SUMMARY
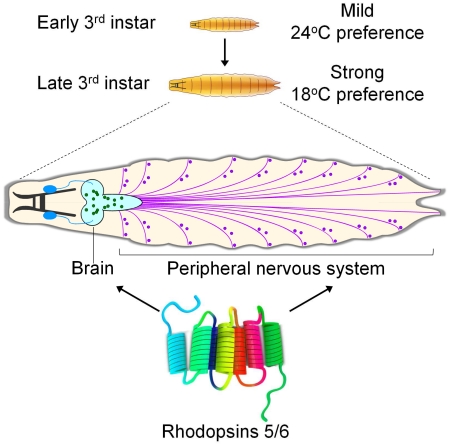
Drosophila 3rd instar larvae exhibit changes in their behavioral responses to gravity and food as they transition from feeding to wandering stages. Using a thermal gradient encompassing the comfortable range (18 to 28°C), we found that 3rd instar larvae exhibit a dramatic shift in thermal preference. Early-3rd instar larvae prefer 24°C, which switches to increasingly stronger biases for 18°—19°C in mid- and late-3rd instar larvae. Mutations eliminating either of two rhodopsins, Rh5 and Rh6, wiped out these age-dependent changes in thermal preference. In larvae, Rh5 and Rh6 are thought to function exclusively in the light-sensing Bolwig organ. However, the Bolwig organ was dispensable for the thermal preference. Rather, Rh5 and Rh6 were required in trpA1-expressing neurons in the brain, ventral nerve cord and body wall. Because Rh1 contributes to thermal selection in the comfortable range during the early- to mid-3rd instar stage, fine thermal discrimination depends on multiple rhodopsins.
eTOC BLURB
Sensing optimal temperatures contributes to animal survival. Sokabe et al. reveal an unconventional role for two rhodopsins that function in the Drosophila brain and in body wall neurons to cause an age-specific switch in thermal preference.
INTRODUCTION
The capacity to sense and avoid acute exposure to noxious heat and cold is critical for survival, and in many animals this ability depends on direct activation of TRP channels (Julius, 2013; Venkatachalam and Montell, 2007). Animals ranging from worms to humans are also sensitive to small temperature differences in the comfortable range, and respond by selecting their preferred temperature zones (Julius, 2013; Venkatachalam et al., 2014; Venkatachalam and Montell, 2007). This behavior is especially acute in poikilothermic organisms such as the fruit fly, Drosophila melanogaster, which equilibrate their body temperature with the environment. As a consequence, Drosophila can respond behaviorally to thermal fluctuations of a fraction of a degree (Fowler and Montell, 2013; Klein et al., 2015). This is best documented in Drosophila larvae (Klein et al., 2015), and we have shown previously that the exquisite sensitivity to small changes in temperature in the comfortable range depends on a thermosensory signaling cascade that is initiated by one of the seven rhodopsins (Rh1) (Kwon et al., 2008; Shen et al., 2011).
The 3rd instar larval stage is a period characterized by dynamic modifications in behavior. Early- to mid-3rd instar larvae are motivated by feeding, while the late-3rd instar larvae must also prepare for the final, wandering stage, when they escape from food and subsequently pupate. Due to these changing needs, 3rd instar larvae transition from positive to negative geotaxis. In addition, attraction to food switches to aversion during 3rd instar larvae (Wu et al., 2003). However, it was unclear whether 3rd instar larvae also exhibit major changes in thermal preference before entering the wandering stage.
In this study, using a linear 18°—28°C thermal gradient, we established that 3rd instar larvae underwent a dramatic shift in their thermal preference over the course of 48 hours. Early-3rd instar larvae had a preference for 24°C, while mid -3rd instar larvae had a bias for 18°C. By the late-3rd instar period, immediately preceding the wandering stage, the animals strongly favored 18°C. Surprisingly, temperature selection in late-3rd instar larvae was normal in mutants missing Rh1, which we previously found was required in mid-3rd instar larvae (Shen et al., 2011). Instead, two other rhodopsins, Rh5 and Rh6, were strictly required in late-3rd instar larvae for choosing 18°C. These two rhodopsins functioned in neurons in the brain and body wall. Thus, the age-dependent change in thermal preference depended on a thermal detection system consisting of multiple rhodopsins as critical components.
RESULTS
Change in temperature preference in 3rd instar larvae
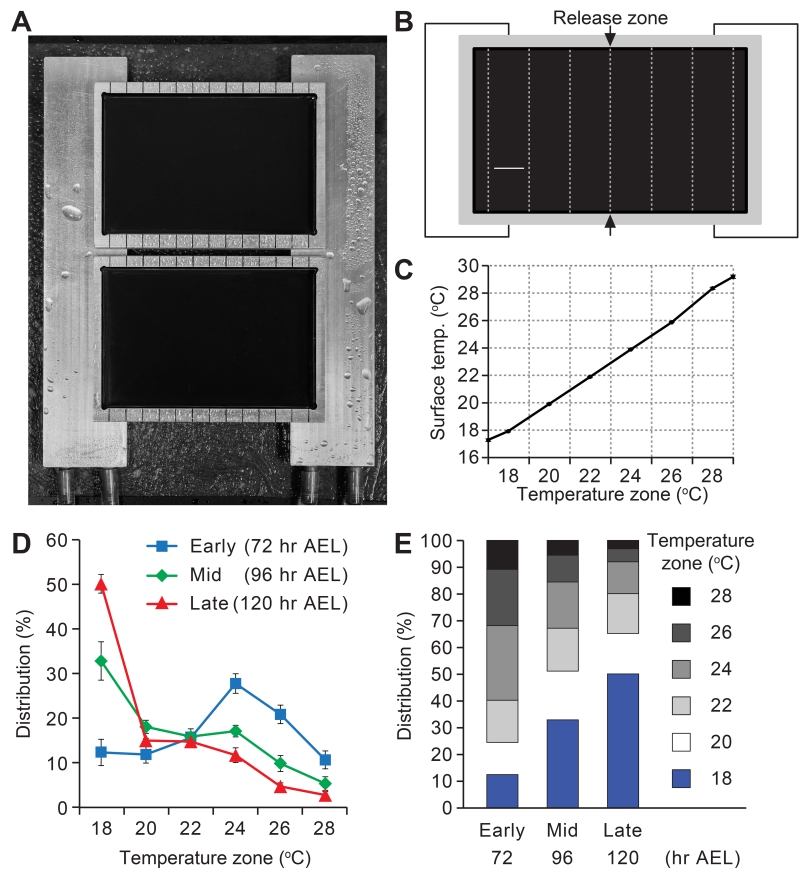
In order to clarify the thermal behavior of larvae we devised an apparatus, which allowed the animals to choose their preferred temperature within a continuous linear gradient (Figures 1A-1C). We focused on the 18°—28° C range, since this included the temperatures that support the most robust growth and survival during the larval period. Furthermore, we restricted the temperatures to 18°C or higher, since at lower temperatures larval locomotion was severely compromised. To characterize changes in thermal preferences in early-, mid- and late-3rd instar larvae, we collected control (w1118) larvae at 72, 96 and 120 hours after egg laying (AEL), respectively. 72 hours AEL coincided with the initiation of the 3rd instar larval period, while 120 hours AEL was immediately prior to the wandering stage when the larvae climb out of food-containing environments and initiate pupation. We did not characterize the wandering larvae (>120 hours AEL), due to their strong motivation to scale the edges of the gradient plate, thereby precluding a reliable analysis of their thermal preference.
