Atherosclerosis, the precursor to acute coronary syndromes (ACS), is a disease of chronic inflammation triggered initially by the focal subendothelial retention of apolipoprotein B100-containing lipoproteins and exacerbated by other risk factors, such as smoking, diabetes, and hypertension 1, 2. The persistence of these stimuli promotes a cycle of nonresolving inflammation that promotes atherosclerotic lesion development and, most importantly, progression to the unique types of necrotic plaques that cause ACS. In order for inflammation to resolve, there must not only be an abatement of initiating risk factors, but also an initiation of pro-resolving molecular and cellular pathways that act to restore tissue homeostasis and promote repair. Based on observations of human and animal atherosclerotic plaque features, genetic and therapy-based causation studies in mice, and cell culture studies with macrophages, researchers over the last two decades have proposed that defective resolution drives atherosclerosis progression. For example, advanced atherosclerosis is characterized by failure to reduce plaque inflammatory cell number, inefficiency in removing dying cells, and suboptimal tissue repair. However, the molecular basis of defective inflammation in atherosclerosis remained to be precisely defined.
The inflammation resolution program is carried out by a number of molecular and cellular effectors. Among these are a superfamily of unsaturated fatty acid-derived lipid mediators referred to as specialized pro-resolving mediators, or SPMs. SPMs are biosynthesized by inflammatory cells during self-limited inflammation and, by interacting with specific cell-surface receptors on immune cells and other cell types, trigger processes that dampen inflammation and promote tissue repair. Moreover, exogenously administered SPMs and other proresolving mediators have shown efficacy in triggering resolution of several types of chronic diseases, including atherosclerosis. Although characterized within human arterial cells in the early 1990’s by Serhan and colleagues 3, a comprehensive and direct cataloguing of SPMs within atherosclerotic lesions is only now coming to light.
Implications for SPMs in atherosclerosis
The major types of SPMs are the lipoxins (LX), resolvins (Rv), protectins (P), and maresins (MaR), each of which is endogenously biosynthesized in resolving exudates and activates specific G-protein coupled receptors 4. The precursors of SPMs include diet-derived polyunsaturated fatty acids that are substrates for SPM biosynthetic enzymes, including lipoxygenases and cyclooxygenases. Lipoxins are derived from omega-6 arachidonic acid, whereas resolvins, protectins, and maresins are derived from omega-3 fatty acids, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). In human plasma, low levels of SPMs are correlated with peripheral and coronary artery disease 5. In mouse models of atherosclerosis, macrophage-specific overexpression of 12/15-lipoxygenase, which increased the local biosynthesis of LXA4, RvD1, and PD1, suppressed lesion development 6. In terms of human evidence, variants in the gene, ALOX5, which can synthesize pro-resolving SPMs in the presence of n-3 fatty acids but pro-inflammatory/pro-atherosclerotic leukotrienes in the presence of n-6 fatty acids, was associated with decreased or increased risk for subjects ingesting diets rich in n-3 vs. n-6 fats, respectively 7. Interestingly, the benefit of fish oils when analyzed in the absence of genetic interactions has yielded conflicting results. In this setting of active investigation, what remained unclear were the levels and temporal patterns of lesional SPMs during atherosclerosis progression.
An imbalance of bioactive lipids in advanced plaque
In a recent study in this issue of Circulation Research, Viola et al. 8 test the hypothesis that atherosclerosis progression is tied to a deficit of arterial SPMs. The investigators performed aortic lipid mediator profiling of advanced atherosclerotic lesions from fat-fed hyperlipidemic mice after carefully snap-freezing aortas and utilizing liquid chromatography in tandem with mass spectrometry. Their findings indicate that the levels of the inflammatory lipids leukotriene B4 (LTB4) and prostaglandin E2 (PGE2) increase as atherosclerotic plaques mature. Conversely, advanced plaques had significantly lower levels of two types of SPMs, RvD2 and Mar1. Importantly, these SPMs declined progressively with longer duration of fat-feeding. Of note, RvD1, LXA4, and protectins were below detection limits. Thus, atheroprogression was associated with an increasing and unbalanced ratio of inflammatory vs. resolving bioactive lipids in plaques.
