, the SOC, and the external and dorsal cortices of IC (LeDoux et al., 1985; Wang et al., 2006; Yasui et al., 1990; Yasui et al., 1992). It is not known what causes a response from these SPF neurons but the connectivity with auditory structures suggests dopamine release from SPF cells may be triggered by input from other auditory neurons. Examining the neurophysiological response properties of SPF neurons to auditory stimuli will be an important step in understanding how the SPF modulates auditory processing with dopamine. While it is known that neural responses to auditory stimuli are modulated by dopamine in the IC (Gittelman et al., 2013) likely via the SPF (Nevue et al., 2016), it is not known how dopamine modulates responses in the SPN and MNTB. Future experiments should address the specific role of the SPF in modulating responses properties in the IC and SOC.
The neurons in the SPF that project to the spinal cord are likely separate from those that project to the IC (Takada, 1993) and the SOC. This combined with the data in the present paper, showing that single dopaminergic neurons in the SPF are innervating the auditory brainstem and midbrain, suggest that there is an auditory specific group of SPF neurons and these neurons play a role in global dopaminergic modulation of auditory processing
As we and others have shown, not all neurons in the SPF are TH positive (Moriizumi and Hattori, 1992; Nevue et al., 2016). The phenotype of the TH-negative neurons in the SPF is unknown but they are likely the same GABAergic neurons that project to the IC (Moriizumi and Hattori, 1992), which would contribute to the overall inhibition in the auditory midbrain and brainstem. In the IC, inhibition plays a variety of roles including shaping selectivity to vocalizations (Mayko et al., 2012; Klug et al., 2002) and it is possible that the SPF plays a role in this modulation.
Other neuropeptides like substance P, enkephalin, and somatostatin are also present in the SPF, but it is unknown if they exist in neurons that project to auditory nuclei. Wang and Robertson (1997) investigated the effect of various neuromodulators on SOC neurons and found that substance P had excitatory effects and enkephalin had inhibitory effects. These modulatory effects could either arise from the SPF or via other projections to the SOC. Somatostatin did not have any effect on SOC neurons so it is unlikely that SPF neurons projecting to the SOC are using somatostatin as a neuropeptide.
5. Conclusion
Neuromodulation via dopamine is of interest throughout the auditory system from the inner ear to the cortex. In the inner ear, dopamine acts via D1 receptors at ribbon synapses to increase neurotransmission in sensory hair cells (Toro et al., 2015; Darrow et al., 2007). In the DCN, dopamine can modulate currents that mediate neuronal excitability (Bender et al., 2010), and it has heterogeneous (although largely depressive) effects on extracellular response properties in the IC (Gittelman et al., 2013) and auditory thalamus (Tebecis, 1970). In auditory cortex, dopamine modulation has been shown to help detect salient stimuli (Happel et al., 2014), auditory memory (Schicknick et al., 2012), and synaptic plasticity (Bao et al., 2001). Thus, it is important to fully understand the mechanisms of dopaminergic modulation in the auditory system. Future studies should explore the specific mechanisms by which the SPF regulates dopaminergic modulation in the auditory system.
Research Highlights.
The subparafascicular thalamic nucleus (SPF) sends dopaminergic projections to the superior olivary complex (SOC)
The same cells in the SPF that project to the SOC also are projecting to the inferior colliculus
The SPF likely acts as a global modulator of auditory processing via dopamine
Acknowledgments
We thank Cameron J. Elde for technical assistance.
Funding: This work was supported by NIDCD R01DC013102 to CVP.
Footnotes
Author Contributions: All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: AAN and CVP. Acquisition of data: AAN and RAF. Analysis and interpretation of data: AAN, RAF, and CVP. Drafting of the manuscript: AAN, RAF, and CVP
Conflict of Interest Statements: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Contributor Information
Alexander A. Nevue, Email: alex.nevue@wsu.edu.
Richard A. Felix, II, Email: rfelix@wsu.edu.
Christine V. Portfors, Email: portfors@wsu.edu.
