Abstract
The ability to culture and expand B cells in vitro has become a useful tool for studying human immunity. A limitation of current methods for human B-cell culture is the capacity to support mature B-cell proliferation. We have developed a culture method to support the efficient activation and proliferation of both naïve and memory human B cells. This culture supports extensive B-cell proliferation, with approximately 103-fold increases following 8 days in culture, and 106-fold increases when cultures are split and cultured for 8 more days. In culture, a significant fraction of naïve B cells undergo isotype switching and differentiate into plasmacytes. Culture-derived (CD) B cells are readily cryopreserved, and when recovered, retain their ability to proliferate and differentiate. Significantly, proliferating CD B cells express high levels of MHCII, CD80, and CD86. CD B cells act as APCs and present both alloantigens and microbial antigens to T cells. We are able to activate and expand antigen-specific memory B cells; these cultured cells are highly effective in presenting antigen to T cells. We have characterized the TCR repertoire of rare antigen-specific CD4+ T cells that proliferated in response to tetanus toxoid (TT) presented by autologous CD B cells. TCR Vβ usage by TT-activated CD4+ T cells differs from both resting and unspecifically activated CD4+ T cells. Moreover, we found that TT-specific TCR Vβ usage by CD4+ T cells was substantially different between donors. This culture method provides a platform for studying the BCR and TCR repertoires within a single individual.
Introduction
B cells are key to adaptive immunity and are now recognized for their multifunctionality: B cells not only produce antibodies, but also present antigens to T cells (1), secrete cytokines (2), and regulate other immunocytes (3). Antigen presentation by B cells is involved, to a significant extent, in both immunoprotection and the pathogenesis of autoimmune diseases (1, 4, 5). The effects of antigen presentation by B cells on T cells depend on the activation state of B cells. Studies show that CD154- or mitogen-activated B cells function as effective antigen presenting cells (APC) to induce T-cell activation (6, 7), while resting B cells are tolerogenic (8).
The antigen presentation function of B cells has long been known (9, 10), and B cells are recognized as professional APC along with dendritic cells, macrophages, and thymic epithelial cells (11). Antigen-presenting B cells participate in the initiation and continuation of autoimmune diseases such as systemic lupus erythematosus (12, 13), rheumatoid arthritis (14, 15), type 1 diabetes (16), and multiple sclerosis (5) in humans and mice. Beyond the scope of autoimmunity, B cells serving as APC are characteristic of atherosclerosis (17), insulin resistance (18), allergy (19), allo-rejection (20), infection, and even immune responses elicited by vaccination (21).
On the whole, professional APC initiate adaptive immune cellular responses by processing and presenting antigens to T cells as well as providing co-stimulatory signals necessary for the activation of T cells. These functional properties of APC have been applied in the clinical assessment of T-cell responses in vitro; for example, to evaluate the efficacy of vaccination (22), to identify the causal allergens for patients (23), and to predict the compatibility of allografts (24). Generally, autologous APC are loaded with target antigens and are co-cultured with T cells; T-cell proliferation or function is then measured (25, 26). To develop effective vaccines that target T cells, epitope mapping of the vaccine antigens is inevitable (22). This is because T-cell responses are generally focused only a few epitopes among the many present on microbial pathogens (27). With ample epitope candidates and multiple rounds of screening, a thorough mapping of T-cell epitopes requires large numbers of APC (22, 28, 29).
Indeed, the availability of autologous APC is often problematic in studies of human T-cell responses (22). Although tetramers of MHC molecules conjugated with peptides provides an alternative option of measuring T-cell responses to specific antigens (30), in practice, only limited numbers of antigens can be assessed using tetramers (31), restricting the application of tetramers in large-scale evaluations of candidate epitopes. For this reason, autologous APC are still the primary choice in T-cell epitope discovery. To overcome the low numbers of APC in the circulating blood, usually the rate-limiting step for mapping human T-cell epitopes, leukapheresis is often required to obtain adequate numbers of APC from patient’s blood (28, 29). Alternatively, APC can be expanded in vitro. The low numbers of circulating dendritic cells (DC) and macrophages in blood and their limited capacity for proliferation in vitro limit their applications (32–34). In contrast, B cells are more abundant in circulating blood and easier to expand in vitro compared to DC and macrophages (35–37). To that end, B cells offer a useful and, potentially, a more convenient source of APC. However, current methods for B-cell culture still do not generate sufficient cell numbers (35–37).
In this study, we adapted the culture methods established by Luo et al. (38) to expand in vitro the numbers of naïve and memory human B cells. This culture method efficiently induces the activation, proliferation, and differentiation of unselected or antigen-binding B cells. Significantly, the culture-derived (CD) B cells express high levels of accessory molecules necessary for effective APC function (MHCII, CD80, and CD86) and effectively present both alloantigens and microbial antigens to human T cells. Expansion of antigen-specific human memory B cells in CD cultures results in the generation of antigen-specific APC activity that is significantly more efficient for the cognate antigen than for unrelated antigens of comparable mass. Using CD cultures, we are able to characterize, globally, TCR repertoire for antigen-specific T cells. Thus, this culture method provides a platform for studying the BCR and TCR repertoires within a single individual.
Material and Methods
Human blood samples
Blood samples were collected from healthy adult donors with informed consent in accordance with guidelines from the Duke Institutional Review Board committee. Mononuclear cells were isolated by Ficoll-paque plus (GE) density gradient centrifugation with SepMate-50 tubes (STEMCELL Technologies). Cells were cryopreserved in liquid nitrogen until use. For microbial antigen-specific T-cell studies, blood samples were collected 2 to 5 weeks after tetanus-diphtheria boost and/or influenza vaccination.
Cryopreservation of human cells
Cells were cryopreserved based on a previous protocol with modifications (39). Briefly, cells were suspended in RPMI 1640 medium (Invitrogen) or neat fetal bovine serum (FBS) (FCS HyClone, Thermo) at a concentration of ≤ 2×107 cells per ml. An equal volume of cooled freezing medium containing 20% DMSO (Sigma) and 80% FBS was added dropwise to the cell suspension to a final concentration of 10% DMSO. Cells were aliquoted into cryovial tubes and placed in a pre-chilled freezing container (Nalgene Mr. Frosty, Sigma). Cryovials were stored at −80 °C for 4 – 24 hours and then were stored in liquid nitrogen until thawing for culture.
Monoclonal antibodies (mAb) and flow cytometry
The following mouse mAbs specific for human surface antigens were used for flow cytometry and cell sorting in this study. Anti-human CD3 allophycocyanin (APC) (clone: HIT3a), CD4 phycoerythrin (PE) (clone: A151A1), CD8 APC-Cy7 (HIT8a), CD19 PE-Cy7 and APC (HIB19), CD24 PE and BV510 (ML5), CD27 BV421 (M-T271), CD38 BV510 (HIT2), CD45 PE-Cy7 (HI30), CD80 PE (5D10), CD86 biotin (IT2.2), IgD FITC APC-Cy7 (IA6-2), IgM FITC and APC-Cy7 (MHM-88), and mouse IgG1 isotype control PE, PE-Cy7, and biotin (MOPC-21) were purchased from BioLegend (San Diego, CA). CD38 biotin (HIT2) was purchased from eBioscience (San Diego, CA). IgM PE-Cy5 (G20–127), IgG APC (G18–145), CD3 PE-Cy5 (UCHT1), and mouse IgG1 isotype control APC (MOPC-21), BV510 (X40) and V450 (MOPC-21) were purchased from BD Biosciences (San Jose, CA). MHC class II FITC (TDR31.3) was purchased from LifeSpan BioSciences. Streptavidin-Pacific Orange was purchased from Invitrogen. Mouse IgG1k isotype control (MG1K) was purchased from Rockland.
Both analysis of cell phenotypes and cell isolation were performed by flow cytometry. Briefly, cells were incubated with fluorochrome-conjugated mAb specific for human surface antigen (listed above) and resuspended in PBS containing 2% FBS prior to analysis. Bound biotin-conjugated Abs were revealed by fluorochrome-labeled streptavidin. Doublets were excluded from our analysis and cell sorting by combination(s) of forward scatter (FSC)-A versus FSC-H, FSC-H versus FSC-W, and side scatter (SSC)-H versus SSC-W gatings. Dead cells were excluded by 7-aminoactinomycin D+ (7-AAD) staining (BD Biosciences). Labeled cells were analyzed on a BD FACSCanto after fixation (BD Cytofix) or sorted on a BD FACSAria using Diva software (BD Biosciences).
Isolation of mature, naïve B cells
Human mature, naïve B cells were isolated from PBMC by negative selection with the EasySep Human Naïve B Cell Enrichment Kit according to the manufacturer’s instructions (STEMCELL Technologies). The purity of mature naïve B cells (CD19+ CD27− IgM+ IgD+) as determined by flow cytometry was > 94%.
CD culture system
Human B cells (1–6 × 103) were plated in 6-well plates or 10-cm tissue culture dishes (BD Falcon) to achieve input cell densities of ~100 B cells/cm2. These culture plates or dishes were pre-seeded overnight with CD154-expressing stromal cells (CD40LLow cell line, gift from David Baltimore) (38). B cells were cultured in R5 medium [RPMI 1640 with 5% human serum (Sigma-Aldrich), 55 µM 2-mercaptoethanol, 2 mM L-glutamine, 100 U/ml penicillin,100 µg/ml streptomycin, 10 mM HEPES, 1 mM sodium pyruvate and 1% MEM nonessential amino acids (all from Invitrogen)], supplemented with recombinant human IL-2 (50 ng/ml), IL-4 (10 ng/ml), IL-21 (10 ng/ml), and BAFF (10 ng/ml) (all from PeproTech, Rocky Hill, NJ) for 8 days unless indicated otherwise. The final volume of B-cell cultures was 2 ml/well for 6-well plates, and 6 ml/dish for 10-cm dishes.
Cells were fed with fresh R5 medium containing cytokines on days 4 and 6 by aspirating half of the old medium without touching the bottom of the wells, and replacing the same volume with pre-warmed, fresh medium containing cytokines. In some experiments when cultures were carried beyond 8 days, cells were split onto new feeder cells with fresh cytokines on day 8, and medium was changed on post-transfer days +4 and +6. At the end of culture, CD B cells were harvested, counted, aliquoted, and cryopreserved in liquid nitrogen until use. In experiments quantifying the kinetics of mature naïve B-cell proliferation, input cell numbers were optimized to facilitate accurate cell counts: input cell numbers on day 0 were 1×104, 2.5×103, and 1×103 per well in 6-well plates for 4-, 6-, and 8-day cultures, respectively; beyond 8 days, cells were split onto new feeders and the input cell numbers on day 8 were 4×104, 1×104, 2.5×103, and 1×103 per well for 10-, 12-, 14-, and 16-day cultures, respectively.
Isolation and culture of antigen-specific human memory B cells
Human PBMC recovered from tetanus-diphtheria vaccinees were incubated with a combination of flowcytometry mAb and tetanus toxoid (TT) conjugated with PE (TT-PE), which had been generated using R-PE-labeling kit-NH2 kit (Dojindo). Single, live B cells (7AAD−CD3−CD19+) were gated, from which IgG memory B cells were defined as CD27+CD24hiIgM−IgD−IgG+. IgG memory B cells that did (TT-PE+) or did not (TT-PE−) bind TT were then sorted into CD cultures at ~100 cells/cm2. Following their activation and proliferation for 8 days, TT-PE+ and TT-PE− CD B cells were harvested and frozen until use. A fraction of day 8 CD B cells were placed into new cultures (~100 CD B cells/cm2) and allowed to expand for another 8 days; these day 16 CD B cells were harvested and cryopreserved until use.
Co-culture of T and B cells
PBMC were thawed and labeled with carboxyfluorescein succinimidyl ester (CFSE, Invitrogen). T cells were isolated by negative selection with the EasySep Human T Cell Enrichment Kit (STEMCELL Technologies) from CFSE-labeled PBMC. The purities of CD3+ T cells (as determined by flow cytometry) were greater than 98% after enrichment. Frozen CD B cells were thawed and counted. Both T and CD B cells were suspended well before co-culture.
