Significance
Heart failure is the leading global cause of death; therefore developing a greater understanding of disease etiology and identifying novel therapeutic targets is critical. Here, we describe the role of the cyclic nucleotide-degrading protein phosphodiesterase 1C (PDE1C) in the context of pathological cardiac remodeling. In cardiac myocytes, we found that PDE1C regulates both cyclic AMP- and cyclic GMP-mediated signaling pathways under different conditions. In both isolated cells and mice we found that inhibition of PDE1C could potentiate protective signaling and prevent the development of many aspects of heart failure, potentially by signaling through multiple cell types. PDE1 inhibition therefore may represent a viable therapeutic strategy for treatment of heart failure.
Keywords: cyclic nucleotide, phosphodiesterase, cardiac remodeling, heart failure
Abstract
Cyclic nucleotide phosphodiesterase 1C (PDE1C) represents a major phosphodiesterase activity in human myocardium, but its function in the heart remains unknown. Using genetic and pharmacological approaches, we studied the expression, regulation, function, and underlying mechanisms of PDE1C in the pathogenesis of cardiac remodeling and dysfunction. PDE1C expression is up-regulated in mouse and human failing hearts and is highly expressed in cardiac myocytes but not in fibroblasts. In adult mouse cardiac myocytes, PDE1C deficiency or inhibition attenuated myocyte death and apoptosis, which was largely dependent on cyclic AMP/PKA and PI3K/AKT signaling. PDE1C deficiency also attenuated cardiac myocyte hypertrophy in a PKA-dependent manner. Conditioned medium taken from PDE1C-deficient cardiac myocytes attenuated TGF-β–stimulated cardiac fibroblast activation through a mechanism involving the crosstalk between cardiac myocytes and fibroblasts. In vivo, cardiac remodeling and dysfunction induced by transverse aortic constriction, including myocardial hypertrophy, apoptosis, cardiac fibrosis, and loss of contractile function, were significantly attenuated in PDE1C-knockout mice relative to wild-type mice. These results indicate that PDE1C activation plays a causative role in pathological cardiac remodeling and dysfunction. Given the continued development of highly specific PDE1 inhibitors and the high expression level of PDE1C in the human heart, our findings could have considerable therapeutic significance.
Heart failure, the inability of the heart to provide sufficient blood to the body, remains one of the leading global causes of death (1). Pathological cardiac remodeling plays a pivotal role in the development of heart failure and is characterized by myocyte hypertrophy and death, fibroblast activation, and extracellular matrix deposition, ultimately leading to functional defects (2). Therefore, identifying novel molecular regulators underlying the pathogenesis of cardiac remodeling is essential for the continued development of therapeutic agents. In the heart, the second messengers cyclic AMP (cAMP) and cyclic GMP (cGMP) are implicated in numerous signaling pathways, from short-term regulation of myocyte contraction/relaxation to long-term functions in growth and survival. In cardiac cells there are multiple functionally distinct pools of cyclic nucleotides. Acutely, activation of β-adrenergic receptor (β-AR) cAMP signaling is procontractile, whereas chronic β1-AR activation elicits detrimental prohypertrophic (2) and proapoptotic effects (3, 4). By contrast, adenosine-derived cAMP signaling can attenuate many of these effects (5). Moreover, cAMP produced by different adenylyl cyclases (ACs) has different cardiac effects: AC5-derived cAMP is detrimental, whereas AC6-derived cAMP is protective in pathological cardiac remodeling (6). cGMP is generated from both the soluble and particulate guanylyl cyclases (GCs), and many studies have shown negative inotropic and cardioprotective effects (7, 8) from cardiac cGMP signaling. Together, these lines of evidence indicate that different cyclic nucleotide signaling modules have distinct, unique, and often even opposing physiological roles.
Cyclic nucleotide phosphodiesterases (PDEs), by catalyzing cyclic nucleotide hydrolysis, play a critical role in regulating the amplitude, duration, and compartmentalization of cyclic nucleotide signaling. PDEs constitute a superfamily of enzymes with 22 different genes and more than 100 mRNAs grouped into 11 broad families (PDE1–PDE11) based on distinct structural, kinetic, regulatory, and inhibitory properties. It has become increasingly clear that cyclic nucleotide degradation by PDEs is not a constitutive process but rather is differentially regulated in response to varying physiological and pathological circumstances. PDEs play diverse roles in cardiac dysfunction: Chronic inhibition or reduced expression of PDE3A (4) and PDE4D (9) worsens the development of cardiac dysfunction, whereas the inhibition of PDE5 (10) and PDE9 (11) protects against it. PDE1 represents a major PDE activity in human myocardium (12), but its role in cardiac biology and disease remains elusive. Three distinct genes, PDE1A, -1B, and -1C, have been reported, with PDE1A and -1C expressed in the heart (13). PDE1A hydrolyzes cGMP with greater affinity than cAMP, whereas PDE1C hydrolyzes both similarly. We previously demonstrated that treatment with a pan-PDE1 inhibitor significantly reduced isoproterenol (ISO)-induced cardiac hypertrophy and fibrosis (13, 14), but these studies were unable to delineate the contributions of individual PDE1 isozymes to this effect. Our preliminary studies indicated that PDE1C expression is increased in failing human and mouse hearts; therefore we investigated the regulation and function of PDE1C in pathological cardiac remodeling through a combination of in vitro and in vivo approaches. Using PDE1C-deficient mice, we determined that PDE1C plays a causative role in cardiac remodeling and dysfunction induced by chronic pressure overload. In vitro, PDE1C inhibition or depletion regulated both cardiac myocyte apoptosis and hypertrophy in response to multiple stimuli in a PKA-dependent manner. Our findings may have significant therapeutic impact and could lead to the development of novel treatment strategies, such as the use of PDE1 inhibitors in treating pathological cardiac remodeling and dysfunction.
Results
PDE1C Expression in Failing Hearts.