References
- Adam TJ, Schwartz DW, Finlayson PG. Firing properties of chopper and delay neurons in the lateral superior olive of the rat. Exp Brain Res. 1999;124(4):489–502. doi: 10.1007/s002210050645. [DOI] [PubMed] [Google Scholar]
- Adams JC. Ascending projections to the inferior colliculus. J Comp Neurol. 1979;183:519–538. doi: 10.1002/cne.901830305. [DOI] [PubMed] [Google Scholar]
- Banks MI, Smith PH. Intracellular recordings from neurobiotin-labeled cells in brain slices of the rat medial nucleus of the trapezoid body. J Neurosci. 1992;12:2819–2837. doi: 10.1523/JNEUROSCI.12-07-02819.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- Bender KJ, Ford CP, Trussell LO. Dopaminergic modulation of axon initial segment calcium channels regulates action potential initiation. Neuron. 2010;68(3):500–511. doi: 10.1016/j.neuron.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricaud O, Chaar V, Dambly-Chaudière C, Ghysen A, Dambly-Chaudie C. Early efferent innervation of the zebrafish lateral-line. J Comp Neurol. 2001;434:253–261. doi: 10.1002/cne.1175. [DOI] [PubMed] [Google Scholar]
- Cant NB, Benson CG. Parallel auditory pathways: projection patterns of the different neuronal populations in the dorsal and ventral cochlear nuclei. Brain Res Bull. 2003;60:457–474. doi: 10.1016/s0361-9230(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Darrow KN, Simons EJ, Dodds L, Liberman MC. Dopaminergic innervation of the mouse inner ear: evidence for a separate cytochemical group of cochlear efferent fibers. J Comp Neurol. 2006;498:403–414. doi: 10.1002/cne.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont JJ. Animal models of auditory temporal processing. Int J Psychophysiol. 2015;95:202–215. doi: 10.1016/j.ijpsycho.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Felix RA, Fridberger A, Leijon S, Berrebi AS, Magnusson AK. Sound rhythms are encoded by postinhibitory rebound spiking in the superior paraolivary nucleus. J Neurosci. 2011;31:12566–78. doi: 10.1523/JNEUROSCI.2450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix RA, Vonderschen K, Berrebi AS, Magnusson AK. Development of on-off spiking in superior paraolivary nucleus neurons of the mouse. J Neurophysiol. 2013;109:2691–2704. doi: 10.1152/jn.01041.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlano PM, Kim SD, Krzyminska ZM, Sisneros JA. Catecholaminergic connectivity to the inner ear, central auditory and vocal motor circuitry in the plainfin midshipman fish Porichthys notatus. J Comp Neurol. 2014;522:2887–2927. doi: 10.1002/cne.23596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittelman JX, Perkel DJ, Portfors CV. Dopamine modulates auditory responses in the inferior colliculus in a heterogeneous manner. J Assoc Res Otolaryngol. 2013;14:719–729. doi: 10.1007/s10162-013-0405-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräber S, Hertrich I, Daum I, Spieker S, Ackermann H. Speech perception deficits in Parkinson’s disease: underestimation of time intervals compromises identification of durational phonetic contrasts. Brain Lang. 2002;82:65–74. doi: 10.1016/s0093-934x(02)00002-0. [DOI] [PubMed] [Google Scholar]
- Grothe B, Pecka M, McAlpine D. Mechanisms of sound localization in mammals. Physiol Rev. 2010;90:983–1012. doi: 10.1152/physrev.00026.2009. [DOI] [PubMed] [Google Scholar]
- Happel MF, Deliano M, Handschuh J, Ohl FW. Dopamine-modulated recurrent corticoefferent feedback in primary sensory cortex promotes detection of behaviorally relevant stimuli. J Neurosci. 2014;34(4):1234–47. doi: 10.1523/JNEUROSCI.1990-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Hall IC. Context-dependent modulation of processing by serotonin. Hear Res. 2011;279(1–2):74–84. doi: 10.1016/j.heares.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda MZ, Jeon SD, Cowell RA, Remage-Healey L. Norepinephrine modulates coding of complex vocalizations in the songbird auditory cortex independent of local neuroestrogen synthesis. J Neurosci. 2015;35(25):9356–9368. doi: 10.1523/JNEUROSCI.4445-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving R, Harrison JM. The superior olivary complex and audition: a comparative study. J Comp Neurol. 1967;130:77–86. doi: 10.1002/cne.901300105. [DOI] [PubMed] [Google Scholar]