Equal numbers (1 × 104 each) of T and CD B cells per well were co-cultured in 96-well U-bottom plates (Fisher Scientific) in 100 µl per well of R5 medium without exogenous cytokines. For alloreactive T-cell proliferation studies, the plates were incubated at 37°C in a 5% CO2- humidified incubator for 5 days. For microbial antigen-specific T-cell proliferation studies, the plates were incubated for 7 days. Tetanus toxoid from Clostridium tetani (List Biological Laboratories), recombinant influenza HA (H3 A/Wisconsin/67/2005, kindly provided by S.C. Harrison), and recombinant B. anthracis PA (BEI Resources) were used in antigen-specific T-cell proliferation studies. T cells were treated with equal numbers of anti-CD3/CD28 Dynabeads® (Invitrogen) as positive controls in both alloreactive and microbial antigen-specific T-cell proliferation studies.
CD B cell immunophenotypic analysis and T-cell proliferation analysis
Analysis of CD B cell phenotypes and T-cell proliferation was performed by flow cytometry. Briefly, cells were incubated with fluorochrome-conjugated mAb specific for human surface antigens (listed above) in PBS containing 2% FBS. Bound biotin-conjugated mAbs were revealed using streptavidin-Pacific Orange. Analysis was performed with FlowJo (TreeStar, Ashland, OR).
CD B cells were phenotyped using flow cytometry. Absolute cell counts were performed with Calibrite beads according to the manufacturer’s instructions (BD Biosciences) or with a hemocytometer and trypan blue exclusion of dead cells. CD B cells were incubated with mAbs for surface staining of CD19, CD45, CD3, MHCII, CD80, CD86, CD27, CD24, CD138, IgG, and IgM. Dead cells were excluded from analysis by 7AAD staining.
T-cell proliferation was assessed as described previously (24). Briefly, cells were washed and resuspended in R5 medium without exogenous cytokines. CFSE dissolved in DMSO was added at a final concentration of 5 µM. Cells were mixed well with CFSE and incubated in the dark at room temperature for 5 min. Cells were washed three times with warm medium to remove excess CFSE and were resuspended in culture medium. After culture, cell proliferation was assessed by flow cytometry. Briefly, cells were harvested and incubated with mAbs for CD3, CD4, CD8, CD19, and 7AAD. Single, live (7AAD−) CD19−CD3+ leukocytes were gated as the T-cell population. The frequency of T-cell proliferation was determined by CFSE dilution.
Deep sequencing for T-cell receptor (TCRβ) repertoire analysis
TCR repertoires of CD4+ T cells were analyzed using the immunoSEQ Analyzer (Adaptive Biotechnologies, Seattle, WA). CFSE-labeled CD4+ T cells isolated from recent Td vaccines (Donors A and D) were cultured with anti-CD3/CD28 Dynabeads® or with autologous CD B cells in the presence of TT (described above). After 7 days of culture, we sorted CFSEdim CD4+ T cells and isolated genomic DNA from the sorted CD4+ T cells by phenol/chloroform extraction (40). Isolated genomic DNA was sent to Adaptive Biotechnologies, which performed amplification of rearranged TCRB genes using multiplex PCR, high-throughput sequencing for the identification of V, D, and J gene segments using the Illumina HiSeq platform, and characterization of TCRβ repertoire using the ImmunoSEQ human TCRβ assay (41, 42).
Data deposition
All TCRβ sequences are available at https://clients.adaptivebiotech.com/pub/9816555b-5673-4316-85e2-244acb293f0b.
Image acquisition
Images of cultured mature, naïve B cells were taken with a Canon EOS20D camera through the eyepiece lens of an Olympus CKX41 microscope; original magnification x200.
Data analysis
Graphs were compiled and statistical analysis was performed using one-way or two-way ANOVA followed by a multiple comparison test using GraphPad Prism software, version 6 (GraphPad Software, San Diego, CA). Results are presented as means ± SD or means ± SEM. Differences between TT-binding enriched and unenriched CD B cells in inducing T-cell proliferation were considered significant if p<0.05.
Results
Extensive proliferation of human B cells in vitro
To generate large numbers of activated human B cells in vitro, we developed a B-cell culture system in which B-cell populations are expanded on feeder cells that express low levels of CD154 in medium containing the recombinant human cytokines IL-2, IL-4, IL-21, and BAFF (38).
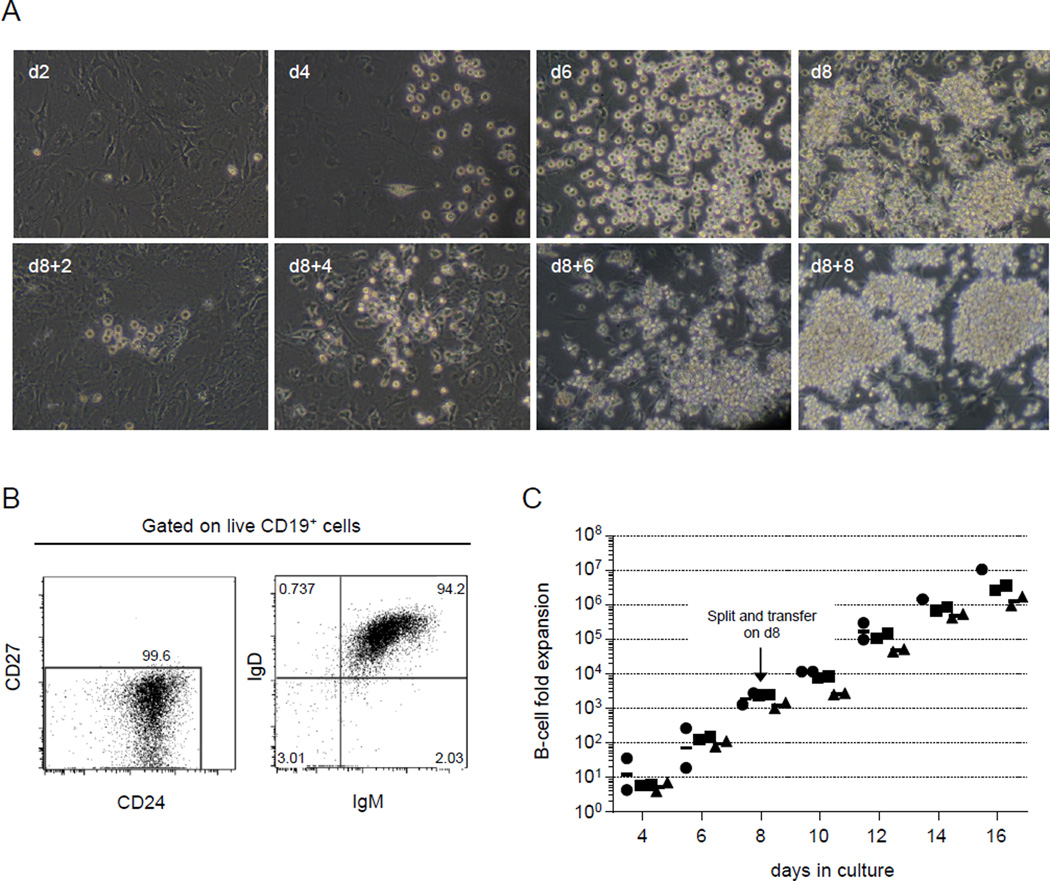
To evaluate the proliferation of these CD B cells, mature, naïve B cells from frozen peripheral blood samples were introduced (day 0) and maintained in cultures for as long as 16 days. B-cell numbers increased substantially in this culture system (CD culture system); starting with B-cell densities of ~100 cells/cm2, we routinely observed clusters of B cells by day 4 of culture that became confluent by day 8 (Fig. 1A). To avoid overcrowding and promote continued proliferation, we split and transferred cultured cells into fresh cultures that contained fresh cytokines and feeder cells (100 cells/cm2). These newly-expanded populations formed B-cell clusters as early as 2 days after transfer (d8+2) and continued to proliferate to confluence by post-transfer day 8 (d8+8) (Fig. 1A). This culture system is capable of supporting vigorous B-cell proliferation for at least 16 days. Indeed, CD B cells are capable of continued proliferation for at least another week in fresh cultures (data not shown).
FIGURE 1.
Vigorous proliferation of human mature, naïve B cells in vitro. Mature, naïve human B cells were isolated from frozen PBMC and cultured (described in Materials and Methods) for as long as 16 days in CD cultures. (A) B-cell proliferation was readily followed by microscopy; representative images of cultured B cells show substantial proliferation over time. Initial plating densities were 6000 cells/dish (~100 cells/cm2) on days 0 and 8. Cultured cell populations were split and transferred to new cultures that contained new feeder cells and fresh cytokines on day 8 and allowed to expand for another 8 days. Original magnification x200. (B) Representative flow cytometry profiles of mature, naïve B cells placed into CD cultures. Single, live B cells (7AAD−CD3−CD19+) that were CD27−CD24+ and expressed surface IgM and IgD were defined as mature, naïve B cells. Typically, >94% of starting B-cell populations expressed this mature, naïve phenotype. (C) The kinetics of B-cell proliferation are shown as fold-increases in viable B-cell (7AAD−CD45+CD19+) numbers compared to the number of input cells (day 0). Input cell numbers were optimized to facilitate accurate cell counts and B-cell numbers were determined by flow cytometry (Materials and Methods). Each symbol represents a donor (n=3); duplicate cultures were established for each donor.
To quantify the proliferative capacity of human B cells in the CD culture system, mature, naïve B cells (CD19+CD27−CD24hiIgM+IgD+) from frozen human peripheral blood (Fig. 1B) were cultured as described above with the adjustment of input cell numbers (see Materials and Methods) to obtain accurate kinetics of B-cell proliferation. With an input of 103 B cells per well in culture, B cells expanded to ≥106 cells per well after 8 days. Subsequently, cultured B-cell populations were split and transferred to fresh cultures (see above) at 103 B cells/well on day 8; these cultured B cells continued proliferation and reached ≥106 cells 8 days after transfer (day 16). Similar expansion capacity was seen in cells cultured for 4, 6, 10, 12, and 14 days. CD cultures supported logarithmic expansion of purified mature, naïve B cells with ≥103-fold increases by day 8 of culture and 106-fold increases by day 16 (Fig. 1C).
Naïve B cells become activated and differentiated in CD cultures
In CD cultures, human mature, naïve B cells soon acquire an activated phenotype that promotes effective antigen presentation, and they eventually differentiate into antibody-secreting plasmablasts and plasmacytes. To characterize the activation and differentiation of CD B cells during culture, we followed the expression of MHCII, CD80, CD86, membrane IgG, CD27, and CD138 on CD B cells over 16 days of culture. Within 4 days, CD B cells exhibited an activated phenotype that manifested in increased expression of MHCII, CD80, and CD86 (Figs. 2A and 2B top panels). Elevated levels of MHCII, CD80, and CD86 were generally sustained through the 16-day culture period although CD80 expression declined somewhat by days 14–16 (Figs. 2A and 2B top panels).
FIGURE 2.
Activation and differentiation of naïve B cells in CD cultures. Activation of cultured B cells was evident based on increased expression of MHCII, CD80, and CD86 during the early culture period, and was followed by differentiation into CD27+ and CD138+ populations of class-switched, IgG+ B cells. Representative histograms are shown in (A), and frequencies of B cells expressing elevated levels of MHCII, CD80, CD86, IgG, CD27, and CD138 during culture are presented as % of CD19+ cells (B). Elevated expression of these surface molecules was defined based on representative flow histograms that quantified expression in input B cells (day 0) and cultured B cells on days 8 and 16; broken black lines indicate thresholds for elevated expression. Each symbol represents a single donor (n=3).
Later, CD cultures supported IgM → IgG class-switch recombination and differentiation to plasmablasts/plasmacytes. In these cultures, IgM → IgG class-switch recombination first became obvious on day 8, with 15 – 20% of cultured naïve mature B cells expressing membrane IgG and increasing gradually to peak at 30% – 40% of CD B cells by day 14 (Figs. 2A and 2B top panels and S1). Expression of CD27, a differentiation marker linked to the human B-cell memory compartments (43, 44), accumulated slowly on CD B cells until day 12 and then sharply increased on days 14–16 (Figs. 2A and 2B top panels and S1). CD138 expression, a marker of specialization for antibody secretion (45), came later, increasing abruptly at days 14–16. Thus, the CD culture system efficiently activates human mature, naïve B cells and induces B-cell proliferation and differentiation.
