Abstract
In recent years, innate lymphoid cells (ILCs) have emerged as innate correlates to T cells. The similarities between ILCs and T cells indicate that lymphocytes of fundamentally distinct lineages can share core “immune modules” that encompass transcriptional circuitry and effector functions, while utilizing non-redundant, complementary mechanisms of pattern recognition to enact these functions. We review modules currently recognized to be shared between ILCs and T cells.
Keywords: ILC, T cell, innate, adaptive, lymphocyte
Introduction
The hematopoietic universe has rapidly expanded in recent years with the identification of a new major class of cells found in both human and mouse, called innate lymphoid cells (1–7). ILCs belong to the lymphoid lineage as they derive from the common lymphoid progenitor (CLP) and depend on the master lymphocyte cytokine receptor Interleukin-2 receptor common gamma chain (IL-2rg, γc) (2, 4). Yet, their lack of recombined antigen-specific receptors and other lineage markers distinguish them from conventional T and B cells as well as innate-like lymphocytes (ILLs) such as Natural Killer T (NKT) cells (1). Remarkably, the functional specializations of ILCs as well as their developmental programs resemble those previously recognized in CD4+ T helper cell subsets (2); therefore ILC1, ILC2 and ILC3 are now considered the innate counterparts of Th1, Th2 and Th17, while natural killer (NK) cells are considered the innate counterpart to CD8+ cytotoxic T cells (2, 8). To date, there is no recognized ILC counterpart to regulatory T cells (Treg) or T follicular helper cells (Tfh). Furthermore, unlike CD4+ T cell polarization from a multipotent naïve cell in the periphery, ILC fate-commitment occurs early in development (9–11) (Figure 1).
Figure 1. Development of T cells and ILCs.
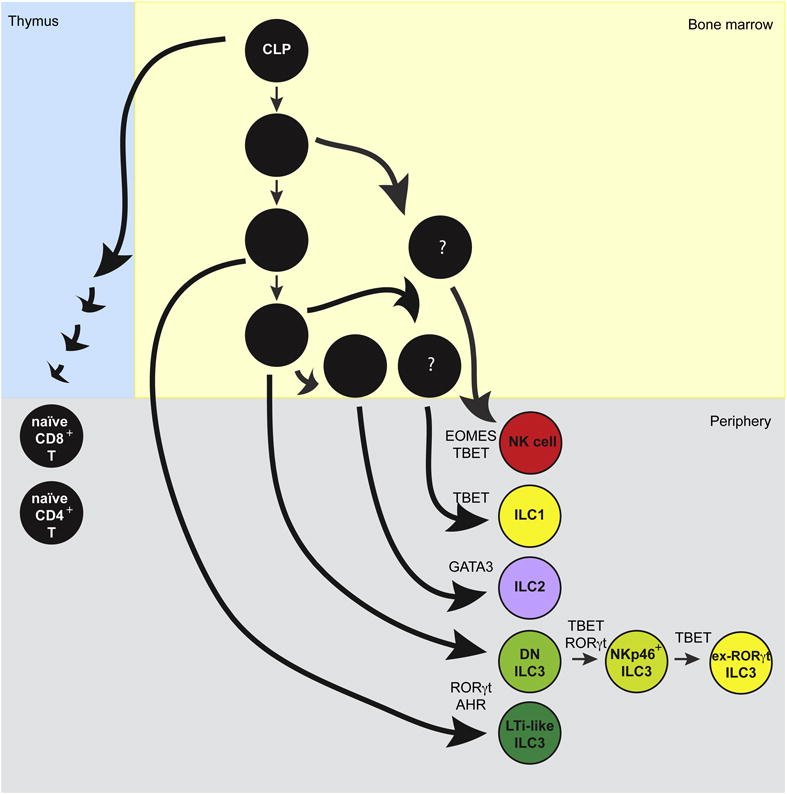
T cells and ILCs both develop downstream of the CLP. T cell progenitors (left) undergo progressive developmental stages in the thymus, which ultimately result in the export of naïve CD8+ and CD4+ T cells to the periphery. In contrast, ILC generation (right) occurs in the bone marrow through a series of progenitors with increasingly restricted developmental potential, resulting in the export of lineage-specified ILCs directly to the periphery. The sequence of recognized ILC progenitors are indicated in black in the bone marrow, while presumed progenitors are denoted by question marks.
ILC subtypes are grouped together based on shared developmental requirements and effector functions. Eomesodermin+ (Eomes) T-bet+ NK cells are cytotoxic and produce IFN-γ and TNF-α; T-bet+ ILC1 produce IFN-γ and TNF-α, like NK cells; GATA3hi ILC2 produce IL-5, IL-13, IL-9 and amphiregulin; and RORγt+ ILC3 produce IL-22, GM-CSF and/or IL-17.
ILC1 and NK cell distinctions are controversial because, as developmental studies in mouse separate these lineages, but their defining cell-surface markers vary between tissues and often overlap (12–15). Thus, mouse ILC1 are currently best defined by their lack of Eomes expression within NKp46+ NK1.1+ cells (15). In human, similar phenotypic heterogeneity in the absence of lineage tracing makes distinctions even more difficult, but two IFN-γ-producing subsets have currently been described: Intraepithelial ILC1 express a CD103+CD56+CD94+NKp44+ phenotype, while CD127+ILC1 express a CD127+CD161+ phenotype and do not express intraepithelial ILC1 markers (16–18).
ILC2 have been identified in many organs in mice and human during homeostasis. Cells from both species express CD25, CD127, the IL-33 receptor ST2, and the inhibitory receptor KLRG1. Human ILC2s along express the prostaglandin D2 receptor-homologous molecule expressed on Th2 cells (CRTH2) (7, 16, 19, 20).