In humans, lesions that are unstable and at risk for rupture are so called “vulnerable.” Vulnerable plaques are marked in part by thin fibrous caps and increased cellular and macrophage death, the effects of which are compounded by defects in dead cell clearance, or efferocytosis. Taking into account these features of unstable plaques, Viola et al. generated an index of plaque instability and found that increases in LTB4 and PGE2 positively correlated with this index. In contrast, RvD2 and MaR1 levels correlated with opposing indices of plaque stability, including the aggregate of smooth muscle cell number, the surface area of collagen deposition, and fibrous cap thickness (Figure 1).
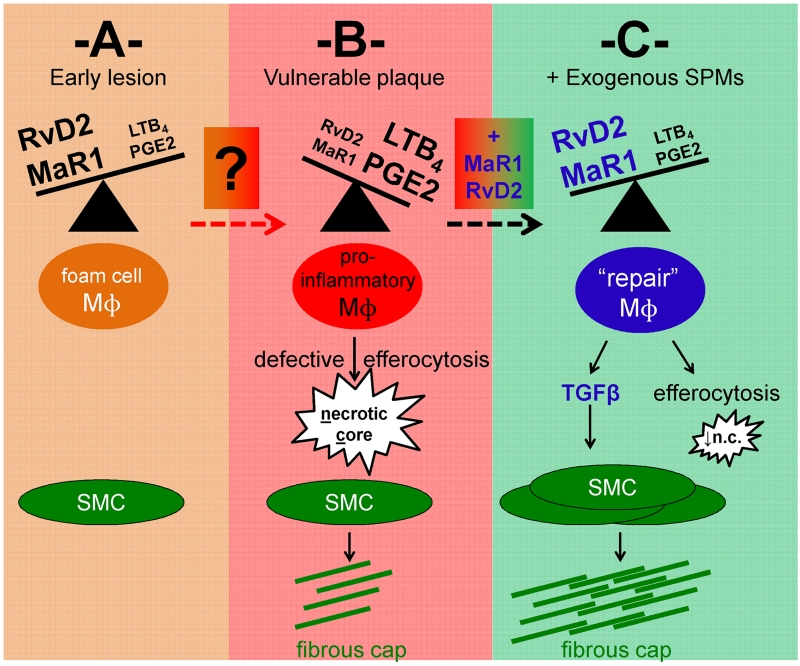
Figure 1. Resolving lipid mediators during atherosclerotic plaque progression.
Working model of the cellular consequences of altered ratios of pro-inflammatory vs. pro-resolving lipid mediators in early (A) versus advanced (B) atherosclerotic plaques. The factors that promote lipid mediator imbalance during the transition from (A) to (B) remain to be fully elucidated. In (B) reduced levels of lipid mediators resolvin D2 (RvD2) and maresin 1 (MaR1), and increased leukotriene LTB4 and prostaglandin PGE2, are associated with features of vulnerable plaques, including inflammatory macrophages, enlarged necrotic cores, and thin fibrous caps. (C) Predicted mechanistic basis of RvD2 and MaR1 therapy in advanced atherosclerosis, leading to macrophage reprogramming, reduced necrotic cores, and macrophage-TGFβ-mediated activation of SMCs. As noted in the commentary, a concurrent study to Viola et al., which showed similar results in mice and also humans [12], together with the results of previous studies showing the benefit of pro-resolving mediator therapy in atherosclerosis, are consistent with the overall principles depicted in this scheme. Mɸ = macrophage. SMC = smooth muscle cell.