Figure 1. Age-dependent changes in thermal preference in 3rd instar larvae.
(A) Apparatus for assaying thermal preference using a temperature gradient. The sides of the test plates are placed on aluminum blocks, each of which is set at a distinct temperature using circulating water from a bath.
(B) Schematic diagram of a test plate divided into 2-cm wide zones. 10—20 min after releasing the larvae between the 22° and 24°C zones, the number of larvae in each of the six zones was tabulated.
(C) Actual temperatures measured in the center of the zones. Data represent mean ±SD. n=7.
(D, E) Mean percentages of control (w1118) early-, mid- and late-stage 3rd instar larvae in the six zones that comprise the 18°—28°C temperature gradient. n=6-7 experiments. The error bars in D represent ±SEMs.
See also Figure S1.
We released the larvae in the border between 22° and 24°C zones (Figure 1B), and allowed the animals to explore the thermal landscape for 10—20 minutes, depending on the stage. We then determined the distribution of the animals within the six temperature zones (Figures 1B and 1C). We found that 3rd instar larvae exhibited a striking shift in their preferred temperature as the animals aged between 72 and 120 hours AEL. The early-3rd instar larvae chose the 24°C zone at the highest frequency (27.9 ±2.2%), and there was virtually no bias for one extreme of the temperature gradient over the other (Figures 1D and 1E; 18°C, 12.5 ±2.9%. 28°C, 10.8 ±2.0%). 24 hours later, the mid-3rd instar larvae altered their temperature preferences significantly. These larvae selected 18°C at the highest frequency (Figures 1D and 1E; 18°C, 32.9 ±4.3%), consistent with the two-way choice assays (Kwon et al., 2008). The late-3rd instar larvae displayed a strong preference for 18°C, and temperatures higher than 24°C became highly aversive (Figures 1D and 1E; 18°C, 50.2 ±2.1%. 28°C, 3.0 ±0.8%). In contrast to the early-3rd instar larvae, the late-3rd instar larvae favored the 18°C over the 28°C zone ~17-fold.
The late-3rd instar larvae were at a stage just prior to when wandering larvae begin climbing up surfaces to escape from food in preparation for pupation. Therefore, to test whether the strong selection of 18°C zone was influenced by the juxtaposition of this zone near the edge of the plate, we created a temperature gradient in which the 18°C zone was in the center, and the warmer zones radiated out bisymmetrically on both sides (Figure S1A). We found that the late-3rd instar larvae still favored the 18°C zone (Figures S1B and S1C). These results indicated that 3rd instar larvae changed their temperature preferences in an age-dependent manner over the course of 48 hours, and selected the lower temperature within the comfortable range as they got older.
Requirements for multiple rhodopsins for transitions in temperature selection
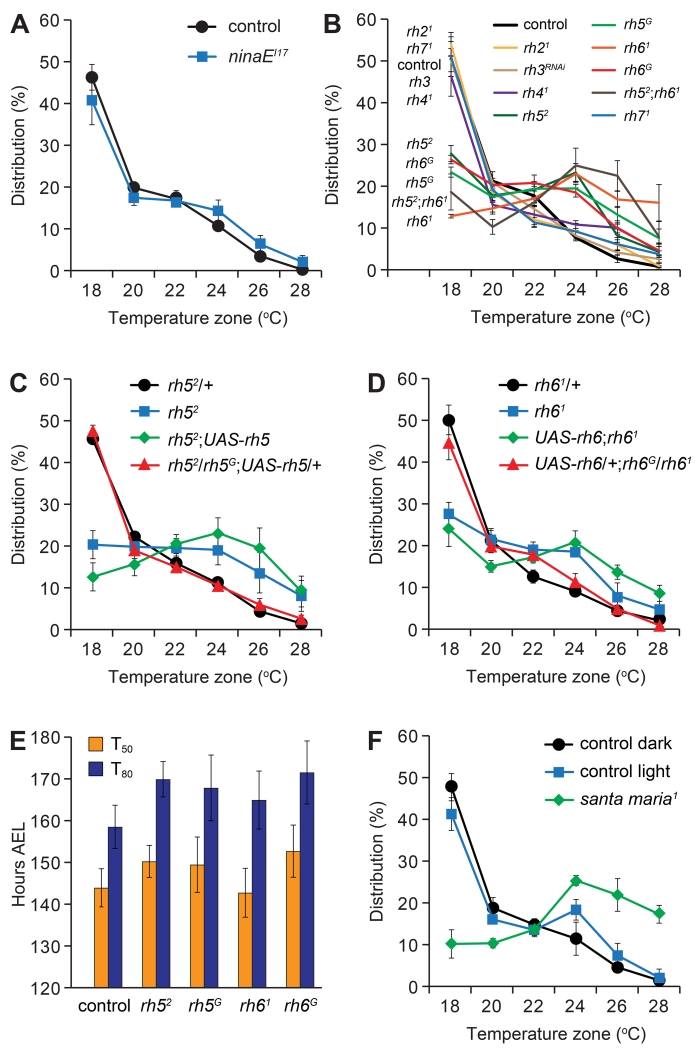
We reported previously that Rh1 was required in 3rd instar larvae for choosing 18°C over other temperatures in the comfortable range (19°—24°C) (Shen et al., 2011). Due to the significant changes in temperature preference during the 3rd instar period, we rechecked rh1 null (ninaEI17) larvae, and found that ninaEI17 larvae at the mid-3rd instar stage (96 hours AEL) were impaired in 18°C selection, consistent with previous results (Figure S2A) (Shen et al., 2011). At 96 hours the ninaEI17 mutant animals slightly favored the 24° and 26°C zones, and exhibited a temperature preference similar to control animals at 72 hours AEL (Figures 1D and S2A). We also characterized early-3rd instar larvae (72 hours AEL), and found that the ninaEI17 distribution pattern on the thermal gradient was shifted slightly towards the warmer temperatures (Figure S2B).