ILC3s are a particularly diverse class of cells in both species. In adult mice these cells include two developmentally distinct lineages, one of which has many subsets. The first lineage, LTi-like cells or NKp46−CCR6+ ILC3 cells, expresses MHC-II and produces IL-22, IL-17a, IL-17f, and LT-α (21–26). The second lineage includes a sequence of cells that progressively differentiate and express different cytokines, cell-surface markers, and variable levels of T-bet: T-betlo NKp46−CCR6− ILC3 express IL-22 and IL-17a (27, 28); T-bet+ NKp46+CCR6− ILC3 express IL-22 and GM-CSF (24, 27–32); and T-bet+NK1.1+NKp46+ “ex-RORγt” ILC3 produce IFN-γ and cannot be distinguished from ILC1 without lineage tracing (13). Recently, single-cell sequencing of human ILC3 has similarly identified 3 cellular clusters based on shared and distinct transcriptional programs, including NKp44+ ILC3, a CD62L+NKp44− ILC3, and HLA-DR+ LTi-like ILC3, reminiscent of diversity found among mouse ILC3 (33). For additional phenotypic information, the cell-surface expression profiles of human (5, 16) and mouse (5) ILCs have recently been reviewed.
Unlike other lymphocytes, ILCs respond directly to cytokine signals in the microenvironment without a need for antigen-specific receptor signaling or preactivation. Signaling specificity is regulated by the cytokine receptors that ILCs express, allowing distinct combinations of myeloid and/or tissue-produced cytokines to activate each ILC class. Although ILCs are a relatively small population of cells compared to adaptive lymphocytes, they appear to play major homeostatic roles including through crosstalk with the epithelium. In mouse, ILCs are now recognized to constitutively produce “adaptive” cytokines including IL-22, GM-CSF, and IL-5 at greater levels during steady-state than conventional T cells (7, 24, 34–38). The similarities between ILCs and T cells indicate that lymphocytes of fundamentally distinct lineages can share core “immune modules” that encompass transcriptional circuitry and effector functions, while utilizing non-redundant, complementary mechanisms of pattern recognition to enact these functions. We review modules currently recognized to be shared between T cells and ILCs.
Cytotoxicity
NK cells and CD8+ T cells are grouped together in the same immune module based on their shared function as professional killers. A core feature of this module is the presence of granular cytotoxicity, characterized in mouse and human by lytic granules that contain perforin and granzmes, which act together on target cells to induce cellular apoptosis (Figure 2). In human, cytotoxic granules also contain an additional anti-microbial factor, granulysin (39). Cells in this module can also kill and induce other effector functions through shared expression of Fas ligand (FasL), TRAIL, TNF-α, and IFN-γ (40). Upstream, cytotoxic cells are activated by IL-12, IL-18, and IL-15.
Figure 2. Effector Modules of T cells and ILCs.
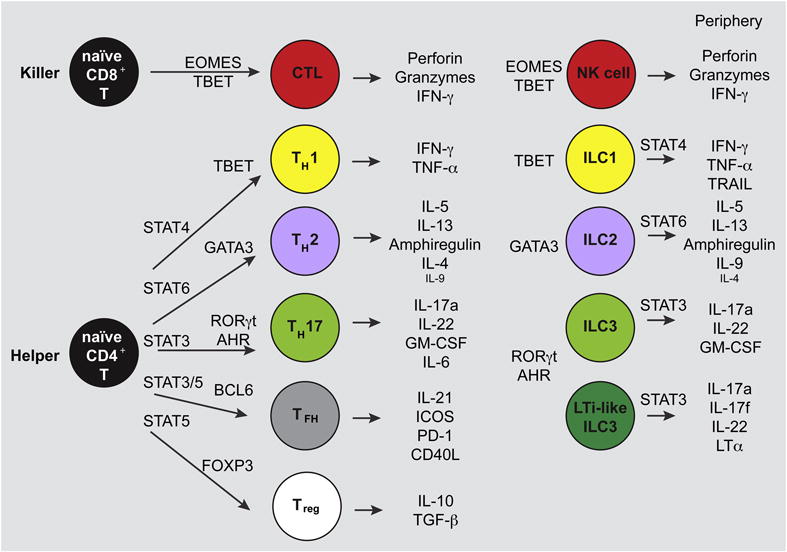
In the periphery, naïve T cells (left) that recognize their specific antigen and are costimulated by antigen presenting cells (APCs) become activated and mature to effector cells. For CD4+ T cells, APC-derived cytokines drive STAT activation and TF induction, leading to Th polarization and the indicated effector functions. Meanwhile, ILCs in the periphery are already mature cells that do not require STAT activation to develop, though STATs are important for some ILC effector functions. The TFs required for Th polarization and ILC development and the effector functions of cells in the same module are strikingly similar. ILC counterparts to Tfh and Treg are not currently recognized.
The TFs Eomes and T-bet are essential regulators of the Cytotoxicity Module in mouse and likely also in human based on their conserved pattern of expression (41) (Figure 2). As members of the same T-box family of TFs, Eomes and T-bet have somewhat redundant functions and can partially compensate for each other (42, 43). Therefore, the cytotoxic program fails to develop only in the absence of both molecules, leading to the selective ablation of CD122hi (marking IL-2/IL-15 receptor beta, IL-15rβ) memory CD8+ T cells and NK cells, and the aberrant expression of IL-17 by remaining CD8+ T cells upon their activation (44, 45).
The regulation and specific functions of Eomes and T-bet appear to differ. In CD8+ T cell effector differentiation, T-bet expression temporally precedes that of Eomes (46), with the highest T-bet to Eomes ratio in early effector cells and the lowest ratio in memory cells. While T-bet better induces IFN-γ and the IL-12 co-receptor IL-12Rβ2, Eomes superiorly induces perforin and granzyme B expression (43, 46, 47). Initially, this developmental sequence appeared to be conserved in NK cell differentiation, with T-bet sustaining immature NK cells and Eomes sustaining mature cells (45). Yet, evaluation of mice deficient for T-bet and Eomes in addition to transfer studies have demonstrated that Eomes is uniquely required for NK cell development, while T-bet is in fact required for the development of ILC1 (13, 48, 49). Moreover, when ectopically expressed, Eomes skews ILC1 to a cytotoxic NK cell profile with enhanced Ly49 expression, while T-bet overexpression similarly results in the increased production of Eomes− phenotypic ILC1 (48, 50). These data suggest that Eomes and T-bet might achieve balance through mutual regulation. Developmentally, the exogenous factors that lead to differential regulation of Eomes and T-bet remain an active area of investigation. These factors likely include tissue factors such as TGF-β, as recently demonstrated in a unique ILC1-like population in the salivary gland that is T-bet- and Eomes- independent (51). Nonetheless, in physiologic conditions, both Eomes and T-bet together define the cytotoxic module, while Eomes is particularly critical for NK cells.