Initially, using quantitative RT-PCR (qRT-PCR), we performed discovery screening for all PDE isoforms in mouse hearts subjected to sham operation or to transverse aortic constriction (TAC) and found that PDE1C expression was increased in TAC hearts. Therefore, we sought to investigate the regulation and function of PDE1C in cardiac disease further. We first confirmed up-regulation of PDE1C protein in mouse TAC hearts relative to sham-operated hearts (Fig. 1 A and B). We also found that PDE1C mRNA is increased in tissue from failing human hearts compared with heart tissue from healthy donors (Fig. 1C). In line with our findings, a recent RNA-sequencing study of samples from failing human hearts reported increased PDE1C expression in both ischemic heart disease and dilated cardiomyopathy (15).
Fig. 1.
PDE1C expression is increased in failing hearts. (A) Representative Western blots showing PDE1C expression in hearts from mice 8 wk after TAC or a sham operation. GAPDH is used as a control. (B) Densitometric quantification of Western blots of PDE1C normalized to GAPDH protein; n = 12 for sham-operated hearts; n = 17 for TAC hearts. (C) PDE1C mRNA levels as assessed by qPCR in heart tissue from human healthy donors or from failing hearts, normalized to GAPDH; n = 23 for tissue from healthy hearts; n = 27 for tissue from failing hearts. *P < 0.05 vs. sham. (D) PDE1C mRNA levels as assessed by qPCR in cardiac myocytes and cardiac fibroblasts isolated from PDE1C-WT and -KO mice; n = 4 for cardiac myocytes; n = 3 for fibroblasts.
Expression of PDE1C in Cardiac Cells.
To determine the cardiac cell types expressing PDE1C, we analyzed PDE1C expression in isolated adult cardiac myocytes and fibroblasts through qPCR. As shown in Fig. 1D, we found that PDE1C is highly expressed in cardiac myocytes but has negligible expression in fibroblasts.
Role of PDE1C in Cardiac Myocyte Death and Apoptosis in Vitro.
To investigate whether PDE1C plays a direct role in regulating cardiac myocyte death, we isolated cardiac myocytes from PDE1C-WT and -KO mice and induced cell death with angiotensin II (Ang II) or ISO treatment. Cell death was detected by Trypan blue staining (Fig. 2A), apoptosis was measured by TUNEL staining (Fig. 2C), and cytotoxicity was evaluated further by lactate dehydrogenase (LDH) colorimetric assay (Fig. 2E). Ang II treatment induced a significant increase in Trypan blue-positive cells in PDE1C-WT myocytes but largely failed to do so in PDE1C-KO myocytes (Fig. 2B). In addition, PDE1 inhibition via IC86340 (a pan-PDE1 inhibitor) almost completely blocked Ang II-induced cell death in PDE1C-WT myocytes but had no further effect on PDE1C-KO myocytes (Fig. 2B), suggesting that the protective effect of IC86340 is achieved primarily by inhibiting PDE1C in cardiac myocytes. Similarly, Ang II-induced apoptosis seen in PDE1C-WT cells was diminished in PDE1C-KO myocytes; IC86340 treatment again was protective only in PDE1C-WT myocytes (Fig. 2D). In line with the results produced by cell biology-based methods, the LDH release assay demonstrated that Ang II induced significant LDH release in PDE1C-WT cardiac myocytes and that this release was blocked by PDE1 inhibition or PDE1C deficiency (Fig. 2E). These findings also confirmed that Trypan blue staining is a valid method for assessing cell death in cardiac myocytes in vitro; we therefore used it for the majority of further experiments investigating cell death. In contrast to PDE1C deficiency, ectopic expression of PDE1C1 through adenoviral vectors in PDE1C-KO myocytes resensitized them to Ang II-stimulated cell death, which could be blocked by IC86340 (Fig. 2F).
Fig. 2.
Role of PDE1C in cardiac myocyte death. (A–D) Cardiac myocytes were isolated from PDE1C-WT or -KO mice, and cell death was stimulated by 200 nM Ang II in the presence of vehicle or IC86340 (15 µM) as indicated. (A) Trypan blue staining after 24 h. White arrows point to Trypan blue-positive myocytes. (B) Quantification of Trypan blue-positive cells. (C) TUNEL staining after 48 h. Nuclei were stained with DAPI (blue). White arrows point to TUNEL-positive apoptotic cells. (D) Quantification of TUNEL-positive nuclei. (E) LDH release assay of PDE1C-WT and -KO cardiac myocytes after treatment for 24 h with Ang II and IC86340 as indicated. (F) Trypan blue staining of PDE1C-KO myocytes transduced with adenovirus expressing LacZ or PDE1C1 for 24 h and stimulated with Ang II for 24 h in the presence of vehicle or IC86340. *P < 0.05 vs. control (B, D, and E) or vs. Ad-LacZ (F); †P < 0.05 vs. Ang II (B, D, and E) or vs. Ad-PDE1C/Ang II (F). n = 3–6 per study.
Furthermore, ISO-induced cell death also was inhibited dose-dependently by IC86340 (Fig. S1A), and although ISO significantly increased cell death in PDE1C-WT cardiac myocytes, it failed to do so in IC86340-treated cells or in PDE1C-KO myocytes (Fig. S1B). These findings suggest that PDE1 plays a critical role in regulating myocyte death/apoptosis and that PDE1C is the major PDE1 isoform responsible for this effect.
Fig. S1.
Effects of PDE1C inhibition and deficiency on ISO-induced cardiomyocyte death. (A) Quantification of Trypan blue staining of WT mouse adult cardiomyocytes pretreated with various doses of the PDE1 inhibitor IC86340, followed by 10 μM ISO to induce cell death. (B) Quantification of Trypan blue staining of mouse adult cardiomyocytes from PDE1C-WT and PDE1C-KO mice pretreated with vehicle or IC86340, followed by 10 μM ISO to induce cell death. *P < 0.05 vs. control; †P < 0.05 vs. ISO alone; n = 3.