Tipping the Balance in favor of SPMs
The imbalance of proresolving to pro-inflammatory mediators in advancing plaques provides a context for previous studies showing benefits of resolving mediator therapy in atherosclerosis 9-11. Based on their findings related to specific SPM deficiencies in advancing plaques, Viola et al. co-administered RvD2 and MaR1 by serial injection to atherosclerotic mice and discovered that this combinatorial SPM cocktail prevented athero-progression, even in the setting of continued high fat-feeding. Specifically, RvD2/MaR1 reduced plaque necrosis and increased both the number of smooth muscle cells and thickness of the fibrous cap. While previous studies identified direct actions of SPMs on both smooth muscle cell function and macrophages 5, Viola et al. showed that MaR1/RvD2-treated macrophages activate smooth muscle collagen production in culture by secreting TGFβ. It is important to note that SPM effects on fibrosis appear organ dependent, as SPM administration can reduce collagen deposition in other tissues. RvD2/MaR1 treatment also reduced plaque macrophage content, and, based on aortic mRNA profiling, promoted anti-inflammatory macrophage polarization. Similar findings were recapitulated after adding RvD2 and MaR1 to isolated cultures of activated macrophages. These data, together with the fact that effects of RVD2/MaR1 on lesions was independent of changes in peripheral plasma lipid levels, circulating inflammatory cells, and markers of endothelial cell activation, suggest that a major mechanism for the suppressive action of SPMs on atherosclerosis is through their direct action on lesional macrophages.
SPMs & efferocytosis
The report of Viola et al. is timely, as a concurrent study by Fredman et al. 12 has also documented an imbalance in pro-resolving to pro-inflammatory lipid mediators in advanced mouse lesions, and, importantly, in advanced vs. early human plaques. This study also showed the benefit of SPM “restoration” therapy in decreasing vulnerable plaque-like features. Whereas Viola et al. focused their mechanistic studies on crosstalk between macrophages and smooth muscle cells, Fredman et al. examined the SPM link to efferocytosis. Defective efferocytosis not only prevents dying cell clearance and thus increases plaque necrosis, but may also may contribute to lipid mediator imbalance, as efferocytosis per se is a signal to initiate SPM biosynthesis 4. In terms of mechanism, a recent study showed that the efferocytosis receptor MerTK, which has been shown to promote plaque stability, is a molecular switch that activates intracellular signaling to trigger SPM biosynthesis 13. The mechanism involves re-localization of 5-lipoxygenase (5-LOX) from the nucleus to cytoplasm, bringing 5-LOX into close proximity to the pro-resolving enzyme 12/15 LOX. In contrast, nuclear 5-LOX, which is proximal to leukotriene A4 hydrolase, leads to pro-inflammatory LTB4 generation.
Unsolved questions
Despite the novel findings of the work discussed herein, the mechanistic underpinnings of how SPM levels are perturbed in advanced atherosclerotic plaque require further study. In one possible scenario, the lesion environment may suppress SPM biosynthesis, which is regulated by various intracellular and transcellular processes and enzymes 4. For example, new findings suggest that reactive oxygen species may be linked to the suppression of DHA-derived resolvins 12. Another mechanism may involve enzymatic or non-enzymatic degradation of SPMs in advanced plaques. On the therapeutic front, it will be interesting to test the effects of SPMs on atherosclerosis under less hyperlipidemic conditions to assess efficacy under current statin-based standards of care and because these conditions may favor endogenous SPM biosynthesis 12, 14. The changes in SPMs during plaque regression and the effect of exogenous SPMs in this setting will also be an interesting topic for future study. Finally, in the context of the SPM cocktail used in this study vs. RvD1 or a pro-resolving peptide used in other studies [10, 12], it will be important to determine whether certain resolving mediators have advantages over others, e.g., due to expression or lack thereof of their cognate receptors in lesional cells.
In sum, the important findings of Viola et al., when viewed together with a concurrent study showing similar results [12], provide direct experimental evidence for the reduction of SPMs during atheroprogression as well as a mechanistic basis for resolution mediator therapy in atherosclerosis. The concept of combining current therapies that inhibit atherosclerotic risk factors, notably statins, with a new type of therapy that actively promotes resolution, might have additive or synergistic benefits in preventing the deadly events caused by advanced atherosclerotic plaque progression.