- Kane KL, Longo-Guess CM, Gagnon LH, Ding D, Salvi RJ, Johnson KR. Genetic background effects on age-related hearing loss associated with Cdh23 variants in mice. Hearing Research. 2012;283:80–88. doi: 10.1016/j.heares.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz JT, Hoptman MJ, Leitman DI, Moreno-Ortega M, Lehrfeld JM, Javitt DC, et al. Neural substrates of auditory emotion recognition deficits in schizophrenia. J Neurosci. 2015;35:14909–14921. doi: 10.1523/JNEUROSCI.4603-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcz A, Hennig MH, Robbins CA, Tempel BL, Rübsamen R, Kopp-Scheinpflug C. Low-voltage activated Kv1.1 subunits are crucial for the processing of sound source location in the lateral superior olive in mice. 2011;589(5):1143–57. doi: 10.1113/jphysiol.2010.203331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JB, Liscum A, van Adel B, Ito M. Projections from the superior olive and lateral lemniscus to tonotopic regions of the rat’s inferior colliculus. Hear Res. 1998;116:43–54. doi: 10.1016/s0378-5955(97)00195-0. [DOI] [PubMed] [Google Scholar]
- Ko KW, Rasbandm MN, Meseguerm V, Kramerm RH, Golding NL. Serotonin modulates spike probability in the axon initial segment through HCN channels. Nat Neurosci. 2016 doi: 10.1038/nn.4293. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Fuchs K, Lippe WR, Tempel BL, Rubsamen R. Decreased temporal precision of auditory signalling in Kcna1-null mice: an electrophysiological study in vivo. J Neurosci. 2003;23(27):9199–207. doi: 10.1523/JNEUROSCI.23-27-09199.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koblinger K, Füzesi T, Ejdrygiewicz J, Krajacic A, Bains JS, Whelan PJ. Characterization of A11 neurons projecting to the spinal cord of mice. PLOS One. 2014;9:10. doi: 10.1371/journal.pone.0109636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesza RJ, Kadner A, Berrebi AS. Distinct roles for glycine and GABA in shaping the response properties of neurons in the superior paraolivary nucleus of the rat. J Neurophysiol. 2007;97:1610–1620. doi: 10.1152/jn.00613.2006. [DOI] [PubMed] [Google Scholar]
- Klug A, Bauer EE, Hanson JT, Hurley L, Meitzen J, Pollak GD. Response selectivity for species-specific calls in the inferior colliculus of Mexican free-tailed bats is generated by inhibition. J Neurophysiol. 2002;88:1941–1954. doi: 10.1152/jn.2002.88.4.1941. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Ruggiero DA, Reis DJ. Projections to the subcortical forebrain from anatomically defined regions of the medial geniculate body in the rat. J Comp Neurol. 1985;242:182–213. doi: 10.1002/cne.902420204. [DOI] [PubMed] [Google Scholar]
- Lennox BR, Park SBG, Medley I, Morris PG, Jones PB. The functional anatomy of auditory hallucinations in schizophrenia. Psychiatry Research: Neuroimaging. 2000;100:13–20. doi: 10.1016/s0925-4927(00)00068-8. [DOI] [PubMed] [Google Scholar]
- Loftus WC, Bishop DC, Saint Marie RL, Oliver DL. Organization of binaural excitatory and inhibitory inputs to the inferior colliculus from the superior olive. J Comp Neurol. 2004;472:330–344. doi: 10.1002/cne.20070. [DOI] [PubMed] [Google Scholar]
- Mayko ZM, Roberts PD, Portfors CV. Inhibition shapes selectivity to vocalizations in the inferior colliculus of awake mice. Frontiers in Neural Circuits. 2012;6:73. doi: 10.3389/fncir.2012.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe WK, Kimmel CB, Schabtach E. Anatomy of the posterior lateral-line system in young larvae of the zebrafish. J Comp Neurol. 1985;233:377–389. doi: 10.1002/cne.902330307. [DOI] [PubMed] [Google Scholar]
- Moriizumi T, Hattori T. Anatomical and functional compartmentalization of the subparafascicular thalamic nucleus in the rat. Exp Brain Res. 1992;90:175–179. doi: 10.1007/BF00229269. [DOI] [PubMed] [Google Scholar]
- Mulders WH, Robertson D. Origin of the noradrenergic innervation of the superior olivary complex in the rat. J Chem Neuroanat. 2001;21(4):313–22. doi: 10.1016/s0891-0618(01)00118-1. [DOI] [PubMed] [Google Scholar]
- Mulders WH, Robertson D. Catecholaminergic innervation of the guinea pig superior olivary complex. J Chem Neuroanat. 2005;30(4):230–42. doi: 10.1016/j.jchemneu.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Muniak M, Mayko ZM, Ryugo DK, Portfors CV. An awake mouse preparation for recording neural response properties and injecting tracers. J Vis Exp. 2012;64:e3755. doi: 10.3791/3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevue AA, Elde CJ, Perkel DJ, Portfors CV. Dopaminergic input to the inferior colliculus in mice. Front Neuroanat. 2016;9:168. doi: 10.3389/fnana.2015.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver DL. Ascending efferent projections of the superior olivary complex. Microsc Res Tech. 2000;51(4):355–63. doi: 10.1002/1097-0029(20001115)51:4<355::AID-JEMT5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Ollo C, Schwartz IR. The superior olivary complex in C57BL/6 mice. Am J Anat. 1979;155:349–374. doi: 10.1002/aja.1001550306. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. 2. San Diego, CA: Academic Press; 2001. [Google Scholar]