Module 1: Intracellular Pathogens and Viruses
ILC1 and Th1 are grouped together based on their shared production of IFN-γ and TNF-α, and their absent or reduced capacity for granular cytotoxicity compared to the Cytotoxicity module, likely due to their absent or reduced levels of perforin and granzyme B (13, 48) (Figure 2). In mouse, some ILC1 additionally appear to share expression of TRAIL (48, 52, 53). Underscoring the similarity in cytokine production with Cytotoxicity, Module 1 is similarly defined by the TF T-bet (48, 49, 54). Cells in this module are also similarly regulated by the cytokines IL-12, IL-18, and IL-15, though in human, CD127+ ILC1 preferentially respond to IL-12 and IL-18 while intraepithelial ILC1 respond to IL-15. While IL-12-mediated STAT4 activation causes Th1 polarization in vivo (55), this pathway is not required for ILC1 development (56). However, it does provide a functional proliferative signal to mouse adipose tissue ILC1 in an inflammatory context (56).
Engagement of Module 1 is physiologically associated with viral and intracellular pathogen immunity, but it is deregulated in Crohn’s disease and some forms of autoimmunity such as Type 1 Diabetes (4). Consistent with this, intraepithelial and CD127+ ILC1 have been reported to be increased in frequency in Crohn’s disease (17, 18, 57, 58). Increased frequencies of ILC1 have also been reported to correlate with chronic obstructive pulmonary disease (COPD) disease severity, presumably linked to viral infection (59).
T-bet has long been recognized as a key driver of Th1 differentiation, and is more recently recognized as a necessary factor for ILC1 differentiation (13, 48, 49, 54, 60). In the absence of T-bet, naïve T cells do not produce IFN-γ, and instead polarize to a Th2 effector program (61). Indeed, T-bet directly represses the development of Th2 (62, 63). The fate of ILC precursors unable to engage T-bet is currently unclear. Functionally, T-bet drives Module 1 effector cytokine production by binding to the promotor and regulatory elements of Il12rb2 and Ifng (64, 65). It also directly induces the expression of Runx3, an additional Ifng-activating TF. Supporting the role of Runx3 in Module 1, Runx3 deficient mice also fail to generate ILC1 (66).
While T-bet clearly has an important role in Module 1, the role of Eomes is more controversial. In mice, ILC1 are currently best defined by their lack of Eomes expression. However, in the absence of an Eomes fate-mapping mouse, it unclear if ILC1 always lack Eomes expression, or if they may sometimes express it. Recently, assay for transposase-accessible chromatin with high-throughput sequencing (ATACseq) data from mouse ILCs demonstrated graded expression of ATAC+ open chromatin surrounding Eomes, with highest expression in NK cells, lower expression in ILC1, and minimal expression in ILC2 and ILC3 (10), suggesting the potential to express Eomes may not be absent in ILC1. Furthermore, the “Eomes negative” definition of this module is not universally true, as some Th1 cells from both mouse and human express Eomes when activated (67, 68). In human, the two identified ILC1 subsets differ in their expression of EOMES and T-BET; CD127+ ILC1 exclusively expresses T-BET, while intraepithelial ILC1 expresses T-BET as well as higher levels of EOMES compared to CD127+ ILC1 and ILC3 cells (17, 18, 57). Notably, intraepithelial lymphocytes are well known to express highly activated phenotypes in steady-state as they are in direct contact with the epithelium and constitutively sense foreign metabolites and antigens (69). Collectively, these data suggest that cells in Module 1 critically rely on T-bet, likely for this TFs preferential induction of cytokine versus cytotoxic machinery, but may express Eomes in some circumstances, such as activation. Yet, unlike T-bet, Eomes is not required for development of Th1 or ILC1 and is unlikely to be a major functional regulator of this module.
Module 2: Barrier Maintenance and Helminthes
ILC2, Th2, and Th9 share the same functional module based on their mutual production of signature cytokines IL-5 and IL-13 (70) (Figure 2). IL-25, IL-33, and TSLP are conserved cytokine regulators. Other factors associated with this module include IL-4, IL-9, GM-CSF, and the epidermal growth factor family member amphiregulin (71, 72). The γc family cytokines IL-4 and IL-9 in particular seem to be differently regulated in this module, as Th2 produce greater quantities of IL-4 (73–75), while ILC2 and Th9 produce IL-9 more readily (72). IL-4 signaling through STAT6 is also an important factor for Th2 and Th9 polarization in vitro, but this pathway is not universally required in vivo and is not required for the development of ILC2 (38, 76). However, STAT6 has a post-developmental role in ILC2 function, as STAT6-deficient ILC2 produce less IL-13 (38). Type 2 cytokines support tissue remodeling and the response to helminth infections, but are deregulated in the development of allergy and asthma (4, 77–79). Indeed, ILC2 are rare population in normal human tissues (33, 57), but are expanded in nasal polyps of allergic patients with chronic rhinosinusitis and the skin of patients with atopic dermatitis (20, 80, 81).