Due to the strong 18°C selection by control late-3rd instar larvae, we tested whether Rh1 was required in these animals. Surprisingly, the distribution pattern of the ninaEI17 late-3rd instar larvae was indistinguishable from the controls (Figure 2A). Therefore, we tested whether mutation or RNAi knockdown of any of the other six rhodopsin genes (rh2-rh7) impaired 18°C selection. We found that rh52 and rh61 mutant larvae displayed severe defects in choosing 18°C (Figure 2B). Other rhodopsin mutants or RNAi knockdown had no impact on thermotactic behavior (Figure 2B). Moreover, the phenotype of rh52;rh61 double mutant flies was similar to the rh52 and rh61 single mutant animals (Figure 2B). During the early- and mid-3rd instar larval stages the rh21, rh3 (RNAi knockdown), rh41 and rh71 also displayed thermal distribution patterns similar to control larvae (Figures S2C and S2D).
Figure 2. Requirements for rh5 and rh6 in late-3rd instar larvae for selecting the optimal temperature.
(A-D, F) Distribution of late-3rd instar larvae (120 hours AEL) of the indicated genotypes over 18°—28°C continuous thermal gradients.
(A) Control (w1118) and rh1 null mutant (ninaEI17). n=4-5.
(B) Rhodopsin mutants were used with the exception of RNAi-mediated knockdown of rh3, which was performed using dicer2;;elav-GAL4/UAS-rh3RNAi. n=3-5.
(C) Testing for rescue of the rh5 thermotaxis phenotype by expressing wild type UAS-rh5 under the control of the GAL4 knocked into the rh5 locus (rh5G). n=4-6.
(D) Testing for rescue of the rh6 mutant phenotype by expressing wild type UAS-rh6 under the control of the GAL4 knocked into the rh6 locus (rh6G). n=4-5.
(E) The time to pupation of the indicated genotypes. T50 and T80 denote the times required for 50% and 80% of larvae to become pupae, respectively. n=6-9.
(F) Thermal distribution of control flies (w1118) maintained under dark conditions (<0.1 μW/cm2) or under ambient light (~ 73 μW/cm2). The santa maria1 larvae were tested in the dark. n=3.
See also Figure S2.
We generated second rh5 and rh6 alleles, each of which included a GAL4 reporter inserted at the position of the normal ATG, in place of the N-terminal 540 and 499 base pairs of rh5 and rh6, respectively (rh5G and rh6G; Figures S2E and S2F). These GAL4 reporters were strongly expressed in the Bolwig organ (Figures S2G and S2H), consistent with previous findings that these rhodopsins are produced and function in this light-sensing organ (Sprecher et al., 2007). Both the rh5G and rh6G larvae showed defects similar to rh52 and rh61 during the mid- and late-3rd instar periods (Figures 2B and S2D). We rescued the rh52 and rh61 mutant phenotypes in late-3rd stage instar with wild-type rh5 and rh6 transgenes (UAS-rh5 and UAS-rh6, respectively; Figures 2C and 2D). These rh5 and rh6 mutant phenotypes did not appear to be due to developmental delays, as the time to pupation was indistinguishable between the mutants and controls (Figure 2E). Moreover, based on the morphology of the mouth hooks and spiracles, the percentages of rh5 and rh6 mutant larvae that entered the early-3rd instar larval stage at 74 hours AEL were not significantly different from the control (Figure S2I). Thus, we conclude that both Rh5 and Rh6 contributed to thermal preference in the mid- and late-stage 3rd instar larvae. Due to the strong temperature preference among control late-3rd instar larvae, we focused the remainder of our study on this larval stage.
Light and the Bolwig organ do not function in optimal temperature selection
Rhodopsins consist of two subunits: a protein moiety referred to as the opsin and a vitamin A derivative that is a chromophore. To test for a requirement for the chromophore, we assayed the temperature selection of a mutant, santa maria1, which disrupts a protein that contributes to retinoid formation (Wang et al., 2007). The santa maria1 late-3rd instar larvae were impaired in 18°C selection similar to the rh5 or rh6 mutants (Figure 2F). This did not appear to be due to a developmental delay as santa maria1 entered the 3rd instar larvae stage with similar timing as control animals (Figure S2J).
In Drosophila photoreceptor cells, the chromophore is required for not only for sensing light, but also for translocation of rhodopsin from the endoplasmic reticulum to the plasma membrane (Ozaki et al., 1993). To determine whether light influenced thermotaxis, we performed gradient assays in the presence or absence of light. We found that control larvae displayed the same strong bias for the 18°C zone in the light or dark (Figure 2F). Because thermotaxis is not dependent on light, we suggest that the impairment exhibited by santa maria1 larvae reflects a requirement for the chromophore for exiting the endoplasmic reticulum (Ozaki et al., 1993).
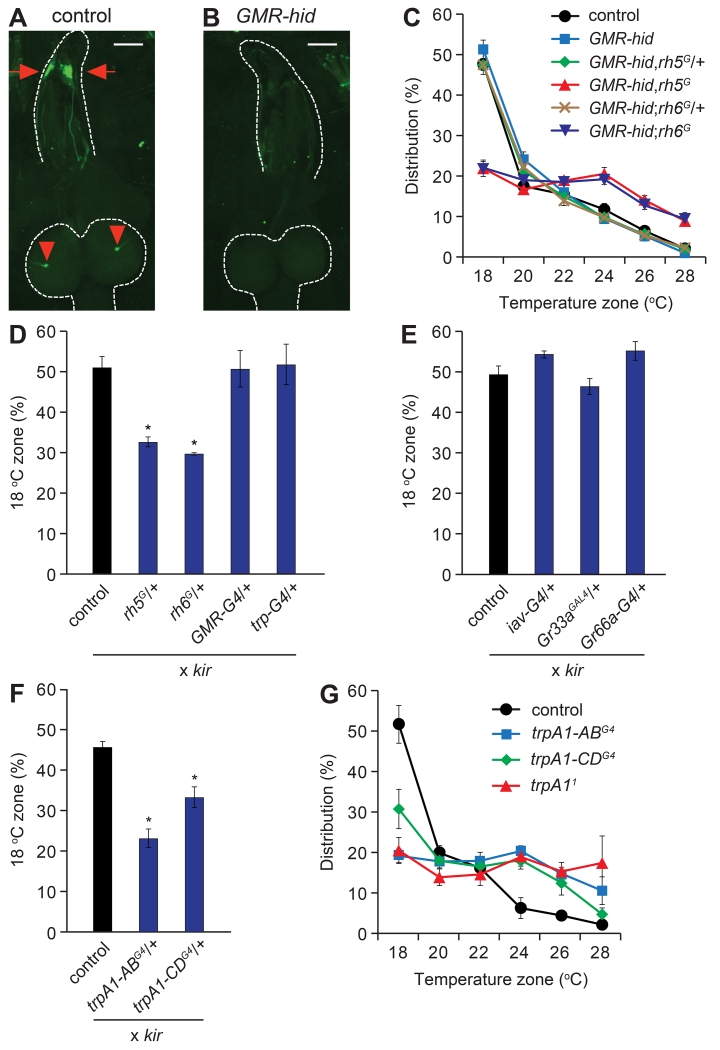
Rh5 and Rh6 are expressed in the Bolwig organ (Sprecher et al., 2007) (Figure 3A), which is required for light avoidance (Mazzoni et al., 2005). To test whether temperature sensation depended on the Bolwig organ, we eliminated this tissue by expressing the pro-apoptotic hid gene under the control of the GMR promoter (Hay et al., 1994). This manipulation was effective in eliminating the Bolwig organ as previously reported (Xiang et al., 2010), since we did not detect anti-Rh6 staining in GMR-hid larvae (Figure 3B). The GMR-hid larvae showed temperature preference behavior similar to control animals (Figure 3C), indicating that Rh5 and Rh6 expression in the Bolwig organ was dispensable for thermotaxis in late-3rd instar larvae.