Given that many human samples are routinely cryopreserved, we also cryopreserved CD B cells, and then recultured these frozen CD B cells to test whether CD B cells retained their proliferative and differentiative capacity after the freeze-thaw process. Frozen aliquots of day 8 CD B cells were thawed and cultured at 103 cells per well for 6 days. Like harvested B cells from cryopreserved peripheral blood (Fig. 1C), the frozen day 8 CD B cells proliferated 2 × 102-fold upon reculture, and maintained high expression of activation markers (data not shown). Thus, CD B cells are readily cryopreserved and retain their activation status and ability to proliferate.
These findings indicate that the CD culture system supports the extensive proliferation of human mature, naïve B cells and upregulates MHCII, CD80, and CD86 on cultured B cells. Taken together, our results suggest that CD B cells may be capable of acting as potent APC for autologous and heterologous T cells.
CD B cells effectively activate allogeneic T-cell proliferation
To test whether CD B cells function as APC to induce antigen-specific T-cell proliferation, we first examined the ability of CD B cells to elicit proliferation of allogeneic T cells in mixed-lymphocyte reactions. T cells from 5 unrelated donors (demographic data in Table I) were co-cultured either with their own (autologous) CD B cells or with CD B cells from the other donors. CFSE-labeled T cells and unlabeled day 8 CD B cells (104 cells each) were co-cultured for 5 days; T-cell proliferation was measured by CFSE dilution at the end of co-culture. T cells cultured alone did not proliferate (~0% CFSEdim), whereas introduction of anti-CD3/CD28 beads resulted in proliferation of most T cells through multiple divisions (64% – 89% CFSEdim) (Fig. 3A). Autologous CD B cells did not induce T-cell proliferation (~1% CFSEdim); in contrast, every CD B-cell cohort induced strong proliferation in allogeneic T cells (26% – 67% CFSEdim) (Fig. 3B and Table II). Of note, allogeneic T-cell proliferation was observed in both the CD4+ and CD8+ compartments (Fig. 3B and Table II).
Table I.
Demographic data of 5 unrelated donors
| Donor | Age | Gender | Recent vaccination(s) |
Duration between vaccination and blood collection |
|---|---|---|---|---|
| A | 47 | F | Td toxoid§ | 5 weeks |
| B | 48 | F | Td toxoid§ | 5 weeks |
| C | 38 | F | Flu shot | 16 days |
| D | 39 | M | Td and Flu shot | 16 days |
| E | 26 | M | None | not applicable |
These donors had received Td vaccination at least once prior to the vaccination listed.
FIGURE 3.
CD B cells effectively activate allogeneic T-cell proliferation. Frozen aliquots of cultured B cells (day 8) were thawed and co-cultured with equal numbers (104) of CFSE-labeled allogeneic or autologous T cells. (A) Representative flow plots of T-cell proliferation in cultures alone (T only) or in the presence of anti-CD3/CD28 beads (aCD3/CD28), or in co-cultures with autologous or allogeneic CD B cells. (B) CFSE-labeled T cells from 5 unrelated donors (A, B, C, D, and E) were co-cultured with their own (autologous) or each other’s CD B cells. Matched, CFSE-labeled T cells were similarly cultured in the presence of anti-CD3/CD28 beads or alone as positive or negative controls, respectively. Five days later, T-cell proliferation was estimated by CFSE dilution. Results for T cells co-cultured with CD B cells from donor A are illustrated as proliferation of CD3+ (left panel), CD4+ (middle panel), and CD8+ (right panel) T-cell populations. Summarized results are shown in Table II. Results are given as mean % CFSEdim ± SD. n=5; 2 independent experiments.
Table II.
Summary of percent T-cell proliferation determined by CFSE dilution in response to autologous or allogeneic CD B cells§
| CD3+ CFSEdim (% of CD3+) |
CD B cells |
T only** | aCD3/CD28 | |||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | ||||
| T cells | A | 3 ± 5††¶ | 39 ± 5 | 26 ± 6 | 35 ± 2 | 28 ± 2 | 0 ± 0 | 64 ± 4 |
| B | 47 ± 7 | 0 ± 0 | 39 ± 3 | 41 ± 10 | 36 ± 6 | 0 ± 0 | 79 ± 8 | |
| C | 36 ± 8 | 43 ± 5 | 0 ± 0 | 58 ± 7 | 47 ± 4 | 0 ± 0 | 76 ± 7 | |
| D | 52 ± 4 | 41 ± 7 | 49 ± 5 | 2 ± 1 | 48 ± 7 | 0 ± 0 | 84 ± 2 | |
| E | 55 ± 5 | 60 ± 6 | 58 ± 8 | 67 ± 3 | 3 ± 1 | 0 ± 0 | 89 ± 3 | |
| CD4+ CFSEdim (% of CD3+) |
CD B cells |
T only | aCD3/CD28 | |||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | ||||
| T cells | A | 3 ± 5 | 28 ± 5 | 22 ± 5 | 27 ± 1 | 21 ± 2 | 0 ± 0 | 53 ± 6 |
| B | 29 ± 7 | 0 ± 0 | 22 ± 5 | 22 ± 7 | 17 ± 4 | 0 ± 0 | 67 ± 4 | |
| C | 23 ± 4 | 25 ± 2 | 0 ± 0 | 31 ± 2 | 20 ± 2 | 0 ± 0 | 62 ± 6 | |
| D | 24 ± 5 | 19 ± 3 | 24 ± 4 | 1 ± 1 | 28 ± 3 | 0 ± 0 | 61 ± 3 | |
| E | 36 ± 5 | 40 ± 5 | 36 ± 7 | 50 ± 4 | 1 ± 1 | 0 ± 0 | 68 ± 3 | |
| CD8+ CFSEdim (% of CD3+) |
CD B cells |
T only | aCD3/CD28 | |||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | ||||
| T cells | A | 0 ± 1 | 9 ± 6 | 3 ± 3 | 7 ± 2 | 7 ± 1 | 0 ± 0 | 9 ± 2 |
| B | 17 ± 6 | 0 ± 0 | 16 ± 4 | 19 ± 6 | 18 ± 4 | 0 ± 0 | 10 ± 3 | |
| C | 12 ± 4 | 17 ± 3 | 0 ± 0 | 25 ± 6 | 25 ± 4 | 0 ± 0 | 12 ± 1 | |
| D | 26 ± 5 | 21 ± 4 | 24 ± 3 | 0 ± 0 | 18 ± 6 | 0 ± 0 | 21 ± 5 | |
| E | 15 ± 4 | 18 ± 2 | 18 ± 1 | 15 ± 3 | 1 ± 1 | 0 ± 0 | 16 ± 5 | |
CFSE-labeled T cells from 5 unrelated donors (A, B, C, D, E) were co-cultured with their own (autologous) or each other’s CD B cells.
Matched, CFSE-labeled T cells were similarly cultured in the presence of anti-CD3/CD28 beads (aCD3/CD28) or alone (T only) as positive or negative controls, respectively.
Percent CFSEdim ± SD cells among CD3+ cells are shown.
Interestingly, the relative intensities of the allogeneic T-cell responses corresponded with those found during treatment with anti-CD3/CD28 beads (Fig. 3B and Table II); for example, T cells from donor E had the highest proliferation rate (CFSEdim 60 ± 5% (mean ± SD)) in response to allogeneic stimulation by CD B cells among T cells from all donors (32 ± 6%, 41 ± 5%, 46 ± 9%, and 47 ± 5% CFSEdim for donors A, B, C, and D, respectively), and also had the most vigorous T-cell division in response to anti-CD3/CD28 treatment (89 ± 3%). Conversely, T cells from donor A had the lowest proliferation frequency in response to allogeneic stimulation and to anti-CD3/CD28 treatment. On the other hand, CD B cells from all donors have a similar capability to induce allogeneic T-cell proliferation (CFSEdim 47 ± 8%, 46 ± 10%, 43 ±14%, 50 ± 15%, and 40 ± 9% by CD B cells from donors A, B, C, D, and E, respectively). Collectively, in vitro-expanded CD B cells efficiently presented alloantigens to induce allogeneic T-cell proliferation but did not activate T-cell proliferation non-specifically.
CD B cells effectively activate antigen-specific autologous T cells
To determine the ability of CD B cells to process and present microbial antigens, we co-cultured CD B cells with autologous T cells from recent vaccinees in the presence of priming and control (unexposed) vaccine antigens and determined T-cell proliferation by CFSE dilution after co-culture for 7 days. Day 8 CD B cells (originating from mature, naïve B cells) were co-cultured with autologous T cells from donors recently immunized with tetanus-diphtheria toxoid (Td) vaccine (donors A and B, 2–5 weeks post-immunization), TIV influenza vaccine (donor C), or both (donor D). Corresponding protein antigens [tetanus toxoid (TT) or recombinant influenza hemagglutinin (HA)], or an irrelevant antigen [recombinant B. anthracis protective antigen (rPA)] (10 µg/ml each) were added into individual co-cultures (Fig. 4). In the absence of added antigen, little or no T-cell proliferation was observed (<5% CFSEdim) whereas cultures containing anti-CD3/CD28 beads supported vigorous (>80% CFSEdim) T-cell proliferation. Significantly, antigen-dependent, autologous T-cell proliferation correlated well with each donor’s recent vaccination history. TT triggered CD3+ T-cell proliferation (≤45% CFSEdim) in donors A, B, and D; HA induced T-cell proliferation (13% – 30% CFSEdim) in all donors, but the highest frequencies of CFSEdim T cells were observed in donors C and D (22% and 30% CFSEdim, respectively) who were recently immunized with influenza vaccine. In contrast, rPA did not induce T-cell proliferation (≤5% CFSEdim), with the single exception of donor D (15% CFSEdim). On inquiry, we discovered that this individual is exposed to rPA due to his occupation. In all cases, T-cell proliferation was most evident in CD4+ T cells: 64% – 90% of the CFSEdim T cells were CD4+ (Fig. 4). CD B cells efficiently take up, process, and present protein antigens to autologous CD4+ T cells and thereby to induce antigen-specific T-cell activation and proliferation.
FIGURE 4.
Antigen presentation by autologous CD B cells results in T-cell proliferation. Frozen aliquots of cultured B cells (day 8) were thawed and co-cultured with equal numbers (104) of CFSE-labeled autologous T cells from recent vaccinees (2 to 5 weeks post-vaccination). Donors A and B received a tetanus-diphtheria booster immunization, donor C received the trivalent influenza vaccine, and donor D was injected with both vaccines simultaneously. Tetanus toxoid (TT), recombinant influenza HA (H3/Wisconsin), or the irrelevant antigen recombinant B. anthracis PA (each, 10 µg/ml) were added into individual co-cultures; cultures without added antigen (UNSTIM) or anti-CD3/CD28 beads served as negative and positive controls, respectively. After 7 days of culture, T-cell proliferation was estimated by CFSE dilution among all (CD3+) T cells, and CD4+ and CD8+ T-cell subsets. Results are compiled from 2–4 independent experiments and are presented as means ± SEM.
Antigen-specific human memory B cells are activated and proliferate in CD cultures
Antigen-specific B cells are supremely efficient APC for their cognate antigen (9). Consequently, we determined whether antigen-specific, memory B cells might proliferate and differentiate in our culture system and whether cultured memory B cells present specific antigens more efficiently than unselected CD B cells. IgG memory B cells (CD19+CD27+CD24hiIgM−IgD−IgG+) from the peripheral blood of Td vaccinees were sorted based on their capacity to avidly bind TT conjugated with PE fluorochrome (TT-PE). Both TT-binding (TT-PE+) and non-TT-binding (TT-PE−) IgG memory B-cell populations were then separately expanded in CD cultures.