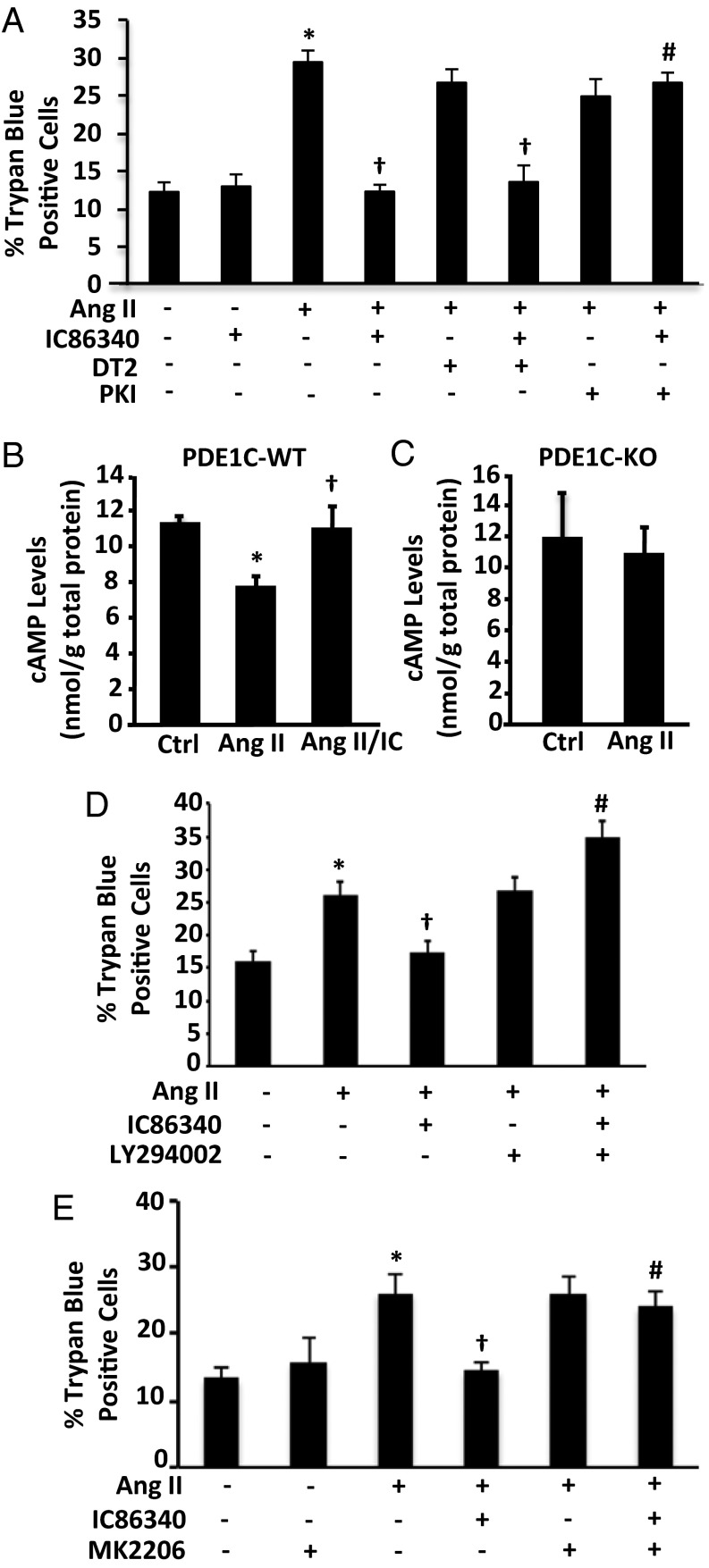
Because PDE1C hydrolyzes both cAMP and cGMP with high affinity in cell-free systems (16), we examined the effect of inhibiting the potential downstream targets, cAMP-dependent protein kinase (PKA, a downstream cAMP effector) and cGMP-dependent protein kinase (PKG, a downstream cGMP effector), on the protective effect of PDE1C inhibition. Although the PKA inhibitor PKI largely abolished the protective effect of IC86340 on Ang II-induced cell death, the peptide PKG inhibitor DT-2 (Fig. 3A) and the cGMP analog PKG inhibitor Rp-8-Br-PET-cGMPs (Fig. S2A) failed to do so. To confirm that PKI did not induce cell death independently of Ang II, we also treated PDE1C-WT cardiac myocytes with PKI alone and found that it failed to induce appreciable cell death (Fig. S2B).
Fig. 3.
Role of PKA/PKG and PI3K/AKT signaling in PDE1C-mediated regulation of cardiac myocyte death. (A) Quantification of Trypan blue staining on myocytes pretreated with the PKG inhibitor DT-2 (1 μM) or the PKA inhibitor PKI (5 μM) in addition to IC86340 (15 µM) and Ang II, as indicated, for 24 h. (B and C) cAMP levels in PDE1C-WT (B) or PDE1C-KO (C) cardiac myocytes pretreated with IC86340 and stimulated with Ang II for 15 min as indicated. (D) WT cardiac myocytes were pretreated with 15 µM IC86340, with the PI3K inhibitor LY294002 (10 nM), or with both, as indicated, followed by treatment with 200 nM Ang II for 24 h to induce cell death. (E) WT cardiac myocytes were pretreated with 15 µM IC86340, with the AKT inhibitor MK-2206 (500 nM), or with both, as indicated. *P < 0.05 vs. control; †P < 0.05 vs. Ang II; #P < 0.05 vs. Ang II/IC86340; n = 3 per experiment.
Fig. S2.
(A) Effects of PKG on PDE1C-mediated regulation of cardiac myocyte death. Quantification of Trypan blue staining on WT mouse adult cardiac myocytes pretreated with the PDE1 inhibitor IC86340 (15 μM), the PKG inhibitor Rp-8-Br-PET-cGMPs (5 μM), or both, followed by treatment with Ang II (200 nM) for 24 h to induce cell death. (B) Effects of PKI on cardiac myocyte survival. Quantification of Trypan blue staining of WT cardiac myocytes pretreated with the PKA inhibitor PKI (5 μM), followed by treatment with Ang II (200 nM) for 24 h. (C) Effects of AKT on PDE1C-mediated regulation of cardiac myocyte death. WT cardiac myocytes were pretreated with the PDE1 inhibitor I86340 (15 µM), the AKT inhibitor MK-2206 (500 nM), or both, followed by treatment with Ang II for 24 h. Medium samples were collected, and LDH levels were measured. *P < 0.05 vs. control; †P < 0.05 vs. Ang II; #P < 0.05 vs. Ang II+IC86340; n = 3.