GATA3 is the master TF regulating Module 2 and is required for the development of ILC2 and Th2 (20, 65, 82–84). In both human and mouse, Il4, Il5, and Il13 are located in a gene cluster on the same chromosome and are mutually regulated by the locus control region within an intervening gene, Rad50 (70, 85, 86); Csf2 (encoding GM-CSF) is also located nearby on the same chromosome. GATA3 binds regulatory elements within this gene cluster and in the Rad50 locus control region as well as directly to the promoter of IL-5 and IL-13 (87). Consistent with the minimal expression of IL-4 by ILC2, GATA3 does not appear to bind directly to the IL-4 promoter and conditional deletion experiments demonstrate that it is not required for expression after Th2 development (87, 88). However, GATA3 conditional deletion demonstrates a common role for this TF in Module 2 production of IL-5, IL-13, and amphiregulin (88, 89).
Module 2 is a feed-forward module with a high degree of lineage stability, which in certain conditions, can be overcome to generate plasticity toward Module 1. Feed-forward loops depend on Module 2 cytokines and, at least for T cells, cell-intrinsic signaling. For T cells, IL-4 production by Th2 facilitates the polarization of more Th2 through STAT6 induction of GATA3 (76). Lineage stability is then enhanced by autoactivation of GATA3 once it overcomes a threshold mediated by the transcriptional regulator FOG-1 (90, 91). Although ILCs appear to develop in the absence of polarizing cytokines in the bone marrow, their activation in the periphery is similarly feed-forward. In mouse, ILC2-generated IL-13 acts on the epithelium to drive the differentiation of tuft cells from LGR5+ stem cells, which in turn generate IL-25 that activates ILC2 to produce more IL-5 and IL-13 (34–36).
Despite Module 2 lineage stability, strong Module 1 stimuli can induce plasticity. For example, viral infection with lymphocytic choriomeningitis virus (LCMV) causes Th2 to upregulate T-bet and subsequently produce IFN-γ in conjunction with IL-4 (92). Recently, mouse ILC2 were also shown to undergo similar T-bet-mediated plasticity in response to infection with respiratory syncytial virus (RSV), influenza virus, Haemophilus influenzae, and Staphylococcus aureus (59). Likewise, human ILC2 cultured in vitro with the danger cytokine IL-1 upregulate T-bet and IL-12Rβ2, prompting responsiveness to IL-12 and the development of IFN-γ and IL-13 dual-producing cells (93). Thus, Module 2 is typically self-perpetuating, but can occasionally be redirected toward Module 1.
Module 3: Extracellular Bacteria and Fungi
All ILC3 subsets, Th17, and Th22 belong to Module 3, based on production of cytokines IL-22, IL-17a, and/or IL-17f (Figure 2). IL-26 is additionally produced in human but is not conserved in mouse. In the T cell lineage, Th22 also produce IL-10, which is not made in substantial quantities but other cells (94). Yet, Module 3 shares additional factors, including inherent plasticity to Module 1 and regulation by the cytokines IL-23 and IL-1. IL-23 activation of STAT3 drives Th17 and Th22 polarization, but it is not required for ILC3 development. However, STAT3-deficient ILC3 are unable to respond appropriately to IL-23 and are functionally impaired (95, 96).
Module 3 is conventionally associated with immunity to extracellular pathogens and fungi, but this module is also recognized to have a role in tissue homeostasis. It is deregulated in the development of psoriasis, Crohn’s disease, and other forms of autoimmunity such as the multiple sclerosis model experimental autoimmune encephalomyelitis (EAE). Consistent with this, investigators have reported ILC3 expansion in: skin and blood of patients with psoriasis (97–99); blood, gut, synovial fluid, and bone marrow of patients with ankylosing spondylitis (100); and blood, lung, and gut of patients with common variable immunodeficiency (CVID) (101). Interestingly, several labs have reported that ILC3 are reduced rather than increased in Crohn’s disease relative to ILC1 (17, 18, 57, 58), and that remaining ILC3 are phenotypically altered and express less MHC-II (102).
The master transcription factor regulating Module 3 identity is RORγt because in its absence, Th17 and ILC3 do not develop (24, 103). After development, however, RORγt directly regulates few genes, though in Th17, these include the signature cytokines IL-17a and IL-17f as well as receptors for key activating cytokines IL-23 and IL-1 (104). In ILC3s, RORγt may have an even smaller effect, as conditional deletion of RORγt as well as pharmacologic blockade revealed no difference in IL-17a production, but did reduce IL-17a production in Th17 (105). Many other factors have been shown to regulate Th17, but the TF AHR in particular appears also to be shared by ILC3 (30, 106, 107). AHR is a ligand-dependent TF that binds pollutants, dietary metabolites, and bacterial metabolic byproducts (108). In conjunction with RORγt, AHR is required for the development of Th-22 and post-natal ILC3 and also directly controls IL-22 production (30, 106, 107, 109).
Both human and mouse Th17 as well as most ILC3 are further characterized by substantial plasticity to Module 1 through the acquisition of T-bet and reduction of RORγt expression. This was originally discovered in patients with Chron’s disease, who harbor a population of RORγt+ T-bet+ cells that produce both IL-17 and IFN-γ (110). Later, mouse lineage tracing experiments of RORγt expression demonstrated that Th17 lose RORγt and IL-17 in IL-12-rich inflammatory environments, but retain the Module 1 TF T-bet and IFN-γ effector profile (111). For the mouse CCR6− lineage of ILC3, similar lineage tracing experiments revealed that plasticity occurs in steady state (13). Unlike transitioning Th17, however, RORγt+ T-bet+ NKp46+ ILC3 have lost the capacity to produce IL-17 and instead produce IL-22 and IFN-γ (24, 27–32). Mouse ILC3 plasticity is enhanced in certain microenvironments, such as the colon, in part through higher levels of IL-23 (27). Human NKp44+ ILC3 also exhibit substantial plasticity in vitro, induced by several cytokines including IL-12, IL-2, and IL-23 (18, 57, 112). Consequently, the reduction of ILC3 and increase in ILC1 in Chron’s disease is may reflect increased ILC3 plasticity that occurs in the inflammatory microenvironment.