A representative example of our sorting strategy to identify TT-specific memory B cells shows that approximately 40% of peripheral blood B cells from donor A exhibit the CD27+CD24hi memory phenotype, among which ~41% have undergone class-switch recombination (IgM−IgD−); 57% of the class-switched memory B cells express surface IgG (Fig. 5A). Among circulating IgG memory B cells, about 2% were TT-PE+ (Fig. 5A). The frequency of the memory phenotype in another donor (donor B) is ~35% of circulating B cells, and around 53% of these memory B cells are IgM−IgD−; IgG memory cells represent 35% of the class-switched memory pool. The TT-PE+ frequency among IgG memory B cells is about 12%, which is higher than in donor A. Combining data from both donors, circulating B cells are composed of 38.4 ± 2.3% (mean ± SD) memory B cells; 47.4 ± 8.5% of these memory B cells are class-switched, and 46.5 ± 15.4% of them are IgG+. The frequency of TT-PE+ cells in the IgG memory B-cell pool varies between donors (12% vs. 2%). Overall, the average frequency of TT-PE+ IgG memory B cells is 0.5% among circulating CD19+ B cells in these two recent Td vaccinees.
FIGURE 5.
In vitro proliferation and activation of antigen-specific human memory B cells. (A) Representative sorting strategy to identify TT-specific memory B cells (from donor A, a tetanus-diphtheria vaccine recipient). Single, live B cells (7AAD−CD3−CD19+) were gated on the CD27+CD24hiIgM−IgD−IgG+ cell population (IgG memory B cells). IgG memory B cells that did (TT-PE+) or did not (TT-PE−) bind TT were then sorted into CD cultures. (B) Expansion of TT-binding and TT-nonbinding memory B cells is shown. On days 8 and 16, cell numbers were determined by flow cytometry (Materials and Methods); B-cell expansion is shown as viable B-cell (7AAD−CD45+ CD19+) numbers over input cells. Data are shown as mean fold-increase ±SD; n=2. (C) Representative flow histograms of MHCII, CD80, and CD86 on cultured IgG memory B cells as well as ex vivo IgG+ memory B cells.
Isolated memory B cells were seeded at ~100 cells/cm2 in CD cultures and allowed to expand for 8 days; subsequently, the proliferating cultured cells were re-seeded in fresh cultures (at ~100 cells/cm2) for another 8 days (total 16 days). In CD cultures, both TT-PE+ and TT-PE− IgG memory B cells proliferated comparably, with ~103-fold increases by day 8, and 2 × 105-fold increases by day 16 over input cell numbers (Fig. 5B). We measured TT binding enrichment by comparing the frequencies of positive TT-PE labeling on these CD B cells using flow cytometry. In donor A, after subtracting the PE-TT signal (~1%) on cultured TT-PE− memory B cells, we found ~ 8% and 6% of CD B cells from the TT-PE+ population after 8 and 16 days of culture, respectively, were positively labeled with TT-PE (data not shown). In donor B, we found ~30% and 15% of CD B cells from 8- and 16-day cultures, respectively, were positively labeled with TT-PE (data not shown), indicating a successful enrichment of TT-binding memory B cells resulting from cell sorting.
To evaluate whether the cultured cells derived from IgG memory B cells also acquire the APC phenotype in CD cultures, the expression levels of surface MHCII, CD80, and CD86 on day 8 and day 16 cultured IgG memory B cells were compared to ex vivo, unselected IgG memory B cells. Both TT-PE+ and TT-PE− IgG memory B cells became activated, increasing expression of MHCII, CD80, and CD86 following 8 days in culture; these expression levels subsequently fell by day 16, but remained higher than starting levels (Fig. 5C). The frequencies of elevated MHCII expression on these cells were approximately 92% and 64% by days 8 and 16, respectively; similar trends were seen in CD80 (74% and 31% by days 8 and 16) and CD86 expressions (92% and 61% by days 8 and 16), although the frequencies of elevated CD80 were generally lower compared to MHCII and CD86 (Fig. 5C). The expression levels of MHCII, CD80, and CD86 [measured as mean fluorescent index (MFI) by flow cytometry] reduced about 45%, 70%, and 65%, respectively, in day 16 cultured cells compared to the frequencies in day 8 cells (Fig. 5C). Surface IgG expression in CD B cells was also assessed. After culture for 8 days, ~75% of cultured IgG memory B cells retained surface IgG expression; the frequency of IgG-expressing cells declined to ~35% on day 16. However, the total expression levels of surface IgG reduced greatly: we observed a 77% and 94% reduction in the MFI of IgG in day 8 and day 16 cultured IgG memory B cells, respectively, as compared to the MFI in input cells (data not shown). The expression of surface MHCII, CD80, CD86, and IgG in CD B cells from sorted TT-PE+ and TT-PE− IgG memory B-cell populations cultured for the same duration were comparable (data not shown).
The presentation efficiency of CD B cells was increased by pre-selecting for antigen-specific BCRs
To evaluate the population of CD B cells enriched for TT-binding for their ability to induce T-cell proliferation in response to cognate antigens, cultured IgG memory B cells were recovered and co-cultured with equal numbers (104) of CFSE-labeled autologous T cells from donors A or B who had recently received a Td booster. Both day 8 and day 16 CD B cells from TT-PE+ and TT-PE− IgG memory B cells were tested for their ability to induce T-cell proliferation in the absence or presence of TT (7 tested concentrations, 5-fold serial dilutions from 10 µg/ml), HA (2 or 10 µg/ml), or irrelevant antigen rPA (10 µg/ml) in the co-cultures. After 7 days of co-culture, total T-cell proliferation was determined by CFSE dilution (Fig. 6).
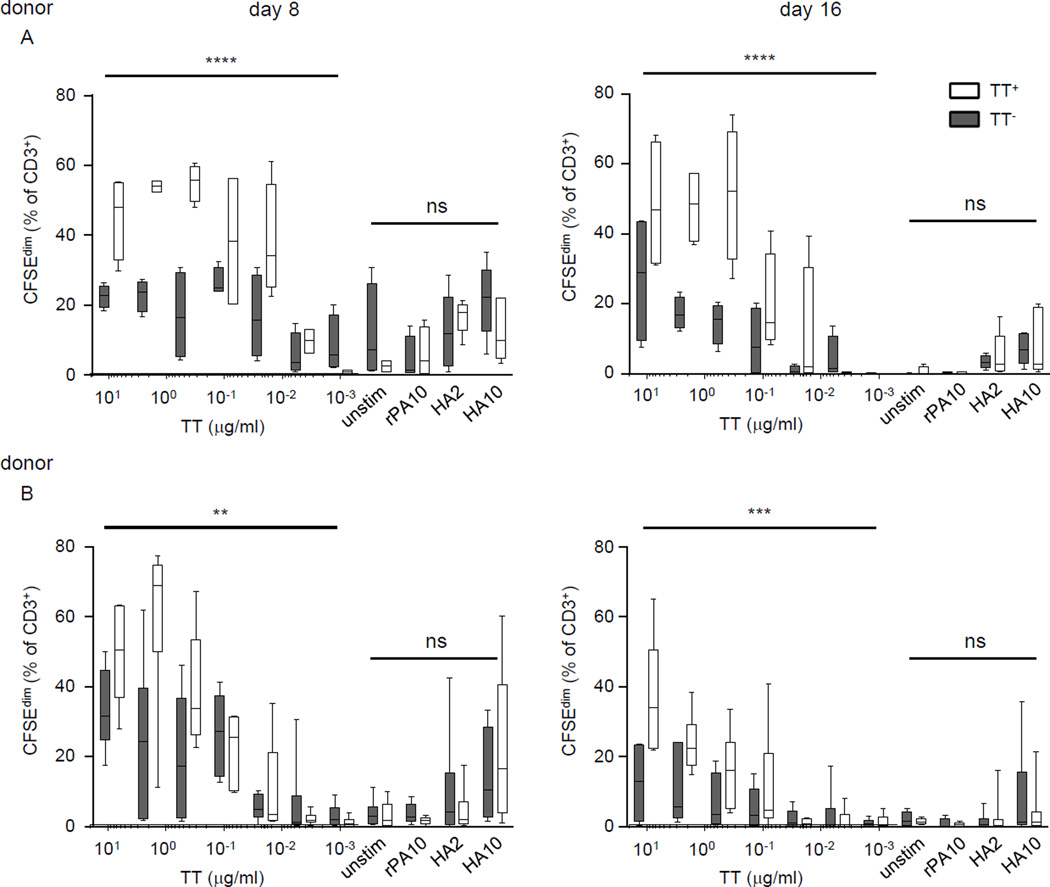
FIGURE 6.
TT-specific cultured memory B cells are more efficient than their non-specific counterparts in inducing the proliferation of TT-specific T cells. Frozen aliquots of day 8 and day 16 cultured cells derived from TT-PE+ and TT-PE IgG memory B cells were thawed and co-cultured with CFSE-labeled autologous T cells for 7 days, and T-cell proliferation was estimated by CFSE dilution among all (CD3+) T cells. Experiments were performed using cells from two healthy donors (A and B) with a recent tetanus-diphtheria booster. TT (10 µg/ml and 5-fold serial dilutions into 6 additional doses), recombinant influenza HA (H3/Wisconsin) (2 or 10 µg/ml), or recombinant B. anthracis PA (10 µg/ml) were added into individual co-cultures; cultures without added antigen (UNSTIM) served as negative controls. Results are compiled from 2–3 independent experiments and are shown as box-and-whisker plots with 5th and 95th percentiles. The proliferation difference in T cells cultured with CD B cells enriched for TT-binding or unenriched CD-B cells was evaluated with two-way ANOVA and a multiple comparison test; **p<0.01, ***p<0.001, ****p<0.0001; ns, no significant difference.
Without the addition of microbial antigens, both CD B cell populations (enriched for TT-binding and for TT non-binding) induced little or no autologous T-cell proliferation (CFSEdim ~3% and ~5% for TT-PE+ and TT-PE− CD B cells, respectively, from donor A; and CFSEdim ~4% and ~3.5% from donor B). A co-culture well using day 8 TT-PE− CD B cells (CFSEdim ~30%) was an exception, which may have been due to the effect of a culture medium component on the T cells. With the addition of rPA, little or no T-cell proliferation was induced by either CD B cell population (CFSEdim ~6% and ~2% for donor A and B, respectively). With either the addition of irrelevant antigen or no antigen, cells derived from IgG memory B cells in CD cultures induced little or no T-cell proliferation, indicating that the CD B cells from the memory pool, similar to those from the naïve mature pool, do not activate T cells nonspecifically (Figs. 3, 4, and 6).
With the addition of TT antigen, T-cell proliferation was more robust in the co-cultures with CD B cells enriched for TT-binding than those with CD B cells enriched for TT non-binding, indicating that antigen-driven T-cell proliferation corresponded with the antigen specificity of CD B cells (Fig. 6, donors A and B). Using day 8 CD B cells from donor A, the population of CD B cells enriched for TT-binding induced more T-cell proliferation (~20% more CFSEdim T cells), as compared to TT non-binding CD B cells in response to TT at 10 µg/ml (CFSEdim ~45% and ~25% when co-cultured with TT-binding and TT non-binding CD B cells, respectively). By serially reducing TT concentrations, we observed about 10% – 38% more CFSEdim T cells in the co-cultures with day 8 CD B cells enriched for TT-binding than in those enriched for TT non-binding in the presence of TT ≥ 0.016 µg/ml (p<0.0001) (Fig. 6, donor A); the superiority of TT-binding-enriched day 8 CD B cells in inducing T-cell proliferation remained until the concentrations of TT were lower than 0.016 µg/ml. Similarly, day 16 CD B cells enriched for TT binding induced more T-cell proliferation (about 10% – 37% more CFSEdim T cells) than CD B cells enriched for TT non-binding (p<0.0001) and the advantage remained until TT < 0.4 µg/ml. Day 8 and day 16 TT-binding enriched CD B cells from donor B also exhibited an enhanced ability to induce T-cell proliferation, with ~10% – 36% more CFSEdim T cells observed at TT ≥ 0.08 µg/ml (p<0.01 and p<0.001 for day 8 and day 16, respectively, in comparing TT-binding enriched and TT non-binding enriched CD B cells).