We next investigated the role of PDE1C in regulating cAMP. Because PDE1C is a Ca2+/calmodulin (CaM)-stimulated PDE (16), we speculated that Ang II treatment could increase its activity in cardiac myocytes. We found that in PDE1C-WT myocytes Ang II induced a reduction in cAMP, which was abolished by inhibiting PDE1 with IC86340 (Fig. 3B). However, Ang II failed to reduce cAMP in PDE1C-KO myocytes (Fig. 3C). These results suggest the involvement of a cAMP-dependent mechanism in PDE1C-mediated regulation of myocyte death.
It is known that stimulation of certain Gs-coupled/cAMP-elevating receptors, such as adenosine type 2 receptors (A2R) (17) and glucagon-like peptide 1 receptors (GLP1R) (18), leads to protection against cardiac myocyte death. Several reports have indicated that PI3K/AKT is critical in mediating these protective effects (18–20). Therefore, we examined the role of PI3K/AKT in PDE1C-mediated myocyte protection. Interestingly, we found that pharmacological inhibition of either PI3K with LY294002 or of AKT with MK2206 blocked the protective effects of PDE1 inhibition as assessed by Trypan blue staining (Fig. 3 D and E) and LDH release (Fig. S2C). This finding suggests that PI3K/AKT signaling plays a critical role in PDE1C-mediated regulation of cell death.
The Role of PDE1C in Cardiac Myocyte Hypertrophy in Vitro.
We next examined whether PDE1C directly regulates cardiac myocyte hypertrophy. Isolated adult mouse cardiac myocytes from PDE1C-WT and -KO mice were stimulated with Ang II or ISO for 72 h in the presence of blebbistatin (a myosin II inhibitor) to block myocyte contraction and to extend their survival during culture (21). Myocyte hypertrophy was assessed by measuring the average cell area. In PDE1C-WT cardiac myocytes, both Ang II and ISO treatment stimulated significant cardiac myocyte hypertrophy (Fig. 4A and Fig. S3A). However, in PDE1C-KO cells, Ang II failed to stimulate hypertrophy (Fig. 4A), but ISO-induced hypertrophy was partially attenuated as assessed by an increase in cell area (Fig. S3A). Additionally, we assessed the expression of the hypertrophic marker atrial natriuretic peptide (ANP) and found that Ang II induced significant ANP elevation in PDE1C-WT cardiac myocytes but failed to do so in the PDE1C-KO cells (Fig. 4B).
Fig. 4.
The role of PDE1C in cardiac myocyte hypertrophy in vitro. (A) Cardiac myocytes were isolated from PDE1C-WT or -KO mice and stimulated with Ang II (100 nM) for 72 h. Cells then were fixed and photographed under a microscope. The cell area was averaged from n > 1,900 cells from five isolations. (B) ANP mRNA levels in PDE1C-WT and -KO cardiomyocytes treated with Ang II for 72 h as indicated; n = 3. (C) Cardiac myocytes were isolated from PDE1C-KO mice and were pretreated with the PKG inhibitor DT-2 (1 µM) or the PKA inhibitor PKI (5 µM), followed by Ang II treatment for 72 h as indicated. Cardiac myocyte areas were quantified from n > 1,000 myocytes from three isolations. *P < 0.05 vs. control; †P < 0.05 vs. + Ang II.
Fig. S3.
(A) The role of PDE1C in ISO-stimulated cardiac myocyte hypertrophy in vitro. Cardiac myocytes were isolated from PDE1C-WT or PDE1C-KO mice and stimulated with ISO (10 µM) for 72 h. Cells then were fixed, and the cell area was quantified. (B) Effects of PKA and PKG inhibitors on basal cardiac myocyte size. WT cardiac myocytes were pretreated with PKI (5 µM) or DT2 (1 µM) followed by ISO treatment for 72 h. Cells then were fixed, and the cell area was quantified. *P < 0.05 vs. control; †P < 0.05 vs. +ISO. n = 3–5 per experiment.
We next determined the role of PKA and PKG in the antihypertrophic effects of PDE1C deficiency with PKA or PKG inhibitors. Consistent with PDE1C’s role in regulating cell death, we found that inhibition of PKA by PKI attenuated the antihypertrophic effect of PDE1C deficiency in response to Ang II treatment (Fig. 4C). Interestingly, PKG inhibition with DT-2 induced hypertrophy under basal conditions in PDE1C-KO cardiac myocytes (Fig. 4C). However, Ang II treatment in combination with DT-2 failed to induce any further cell hypertrophy compared with DT-2 alone (Fig. 4C). To determine whether this effect was specific to PDE1C-KO cardiac myocytes, we also treated WT cardiac myocytes with DT-2 and PKI and found that neither treatment alone had any significant effect on cell hypertrophy (Fig. S3B). These results suggest PKA mediates the inhibitory effects of PDE1C deficiency on Ang II-stimulated myocyte hypertrophy, although it is not yet clear how DT-2 treatment alone induces myocyte hypertrophy specifically in PDE1C-KO myocytes.
PDE1C Deficiency Ameliorates TAC-Induced Cardiac Dysfunction in Vivo.
To investigate whether PDE1C plays a causative role in pathological cardiac remodeling in vivo, we subjected PDE1C-WT and -KO mice to TAC or to a sham operation as control. PDE1C-KO mice are physiologically normal aside from a defect in sensitivity and adaptation in olfactory neurons (22). We did not observe any significant difference in heart rate (Table 1) or blood pressure between PDE1C-WT and -KO mice, as is consistent with our finding that PDE1C is not expressed in normal vessels (23). Cardiac function was monitored by echocardiography throughout the study (Fig. 5A). As expected, PDE1C-WT mice experienced a time-dependent loss of contractile function, as assessed by percentage of fractional shortening (FS) and ejection fraction (EF), with progression into heart failure (FS <20%) usually occurring within 8–10 wk after surgery (Fig. 5 B and C and Table 1). However, this loss of cardiac function was markedly attenuated in PDE1C-KO mice. Furthermore, TAC-induced increases in left ventricular (LV) diameter at systole (LVID, s) and diastole (LVID, d), indicators of chamber dilation, were also drastically attenuated in PDE1C-KO mice relative to PDE1C-WT (Fig. 5 D and E).