Concluding Remarks
The identification of ILCs was a surprise in part because an entire lineage of cells was missed for many years, but also because it suggested that cytokines conventionally thought to be produced only by T cells could also be expressed by innate immune cells (113). Over recent years, the similarity between T cell and ILC development and effector programs has been increasingly appreciated. For ILCs, engagement of master TFs necessary for T helper cell polarization explains the convergent function of these cells in a single module, which notably each directly activate module-defining effector cytokines. In the future, emerging unique functions for ILCs are likely to be mediated by lineage-defining TFs, such as the master-ILC regulator Id2 and Ets family members (114), either directly or through reorganization of the accessible chromatin landscape.
Can ILCs perform all the functions of T cells? Thus far, there appear to be three functions that ILCs lack. First, ILCs by definition do not express the T cell receptor (TCR) and therefore cannot signal through it. Though obvious, this point is notable because gene expression and regulatory differences between ILCs and T cells may involve signaling factors differentially associated with TCR but not cytokine receptors. Consistent with this, we recently reported that the regulomes of human T helper cells were characterized by AP-1 and NFAT TF motifs compared to ILCs, core pathways activated by TCR signaling (115). Indeed, Il4 expression, which is increased in Th2 compared to ILC2, is well known to be regulated by AP-1 and NFAT (116).
Second, as immediately functional cells, ILCs lack the capacity for naivety, leading one group to propose that the naïve state is the defining feature of adaptive immune cells, rather than any particular effector function (117). As ILCs develop, they appear to acquire modular pathways de novo in the bone marrow, whereas naïve CD4+ T cells uniquely require TCR signaling and cytokine-mediated STAT-activation to generate chromatin landscapes supportive of Th- polarization (Figure 1). Interestingly, in-vitro generated Th cells also have the capacity to respond directly to cytokines via innate-like STAT- and NFκB-dependent, NFAT-independent mechanisms (118–122). In vivo, tissue-resident memory CD4+ T cell populations can also demonstrate similar innate-like cytokine activation (123, 124). Future comparisons between effector profiles of in-vivo generated T cells of different stages of maturity/tissue-residence and ILCs will likely be informative to further define the scope of shared immune modules.
Finally, to date, no NKT or ILC subset has been discovered to express the master regulatory TF FOXP3, which drives Treg development. In the future, it will be interesting to determine if a Regulatory Module driven by FOXP3 expression is incompatible with NKT and ILC lineage, and if so, the mechanism by which this occurs.
What do we know?
ILCs and T cells share key transcription factors and effector functions.
Transcription factors drive effector functions and define ILC and T cell subtypes.
T helper cells go through a naive state while ILCs do not.
ILCs and T cell functions are similar but not identical.
What is still unknown?
To what extent do differences in cell surface receptor signaling produce functional differences between ILCs and T cells?
How do effector programs of ILCs compare with those of naive, effector, and memory T cell subsets?
Are there ILC counterparts to Tfh and Treg, and if not, why?
What is the contribution of ILCs to human biology in homeostasis and disease?
Acknowledgments
Supported by the US National Institutes of Health (1U01AI095542, R01DE021255 and R21CA16719 to the Colonna laboratory; 1F30DK107053-01 to M.L.R.). The authors thank J. Bando for critical comments.
List with all current abbreviations
- APC
Antigen Presenting Cell
- ATACseq
Assay for Transposase-Accessible Chromatin with high throughput sequencing
- CLP
Common Lymphoid Progenitor
- COPD
Chronic Obstructive Pulmonary Disease
- CVID
Common Variable Immunodeficiency
- EAE
Experimental Autoimmune Encephalomyelitis
- Eomes
Eomesodermin
- FasL
Fas Ligand
- γc
Common gamma chain, IL-2rg
- ILC
Innate Lymphoid Cell
- ILL
Innate-Like Lymphocyte
- LCMV
Lymphocytic Choriomeningitis Virus
- NK
Natural Killer
- NKT
Natural Killer T
- RSV
Respiratory Syncytial Virus
- TCR
T Cell Receptor
- TF
Transcription Factor
- Tfh
T follicular helper cell
- Th
T helper
- Treg
Regulatory T cell
References
- 1.Spits H, et al. Innate lymphoid cells–a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13(2):145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 2.Diefenbach A, Colonna M, Koyasu S. Development, differentiation, and diversity of innate lymphoid cells. Immunity. 2014;41(3):354–365. doi: 10.1016/j.immuni.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortez VS, Robinette ML, Colonna M. Innate lymphoid cells: new insights into function and development. Curr Opin Immunol. 2015;32:71–77. doi: 10.1016/j.coi.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eberl G, Colonna M, Di Santo JP, McKenzie AN. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science. 2015;348(6237):aaa6566. doi: 10.1126/science.aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serafini N, Vosshenrich CA, Di Santo JP. Transcriptional regulation of innate lymphoid cell fate. Nat Rev Immunol. 2015;15(7):415–428. doi: 10.1038/nri3855. [DOI] [PubMed] [Google Scholar]
- 6.Cording S, Medvedovic J, Aychek T, Eberl G. Innate lymphoid cells in defense, immunopathology and immunotherapy. Nat Immunol. 2016;17(7):755–757. doi: 10.1038/ni.3448. [DOI] [PubMed] [Google Scholar]
- 7.Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016;17(7):765–774. doi: 10.1038/ni.3489. [DOI] [PubMed] [Google Scholar]
- 8.Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8(+) T cells. Nat Rev Immunol. 2011;11(10):645–657. doi: 10.1038/nri3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishizuka IE, et al. Single-cell analysis defines the divergence between the innate lymphoid cell lineage and lymphoid tissue-inducer cell lineage. Nat Immunol. 2016;17(3):269–276. doi: 10.1038/ni.3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shih HY, et al. Developmental Acquisition of Regulomes Underlies Innate Lymphoid Cell Functionality. Cell. 2016;165(5):1120–1133. doi: 10.1016/j.cell.2016.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bando JK, Liang HE, Locksley RM. Identification and distribution of developing innate lymphoid cells in the fetal mouse intestine. Nat Immunol. 2015;16(2):153–160. doi: 10.1038/ni.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. A committed precursor to innate lymphoid cells. Nature. 2014;508(7496):397–401. doi: 10.1038/nature13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klose CS, et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014;157(2):340–356. doi: 10.1016/j.cell.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 14.Constantinides MG, et al. PLZF expression maps the early stages of ILC1 lineage development. Proc Natl Acad Sci U S A. 2015;112(16):5123–5128. doi: 10.1073/pnas.1423244112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spits H, Bernink JH, Lanier L. NK cells and type 1 innate lymphoid cells: partners in host defense. Nat Immunol. 2016;17(7):758–764. doi: 10.1038/ni.3482. [DOI] [PubMed] [Google Scholar]