Furthermore, the population of CD B cells enriched for TT-binding induced measurable T-cell proliferation with the addition of the non-cognate antigen, HA at 10 µg/ml (13% – 23% CFSEdim T cells observed using day 8 CD B cells, and 4% – 9% CFSEdim T cells using day 16 CD B cells, both donors), and at 2 µg/ml (5% – 17% and 2% – 5% CFSEdim T cells detected using day 8 and day 16 CD B cells, respectively) (Fig. 6), suggesting that these B cells can acquire antigens through a BCR-independent pathway; however, the HA-presenting capacity of TT-PE+ enriched CD B cells was less efficient as compared to their presenting capacity for the TT cognate antigen (Fig. 6). On the other hand, TT non-binding enriched CD B cells exhibited an antigen-presenting capability that was similar to the capability of TT-binding enriched cells in the presence of HA at 10 µg/ml (14% – 22% and 7% – 9% CFSEdim T cells observed using day 8 and day 16 unenriched CD B cells, respectively) and at 2 µg/ml (10% – 12% and 2% – 3% CFSEdim T cells observed using day 8 and day 16 unenriched CD B cells, respectively) (Fig. 6). Taken together, we found comparable antigen-presenting capacities by CD B-cell populations, regardless of TT-binding enrichment, in presenting non-TT antigens (HA and rPA) to autologous T cells (p≥0.59 and p≥0.49 for donor A and B, respectively). From the above T-cell proliferation results for all tested antigens (Fig. 6), we conclude that the expression of TT-specific BCRs on TT-PE+ enriched CD B cells contributed to their superior ability for TT presentation as compared to TT-PE− enriched CD B cells.
Taken together, these results indicate that cells expanded from IgG memory B cells in vitro can function as APC that take up antigens through both BCR-dependent and BCR-independent pathways. Furthermore, the BCR-dependent antigen uptake pathway significantly enhances the antigen-presenting function of these CD B cells in inducing autologous T-cell proliferation.
TCR Vβ gene segment usage of TT-specific, human CD4+ T cells
To demonstrate the utility of our CD culture system in analyzing the human TCR repertoire, we characterized TCR Vβ usage of TT-specific CD4+ T cells from two donors. We isolated genomic DNA from CFSEdim CD4+ T cells that proliferated in response to TT presented by autologous CD B cells, and then amplified TCRβ VDJ rearrangements by PCR. For comparison, we amplified TCRβ VDJ rearrangements from genomic DNA of unstimulated CD4+ T cells and CFSEdim CD4+ T cells activated by anti-CD3/CD28 from the same donors. From the two unrelated donors (A and D), we obtained total 31,019, 41,265, and 8,791 productive TCRβ VDJ rearrangements by deep sequencing from freshly isolated CD4+ T cells, anti-CD3/CD28 activated CD4+ T cells, and TT-activated CD4+ T cells, respectively.
Vβ gene segment usage of freshly isolated CD4+ T cells was diverse in both donors (Figs. 7A and 7B; gray bars). As expected for unspecific expansion of CD4+ T cells, in each donor Vβ gene segment usage was virtually identical between freshly isolated CD4+ T cells and anti-CD3/CD28-stimulated CD4+ T cells (Figs. 7A and 7B; gray and blue bars). By contrast, Vβ usage of TT-activated CD4+ T cells was clearly distinct from freshly isolated CD4+ T cells and from unspecifically activated CD4+ T cells (Figs. 7A and 7B; red bars). In donor A, Vβ2-1, Vβ4-3, Vβ5-4, Vβ6-1, and Vβ19-1 gene segments were particularly frequent in TT-activated CD4+ T cells compared to resting or unspecifically activated CD4+ T cells (Fig. 7A). In donor D, Vβ2-1, Vβ5-1, Vβ18-1, and Vβ29-1 gene segments were particularly enriched in TT-activated CD4+ T cells (Fig. 7B).
FIGURE 7.
Vβ gene segment usage of TT-specific CD4+ T cells. Distributions of Vβ gene segment usage for freshly isolated CD4+ T cells (day 0, gray), anti-CD3/CD28-activated CD4+ T cells (blue), and TT-activated CD4+ T cells (TT-specific, red) isolated from PBMCs of donor A (A and C) and donor D (B and D) are shown. CFSE-labeled T cells were co-cultured with equal numbers of autologous CD B cells in the presence of TT or were cultured with anti-CD3/CD28 beads in the absence of CD B cells for 7 days (see also legend of Fig. 4). After culture, CFSEdim CD4+ T cells were purified for isolation of genomic DNA. Characterization of TCRβ was performed by Adaptive Technologies using the ImmunoSEQ human TCRβ assay (C and D). Top 10 unique VDJ rearrangements most frequently recovered from each T-cell group are selected and percentage of individual rearrangement among all productive VDJ rearrangements is shown. ***p<0.001, ****p<0.0001; ns, no significant difference by one-way ANOVA test.
In general, whereas Vβ usage by TT-activated CD4+ T cells differed between donors A and D, the common use of the Vβ2-1 gene segment may represent a general structural solution for the TCR of TT-specific CD4+ T cells (Figs. 7A and 7B red bars). The frequency of Vβ2-1 in resting and unspecifically activated CD4+ T cells was 5.2% and 4.6%, respectively for donor A and 8.8% and 6.6% for donor D (Fig. 7A and B).Frequency of Vβ2-1rearrangements in TT-activated cohorts doubled these values in both donors at 9.9% (donor A) and 14.1% (donor D) (Fig. 7A and B).
This structural selection is evident in the decreased diversity of independent TCRβ rearrangements recovered from TT-activated T cells (Fig. 7C). Whereas the 10 most common TCRβ rearrangements in resting and unspecifically activated CD4+ T cells constitute <6% (1.1% – 5.5%) of all TCRβ sequences, the top 10 rearrangements from TT-activated cells represent ≈25% (24%, donor D and 26.9%, donor A) (Fig. 7C). Rearrangements of Vβ2-1 are notably represented ( among the top 10 TCRβ sequences in both donors; in donor A, Vβ2-1 rearrangements are ranked third and eighth in abundance and in donor D, first (Table SI). The increased frequencies of a handful of TCRβ rearrangements are consistent with clonal proliferation and dominance in response to TT; in donor A, two Vβ2-1 rearrangements account for 56% of all Vβ2-1 rearrangements in the TT-activated cohort, in donor D, a single rearrangement accounts for 47% of all Vβ2-1 rearrangements.
Antigen-specific selection is also evident in sequence of dominant TCRβ rearrangements. The two most frequent Vβ2-1 rearrangements from TT-activated T cells for donor A (third and eighth) share an virtually identical CDR3 amino acid sequence motifs (ASRPGQPPYEQY and ASSGGQPPYEQY, respectively). This near identity implies convergent selection, presumably for a common peptide/MHC II epitope. We conclude that TCR sequences from TT-activated CD4+ T cells reflect the structural restriction inherent in Antigen-specific receptors preferentially expanded in response to TT presented by autologous CD B cells.
Discussion
The value of the CD culture system in evaluating T-cell specificity and TCR repertoire is demonstrated by the efficient and large yield of CD B cells that are capable of acting as APC to induce T-cell proliferation against alloantigens or pathogenic antigens. Expanded, antigen-specific human CD4+ T cells in CD culture system were subsequently analyzed for the use of TCR Vβ gene segments. Furthermore, we showed that human antigen-specific memory B cells can be expanded efficiently in vitro and function as highly-effective APC, which should allow them to serve as a valuable tool for studying the interaction between cognate T and B cells.
The utility of CD B cells includes evaluating a B-cell repertoire that changes during exposure to antigens in chronic inflammatory conditions (46–49). Mapping the alterations in B-cell repertoires during the courses of diseases may provide insights into the pathogenesis of these diseases, and subsequently, potential therapeutic strategies for them. For example, the existence of B cells secreting broadly-neutralizing antibodies against HIV demonstrates co-evolutionary changes between the B-cell repertoire and viral variants (46), and suggests that the timing of humoral immune responses does not correspond well with the progression of mutations in HIV antigens. Furthermore, B-cell repertoire studies in patients with autoimmune diseases reveal that autoreactive B-cell clones are generated as a result of defective tolerance checkpoints as well as persistent antigen stimulation (47–49). In our lab, we are currently studying human B-cell repertoires using the CD culture method.
Along with humoral responses, the repertoire of T cells theoretically changes upon persistent exposure to antigens (14, 50). This is because T cells are interacting with B cells that can function as APC during chronic inflammatory diseases (51–53). Cognate T and B cells interact and provide reciprocal help required for activation and differentiation of both cell types, which results in alterations in both T- and B-cell populations (9, 52). In contrast to studies of B-cell repertoire dynamics, relatively few studies of co-evolutional changes between human T-cell repertoires and chronic pathogenic antigens have been reported (54, 55). Furthermore, studies directly determining TCR specificity have been rare (56), at least in part because sufficient numbers of autologous APC are infrequently available. Here, we have developed and explored a method that provides abundant autologous APC using CD B cells and could be used to study T-cell repertoire progression.
In our CD culture system, cytokines and CD40:CD154 interaction between B cells and feeder cells support activation, vigorous expansion (Figs. 1 and 5) (57–59), and APC function of human naïve and memory B cells (Figs 4–7) (6, 60, 61). Among cytokines present in our culture medium (IL-2, IL-4, IL-21, and BAFF), IL-21 is crucial to induce robust B-cell proliferation (Fig. S2), an observation consistent with other studies (62–64). BAFF supports B-cell survival (65), the differentiation of human memory B cells into antibody-secreting cells (66), and class-switch recombination in human B cells (67, 68). As IL-4 and IL-21 differentially regulate class-switching to certain Ig isotypes (64, 69), one might consider the combinations of cytokines for CD cultures depending on their own purposes.
Although studies have also used CD40-mediated activation to induce proliferation of human B cells (6, 60, 61, 63), our cultures supported more robust and sustained proliferation of B cells than previous culture methods. In the CD culture system, B cells expanded by approximately 106-fold after 16 days of culture: the rate of B-cell expansion was stable over a 16-day culture period after an initial lag phase (Fig. 1C). Beginning in day 4 of culture, nearly a log increase in cell numbers was observed every 2 days; the population doubling time in day 4-day 16 CD B cells was approximately 15 hours [calculated using the logarithmic least-squares fitting technique (70)]. We believe that our CD culture may be the most efficient system to induce primary human B-cell division in vitro (6, 60, 61).
In the CD culture system, mature, naïve human B cells are activate and express elevated levels of MHCII, CD80, and CD86 as early as day 4 (Fig. 2A), undergo IgM → IgG class-switch recombination (day 8 through day 16), and later differentiate into plasmablasts/plasmacytes on around day 14–16 after culture (Fig. 2B). By day 16, frequency of CD138+ plasmablasts/plasmacytes populations reaches to 10 – 30% in the CD culture system (Fig. 2B). We note that our estimate of plasmablasts/plasmacytes frequencies in CD culture system might vary depending on the use of surface markers (e.g., CD27hiCD38hi) to define plasmablasts/plasmacytes (44, 62).
The observation that the CD culture system supports activation and extensive proliferation of memory B cells (Fig. 5B) is consistent with previous reports that human memory B cells can be activated and differentiate through a pathway that bypasses BCR signaling (62, 63). CD B cells originating from memory B cells exhibited slightly less cell expansion and more frequent CD138 expression compared to those originating from naïve B cells although both were statistically-insignificant (Figs. 1C and 5B). Furthermore, the elevated expression of MHCII, CD80, and CD86 on cultured memory B cells declined faster than in cultured naïve B cells (Figs. 2A and 5C). These findings may be due to fundamental differences in the activation capacity and differentiation potential of naïve and memory B cells (63, 71).
CD B cells from day 8 cultures displayed more efficient antigen-presenting function than CD B cells from day 16 cultures (p≤0.0067 and p≤0.0011 for TT-PE+ and TT-PE− enriched CD B cells, respectively) (Fig. 6). This loss of functional was correlated with the higher levels of MHCII, CD80, and CD86 expressed by day 8 CD B cells compared to CD B cells from day 16 cultures (Fig. 5C). Although plasmacytes may retain the expression of surface MHCII, CD80, and CD86 and function as APC (72), the expression of mRNA encoding MHCII, CD80, and CD86 molecules is very low in plasmacytes (72, 73). Thus, plasmacytic differentiation of CD B cells likely contributes also to losses in APC function.