Table 1.
Cardiac function of PDE1C-WT and -KO mice
| WT sham operated, n = 6 | WT TAC, n = 10 | PDE1C-KO sham operated, n = 5 | PDE1C- KO TAC, n = 10 | |||||
| Parameters | Baseline | 10 wk post surgery | Baseline | 10 wk post surgery | Baseline | 10 wk post surgery | Baseline | 10 wk post surgery |
| EF (%) | 81.50 ± 0.28 | 80.16 ± 0.65 | 80.95 ± 0.27 | 30.97 ± 4.56* | 80.44 ± 0.99 | 78.35 ± 0.63 | 80.32 ± 0.66 | 69.33 ± 4.73† |
| LVAW,d (mm) | 0.81 ± 0.04 | 0.75 ± 0.03 | 0.77 ± 0.02 | 1.04 ± 0.07* | 0.86 ± 0.03 | 0.90 ± 0.05 | 0.81 ± 0.02 | 1.21 ± 0.05† |
| LVAW,s (mm) | 1.20 ± 0.05 | 1.24 ± 0.01 | 1.19 ± 0.02 | 1.25 ± 0.08 | 1.25 ± 0.04 | 1.30 ± 0.03 | 1.22 ± 0.03 | 1.60 ± 0.06† |
| LVPW,d (mm) | 0.67 ± 0.02 | 0.64 ± 0.03 | 0.69 ± 0.02 | 1.06 ± 0.06* | 0.68 ± 0.03 | 0.76 ± 0.05 | 0.69 ± 0.02 | 0.99 ± 0.06 |
| LVPW,s (mm) | 1.05 ± 0.03 | 1.14 ± 0.02 | 1.07 ± 0.03 | 1.13 ± 0.07 | 1.07 ± 0.05 | 1.08 ± 0.03 | 1.05 ± 0.02 | 1.28 ± 0.07 |
| LVV,d (µL) | 38.2 ± 1.69 | 55.46 ± 4.02 | 41.38 ± 1.77 | 121.44 ± 17.78* | 42.89 ± 1.48 | 48.43 ± 5.18 | 37.65 ± 1.88 | 48.88 ± 5.83† |
| LVV,s (µL) | 7.07 ± 0.33 | 11.08 ± 1.07 | 7.90 ± 0.39 | 90.11 ± 17.21* | 8.40 ± 0.54 | 10.56 ± 1.29 | 7.48 ± 0.54 | 17.3 ± 5.18† |
| Body weight (g) | 24.37 ± 0.98 | 27.98 ± 01.04 | 25.45 ± 0.56 | 27.19 ± 0.86 | 27.06 ± 0.56 | 30.62 ± 0.78 | 24.05 ± 0.53 | 27.77 ± 0.72 |
| Heart rate (beats/min) | 551.79 ± 0.79 | 597.24 ± 7.05 | 551.27 ± 7.53 | 543.16 ± 12.54* | 546.83 ± 6.12 | 565.71 ± 7.38 | 553.10 ± 10.45 | 549.49 ± 7.60 |
LVAW, d/s: left ventricular anterior wall diameter at diastole/systole; LVPW, d/s: left ventricular posterior wall diameter at diastole/systole; LVV, d/s: left ventricular volume at diastole/systole. Values are expressed as mean ± SEM.
P < 0.05 vs. WT/sham.
P < 0.05 vs. WT/TAC.
Fig. 5.
Genetic deletion of PDE1C attenuates TAC-induced cardiac dysfunction. PDE1C-WT and PDE1C-KO mice at 8–12 wk of age were subjected to TAC or to a sham operation. Cardiac function was monitored via echocardiography at baseline and at 2, 4, 8, and 10 wk after surgery. (A) Representative M-mode echocardiographic images of each study group. (B–E) Summaries of the echocardiographic data of each group. *P < 0.05 WT TAC vs. WT sham; †P < 0.05; KO TAC vs. WT TAC. Animal numbers: PDE1C-WT sham: n = 6; PDE1C-WT TAC: n = 10; PDE1C-KO sham: n = 5; and PDE1C-KO-TAC: n = 10.
PDE1C Deficiency Attenuates TAC-Induced Cardiac Structural Remodeling in Vivo.
We next evaluated morphological and structural changes in TAC-treated hearts, including global heart size (Fig. 6A) and the ratio of ventricular weight to tibia length (Fig. 6B) or body weight (Table 1). TAC-induced increases in heart size and weight were markedly reduced in PDE1C-KO mice. The ratio of LV mass to body weight, as calculated by echocardiography, was also significantly reduced in PDE1C-KO mice (Fig. 6C). Furthermore, cardiac expression of the prohypertrophic markers ANP, B-type natriuretic peptide (BNP), and β-myosin heavy chain (β-MHC) were induced by TAC in PDE1C-WT mice but were significantly reduced in PDE1C-KO TAC mice (Fig. 6 D and F). Although we observed a considerable reduction in global cardiac hypertrophy in PDE1C-KO TAC compared with PDE1C-WT TAC mice, we did not see a reduction in either anterior or posterior LV wall thickness in PDE1C-KO mice (Table 1). This difference likely can be explained by the considerable LV dilation observed in PDE1C-WT TAC mice. Although wall thickness was similar in the two genotypes, PDE1C-WT TAC hearts had a much greater internal ventricular diameter and volume; therefore their overall heart mass was drastically increased.
Fig. 6.