Figure 3. rh5- and rh6-dependent temperature selection depends on trpA1-neurons, but not the Bolwig organ.
(A) Staining of control (w1118) larvae with anti-Rh6. The antibodies labeled neurons in the Bolwig organ (arrows) and their processes that extend to the brain (arrowheads). The scale bars in A and B represent 100 μm.
(B) Anti-Rh6 staining was not detectable after elimination of photoreceptor cells in the Bolwig organ with GMR-hid.
(C) Thermal preferences of control (w1118), GMR-hid, and GMR-hid larvae carrying either the rh5G or rh6G mutations. n=6-7.
(D, E, F) Fraction of larvae in the 18°C zone of an 18°—28°C thermal gradient after expressing UAS-kir using the indicated GAL4 lines. The control larvae harbored the UAS-kir (UAS-kir/+) transgene only. n=4-5.
(G) Distribution of the indicated trpA1 mutants in the six indicated temperature zones. n=3-4.
See also Figure S3.
Because elimination of Rh5 or Rh6 disrupts signaling in the Bolwig organ, it was possible that the altered function of the photoreceptor cells in the Bolwig organ was disrupting thermotaxis behavior. If so, then elimination of the Bolwig organ might suppress the rh5 or rh6 mutant phenotypes. To test this possibility, we generated GMR-hid larvae carrying either the rh5G or rh6G mutations. We found that GMR-hid,rh5G and GMR-hid;rh6G flies displayed defects in 18°C selection (Figure 3C) similar to the rhodopsin mutants (Figure 2B). The GMR-hid transgene alone or GMR-hid in combination with the rh5G/+ or rh6G/+ heterozygous backgrounds had no effect on thermotaxis, as these animals showed normal 18°C preference similar to control larvae (Figure 3C). Therefore, elimination of the Bolwig organ did not suppress the rh5G or rh6G thermotaxis phenotypes.
rh5 and rh6 required in trpA1 neurons for optimal temperature selection
The preceding data indicated that rh5 and rh6 functioned in thermotaxis through cells external to the Bolwig organ. To address the cellular requirements for rh5 and rh6, we inactivated a variety of neurons by expressing kir2.1 (UAS-kir2.1) under control of the GAL4-UAS system. Introduction of kir2.1 in rh5- or rh6-expressing neurons using the rh5G/+ and rh6G/+ GAL4s decreased the proportion of larvae attracted to the 18°C zone (Figure 3D). The GAL4 drivers alone (rh5G/+, rh6G/+) had no impact on temperature selection (Figure S3A). In contrast, silencing Bolwig neurons with either the trp-GAL4 (Petersen and Stowers, 2011) or the GMR-GAL4 (Hay et al., 1994) did not change the preference for this temperature zone (Figure 3D). The chordotonal and terminal organs participate in discriminating 18°C from cooler temperatures (Kwon et al., 2010; Liu et al., 2003). Inactivation of neurons in these organs using the iav-GAL4, Gr33aGAL4 and Gr66a-GAL4 (Kwon et al., 2011; Kwon et al., 2010) to drive UAS-kir2.1 did not impact on selection of the 18°C zone in the thermal gradient (Figure 3E). Thus, we conclude that the Bolwig, chordotonal and terminal organs are all dispensable for late-3rd instar larvae to choose the optimal temperature.
A subset of trpA1-expressing neurons were candidates for requiring rh5 and rh6, since mutation of trpA1 prevented mid-3rd instar larvae from discriminating 18°C from other temperatures in the comfortable range (Kwon et al., 2008). Thus, we considered whether trpA1-expressing neurons were required in the late-3rd instar larvae. There are at least four trpA1 mRNA isoforms (Figure S3B). The trpA1-A and trpA1-B isoforms (trpA1-AB) are expressed under the control of one promoter and trpA1-C and trpA1-D (trpA1-CD) are synthesized using a second promoter (Figure S3B). In addition, each pair of isoforms differs through alternative splicing. We found that when we drove UAS-kir2.1 expression using the trpA1-ABG4/+ or the trpA1-CDG4/+ reporter, the larvae were impaired in selecting 18°C (Figure 3F). Introduction of the reporters alone (trpA1-ABG4/+ or trpA1-CDG4/+) had no impact on temperature selection (Figure S3A). The trpA1-AB and trpA1-CD mutations each include either GAL4 or LexA reporters inserted at the site of the original translation start codon (Figure S3B). The trpA1-AB reporter was expressed predominately in the brain, and to a lesser extent in the ventral nerve cord (VNC), while the trpA1-CD reporter stained multidendritic type IV neurons and external sensory organ neurons in the body wall, which extended axons to the VNC (Figures S3C and S3D) (Zhong et al., 2012). We found that a null mutation in trpA1 (trpA11) eliminated the preference of late-3rd instar larvae for the 18°C zone (Figure 3G). In addition, mutation of trpA1-AB (trpA1-ABG4) prevented 18°C selection, while mutation of trpA1-CD (trpA1-CDG4) significantly impaired 18°C thermotaxis (Figure 3G). These results indicate strongly that trpA1 neurons were required for 18°C preference during the late-3rd instar period.