Potent allo-responses by T cells are induced by CD B cells from allogeneic donors (Fig. 3 and Table II). We observed alloreactivity among both CD4+ and CD8+ T-cell subsets, indicating that antigen-display functions of MHCI and MHCII are normal in CD B cells (27). This suggests that CD B cells acquire antigens derived from dying cells and/or through autophagy in an MHCII-dependent pathway (27, 74); conversely, the antigens for MHCI-dependent presentation can be acquired from the intracellular space for canonical presentation and/or from the extracellular space for cross-presentation (27, 75). Unlike allogeneic co-cultures, little or no T-cell proliferation was observed in the autologous T-B co-cultures (Figs. 3, 4, and 6, and Table II). These responses may have contained some minor component of xeno-activation, because we did not isolate CD B cells from the CD154-expressing mouse stromal cells after culture. Nonetheless, as expected (76), xeno-reactivity of T cells against the mouse stromal cell line appeared to be negligible in these conditions (Figs. 3–4, and 6, and Table II).
CD B cells efficiently induced the proliferation of autologous T cells against microbial antigens when these cells were prepared from donors who had recently been vaccinated with (components of) the same microbial antigens (Fig. 4). Presumably, this reflects the increased numbers of specific T cells elicited by homologous vaccination (56, 77). CD B cells expanded from non-selected, naïve mature B cells are capable of acquiring antigens through BCR-independent fluid-phase endocytosis (78) and presenting these antigens to T lymphocytes. As expected (79, 80), the presentation of exogenous antigens (TT and HA) by CD B cells to T cells mainly occurs through the MHCII-dependent pathway that induces CD4+ T-cell responses (Fig. 4). Interestingly, we observed T-cell responses against rPA in the individual (donor D) who had a history of occupation-related exposure to rPA (Fig. 4); a low level of antibodies against rPA could also be detected in this individual’s plasma (data not shown). Together with the co-culture results, this indicates the presence of both humoral and cellular immune responses against rPA in donor D (81, 82).
It is applicable to use CD B cells with sets of antigens to determine the antigen specificity of T cells and to evaluate post-vaccine cellular responses. The identified antigen-responding T cells can be isolated for subsequent determination of the TCR repertoire [Fig. 7, (56)]. Alternatively, many studies have used tetramers of MHC molecules conjugated with peptides and fluorochromes to separate the target T cells (30). However, some limitations of this method should be considered. First, tetramers allow for the identification of T cells specific for pathogens with a few immunodominant peptides, not against pathogens with a complex set of epitopes (30, 83). Second, tetramers are not primarily designed to measure the breadth of cellular immune responses induced by vaccination (84). Third, each individual needs an MHC test to find MHC-matched tetramers (if available) (85). In contrast, CD B cells would allow for the identification (and subsequent isolation) of T cells against pathogens using a complex set of antigens. Because the tested antigens would not be limited to peptide forms (Figs. 4, 6, and 7), CD B cells would allow more antigens to be tested and would provide a suitable method for determining both intensity and breadth of vaccine responses. Moreover, an MHC test would not be necessary because both T and B cells could be isolated from the same individuals (Figs. 4, 6, and 7). In addition to specific T-cell isolation, CD B cells could be valuable in defining the characteristics of antigen-specific memory T cells, such as their expression of surface molecules and effector molecules.
Using autologous CD B cells and CD4+ T cells, we characterized the TCR repertoire for TT-reactive CD4+ T cells in two unrelated donors. Analysis of some 8,800 rearranged TCRB gene sequences from TT-reactive CD4+ T cells showed that in each donor distributions of TCR Vβ usage of TT-activated CD4+ T cells was distinct from resting CD4+ T cells or CD4+ T cells unspecifically activated by anti-CD3/CD28 (Fig. 7). Whereas TCR Vβ usage of TT-activated CD4+ T cells substantially differed between donors, presumably due to dissimilar HLA types and/or differences in vaccination and exposure histories (86), we noted selective expansion of small sets of Vβ rearrangements such that the top 10 rearrangements in either donor constituted at least 25% of all TCRβ amplicands. This decreased diversity is consistent with TT-specific clonal expansion.
While genetically diverse, human TT-specific TCRαβ may share some general structural(s) characteristics, as we observed overrepresentation of Vβ2-1 rearrangements in both donors (Fig. 7 and Table SI). Frequencies of Vβ2-1 usage were doubled in the TT-activated cohorts of both donor A and D (Fig. 7) and this increase was the consequence of clonal expansion. Interestingly, in donor A, two Vβ2-1 TCR rearrangements, ranked third and eighth among the top 10, shared a CDR3 amino acid sequence independently generated by distinct VDJ rearrangements (Table SI). We hypothesize that this convergence represents a common structural solution for a single peptide/MHC II epitope.
One unique feature of antigen-presenting B cells is that B cells uptake antigen in both BCR-independent and dependent manners (1, 9, 87). While non-antigen-specific B cells uptake antigens in a BCR-independent manner, antigen-specific B cells uptake antigens more efficiently through their high-affinity BCRs (9). Our results (Fig. 6) indicate that TT-binding memory CD B cells could present TT more efficiently than TT non-binding memory CD B cells, which is consistent with previous reports using EBV-transformed B cells (9). Our data show that the ability of HA presentation was comparable in cultured memory B cells with or without TT binding-enrichment (Fig. 6). In addition, the comparable expression levels of MHCII, CD80, CD86, and IgG on both CD B cell groups (data not shown) suggest that BCR-independent antigen-presenting function was similar in both CD B cell groups. We therefore conclude that the superior ability of TT-binding enriched CD B cells to induce T-cell proliferation in the presence of TT was related to their harboring of TT-specific BCRs, which uptake TT more efficiently.
Consistent with our own and earlier observations (9), TT-binding memory CD B cells induced T-cell proliferation more efficiently than did naive CD B cells in the presence of TT (Fig. S3). We also noted that APC functions of naïve CD B cells was generally less efficient compared to those of memory CD B cells (TT-PE+ or TT-PE−) regardless of antigen types (Fig. S3), a finding also noted by others (88). Further studies, such as transcriptome analysis for memory and naive CD B cells would help to understand differential APC function by these CD B cells.
Several studies have shown that human memory B cells act as APC and activate allogeneic T cells (88–90). By contrast, the studies in human antigen-specific memory B cells are often limited due to low cell numbers (91, 92). To obtain sufficient numbers of specific B cells, EBV-transformed B-cell lines are commonly used in studies of the interaction between human cognate T and B cells (9). However, there are potential T-cell responses to the EBV-infected B cells (93, 94), and thus EBV-transformed B cells are not suitable for this type of study. Our culture system would enable the expansion of antigen-specific human memory B cells in vitro without EBV transformation (Fig. 5B), thus offering the opportunity to use activated and culture-expanded primary cells to evaluate the interaction between cognate T and B cells.
Our results suggest utility and immediate application of CD B cells for establishing T-cell lines/clones and epitope mapping. Numerous CD B cells originating from naïve or memory subsets would provide sufficient numbers of APC without the requirement for leukapheresis or EBV transformation. Expanded CD B cells retain surface expression of MHCII and co-stimulatory molecules (CD80 and CD86), and importantly, CD B cells can serve as APC to induce T-cell proliferation against microbial antigens. Furthermore, samples from patients with infectious diseases are infrequent accompanied by leukapheresis and abundant numbers of APC. Consequently, this new method represents a potentially consequential methodological advance. Thus, the use of CD B cells as a source of autologous APC for expanding antigen-specific T cells and studying their epitope specificity would be a useful strategy for the development of effective T-cell based vaccines and revealing the complexity of co-evolutional changes in the T-cell repertoire during chronic inflammation. In conclusion, this CD culture system provides a platform for studying human B- and T-cell repertoires.
Supplementary Material
Acknowledgments
We thank David Baltimore for providing the CD154-expressing cell line. We thank Stephen Harrison for providing the recombinant influenza HA. We thank the blood donors. We thank Elizabeth Wong and Alexander Reynolds for their editorial assistance. We thank Dongmei Liao and Xiaoe Liang for their technical assistance.
This work was supported in part by Duke Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery (CHAVI-ID) grant AI100645-02 and Autoimmunity Center of Excellence (ACE) grant AI56363.
Glossary
Abbreviations used in this article
- APC
antigen-presenting cells
- BCR
B-cell receptor
- CD B cells
culture-derived B cells
- CFSE
carboxyfluorescein succinimidyl ester
- HA
recombinant influenza hemagglutinin
- rPA
recombinant B. anthracis protective antigen
- TT
tetanus toxoid
References
- 1.Rodríguez-Pinto D. B cells as antigen presenting cells. Cellular Immunology. 2005;238:67–75. doi: 10.1016/j.cellimm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Lund FE. Cytokine-producing B lymphocytes - key regulators of immunity. Current opinion in immunology. 2008;20:332–338. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshizaki A, Miyagaki T, DiLillo DJ, Matsushita T, Horikawa M, Kountikov EI, Spolski R, Poe JC, Leonard WJ, Tedder TF. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature. 2012;491:264–268. doi: 10.1038/nature11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perera J, Meng L, Meng F, Huang H. Autoreactive thymic B cells are efficient antigen-presenting cells of cognate self-antigens for T cell negative selection. Proceedings of the National Academy of Sciences. 2013;110:17011–17016. doi: 10.1073/pnas.1313001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molnarfi N, Schulze-Topphoff U, Weber MS, Patarroyo JC, Prod’homme T, Varrin-Doyer M, Shetty A, Linington C, Slavin AJ, Hidalgo J, Jenne DE, Wekerle H, Sobel RA, Bernard CCA, Shlomchik MJ, Zamvil SS. MHC class II-dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. J. Exp. Med. 2013;210:2921–2937. doi: 10.1084/jem.20130699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Bergwelt-Baildon MS, Vonderheide RH, Maecker B, Hirano N, Anderson KS, Butler MO, Xia Z, Zeng WY, Wucherpfennig KW, Nadler LM, Schultze JL. Human primary and memory cytotoxic T lymphocyte responses are efficiently induced by means of CD40-activated B cells as antigen-presenting cells: potential for clinical application. Blood. 2002;99:3319–3325. doi: 10.1182/blood.v99.9.3319. [DOI] [PubMed] [Google Scholar]
- 7.Jiang W, Lederman MM, Harding CV, Rodriguez B, Mohner RJ, Sieg SF. TLR9 stimulation drives naïve B cells to proliferate and to attain enhanced antigen presenting function. European Journal of Immunology. 2007;37:2205–2213. doi: 10.1002/eji.200636984. [DOI] [PubMed] [Google Scholar]