PDE1C deficiency attenuates TAC-induced cardiac hypertrophy. (A) Representative H&E-stained heart cross-sections. (Scale bars: 1,000 μm.) (B) Heart weight (HW)-to-tibia length (TL) ratios in sham- and TAC-operated mice. (C) Ratio of LV mass, as assessed by echocardiography, to body weight throughout the duration of TAC studies. (D–F) ANP (D), BNP (E), and β-MHC (F) levels in the indicated ventricular samples assessed by qPCR and normalized to GAPDH. *P < 0.05 vs. PDE1C-WT sham-operated mice; †P < 0.05 vs. WT TAC-treated mice. Animal numbers: PDE1C-WT sham-operated: n = 6; PDE1C-WT TAC: n = 10; PDE1C-KO sham-operated: n = 5; and PDE1C-KO-TAC: n = 10.
We further analyzed myocyte hypertrophy specifically in vivo by assessing myocardial cross-sectional area (CSA) using wheat germ agglutinin (WGA) staining (Fig. 7A). TAC induced a significant increase in CSA in PDE1C-WT hearts, but this effect was markedly reduced in PDE1C-KO hearts (Fig. 7B). We also assessed cardiac fibrosis using Masson's trichrome staining (Fig. 7C). Although TAC-induced interstitial fibrosis was significantly reduced in PDE1C-KO hearts compared with PDE1C-WT hearts, perivascular fibrosis was induced similarly in the two genotypes (Fig. 7D), suggesting that PDE1C regulates interstitial fibrosis specifically. Furthermore, myocyte apoptosis was evaluated by double staining for the myocyte marker α-actinin and TUNEL-positive nuclei (Fig. 7E). TAC-treated PDE1C-WT hearts displayed a significant increase in cardiac myocyte apoptosis (marked with arrows in Fig. 7E) relative to sham-operated hearts, but, again, this effect was largely suppressed in PDE1C-KO hearts (Fig. 7F). To determine whether compensatory up-regulation of other PDEs occurred in response to PDE1C depletion, we also examined the expression of a number of other cAMP-hydrolyzing PDEs previously reported in the heart, including PDE1A, -2A, -3A, -3B, -4D, and -8A (Fig. S4A). We did not observe any significant changes in the expression of other PDE isozymes between PDE1C-WT and -KO hearts. We also assessed PDE activities in PDE1C-WT and -KO hearts. As expected, in WT heart, PDE1 cAMP-hydrolyzing activity was much higher in the presence of Ca2+/CaM than in the presence of EGTA, and this higher activity was largely suppressed in PDE1C-KO heart. This result indicates that PDE1C represents the major cardiac cAMP-hydrolyzing activity when Ca2+ signaling is stimulated. Although most PDE activities were similar in the two genotypes, we observed a modest increase in PDE4 activity in PDE1C-KO hearts (Fig. S4B).
Fig. 7.
Genetic deletion of PDE1C attenuates TAC-induced cardiac structural remodeling. PDE1C-WT and PDE1C-KO mice at 8–12 wk of age were subjected to TAC for 10 wk or to a sham operation. (A) Representative images of WGA staining in mouse hearts after the sham operation or TAC. (B) Cardiac myocyte hypertrophy was quantified by assessing cross-sectional cell-surface areas from WGA staining. At least 300 myocytes were quantified per heart. (C) Representative images of heart sections stained with Masson’s trichrome. Large images show interstitial fibrosis; Insets show perivascular fibrosis. Blue staining shows fibrotic areas. (D) Quantification of cardiac fibrosis. (E) Representative images of cardiac myocyte apoptosis in hearts subjected to the sham operation or TAC. Green indicates TUNEL-positive (apoptotic) nuclei, red indicates α-actinin (myocyte) staining, and blue represents DAPI staining for nuclei. TUNEL-positive nuclei occurring in α-actinin–positive cells were considered to be apoptotic cardiac myocytes (white arrows). (F) Quantification of TUNEL-positive cardiac myocytes. (All scale bars: 50 µm.) *P < 0.05 vs. WT sham; †P < 0.05 vs. WT TAC. Animal numbers: PDE1C-WT sham: n = 6 in A–F; PDE1C-WT TAC: n = 10 in A–D, and n = 8 in E and F; PDE1C-KO sham: n = 5 in A–F; PDE1C-KO-TAC: n = 9 in A and B, n = 10 in C and D, and n = 5 in E and F.
Fig. S4.
Comparison of the expression and activity of cAMP-hydrolyzing PDE isozymes in PDE1C-WT and -KO hearts. (A) mRNA levels of PDE1A, -1C, -2A, -3A, -3B, -4D, and -8A in PDE1C-WT and -KO hearts measured by qRT-PCR and normalized to GAPDH. (B) PDE activities in PDE1C-WT and -KO hearts with and without Ca2+/CaM stimulation as indicated. Results are representative of three separate experiments. *P < 0.05 vs. the corresponding WT.
The Role of Myocyte PDE1C in the Regulation of Fibroblast Activation.