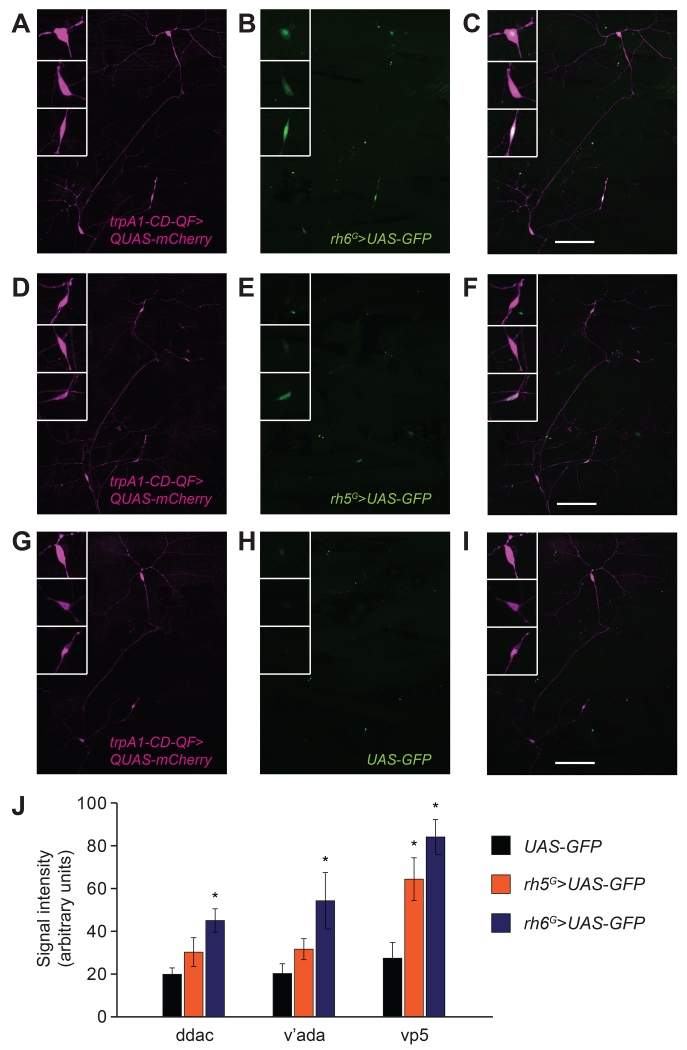
Our results led us to test whether rh5 and rh6 were expressed in trpA1-expressing neurons. The trpA1-CD-QF reporter (Petersen and Stowers, 2011) drove expression of QUAS-mCherry in class IV neurons (ddaC and v’ada) and external sensory organ neurons vp5 (Figures 4A, 4D and 4G)—an expression pattern consistent with the cellular distribution of the trpA1-CDG4 reporter (Figures S3D and S4) (Zhong et al., 2012). Our initial attempts to detect rh5 and rh6 reporter expression in the body wall were unsuccessful. Therefore, we used GAL4 reporters (rh5G/+ and rh6G/+) to drive two copies of a transgene encoding six tandem copies of GFP (20XUAS-6XGFP), and enhanced the signals using the tyramide signal amplification (TSA) method (Chao et al., 1996). We detected rh5G/+ and rh6G/+-driven GFP signals (Figures 4B and 4E), which co-localized with the trpA1-CD staining (Figures 4C and 4F). We then wrote a script using MATLAB to automatically identify regions of interest (ROI) based on the trpA1-CD-expressing cell bodies, and to measure pixel intensities of the GFP signals. While the TSA approach resulted in some random background signals that occurred when using UAS-GFP only (Figures 4H and 4J), we detected significantly stronger signals in the presence of the rh6G/+ reporter (ddac, v’ada and vp5; Figures 4B and 4J). We also detected stronger signals in the same cells of larvae expressing the rh5G/+ reporter. However, the increase in signal over background was statistically significant in vp5 only (Figures 4E and 4J). We also attempted to detect rh5 and rh6 reporter signals in trpA1-AB neurons but this was impeded by high background staining in the brain and ventral nerve cord when using the TSA approach. Nevertheless, the results with the trpA1-CD reporter indicate that rh5 and rh6 are co-expressed with trpA1.
Figure 4. Co-expression of rh5 and rh6 with trpA1 in the body wall.
Representative confocal images of rh5, rh6 and trpA1-CD reporter expression from a 3rd instar larval body segment. The rh5 GAL4 (rh5G/+) and the rh6 GAL4 (rh6G/+) drove expression of two copies of 20XUAS-6XGFP (UAS-GFP;anti-GFP, green). The trpA1-CD reporter (trpA1-CD-QF) drove expression of one copy of mCherry (QUAS-mCherry;anti-dsRed, magenta). The GFP signals were enhanced using the TSA approach. The arrowheads labeled 1, 2 and 3 indicate the cell bodies of trpA1-CD-positive neurons. Arrowheads 1 and 2 correspond to the type IV neurons ddaC and v’ada, and arrowhead 3 corresponds to the external sensory organ neuron vp5. The boxes in the upper left of each panel labeled 1, 2 and 3 show 3-fold magnifications of ddaC, v’ada and vp5, respectively. See Figure S4 for depictions of the locations of the ddaC, v’ada and vp5 neurons. In all pictures, the left is anterior side and the top is dorsal side.
(A-C) Co-expression of rh6 and trpA1-CD reporters. (A) trpA1-CD reporter staining (trpA1-CD-QF/+;QUAS-mCherry/+). (B) rh6 reporter staining (20XUAS-6XGFP/+;rh6G,20XUAS-6XGFP/+). (C) Merge of (A and B).
(D-F) Co-expression of rh5 and trpA1-CD reporters. (D) trpA1-CD reporter staining (trpA1-CD-QF/+;QUAS-mCherry/+). (E) rh5 reporter staining (rh5G,20XUAS-6XGFP/+;20XUAS-6XGFP/+). (F) Merge of (D and E).
(G-I) Control showing typical background staining in flies harboring the UAS-GFP transgene without a GAL4 driver line. (G) trpA1-CD reporter staining (trpA1-CD-QF/+;QUAS-mCherry/+). (H) UAS-GFP only (20XUAS-6XGFP/+;20XUAS-6XGFP/+). (I) Merge of (G and H).
The scale bars in C, F and I represent 100 μm.
(J) Quantification of the GFP signals in the body wall neurons driven by rh5 (orange) or rh6 (blue) reporter, or UAS-GFP only (black). The ROI and quantifications were performed automatically in an unbiased fashion using a MATLAB program. n=9-11.
See also Figure S4.