- 16.Juelke K, Romagnani C. Differentiation of human innate lymphoid cells (ILCs) Curr Opin Immunol. 2016;38:75–85. doi: 10.1016/j.coi.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Fuchs A, et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-gamma-producing cells. Immunity. 2013;38(4):769–781. doi: 10.1016/j.immuni.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernink JH, et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol. 2013;14(3):221–229. doi: 10.1038/ni.2534. [DOI] [PubMed] [Google Scholar]
- 19.Molofsky AB, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210(3):535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mjosberg J, et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37(4):649–659. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Crellin NK, Trifari S, Kaplan CD, Cupedo T, Spits H. Human NKp44+IL-22+ cells and LTi-like cells constitute a stable RORC+ lineage distinct from conventional natural killer cells. J Exp Med. 2010;207(2):281–290. doi: 10.1084/jem.20091509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cella M, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457(7230):722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hepworth MR, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013;498(7452):113–117. doi: 10.1038/nature12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawa S, et al. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science. 2010;330(6004):665–669. doi: 10.1126/science.1194597. [DOI] [PubMed] [Google Scholar]
- 25.Vonarbourg C, et al. Regulated expression of nuclear receptor RORgammat confers distinct functional fates to NK cell receptor-expressing RORgammat(+) innate lymphocytes. Immunity. 2010;33(5):736–751. doi: 10.1016/j.immuni.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buonocore S, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464(7293):1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klose CS, et al. A T-bet gradient controls the fate and function of CCR6-RORgammat+ innate lymphoid cells. Nature. 2013;494(7436):261–265. doi: 10.1038/nature11813. [DOI] [PubMed] [Google Scholar]
- 28.Song C, et al. Unique and redundant functions of NKp46+ ILC3s in models of intestinal inflammation. J Exp Med. 2015;212(11):1869–1882. doi: 10.1084/jem.20151403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satoh-Takayama N, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29(6):958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Lee JS, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol. 2012;13(2):144–151. doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sciume G, et al. Distinct requirements for T-bet in gut innate lymphoid cells. J Exp Med. 2012;209(13):2331–2338. doi: 10.1084/jem.20122097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rankin LC, et al. The transcription factor T-bet is essential for the development of NKp46+ innate lymphocytes via the Notch pathway. Nat Immunol. 2013;14(4):389–395. doi: 10.1038/ni.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bjorklund AK, et al. The heterogeneity of human CD127(+) innate lymphoid cells revealed by single-cell RNA sequencing. Nat Immunol. 2016;17(4):451–460. doi: 10.1038/ni.3368. [DOI] [PubMed] [Google Scholar]
- 34.Gerbe F, et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature. 2016;529(7585):226–230. doi: 10.1038/nature16527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howitt MR, et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science. 2016;351(6279):1329–1333. doi: 10.1126/science.aaf1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Moltke J, Ji M, Liang HE, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2016;529(7585):221–225. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mortha A, et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 2014;343(6178):1249288. doi: 10.1126/science.1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nussbaum JC, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502(7470):245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voskoboinik I, Whisstock JC, Trapani JA. Perforin and granzymes: function, dysfunction and human pathology. Nat Rev Immunol. 2015;15(6):388–400. doi: 10.1038/nri3839. [DOI] [PubMed] [Google Scholar]
- 40.Berke G. The binding and lysis of target cells by cytotoxic lymphocytes: molecular and cellular aspects. Annu Rev Immunol. 1994;12:735–773. doi: 10.1146/annurev.iy.12.040194.003511. [DOI] [PubMed] [Google Scholar]
- 41.McLane LM, et al. Differential localization of T-bet and Eomes in CD8 T cell memory populations. J Immunol. 2013;190(7):3207–3215. doi: 10.4049/jimmunol.1201556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12(11):749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pearce EL, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302(5647):1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 44.Intlekofer AM, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6(12):1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 45.Gordon SM, et al. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity. 2012;36(1):55–67. doi: 10.1016/j.immuni.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cruz-Guilloty F, et al. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J Exp Med. 2009;206(1):51–59. doi: 10.1084/jem.20081242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Townsend MJ, et al. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20(4):477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 48.Daussy C, et al. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. J Exp Med. 2014;211(3):563–577. doi: 10.1084/jem.20131560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sojka DK, et al. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. Elife. 2014;3:e01659. doi: 10.7554/eLife.01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pikovskaya O, et al. Cutting Edge: Eomesodermin Is Sufficient To Direct Type 1 Innate Lymphocyte Development into the Conventional NK Lineage. J Immunol. 2016;196(4):1449–1454. doi: 10.4049/jimmunol.1502396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cortez VS, et al. Transforming Growth Factor-beta Signaling Guides the Differentiation of Innate Lymphoid Cells in Salivary Glands. Immunity. 2016;44(5):1127–1139. doi: 10.1016/j.immuni.