Acknowledgments
Sources of Funding: R01HL122309 to ET.
Footnotes
Disclosures: None
References
- 1.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–51. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15:551–61. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brezinski DA, Nesto RW, Serhan CN. Angioplasty triggers intracoronary leukotrienes and lipoxin A4. Impact of aspirin therapy. Circulation. 1992;86:56–63. doi: 10.1161/01.cir.86.1.56. [DOI] [PubMed] [Google Scholar]
- 4.Buckley CD, Gilroy DW, Serhan CN. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity. 2014;40:315–27. doi: 10.1016/j.immuni.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho KJ, Spite M, Owens CD, Lancero H, Kroemer AH, Pande R, Creager MA, Serhan CN, Conte MS. Aspirin-triggered lipoxin and resolvin E1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis. The American journal of pathology. 2010;177:2116–23. doi: 10.2353/ajpath.2010.091082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. Faseb j. 2008;22:3595–606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dwyer JH, Allayee H, Dwyer KM, Fan J, Wu H, Mar R, Lusis AJ, Mehrabian M. Arachidonate 5-lipoxygenase promoter genotype, dietary arachidonic acid, and atherosclerosis. N Engl J Med. 2004;350:29–37. doi: 10.1056/NEJMoa025079. [DOI] [PubMed] [Google Scholar]
- 8.Viola J, Lemnitzer P, Jansen Y, Csaba G, Winter C, Neideck C, Silvestre-Roig C, Dittmar G, Doring Y, Drechsler M, Weber C, Zimmer R, Cenac N, Soehnlein O. Resolving Lipid Mediators Maresin 1 and Resolvin D2 Prevent Atheroprogression in Mice. Circulation research. 2016 doi: 10.1161/CIRCRESAHA.116.309492. [DOI] [PubMed] [Google Scholar]
- 9.Drechsler M, de Jong R, Rossaint J, Viola JR, Leoni G, Wang JM, Grommes J, Hinkel R, Kupatt C, Weber C, Doring Y, Zarbock A, Soehnlein O. Annexin A1 counteracts chemokine-induced arterial myeloid cell recruitment. Circulation research. 2015;116:827–35. doi: 10.1161/CIRCRESAHA.116.305825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fredman G, Kamaly N, Spolitu S, Milton J, Ghorpade D, Chiasson R, Kuriakose G, Perretti M, Farokhzad O, Tabas I. Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci Transl Med. 2015;7:275ra20. doi: 10.1126/scitranslmed.aaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasturk H, Abdallah R, Kantarci A, Nguyen D, Giordano N, Hamilton J, Van Dyke TE. Resolvin E1 (RvE1) Attenuates Atherosclerotic Plaque Formation in Diet and Inflammation-Induced Atherogenesis. Arterioscler Thromb Vasc Biol. 2015;35:1123–33. doi: 10.1161/ATVBAHA.115.305324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fredman G, Hellmann J, Proto J, Kuriakose G, Colas RA, Dorweiler B, Connolly ES, Solomon R, Jones DM, Heyer EJ, Spite M, Tabas I. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nature Commun. 2016 doi: 10.1038/ncomms12859. doi: 10.1038/ncomms12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai B, Thorp EB, Doran AC, Subramanian M, Sansbury BE, Lin CS, Spite M, Fredman G, Tabas I. MerTK cleavage limits proresolving mediator biosynthesis and exacerbates tissue inflammation. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1524292113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merched AJ, Serhan CN, Chan L. Nutrigenetic disruption of inflammation-resolution homeostasis and atherogenesis. Journal of nutrigenetics and nutrigenomics. 2011;4:12–24. doi: 10.1159/000326890. [DOI] [PMC free article] [PubMed] [Google Scholar]