Signaling pathway required for thermotaxis in late-3rd instar larvae
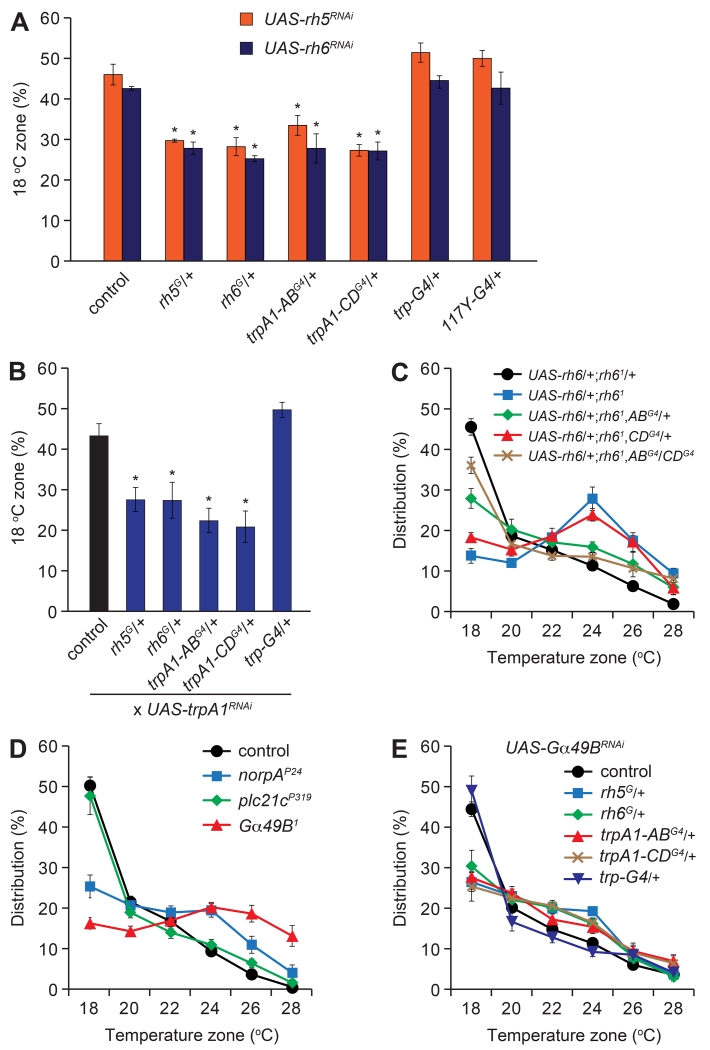
To address whether rh5, rh6 and trpA1 functioned in the same cells, we performed RNAi-mediated knockdown of rh5 and rh6 (UAS-rh5RNAi and UAS-rh6RNAi) using a series of GAL4 lines. Knockdown of either rh5 or rh6 using the rh5- or rh6-GAL4 lines (rh5G/+ and rh6G/+, respectively) resulted in a significant reduction in 18°C selection (Figure 5A). We obtained similar reductions in larval distribution in the 18°C zone after suppressing either rh5 or rh6 under control of the trpA1-ABG4 or the trpA1-CDG4 (trpA1-ABG4/+ or trpA1-CDG4/+; Figure 5A). Conversely, there were no significant effects resulting from RNAi-mediated knockdown of rh5 or rh6 using GAL4 lines expressed in the Bolwig organ (trp-GAL4) or the mushroom bodies (117Y-GAL4; Figure 5A).
Figure 5. Requirements for rh5, rh6, norpA and Gα49B in trpA1 neurons for thermotaxis.
Larvae of the indicated genotypes were assayed on 18°—28°C thermal gradients.
(A) Percentages of larvae expressing UAS-rh5RNAi (orange) or UAS-rh6RNAi (blue) transgenes under control of the indicated GAL4 lines in the 18°C zone. n=5-7.
(B) Percentages of larvae expressing dicer2;UAS-trpA1RNAi under control of the indicated GAL4 lines in the 18°C zone. n=4-5.
(C) Testing for rescue of the rh61 mutant phenotype by expressing UAS-rh6 under control of trpA1-ABG4 (ABG4/+), trpA1-CDG4 (CDG4/+) or both GAL4s (ABG4/CDG4). n=5-6.
(D) Assaying thermotactic behavior of plc mutants (norpAP24 and plc21cP319) and the Gqα mutant (Gα49B1). n=4-6.
(E) Thermal distribution of larvae expressing UAS-Gα49BRNAi under control of the indicated GAL4 lines. n=4-6.
See also Figure S5.
We also performed RNAi mediated knockdown of trpA1 using the rh5- and rh6-GAL4 drivers. Knockdown of trpA1 (UAS-trpA1RNAi) using either the rh5G or rh6G (rh5G/+ or rh6G/+), resulted in a significant reduction in 18°C selection, which was similar to trpA1-ABG4/+ and trpA1-CDG4/+-induced trpA1 knockdown (Figure 5B). RNAi-mediated knockdown of trpA1 using the trp-GAL4, which is expressed in the Bolwig organ (Petersen and Stowers, 2011), had no effect (Figure 5B). Moreover, we performed rescue experiments focusing on rh6, and found that the rh61 phenotype was suppressed by expression of rh6 in combination with both the trpA1-ABG4/+ and trpA1-CDG4/+ drivers (Figure 5C). However, the rescue was reduced when we expressed rh6 in trpA1-AB neurons alone, and there was no suppression resulting from rh6 expression in trpA1-CD neurons alone (Figure 5C). The combination of these results indicate that rh5 and rh6 function together in 18°C temperature selection in trpA1 neurons.
To address whether the rhodopsin mutations affected the gross morphology of trpA1-CD neurons, we compared the appearance of the peripheral neurons expressing the trpA1-CD reporter in control and rh5 and rh6 mutant larvae. We found that the morphology of trpA1-CD neurons was indistinguishable in the mutants and heterozygous animals (Figures S5A-D). To test the possibility that the temperature preference phenotypes exhibited by the rh5 and rh6 mutants might be caused by a general deficit in thermotaxis or locomotor defects we performed additional controls. We found that when the larvae were given a choice between 18° and 28°C, the rh5 and rh6 mutants avoided 28°C and accumulated on the 18°C side similar to control larvae (Figure S5E). These latter results suggest that the deficits in temperature selection exhibited by the rhodopsin mutants was not due to general impairment in thermotaxis, or reductions in locomotor activities. In further support of this latter conclusion, the moving speeds of the rh5 and rh6 mutant larvae were comparable to the control, except for a slight elevation in rh6G allele (Figure S5F).
Drosophila rhodopsins couple to a Gq/phospholipase Cβ (PLC)/TRP channel signaling cascade in photoreceptor cells (Montell, 2012). Moreover, we showed previously that Rh1 functions in thermotaxis during the mid-3rd instar larval period in collaboration with a Gq/PLC/TRPA1 signaling cascade (Kwon et al., 2008; Shen et al., 2011). Drosophila encodes one Gqα (Gα49B) and two PLCs (NORPA and PLC21c). We found that late-3rd instar larvae carrying either the Gα49B1 or norpAP24 mutation exhibited defects in 18°C thermotaxis, whereas the behavior of plc21CP319 mutant larvae was indistinguishable from controls (Figure 5D). We also observed a thermotaxis defect resulting from RNAi-mediated knockdown of Gα49B using GAL4 lines that directed expression in rh5, rh6, trpA1-AB or trpA1-CD neurons (Figure 5E).