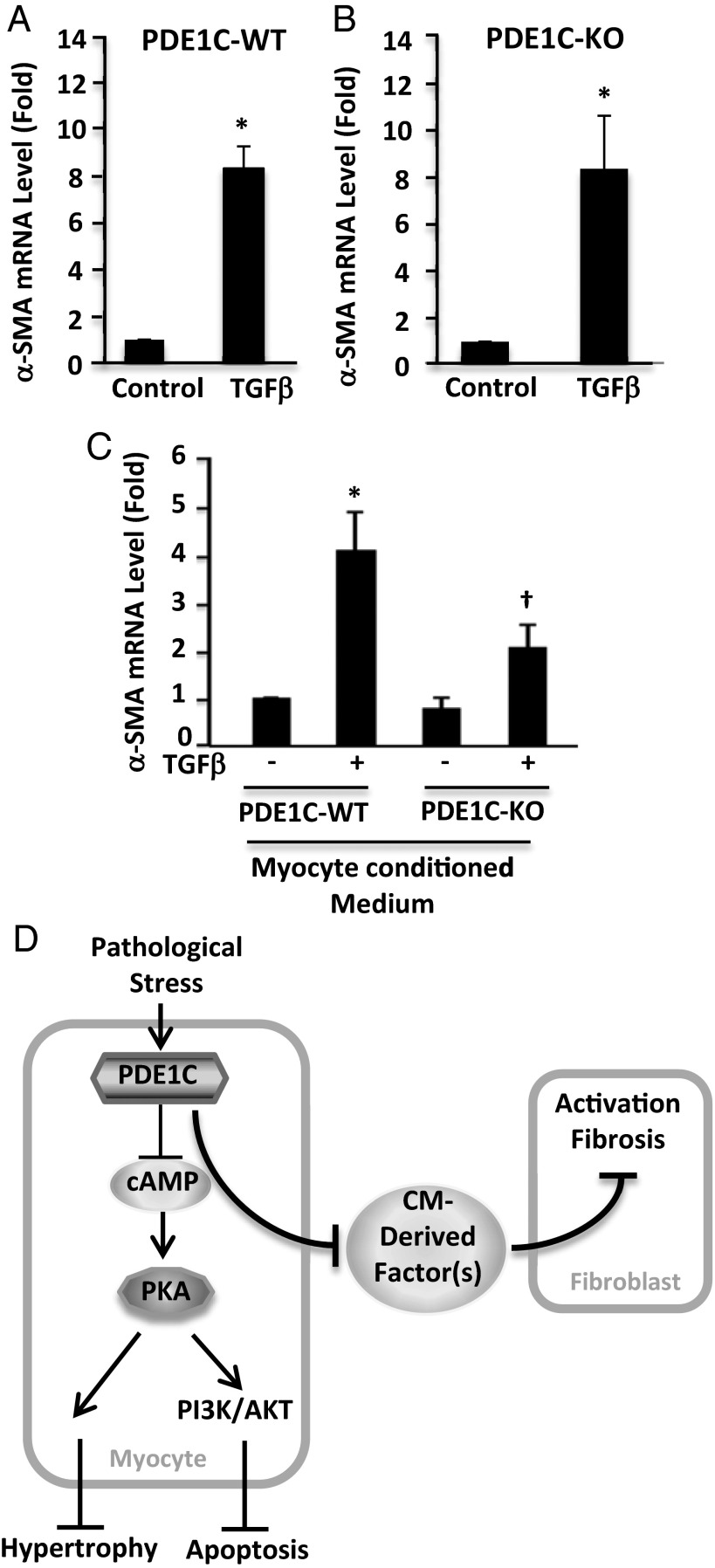
Although we failed to detect PDE1C in cardiac fibroblasts, because we observed reduced cardiac fibrosis in PDE1C-KO hearts, we examined the effect of PDE1C deficiency on TGF-β–stimulated cardiac fibroblast activation by assessing the expression of smooth muscle α-actin (α-SMA), a marker of fibroblast-to-myofibroblast conversion. We found that TGF-β treatment stimulated α-SMA expression similarly in PDE1C-WT and -KO fibroblasts (Fig. 8 A and B), further indicating that PDE1C does not function directly in cardiac fibroblasts. This result is in line with the lack of PDE1C expression in fibroblasts and myofibroblasts (Fig. 1D). Because crosstalk between myocytes and fibroblasts has been shown to be important in the development of cardiac fibrosis (24), we hypothesized that the antifibrotic effect of PDE1C deficiency seen in vivo (Fig. 7D) could be a secondary effect of PDE1C depletion in myocytes. Potentially, such an effect could be mediated by a cardiac myocyte-derived secreted factor. To test this hypothesis, we collected conditioned medium from PDE1C-WT and -KO cardiac myocyte cultures and tested whether treatment of fibroblasts with this medium could modulate their activation by TGF-β. Interestingly, TGF-β stimulation elicited a robust increase in α-SMA expression in cardiac fibroblasts treated with medium from PDE1C-WT myocytes but resulted in significantly lower α-SMA expression in fibroblasts treated with medium from PDE1C-KO myocytes (Fig. 8C). Therefore it appears likely that a cardiac myocyte-derived factor is capable of mediating fibroblast activation, perhaps accounting for the antifibrotic effects of PDE1C deficiency in vivo.
Fig. 8.
The role of myocyte PDE1C in regulating cardiac fibroblast activation. (A and B) Cardiac fibroblasts were isolated from PDE1C-WT (A) or PDE1C-KO (B) mice as indicated, serum starved for 24 h, and treated with TGF-β (10 ng/mL) for 24 h to induce activation. Fibroblast activation was assessed by α-SMA mRNA levels via qPCR. (C) Conditioned medium was collected from PDE1C-WT and -KO cardiac myocytes cultured in vitro for 1 h and were used to treat WT cardiac fibroblasts. Fibroblasts then were stimulated with TGF-β for 24 h. *P < 0.05 vs. control; †P < 0.05 vs. TGF-β with WT conditioned medium, n = 3. (D) Proposed model.
Discussion
In this study, we used genetic and pharmacological approaches both in vitro and in vivo to explore the role and mechanism of PDE1C in pathological cardiac remodeling and dysfunction. We found that PDE1C deficiency or inhibition attenuates cardiac remodeling and dysfunction by antagonizing cardiac myocyte hypertrophy and death as well as cardiac fibroblast activation (Fig. 8D). This study includes the following major findings: (i) we showed that PDE1C expression is up-regulated in failing animal and human hearts and that PDE1C-deficient mice subjected to TAC displayed attenuated myocardial hypertrophy, myocardial death, chamber dilation, and contractile dysfunction. This finding suggests that PDE1C plays a detrimental role in the development of heart failure induced by chronic pressure overload. (ii) In cardiac myocytes, PDE1C negatively regulates protective cAMP/PKA signaling that antagonizes myocyte death as well as hypertrophic growth. Activation of PI3K/AKT appears to be necessary for mediating the protective effects of PDE1C depletion on cell death. (iii) We found that although PDE1C is expressed exclusively in cardiac myocytes and not in fibroblasts, PDE1C in myocytes likely regulates myocyte production of secreted factor(s) that are important for fibroblast activation and fibrosis. Thus, PDE1C may represent a compelling therapeutic target, because inhibition of this enzyme may prevent diverse aspects of pathological cardiac remodeling in multiple cell types. In addition, we have shown previously that the PDE1A isoform inhibition attenuates cardiac myocyte hypertrophy (13) and fibroblast activation (14) largely through a cGMP-dependent mechanism. Thus, our previous and current findings may have considerable therapeutic impact, because they may lead to the development of novel therapeutic strategies using PDE1 inhibitors to combat pathological cardiac remodeling and dysfunction. Currently, a pan-PDE1 inhibitor is under development for treating schizophrenia by targeting the PDE1B isozyme in the brain (25), suggesting the potential safety of using PDE1 inhibitors as therapeutic agents. Therefore, the therapeutic effects of PDE1 inhibitors in cardiac remodeling and failure deserve further investigation in preclinical animal models.