- 8.Bennett SRM, Carbone FR, Toy T, Miller JFAP, Heath WR. B Cells Directly Tolerize CD8+ T Cells. The Journal of Experimental Medicine. 1998;188:1977–1983. doi: 10.1084/jem.188.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 10.Rock KL, Benacerraf B, Abbas AK. Antigen presentation by hapten-specific B lymphocytes. I. Role of surface immunoglobulin receptors. J. Exp. Med. 1984;160:1102–1113. doi: 10.1084/jem.160.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roche PA, Furuta K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat. Rev. Immunol. 2015;15:203–216. doi: 10.1038/nri3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan J, Harvey BP, Gee RJ, Shlomchik MJ, Mamula MJ. B Cells Drive Early T Cell Autoimmunity In Vivo prior to Dendritic Cell-Mediated Autoantigen Presentation. The Journal of Immunology. 2006;177:4481–4487. doi: 10.4049/jimmunol.177.7.4481. [DOI] [PubMed] [Google Scholar]
- 13.Sfikakis PP, Boletis JN, Lionaki S, Vigklis V, Fragiadaki KG, Iniotaki A, Moutsopoulos HM. Remission of proliferative lupus nephritis following B cell depletion therapy is preceded by down-regulation of the T cell costimulatory molecule CD40 ligand: An open-label trial. Arthritis & Rheumatism. 2005;52:501–513. doi: 10.1002/art.20858. [DOI] [PubMed] [Google Scholar]
- 14.O’Neill SK, Cao Y, Hamel KM, Doodes PD, Hutas G, Finnegan A. Expression of CD80/86 on B Cells Is Essential for Autoreactive T Cell Activation and the Development of Arthritis. The Journal of Immunology. 2007;179:5109–5116. doi: 10.4049/jimmunol.179.8.5109. [DOI] [PubMed] [Google Scholar]
- 15.Takemura S, Klimiuk PA, Braun A, Goronzy JJ, Weyand CM. T Cell Activation in Rheumatoid Synovium Is B Cell Dependent. The Journal of Immunology. 2001;167:4710–4718. doi: 10.4049/jimmunol.167.8.4710. [DOI] [PubMed] [Google Scholar]
- 16.Noorchashm H, Lieu YK, Noorchashm N, Rostami SY, Greeley SAS, Schlachterman A, Song HK, Noto LE, Jevnikar AM, Barker CF, Naji A. I-Ag7-Mediated Antigen Presentation by B Lymphocytes Is Critical in Overcoming a Checkpoint in T Cell Tolerance to Islet β Cells of Nonobese Diabetic Mice. The Journal of Immunology. 1999;163:743–750. [PubMed] [Google Scholar]
- 17.Ait-Oufella H, Herbin O, Bouaziz J-D, Binder CJ, Uyttenhove C, Laurans L, Taleb S, Van Vré E, Esposito B, Vilar J, Sirvent J, Van Snick J, Tedgui A, Tedder TF, Mallat Z. B cell depletion reduces the development of atherosclerosis in mice. The Journal of Experimental Medicine. 2010;207:1579–1587. doi: 10.1084/jem.20100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, Leong HX, Glassford A, Caimol M, Kenkel JA, Tedder TF, McLaughlin T, Miklos DB, Dosch HM, Engleman EG. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 2011;17:610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.León B, Ballesteros-Tato A, Lund FE. Dendritic Cells and B Cells: Unexpected Partners in Th2 Development. The Journal of Immunology. 2014;193:1531–1537. doi: 10.4049/jimmunol.1400149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng Q, Ng Y-H, Singh T, Jiang K, Sheriff KA, Ippolito R, Zahalka S, Li Q, Randhawa P, Hoffman RA, Ramaswami B, Lund FE, Chalasani G. B cells mediate chronic allograft rejection independently of antibody production. The Journal of Clinical Investigation. 2014;124:1052–1056. doi: 10.1172/JCI70084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnett LG, Simkins HMA, Barnett BE, Korn LL, Johnson AL, Wherry EJ, Wu GF, Laufer TM. B Cell Antigen Presentation in the Initiation of Follicular Helper T Cell and Germinal Center Differentiation. The Journal of Immunology. 2014;192:3607–3617. doi: 10.4049/jimmunol.1301284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sette A, Peters B. Immune epitope mapping in the post-genomic era: lessons for vaccine development. Current Opinion in Immunology. 2007;19:106–110. doi: 10.1016/j.coi.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Zeiler T, Virtanen T. Mapping of Human T-Cell Epitopes of Allergens. In: Jones M, Lympany P, editors. Allergy Methods and Protocols. Humana Press; 2008. pp. 51–56. [DOI] [PubMed] [Google Scholar]
- 24.Muul LM, Heine G, Silvin C, James SP, Candotti F, Radbruch A, Worm M. Current Protocols in Immunology. John Wiley & Sons, Inc; 2011. Measurement of Proliferative Responses of Cultured Lymphocytes. [DOI] [PubMed] [Google Scholar]
- 25.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 26.Bacher P, Schink C, Teutschbein J, Kniemeyer O, Assenmacher M, Brakhage AA, Scheffold A. Antigen-reactive T cell enrichment for direct, high-resolution analysis of the human naive and memory Th cell repertoire. The Journal of Immunology. 2013;190:3967–3976. doi: 10.4049/jimmunol.1202221. [DOI] [PubMed] [Google Scholar]
- 27.Blum JS, Wearsch PA, Cresswell P. Pathways of Antigen Processing. Annual Review of Immunology. 2013;31:443–473. doi: 10.1146/annurev-immunol-032712-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hosken N, McGowan P, Meier A, Koelle DM, Sleath P, Wagener F, Elliott M, Grabstein K, Posavad C, Corey L. Diversity of the CD8+ T-Cell Response to Herpes Simplex Virus Type 2 Proteins among Persons with Genital Herpes. Journal of Virology. 2006;80:5509–5515. doi: 10.1128/JVI.02659-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cannella AP, Arlehamn CSL, Sidney J, Patra KP, Torres K, Tsolis RM, Liang L, Felgner PL, Saito M, Gotuzzo E, Gilman RH, Sette A, Vinetz JM. Brucella melitensis T Cell Epitope Recognition in Humans with Brucellosis in Peru. Infection and Immunity. 2014;82:124–131. doi: 10.1128/IAI.00796-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klenerman P, Cerundolo V, Dunbar PR. Tracking T cells with tetramers: new tales from new tools. Nat. Rev. Immunol. 2002;2:263–272. doi: 10.1038/nri777. [DOI] [PubMed] [Google Scholar]
- 31.Cole DK, Pumphrey NJ, Boulter JM, Sami M, Bell JI, Gostick E, Price DA, Gao GF, Sewell AK, Jakobsen BK. Human TCR-binding affinity is governed by MHC class restriction. The Journal of Immunology. 2007;178:5727–5734. doi: 10.4049/jimmunol.178.9.5727. [DOI] [PubMed] [Google Scholar]
- 32.Haruta M, Tomita Y, Imamura Y, Matsumura K, Ikeda T, Takamatsu K, Nishimura Y, Senju S. Generation of a large number of functional dendritic cells from human monocytes expanded by forced expression of cMYC plus BMI1. Human Immunology. 2013;74:1400–1408. doi: 10.1016/j.humimm.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 33.Ardeshna, Pizzey, Thomas, Orr, Linch, Devereux Monocyte-derived dendritic cells do not proliferate and are not susceptible to retroviral transduction. British Journal of Haematology. 2000;108:817–824. doi: 10.1046/j.1365-2141.2000.01956.x. [DOI] [PubMed] [Google Scholar]
- 34.Landis RC, Yagnik DR, Florey O, Philippidis P, Emons V, Mason JC, Haskard DO. Safe disposal of inflammatory monosodium urate monohydrate crystals by differentiated macrophages. Arthritis & Rheumatism. 2002;46:3026–3033. doi: 10.1002/art.10614. [DOI] [PubMed] [Google Scholar]
- 35.Néron S, Roy A, Dumont N. Large-Scale In Vitro Expansion of Polyclonal Human Switched-Memory B Lymphocytes. PLoS ONE. 2012;7:e51946. doi: 10.1371/journal.pone.0051946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kondo E, Gryschok L, Klein-Gonzalez N, Rademacher S, Weihrauch MR, Liebig T, Shimabukuro-Vornhagen A, Kochanek M, Draube A, Von Bergwelt-Baildon MS. CD40-activated B cells can be generated in high number and purity in cancer patients: analysis of immunogenicity and homing potential. Clinical & Experimental Immunology. 2009;155:249–256. doi: 10.1111/j.1365-2249.2008.03820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-Marquez MA, Shimabukuro-Vornhagen A, Theurich S, Kochanek M, Weber T, Wennhold K, Dauben A, Dzionek A, Reinhard C, Bergwelt-Baildon Mv. A multimerized form of recombinant human CD40 ligand supports long-term activation and proliferation of B cells. Cytotherapy. 2014;16:1537–1544. doi: 10.1016/j.jcyt.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 38.Luo XM, Maarschalk E, O’Connell RM, Wang P, Yang L, Baltimore D. Engineering human hematopoietic stem/progenitor cells to produce a broadly neutralizing anti-HIV antibody after in vitro maturation to human B lymphocytes. Blood. 2009;113:1422–1431. doi: 10.1182/blood-2008-09-177139. [DOI] [PubMed] [Google Scholar]
- 39.Disis ML, dela Rosa C, Goodell V, Kuan L-Y, Chang JCC, Kuus-Reichel K, Clay TM, Kim Lyerly H, Bhatia S, Ghanekar SA, Maino VC, Maecker HT. Maximizing the retention of antigen specific lymphocyte function after cryopreservation. Journal of Immunological Methods. 2006;308:13–18. doi: 10.1016/j.jim.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 40.Kuraoka M, Liao D, Yang K, Allgood SD, Levesque MC, Kelsoe G, Ueda Y. Activation-induced cytidine deaminase expression and activity in the absence of germinal centers: insights into hyper-IgM syndrome. J Immunol. 2009;183:3237–3248. doi: 10.4049/jimmunol.0901548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robins HS, Campregher PV, Srivastava SK, Wacher A, Turtle CJ, Kahsai O, Riddell SR, Warren EH, Carlson CS. Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood. 2009;114:4099–4107. doi: 10.1182/blood-2009-04-217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suessmuth Y, Mukherjee R, Watkins B, Koura DT, Finstermeier K, Desmarais C, Stempora L, Horan JT, Langston A, Qayed M, Khoury HJ, Grizzle A, Cheeseman JA, Conger JA, Robertson J, Garrett A, Kirk AD, Waller EK, Blazar BR, Mehta AK, Robins HS, Kean LS. CMV reactivation drives posttransplant T-cell reconstitution and results in defects in the underlying TCRbeta repertoire. Blood. 2015;125:3835–3850. doi: 10.1182/blood-2015-03-631853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jung J, Choe J, Li L, Choi YS. Regulation of CD27 expression in the course of germinal center B cell differentiation: the pivotal role of IL-10. Eur. J. Immunol. 2000;30:2437–2443. doi: 10.1002/1521-4141(2000)30:8<2437::AID-IMMU2437>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 44.Avery DT, Ellyard JI, Mackay F, Corcoran LM, Hodgkin PD, Tangye SG. Increased expression of CD27 on activated human memory B cells correlates with their commitment to the plasma cell lineage. The Journal of Immunology. 2005;174:5885. doi: 10.4049/jimmunol.174.7.4034. [DOI] [PubMed] [Google Scholar]
- 45.Costes V, Magen V, Legouffe E, Durand L, Baldet P, Rossi J-F, Klein B, Brochier J. The Mi15 monoclonal antibody (anti-syndecan-1) is a reliable marker for quantifying plasma cells in paraffin-embedded bone marrow biopsy specimens. Human Pathology. 1999;30:1405–1411. doi: 10.1016/s0046-8177(99)90160-0. [DOI] [PubMed] [Google Scholar]
- 46.Liao H-X, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang Z, Zhu J, Shapiro L, Mullikin JC, Gnanakaran S, Hraber P, Wiehe K, Kelsoe G, Yang G, Xia S-M, Montefiori DC, Parks R, Lloyd KE, Scearce RM, Soderberg KA, Cohen M, Kamanga G, Louder MK, Tran LM, Chen Y, Cai F, Chen S, Moquin S, Du X, Joyce MG, Srivatsan S, Zhang B, Zheng A, Shaw GM, Hahn BH, Kepler TB, Korber BTM, Kwong PD, Mascola JR, Haynes BF. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013;496:469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meffre E, Wardemann H. B-cell tolerance checkpoints in health and autoimmunity. Curr. Opin. Immunol. 2008;20:632–638. doi: 10.1016/j.coi.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Schaller M, Bigler C, Danner D, Ditzel HJ, Trendelenburg M. Autoantibodies against C1q in Systemic Lupus Erythematosus Are Antigen-Driven. The Journal of Immunology. 2009;183:8225–8231. doi: 10.4049/jimmunol.0902642. [DOI] [PubMed] [Google Scholar]