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seillet C, et al. Nfil3 is required for the development of all innate lymphoid cell subsets. J Exp Med. 2014;211(9):1733–1740. doi: 10.1084/jem.20140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robinette ML, et al. Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat Immunol. 2015;16(3):306–317. doi: 10.1038/ni.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szabo SJ, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100(6):655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 55.Hsieh CS, et al. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260(5107):547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 56.O’Sullivan TE, R M, Fan X, Weizman OE, Bhardwaj P, Adams NM, Walzer T, Dannenberg AJ, Sun JC. Adipose-Resident Group 1 Innate Lymphoid Cells Promote Obesity-Associated Insulin Resistance. Immunity. 2016 doi: 10.1016/j.immuni.2016.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bernink JH, et al. Interleukin-12 and -23 Control Plasticity of CD127(+) Group 1 and Group 3 Innate Lymphoid Cells in the Intestinal Lamina Propria. Immunity. 2015;43(1):146–160. doi: 10.1016/j.immuni.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 58.Li J, Doty AL, Iqbal A, Glover SC. The differential frequency of Lineage(−)CRTH2(−)CD45(+)NKp44(−)CD117(−)CD127(+)ILC subset in the inflamed terminal ileum of patients with Crohn’s disease. Cell Immunol. 2016:304–305. 63–68. doi: 10.1016/j.cellimm.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 59.Silver JS, et al. Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs. Nat Immunol. 2016;17(6):626–635. doi: 10.1038/ni.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peng H, et al. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J Clin Invest. 2013;123(4):1444–1456. doi: 10.1172/JCI66381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szabo SJ, et al. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295(5553):338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 62.Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science. 2005;307(5708):430–433. doi: 10.1126/science.1103336. [DOI] [PubMed] [Google Scholar]
- 63.Zhu J, et al. The transcription factor T-bet is induced by multiple pathways and prevents an endogenous Th2 cell program during Th1 cell responses. Immunity. 2012;37(4):660–673. doi: 10.1016/j.immuni.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Djuretic IM, et al. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nat Immunol. 2007;8(2):145–153. doi: 10.1038/ni1424. [DOI] [PubMed] [Google Scholar]
- 65.Zhu J, Yamane H, Cote-Sierra J, Guo L, Paul WE. GATA-3 promotes Th2 responses through three different mechanisms: induction of Th2 cytokine production, selective growth of Th2 cells and inhibition of Th1 cell-specific factors. Cell Res. 2006;16(1):3–10. doi: 10.1038/sj.cr.7310002. [DOI] [PubMed] [Google Scholar]
- 66.Ebihara T, et al. Runx3 specifies lineage commitment of innate lymphoid cells. Nat Immunol. 2015;16(11):1124–1133. doi: 10.1038/ni.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Knox JJ, Cosma GL, Betts MR, McLane LM. Characterization of T-bet and eomes in peripheral human immune cells. Front Immunol. 2014;5:217. doi: 10.3389/fimmu.2014.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lupar E, et al. Eomesodermin Expression in CD4+ T Cells Restricts Peripheral Foxp3 Induction. J Immunol. 2015;195(10):4742–4752. doi: 10.4049/jimmunol.1501159. [DOI] [PubMed] [Google Scholar]
- 69.Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol. 2011;11(7):445–456. doi: 10.1038/nri3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu J. T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine. 2015;75(1):14–24. doi: 10.1016/j.cyto.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Monticelli LA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12(11):1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Turner JE, et al. IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. J Exp Med. 2013;210(13):2951–2965. doi: 10.1084/jem.20130071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moro K, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463(7280):540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 74.Neill DR, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464(7293):1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Price AE, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107(25):11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paul WE. What determines Th2 differentiation, in vitro and in vivo? Immunol Cell Biol. 2010;88(3):236–239. doi: 10.1038/icb.2010.2. [DOI] [PubMed] [Google Scholar]
- 77.Gold MJ, et al. Group 2 innate lymphoid cells facilitate sensitization to local, but not systemic, TH2-inducing allergen exposures. J Allergy Clin Immunol. 2014;133(4):1142–1148. doi: 10.1016/j.jaci.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 78.Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36(3):451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 79.Halim TY, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40(3):425–435. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim BS, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5(170):170ra116. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salimi M, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210(13):2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoyler T, et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37(4):634–648. doi: 10.1016/j.immuni.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang DH, Cohn L, Ray P, Bottomly K, Ray A. Transcription factor GATA-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gene. J Biol Chem. 1997;272(34):21597–21603. doi: 10.1074/jbc.272.34.21597. [DOI] [PubMed] [Google Scholar]
- 84.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89(4):587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 85.Smirnov DV, Smirnova MG, Korobko VG, Frolova EI. Tandem arrangement of human genes for interleukin-4 and interleukin-13: resemblance in their organization. Gene. 1995;155(2):277–281. doi: 10.1016/0378-1119(94)00720-d. [DOI] [PubMed] [Google Scholar]
- 86.Lee DU, Rao A. Molecular analysis of a locus control region in the T helper 2 cytokine gene cluster: a target for STAT6 but not GATA3. Proc Natl Acad Sci U S A. 2004;101(45):16010–16015. doi: 10.1073/pnas.0407031101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yagi R, Zhu J, Paul WE. An updated view on transcription factor GATA3-mediated regulation of Th1 and Th2 cell differentiation. Int Immunol. 2011;23(7):415–420. doi: 10.1093/intimm/dxr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu J, et al. Conditional deletion of Gata3 shows its essential function in T(H)1–T(H)2 responses. Nat Immunol. 2004;5(11):1157–1165. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