Discussion
We conclude that 3rd instar Drosophila larvae undergo an age-dependent change in their thermal preference, and this behavioral modification requires several rhodopsins. Rh5 and Rh6 were the most important since the stage-dependent alteration in temperature selection was eliminated in either rh5 and rh6 mutant flies. Several observations support the conclusion that the thermotaxis phenotypes exhibited by the rh5 and rh6 mutants are not secondary consequences of developmental defects or motor problems. We found that the percentage of larvae that entered the 3rd instar larval stage at 74 hours AEL were similar to controls, as were the times to pupation. Furthermore, the morphology of the peripheral trpA1-positive neurons that normally express rh5 and rh6 were indistinguishable between the rh5 and rh6 mutants and controls. In addition, the movement speeds of the rh5 and rh6 mutants were not reduced, and they were able to choose 18° over 28°C normally in two-way choice assays.
The requirements for Rh5 and Rh6 were light independent since the thermotaxis occurred equally well in the light or dark, and was not dependent on the Bolwig organ, which is the rhodopsin-expressing light sensitive tissue in larvae. Rhodopsins are comprised of the protein subunit, opsin, and a vitamin A-derived chromophore, which senses light. In Drosophila photoreceptor cells, the chromophore also functions as a molecular chaperone to facilitate transport of the opsin out of the endoplasmic reticulum (Ozaki et al., 1993). We found that thermotaxis in late-3rd instar larvae was impaired in a mutant that disrupts chromophore biosynthesis. However, we suggest that this phenotype is due to the second function of the chromophore as a molecular chaperone.
Our findings lead us to conclude that Rh5 and Rh6 function upstream of a Gq/PLC/TRPA1 signaling cascade, which allows late-3rd instar larvae to select their favorite temperature in the comfortable range. We propose that this pathway enables the animals to sense minute temperature differences over a shallow thermal gradient through signal amplification, similar to role of these proteins in phototransduction. If the perfect option is not available in the thermal landscape, the thermosensory signaling cascade may facilitate adaptation to hospitable temperatures that deviate slightly from their preferred temperature.
Because of the exquisite effectiveness of rhodopsin in photon capture, we suggest that Rh5 and Rh6 are expressed outside the Bolwig organ at extremely low levels, to prevent light from interfering with temperature sensation. Nevertheless, we detected expression of the rh5 and rh6 reporters in a subset of trpA1-CD neurons in the body wall. Using the GAL4/UAS system, we provided evidence that rh5 and rh6 both function in trpA1-CD as well as trpA1-AB expressing neurons outside of the Bolwig organ. In addition, rh5 GAL4-mediated RNAi knockdown of rh6, and rh6 GAL4-mediated knockdown of rh5 resulted in defects in 18°C selection. RNAi based knockdown of trpA1 with either of the rh5- and rh6-GAL4 drivers caused similar thermotaxis defects. Although, these drivers are expressed at very low levels, we suggest that they are still effective since trpA1 is also expressed at very low levels in the periphery (Xiang et al., 2010). The effects of the rh5- and rh6-GAL4 drivers in suppressing trpA1 were not non-specific, as we did not observe a thermotaxis phenotype using the trp-GAL4 driver. We also found that the rh5- and rh6-GAL4s silenced the thermosensory neurons in combination with UAS-kir2.1. We propose that this was effective since small increases in hyperpolarization due to slight elevation of Kir2.1 cannot be overcome by the slight depolarization mediated by the low levels of TRPA1.
The combination of these findings indicate that both rh5 and rh6 are co-expressed and function in the same or an overlapping subsets of neurons required for thermotaxis. These findings raise the possibility that Rh5 and Rh6 may form heterodimers in vivo. Another key question is whether rhodopsins are direct thermosensors, an issue that remains unresolved due to challenges inherent in expressing these and most invertebrate rhodopsins in vitro.
The observation that multiple rhodopsins function in thermotaxis in Drosophila raise the question as to whether rhodopsin-dependent thermosensory signaling cascades are employed in other animals, including mammals. We suggest that mammalian cells that undergo thermotaxis over very small temperature gradients may rely on opsin-coupled amplification cascades. Intriguing possibilities include leukocytes, which thermotax to sites of inflammation (Kessler et al., 1979), and mammalian sperm, which undergo thermotaxis to the egg over temperature gradients of ~1°C, and require PLC for this cellular behavior (Bahat and Eisenbach, 2010; Bahat et al., 2003). Intriguingly, mammalian TRP channels and non-visual rhodopsins appear to be expressed in sperm and have been suggested to function in sperm thermotaxis (Kumar and Shoeb, 2011; Kumbalasiri and Provencio, 2005; Perez-Cerezales et al., 2015).
Experimental Procedures
Generation of rh5G and rh6G flies
We generated rh5G and rh6G by ends-out homologous recombination (Gong and Golic, 2003).
Temperature gradient assays
We reared the larvae under standard 12-hour light/12-hour dark cycles. We prepared synchronized larvae and assayed the distribution of ~150 larvae on linear 18°—28°C continuous gradients after allowing them to explore for 11—20 minutes, depending on their age. We tabulated the larvae in each of the six temperature zones and calculated the distribution as follows: (number of larvae in a given 2-cm zone)/(total number of larvae in 6 zones) × 100%.
Evaluation of developmental rate
The percentages of pupae were calculated based on the maximum number at 227 hours AEL. T50 and T80 were the times at which 50% and 80% of the animals underwent pupation, respectively.
Immunostaining
To perform immunostaining, 3rd instar larvae were dissected and stained, followed in some cases by signal amplification using the TSA method. Samples were imaged using a Zeiss LSM 700 confocal laser scanning microscope and a 20×/0.8 Plan-Apochromat DIC objective. The images were analyzed using Zen software.
Statistics
Multiple comparisons between the wild-type control and test groups were performed using one-way ANOVA followed by the Dunnett’s post-hoc test. Values are shown as mean ±SEM, unless indicated otherwise. A p value <0.05 was considered significant.
Supplementary Material
HIGHLIGHTS.
Drosophila larvae undergo age-dependent transitions in temperature selection.
Temperature selection in mid- and late-3rd instar larvae depends on rh5 and rh6.
rh5 and rh6 are required in trpA1-positive neurons in the brain and the periphery.
Rh5 and Rh6 function in thermotaxis through a Gq/PLC/TRPA1 signaling cascade.
ACKNOWLEDGMENTS
This work was supported by a grant to C.M. from the National Eye Institute (EY008117).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Information, which includes Supplemental Experimental Procedures, five Supplemental Figures, Supplemental Legends and Supplemental References, can be found with this article online at:_____________.