- Saldaña E, Aparicio MA, Fuentes-Santamaría V, Berrebi AS. Connections of the superior paraolivary nucleus of the rat: projections to the inferior colliculus. Neuroscience. 2009;163:372–387. doi: 10.1016/j.neuroscience.2009.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schicknick H, Reichenbach N, Smalla KH, Scheich H, Gundelfinger ED, Tischmeyer W. Dopamine modulates memory consolidation of discrimination learning in auditory cortex. Eur J Neurosci. 2012;35(5):763–74. doi: 10.1111/j.1460-9568.2012.07994.x. [DOI] [PubMed] [Google Scholar]
- Schröder C, Nikolova ZT, Dengler R. Changes of emotional prosody in Parkinson’s disease. J Neurol Sci. 2010;289:32–35. doi: 10.1016/j.jns.2009.08.038. [DOI] [PubMed] [Google Scholar]
- Schofield BR. The Inferior Colliculus. New York: Springer; 2005. Olivary and lemniscal projections. [Google Scholar]
- Sommer I, Lingenhöhl K, Friauf E. Principal cells of the rat medial nucleus of the trapezoid body: an intracellular in vivo study of their physiology and morphology. Exp Brain Res. 1993;95:223–239. doi: 10.1007/BF00229781. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Takada M, Kaneko T, Mizuno N. Substance P-positive thalamocaudate neurons in the center median-parafascicular complex in the cat. Brain Res. 1984;323:181–184. doi: 10.1016/0006-8993(84)90285-3. [DOI] [PubMed] [Google Scholar]
- Takada M. Widespread dopaminergic projections of the subparafascicular thalamic nucleus in the rat. Brain Res Bull. 1993;32:301–309. doi: 10.1016/0361-9230(93)90191-d. [DOI] [PubMed] [Google Scholar]
- Takada M, Li ZK, Hattori T. Single thalamic dopaminergic neurons project to both the neocortex and spinal cord. Brain Res. 1988;455:346–352. doi: 10.1016/0006-8993(88)90093-5. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Schofield BR. Afferent projections of the superior olivary complex. Microsc Res Tech. 2000;51(4):330–54. doi: 10.1002/1097-0029(20001115)51:4<330::AID-JEMT4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Tebecis AK. Effects of monoamines and amino acids on medial geniculate neurones of the cat. Neuropharmacology. 1970;9:381–390. doi: 10.1016/0028-3908(70)90035-3. [DOI] [PubMed] [Google Scholar]
- Tollin DJ. The lateral superior olive: a functional role in sound source localization. Neuroscientist. 2003;9:127–143. doi: 10.1177/1073858403252228. [DOI] [PubMed] [Google Scholar]
- Toro C, Trapani JG, Pacentine I, Maeda R, Sheets L, Mo W, Nicolson T. Dopamine Modulates the Activity of Sensory Hair Cells. J Neurosci. 2015;35(50):16494–16503. doi: 10.1523/JNEUROSCI.1691-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley JK, Young WS, III, Kuhar MJ. Immunohistochemical localization of enkephalin in rat forebrain. Brain Res. 1980;190:153–174. doi: 10.1016/0006-8993(80)91166-x. [DOI] [PubMed] [Google Scholar]
- Wang X, Robertson D. Effects of bioamines and peptides on neurones in the ventral nucleus of the trapezoid body and rostral periolivary regions of the rat superior olivary complex: an in vitro investigation. Hear Res. 1997;106:20–28. doi: 10.1016/s0378-5955(96)00211-0. [DOI] [PubMed] [Google Scholar]
- Wang J, Palkovits M, Usdin TB, Dobolyi A. Afferent connections of the subparafascicular area in rat. Neuroscience. 2006;138:197–220. doi: 10.1016/j.neuroscience.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Kayahara T, Nakano K, Mizuno N. The subparafascicular thalamic nucleus of the rat receives projection fibers from the inferior colliculus and auditory cortex. Brain Res. 1990;537:323–327. doi: 10.1016/0006-8993(90)90378-o. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Nakano K, Mizuno N. Descending projections from the subparafascicular thalamic nucleus to the lower brainstem in the rat. Exp Brain Res. 1992;90:508–518. doi: 10.1007/BF00230933. [DOI] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hearing Research. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]