- 89.Yagi R, et al. The transcription factor GATA3 is critical for the development of all IL-7Ralpha-expressing innate lymphoid cells. Immunity. 2014;40(3):378–388. doi: 10.1016/j.immuni.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou M, et al. Friend of GATA-1 represses GATA-3-dependent activity in CD4+ T cells. J Exp Med. 2001;194(10):1461–1471. doi: 10.1084/jem.194.10.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ouyang W, et al. Stat6-independent GATA-3 autoactivation directs IL-4-independent Th2 development and commitment. Immunity. 2000;12(1):27–37. doi: 10.1016/s1074-7613(00)80156-9. [DOI] [PubMed] [Google Scholar]
- 92.Hegazy AN, et al. Interferons direct Th2 cell reprogramming to generate a stable GATA-3(+)T-bet(+) cell subset with combined Th2 and Th1 cell functions. Immunity. 2010;32(1):116–128. doi: 10.1016/j.immuni.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 93.Ohne Y, et al. IL-1 is a critical regulator of group 2 innate lymphoid cell function and plasticity. Nat Immunol. 2016;17(6):646–655. doi: 10.1038/ni.3447. [DOI] [PubMed] [Google Scholar]
- 94.Eyerich S, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119(12):3573–3585. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guo X, et al. Induction of innate lymphoid cell-derived interleukin-22 by the transcription factor STAT3 mediates protection against intestinal infection. Immunity. 2014;40(1):25–39. doi: 10.1016/j.immuni.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rankin LC, et al. Complementarity and redundancy of IL-22-producing innate lymphoid cells. Nat Immunol. 2016;17(2):179–186. doi: 10.1038/ni.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Teunissen MB, et al. Composition of innate lymphoid cell subsets in the human skin: enrichment of NCR(+) ILC3 in lesional skin and blood of psoriasis patients. J Invest Dermatol. 2014;134(9):2351–2360. doi: 10.1038/jid.2014.146. [DOI] [PubMed] [Google Scholar]
- 98.Villanova F, et al. Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ ILC3 in psoriasis. J Invest Dermatol. 2014;134(4):984–991. doi: 10.1038/jid.2013.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dyring-Andersen B, et al. Increased number and frequency of group 3 innate lymphoid cells in nonlesional psoriatic skin. Br J Dermatol. 2014;170(3):609–616. doi: 10.1111/bjd.12658. [DOI] [PubMed] [Google Scholar]
- 100.Ciccia F, et al. Type 3 innate lymphoid cells producing IL-17 and IL-22 are expanded in the gut, in the peripheral blood, synovial fluid and bone marrow of patients with ankylosing spondylitis. Ann Rheum Dis. 2015;74(9):1739–1747. doi: 10.1136/annrheumdis-2014-206323. [DOI] [PubMed] [Google Scholar]
- 101.Cols M, et al. Expansion of inflammatory innate lymphoid cells in patients with common variable immune deficiency. J Allergy Clin Immunol. 2016;137(4):1206–1215. e1201–1206. doi: 10.1016/j.jaci.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hepworth MR, et al. Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4(+) T cells. Science. 2015;348(6238):1031–1035. doi: 10.1126/science.aaa4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 104.Ciofani M, et al. A validated regulatory network for Th17 cell specification. Cell. 2012;151(2):289–303. doi: 10.1016/j.cell.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Withers DR, et al. Transient inhibition of ROR-gammat therapeutically limits intestinal inflammation by reducing TH17 cells and preserving group 3 innate lymphoid cells. Nat Med. 2016;22(3):319–323. doi: 10.1038/nm.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kiss EA, et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334(6062):1561–1565. doi: 10.1126/science.1214914. [DOI] [PubMed] [Google Scholar]
- 107.Qiu J, et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36(1):92–104. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cella M, Colonna M. Aryl hydrocarbon receptor: Linking environment to immunity. Semin Immunol. 2015;27(5):310–314. doi: 10.1016/j.smim.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Basu R, et al. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity. 2012;37(6):1061–1075. doi: 10.1016/j.immuni.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Annunziato F, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204(8):1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee YK, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30(1):92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cella M, Otero K, Colonna M. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1beta reveals intrinsic functional plasticity. Proc Natl Acad Sci U S A. 2010;107(24):10961–10966. doi: 10.1073/pnas.1005641107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells–how did we miss them? Nat Rev Immunol. 2013;13(2):75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- 114.Zook EC, Kee BL. Development of innate lymphoid cells. Nat Immunol. 2016;17(7):775–782. doi: 10.1038/ni.3481. [DOI] [PubMed] [Google Scholar]
- 115.Koues OI, et al. Distinct Gene Regulatory Pathways for Human Innate versus Adaptive Lymphoid Cells. Cell. 2016;165(5):1134–1146. doi: 10.1016/j.cell.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li-Weber M, Krammer PH. Regulation of IL4 gene expression by T cells and therapeutic perspectives. Nat Rev Immunol. 2003;3(7):534–543. doi: 10.1038/nri1128. [DOI] [PubMed] [Google Scholar]
- 117.Bedoui S, Gebhardt T, Gasteiger G, Kastenmuller W. Parallels and differences between innate and adaptive lymphocytes. Nat Immunol. 2016;17(5):490–494. doi: 10.1038/ni.3432. [DOI] [PubMed] [Google Scholar]
- 118.Guo L, et al. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc Natl Acad Sci U S A. 2009;106(32):13463–13468. doi: 10.1073/pnas.0906988106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Robinson D, et al. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-gamma production and activates IRAK and NFkappaB. Immunity. 1997;7(4):571–581. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- 120.Yang J, Zhu H, Murphy TL, Ouyang W, Murphy KM. IL-18-stimulated GADD45 beta required in cytokine-induced, but not TCR-induced, IFN-gamma production. Nat Immunol. 2001;2(2):157–164. doi: 10.1038/84264. [DOI] [PubMed] [Google Scholar]
- 121.Chung Y, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30(4):576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Meisel C, et al. Regulation and function of T1/ST2 expression on CD4+ T cells: induction of type 2 cytokine production by T1/ST2 cross-linking. J Immunol. 2001;166(5):3143–3150. doi: 10.4049/jimmunol.166.5.3143. [DOI] [PubMed] [Google Scholar]
- 123.Coquet JM, et al. Interleukin-21-Producing CD4(+) T Cells Promote Type 2 Immunity to House Dust Mites. Immunity. 2015;43(2):318–330. doi: 10.1016/j.immuni.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 124.Guo L, et al. Innate immunological function of TH2 cells in vivo. Nat Immunol. 2015;16(10):1051–1059. doi: 10.1038/ni.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]