AUTHOR CONTRIBUTIONS
T.S., H.C.C. and C.M. designed the study, analyzed the data and wrote the manuscript. T.S. and H.C.C. performed most of the experiments. J.L. prepared a MATLAB programs for performing and quantifying immunostaining and larval locomotion. H.C.C. generated the rh5G and rh6G mutants and J.L. generated the trpA1-ABLexA and trpA1-CDG4 mutants.
COMPETING INTERESTS STATEMENT
Authors declare no competing interests.
REFERENCES
- Bahat A, Eisenbach M. Human sperm thermotaxis is mediated by phospholipase C and inositol trisphosphate receptor Ca2+ channel. Biol. Reprod. 2010;82:606–616. doi: 10.1095/biolreprod.109.080127. [DOI] [PubMed] [Google Scholar]
- Bahat A, Tur-Kaspa I, Gakamsky A, Giojalas LC, Breitbart H, Eisenbach M. Thermotaxis of mammalian sperm cells: a potential navigation mechanism in the female genital tract. Nat. Med. 2003;9:149–150. doi: 10.1038/nm0203-149. [DOI] [PubMed] [Google Scholar]
- Chao J, DeBiasio R, Zhu Z, Giuliano KA, Schmidt BF. Immunofluorescence signal amplification by the enzyme-catalyzed deposition of a fluorescent reporter substrate (CARD) Cytometry. 1996;23:48–53. doi: 10.1002/(SICI)1097-0320(19960101)23:1<48::AID-CYTO7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Fowler MA, Montell C. Drosophila TRP channels and animal behavior. Life Sci. 2013;92:394–403. doi: 10.1016/j.lfs.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong WJ, Golic KG. Ends-out, or replacement, gene targeting in Drosophila. Proc. Natl. Acad. Sci. USA. 2003;100:2556–2561. doi: 10.1073/pnas.0535280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay BA, Wolff T, Rubin GM. Expression of baculovirus P35 prevents cell death in Drosophila. Development. 1994;120:2121–2129. doi: 10.1242/dev.120.8.2121. [DOI] [PubMed] [Google Scholar]
- Julius D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- Kessler JO, Jarvik LF, Fu TK, Matsuyama SS. Thermotaxis, chemotaxis and age. Age. 1979;2:5–11. [Google Scholar]
- Klein M, Afonso B, Vonner AJ, Hernandez-Nunez L, Berck M, Tabone CJ, Kane EA, Pieribone VA, Nitabach MN, Cardona A, et al. Sensory determinants of behavioral dynamics in Drosophila thermotaxis. Proc. Natl. Acad. Sci. USA. 2015;112:E220–229. doi: 10.1073/pnas.1416212112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar PG, Shoeb M. The role of TRP ion channels in testicular function. Adv. Exp. Med. Biol. 2011;704:881–908. doi: 10.1007/978-94-007-0265-3_46. [DOI] [PubMed] [Google Scholar]
- Kumbalasiri T, Provencio I. Melanopsin and other novel mammalian opsins. Exp. Eye Res. 2005;81:368–375. doi: 10.1016/j.exer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Kwon JY, Dahanukar A, Weiss LA, Carlson JR. Molecular and cellular organization of the taste system in the Drosophila larva. J. Neurosci. 2011;31:15300–15309. doi: 10.1523/JNEUROSCI.3363-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Shen WL, Shim HS, Montell C. Fine thermotactic discrimination between the optimal and slightly cooler temperatures via a TRPV channel in chordotonal neurons. J. Neurosci. 2010;30:10465–10471. doi: 10.1523/JNEUROSCI.1631-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Shim HS, Wang X, Montell C. Control of thermotactic behavior via coupling of a TRP channel to a phospholipase C signaling cascade. Nat. Neurosci. 2008;11:871–873. doi: 10.1038/nn.2170. [DOI] [PubMed] [Google Scholar]
- Liu L, Yermolaieva O, Johnson WA, Abboud FM, Welsh MJ. Identification and function of thermosensory neurons in Drosophila larvae. Nat. Neurosci. 2003;6:267–273. doi: 10.1038/nn1009. [DOI] [PubMed] [Google Scholar]
- Mazzoni EO, Desplan C, Blau J. Circadian pacemaker neurons transmit and modulate visual information to control a rapid behavioral response. Neuron. 2005;45:293–300. doi: 10.1016/j.neuron.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Montell C. Drosophila visual transduction. Trends Neurosci. 2012;35:356–363. doi: 10.1016/j.tins.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki K, Nagatani H, Ozaki M, Tokunaga F. Maturation of major Drosophila rhodopsin, ninaE, requires chromophore 3-hydroxyretinal. Neuron. 1993;10:1113–1119. doi: 10.1016/0896-6273(93)90059-z. [DOI] [PubMed] [Google Scholar]
- Perez-Cerezales S, Boryshpolets S, Afanzar O, Brandis A, Nevo R, Kiss V, Eisenbach M. Involvement of opsins in mammalian sperm thermotaxis. Sci. Rep. 2015;5:16146. doi: 10.1038/srep16146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen LK, Stowers RS. A Gateway MultiSite recombination cloning toolkit. PLoS ONE. 2011;6:e24531. doi: 10.1371/journal.pone.0024531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen WL, Kwon Y, Adegbola AA, Luo J, Chess A, Montell C. Function of rhodopsin in temperature discrimination in Drosophila. Science. 2011;331:1333–1336. doi: 10.1126/science.1198904. [DOI] [PubMed] [Google Scholar]
- Sprecher SG, Pichaud F, Desplan C. Adult and larval photoreceptors use different mechanisms to specify the same Rhodopsin fates. Genes Dev. 2007;21:2182–2195. doi: 10.1101/gad.1565407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K, Luo J, Montell C. Evolutionarily conserved, multitasking TRP channels: lessons from worms and flies. Handb. Exp. Pharmacol. 2014;223:937–962. doi: 10.1007/978-3-319-05161-1_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annu. Rev. Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Jiao Y, Montell C. Dissection of the pathway required for generation of vitamin A and for Drosophila phototransduction. J. Cell Biol. 2007;177:305–316. doi: 10.1083/jcb.200610081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Wen T, Lee G, Park JH, Cai HN, Shen P. Developmental control of foraging and social behavior by the Drosophila neuropeptide Y-like system. Neuron. 2003;39:147–161. doi: 10.1016/s0896-6273(03)00396-9. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Yuan Q, Vogt N, Looger LL, Jan LY, Jan YN. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature. 2010;468:921–926. doi: 10.1038/nature09576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Bellemer A, Yan H, Honjo K, Robertson J, Hwang RY, Pitt GS, Tracey WD. Thermosensory and non-thermosensory isoforms of Drosophila melanogaster TRPA1 reveal heat sensor domains of a thermoTRP channel. Cell Rep. 2012;1:43–55. doi: 10.1016/j.celrep.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.