- 49.Wellmann U, Letz M, Herrmann M, Angermüller S, Kalden JR, Winkler TH. The evolution of human anti-double-stranded DNA autoantibodies. Proc. Natl. Acad. Sci. USA. 2005;102:9258–9263. doi: 10.1073/pnas.0500132102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alugupalli KR, Abraham D. B Cell Multitasking Is Required to Control Nematode Infection. Immunity. 2009;30:317–319. doi: 10.1016/j.immuni.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 51.Deenick EK, Chan A, Ma CS, Gatto D, Schwartzberg PL, Brink R, Tangye SG. Follicular Helper T Cell Differentiation Requires Continuous Antigen Presentation that Is Independent of Unique B Cell Signaling. Immunity. 2010;33:241–253. doi: 10.1016/j.immuni.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baumjohann D, Preite S, Reboldi A, Ronchi F, Ansel KM, Lanzavecchia A, Sallusto F. Persistent Antigen and Germinal Center B Cells Sustain T Follicular Helper Cell Responses and Phenotype. Immunity. 2013;38:596–605. doi: 10.1016/j.immuni.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 53.Ciechomska M, Wilson CL, Floudas A, Hui W, Rowan AD, van Eden W, Robinson JH, Knight AM. Antigen-specific B lymphocytes acquire proteoglycan aggrecan from cartilage extracellular matrix resulting in antigen presentation and CD4+T-cell activation. Immunology. 2014;141:70–78. doi: 10.1111/imm.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Russi S, Lauletta G, Serviddio G, Sansonno S, Conteduca V, Sansonno L, De Re V, Sansonno D. T cell receptor variable β gene repertoire in liver and peripheral blood lymphocytes of chronically hepatitis C virus-infected patients with and without mixed cryoglobulinaemia. Clinical & Experimental Immunology. 2013;172:254–262. doi: 10.1111/cei.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wagner UG, Koetz K, Weyand CM, Goronzy JJ. Perturbation of the T cell repertoire in rheumatoid arthritis. Proceedings of the National Academy of Sciences. 1998;95:14447–14452. doi: 10.1073/pnas.95.24.14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Becattini S, Latorre D, Mele F, Foglierini M, De Gregorio C, Cassotta A, Fernandez B, Kelderman S, Schumacher TN, Corti D, Lanzavecchia A, Sallusto F. Functional heterogeneity of human memory CD4+ T cell clones primed by pathogens or vaccines. Science. 2015;347:400–406. doi: 10.1126/science.1260668. [DOI] [PubMed] [Google Scholar]
- 57.Clark EA, Ledbetter JA. Activation of human B cells mediated through two distinct cell surface differentiation antigens, Bp35 and Bp50. Proc Natl Acad Sci U S A. 1986;83:4494–4498. doi: 10.1073/pnas.83.12.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Banchereau J, de Paoli P, Valle A, Garcia E, Rousset F. Long-term human B cell lines dependent on interleukin-4 and antibody to CD40. Science. 1991;251:70–72. doi: 10.1126/science.1702555. [DOI] [PubMed] [Google Scholar]
- 59.Banchereau J, Rousset F. Growing human B lymphocytes in the CD40 system. Nature. 1991;353:678–679. doi: 10.1038/353678a0. [DOI] [PubMed] [Google Scholar]
- 60.Zheng J, Liu Y, Lau Y-L, Tu W. CD40-activated B cells are more potent than immature dendritic cells to induce and expand CD4+ regulatory T cells. Cell. Mol. Immunol. 2010;7:44–50. doi: 10.1038/cmi.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mathieu M, Cotta-Grand N, Daudelin J-F, Boulet S, Lapointe R, Labrecque N. CD40-activated B cells can efficiently prime antigen-specific naïve CD8+ T cells to generate effector but not memory T cells. PLoS ONE. 2012;7:e30139. doi: 10.1371/journal.pone.0030139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ettinger R, Sims GP, Fairhurst A-M, Robbins R, da Silva YS, Spolski R, Leonard WJ, Lipsky PE. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. The Journal of Immunology. 2005;175:7867–7879. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 63.Good KL, Bryant VL, Tangye SG. Kinetics of Human B Cell Behavior and Amplification of Proliferative Responses following Stimulation with IL-21. The Journal of Immunology. 2006;177:5236–5247. doi: 10.4049/jimmunol.177.8.5236. [DOI] [PubMed] [Google Scholar]
- 64.Ettinger R, Kuchen S, Lipsky PE. The role of IL-21 in regulating B-cell function in health and disease. Immunol Rev. 2008;223:60–86. doi: 10.1111/j.1600-065X.2008.00631.x. [DOI] [PubMed] [Google Scholar]
- 65.Mackay F, Browning JL. BAFF: a fundamental survival factor for B cells. Nat Rev Immunol. 2002;2:465–475. doi: 10.1038/nri844. [DOI] [PubMed] [Google Scholar]
- 66.Avery DT, Kalled SL, Ellyard JI, Ambrose C, Bixler SA, Thien M, Brink R, Mackay F, Hodgkin PD, Tangye SG. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J Clin Invest. 2003;112:286–297. doi: 10.1172/JCI18025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamada T, Zhang K, Yamada A, Zhu D, Saxon A. B lymphocyte stimulator activates p38 mitogen-activated protein kinase in human Ig class switch recombination. Am J Respir Cell Mol Biol. 2005;32:388–394. doi: 10.1165/rcmb.2004-0317OC. [DOI] [PubMed] [Google Scholar]
- 68.Cerutti A. The regulation of IgA class switching. Nat Rev Immunol. 2008;8:421–434. doi: 10.1038/nri2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Avery DT, Bryant VL, Ma CS, de Waal Malefyt R, Tangye SG. IL-21-induced isotype switching to IgG and IgA by human naive B cells is differentially regulated by IL-4. J Immunol. 2008;181:1767–1779. doi: 10.4049/jimmunol.181.3.1767. [DOI] [PubMed] [Google Scholar]
- 70.Roth V. 2006 < http://www.doubling-time.com/compute.php>.
- 71.Deenick EK, Avery DT, Chan A, Berglund LJ, Ives ML, Moens L, Stoddard JL, Bustamante J, Boisson-Dupuis S, Tsumura M, Kobayashi M, Arkwright PD, Averbuch D, Engelhard D, Roesler J, Peake J, Wong M, Adelstein S, Choo S, Smart JM, French MA, Fulcher DA, Cook MC, Picard C, Durandy A, Klein C, Holland SM, Uzel G, Casanova J-L, Ma CS, Tangye SG. Naive and memory human B cells have distinct requirements for STAT3 activation to differentiate into antibody-secreting plasma cells. The Journal of Experimental Medicine. 2013;210:2739–2753. doi: 10.1084/jem.20130323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pelletier N, McHeyzer-Williams LJ, Wong KA, Urich E, Fazilleau N, McHeyzer-Williams MG. Plasma cells negatively regulate the follicular helper T cell program. Nat. Immunol. 2010;11:1110–1118. doi: 10.1038/ni.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Piskurich JF, Lin KI, Lin Y, Wang Y, Ting JPY, Calame K. BLIMP-1 mediates extinction of major histocompatibility class II transactivator expression in plasma cells. Nat. Immunol. 2000;1:526–532. doi: 10.1038/82788. [DOI] [PubMed] [Google Scholar]
- 74.Crotzer VL, Blum JS. Autophagy and its role in MHC-mediated antigen presentation. The Journal of Immunology. 2009;182:3335–3341. doi: 10.4049/jimmunol.0803458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nagy ZA. Alloreactivity: An Old Puzzle Revisited. Scandinavian Journal of Immunology. 2012;75:463–470. doi: 10.1111/j.1365-3083.2012.02680.x. [DOI] [PubMed] [Google Scholar]
- 76.Wilson DB, Nowell PC. Quantitative studies on the mixed lymphocyte interaction. IV. Immunological potentiality of the responding cells. The Journal of Experimental Medicine. 1970;131:391–407. doi: 10.1084/jem.131.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.He J, Tsai Louis M, Leong Yew A, Hu X, Ma Cindy S, Chevalier N, Sun X, Vandenberg K, Rockman S, Ding Y, Zhu L, Wei W, Wang C, Karnowski A, Belz Gabrielle T, Ghali Joanna R, Cook Matthew C, Riminton DS, Veillette A, Schwartzberg Pamela L, Mackay F, Brink R, Tangye Stuart G, Vinuesa Carola G, Mackay Charles R, Li Z, Yu D. Circulating precursor CCR7loPD-1hi CXCR5+ CD4+ T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity. 2013;39:770–781. doi: 10.1016/j.immuni.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 78.Kamphorst AO, Guermonprez P, Dudziak D, Nussenzweig MC. Route of antigen uptake differentially impacts presentation by dendritic cells and activated monocytes. J. Immunol. 2010;185:3426–3435. doi: 10.4049/jimmunol.1001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhong G, Sousa CRe, Germain RN. Antigen-unspecific B cells and lymphoid dendritic cells both show extensive surface expression of processed antigen-major histocompatibility complex class II complexes after soluble protein exposure in vivo or in vitro. The Journal of Experimental Medicine. 1997;186:673–682. doi: 10.1084/jem.186.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rodríguez-Pinto D, Moreno J. B cells can prime naive CD4+ T cells in vivo in the absence of other professional antigen-presenting cells in a CD154-CD40-dependent manner. European Journal of Immunology. 2005;35:1097–1105. doi: 10.1002/eji.200425732. [DOI] [PubMed] [Google Scholar]
- 81.Leppla SH, Robbins JB, Schneerson R, Shiloach J. Development of an improved vaccine for anthrax. The Journal of Clinical Investigation. 2002;110:141–144. doi: 10.1172/JCI16204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laughlin EM, Miller JD, James E, Fillos D, Ibegbu CC, Mittler RS, Akondy R, Kwok W, Ahmed R, Nepom G. Antigen-specific CD4+ T cells recognize epitopes of protective antigen following vaccination with an anthrax vaccine. Infection and Immunity. 2007;75:1852–1860. doi: 10.1128/IAI.01814-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nepom GT. MHC class II tetramers. The Journal of Immunology. 2012;188:2477–2482. doi: 10.4049/jimmunol.1102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Long HM, Chagoury OL, Leese AM, Ryan GB, James E, Morton LT, Abbott RJM, Sabbah S, Kwok W, Rickinson AB. MHC II tetramers visualize human CD4+ T cell responses to Epstein-Barr virus infection and demonstrate atypical kinetics of the nuclear antigen EBNA1 response. The Journal of Experimental Medicine. 2013;210:933–949. doi: 10.1084/jem.20121437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.James EA, Bui J, Berger D, Huston L, Roti M, Kwok WW. Tetramer-guided epitope mapping reveals broad, individualized repertoires of tetanus toxin-specific CD4+ T cells and suggests HLA-based differences in epitope recognition. International Immunology. 2007;19:1291–1301. doi: 10.1093/intimm/dxm099. [DOI] [PubMed] [Google Scholar]
- 86.Schmidt AG, Do KT, McCarthy KR, Kepler TB, Liao HX, Moody MA, Haynes BF, Harrison SC. Immunogenic Stimulus for Germline Precursors of Antibodies that Engage the Influenza Hemagglutinin Receptor-Binding Site. Cell Rep. 2015;13:2842–2850. doi: 10.1016/j.celrep.2015.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Avalos AM, Ploegh H. Early BCR events and antigen capture, processing and loading on MHC class II on B cells. Front. Immunol. 2014;5:1–5. doi: 10.3389/fimmu.2014.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Good KL, Avery DT, Tangye SG. Resting human memory B cells are intrinsically programmed for enhanced survival and responsiveness to diverse stimuli compared to naive B cells. J Immunol. 2009;182:890–901. doi: 10.4049/jimmunol.182.2.890. [DOI] [PubMed] [Google Scholar]
- 89.Liu YJ, Barthelemy C, de Bouteiller O, Arpin C, Durand I, Banchereau J. Memory B cells from human tonsils colonize mucosal epithelium and directly present antigen to T cells by rapid up-regulation of B7-1 and B7-2. Immunity. 1995;2:239–248. doi: 10.1016/1074-7613(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 90.Morbach H, Wiegering V, Richl P, Schwarz T, Suffa N, Eichhorn E-M, Eyrich M, Girschick HJ. Activated memory B cells may function as antigen-presenting cells in the joints of children with juvenile idiopathic arthritis. Arthritis & Rheumatism. 2011;63:3458–3466. doi: 10.1002/art.30569. [DOI] [PubMed] [Google Scholar]
- 91.Pinna D, Corti D, Jarrossay D, Sallusto F, Lanzavecchia A. Clonal dissection of the human memory B-cell repertoire following infection and vaccination. European Journal of Immunology. 2009;39:1260–1270. doi: 10.1002/eji.200839129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Franz B, May KF, Dranoff G, Wucherpfennig K. Ex vivo characterization and isolation of rare memory B cells with antigen tetramers. Blood. 2011;118:348–357. doi: 10.1182/blood-2011-03-341917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bhaduri-McIntosh S, Rotenberg MJ, Gardner B, Robert M, Miller G. Repertoire and frequency of immune cells reactive to Epstein-Barr virus-derived autologous lymphoblastoid cell lines. Blood. 2008;111:1334–1343. doi: 10.1182/blood-2007-07-101907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nikiforow S, Bottomly K, Miller G. CD4+ T-Cell Effectors Inhibit Epstein-Barr Virus-Induced B-Cell Proliferation. Journal of Virology. 2001;75:3740–3752. doi: 10.1128/JVI.75.8.3740-3752.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.