Increasing evidence indicates that in cardiac myocytes multiple spatially and functionally distinct cAMP/PKA-signaling modules are activated through different G protein-coupled receptors (GPCRs) and regulated by different PDEs. For example, chronic stimulation of β-AR, particularly β1-AR, activates cAMP/PKA signaling, which promotes myocyte death and hypertrophy and cardiac remodeling (3, 4). In contrast, activating cAMP/PKA signaling by stimulating A2R appears to be cardioprotective. For example, A2R activation attenuated TAC-induced cardiac dysfunction (26) and prevented pathological remodeling in response to myocardial infarction (27). A2R activation also is necessary for the cardioprotective effects of ischemic postconditioning (20). Stimulating GLP1R and cAMP/PKA signaling also can prevent cardiac myocyte death (18, 28). PDE3 appears to be important in regulating β-AR–stimulated cAMP-signaling modules. Chronic inhibition of PDE3A function triggered cardiac myocyte apoptosis (4, 29), whereas myocardial overexpression of PDE3A1 prevented cardiac myocyte apoptosis and ischemia/reperfusion-induced myocardial injury (30). In this study we found that PDE1C deficiency and inhibition increased cAMP and exhibited antiapoptotic and antihypertrophic effects through a PKA-dependent mechanism. This finding suggests that PDE1C may couple specifically to a protective cAMP-signaling module in cardiac myocytes. Thus, it would be reasonable to determine in the future whether PDE1C modulates A2R- or GLP1R-mediated cAMP signaling modules or other cAMP-mediated cardiac-protective pathways. We found that PI3K or AKT inhibition blocked PDE1’s protective inhibition of cardiac myocytes’ death (Fig. 3 D and E and Fig. S2C), potentially suggesting the involvement of PI3K/AKT signaling. Although the relationship between PKA and PI3K/AKT in cardiomyocytes is not well established, PKA-mediated phosphorylation of the PI3K regulatory subunit p85α (p85αPI3K) and subsequent activation of AKT has been reported to be responsible for the prosurvival effects of cAMP in FRTL-5 thyroid (31) and MCF-7 breast cancer cells (32). Therefore, it is possible that cAMP/PKA could modulate PI3K/AKT signaling directly in cardiac myocytes as well; this possibility deserves further characterization in the future.
Genetic compensation may occur because of chronic depletion of PDE1C. To address this issue in PDE1C-deficient mice, we analyzed the levels of cardiac expression and enzyme activity for PDE1A and a number of cAMP-hydrolyzing PDEs that have been reported in the heart. We did not detect a significant change in gene expression and activity for most of these PDEs, aside from a modest increase in PDE4 activity (Fig. S4B). Increasing PDE4 activity potentially could be cardiac protective, because loss of PDE4B results in tachycardia and arrhythmias (33) and because loss of PDE4D function promotes cardiac arrhythmogenesis and heart failure (9). However, we previously found that PDE4 inhibitors did not have significant effects on cardiac myocyte apoptosis (4, 29). Therefore it is unlikely that the up-regulation of PDE4 activity seen in PDE1C-KO hearts contributes to the antiapoptotic effect of PDE1C deficiency. It is not surprising that depletion of PDE1C did not drastically alter the expression and activity of other PDE isozymes, because different PDE isozymes likely regulate multiple, discretely compartmentalized cyclic nucleotide-signaling modules. Another potential concern of this study is that the noncardiac effects of PDE1C depletion might modulate cardiac remodeling indirectly. Although we cannot rule out this possibility when using global PDE1C-KO mice, our findings obtained using isolated cardiac myocytes from PDE1C-WT and -KO mice strongly support the notion that PDE1C functions directly in cardiac myocytes. Nevertheless, the generation of mice with cardiac myocyte-specific depletion of PDE1C in the future will be helpful.
Although PDE1C hydrolyzes both cAMP and cGMP with high affinity in cell-free conditions (16), most cellular studies to date have shown that PDE1C regulates cAMP signaling primarily in cells such as proliferating vascular smooth muscle cells (23, 34) and pancreatic B cells (35). In this study we also demonstrated that PDE1C regulates cAMP levels, particularly Ang II-mediated suppression of cAMP (Fig. 3 B and C), in cardiac myocytes. This finding suggests that Ang II activates PDE1C, likely by elevating Ca2+, given that PDE1C is a Ca2+/CaM-stimulated PDE. Although the source of Ca2+-activating PDE1C is not established, possible candidates are the transient receptor potential canonical (TRPC) channels. These nonselective Ca2+ channels play an established, maladaptive role in pathological cardiac remodeling (36), and TRPC3 and TRPC6 can become activated in response to Ang II signaling via DAG production in cardiac myocytes (37) and in other cell types (38). Potentially, one of the mechanisms by which TRPC channel activation worsens pathological remodeling could be via PDE1C activation and the suppression of protective cAMP signaling, a notion that deserves future investigation. The role of PDE1C in cGMP signaling still is not clear. Surprisingly, in this study we observed a significant increased basal myocyte area in response to treatment with the PKG inhibitor DT-2 alone in PDE1C-KO, but not in PDE1C-WT, myocytes (Fig. 4C and Fig. S3B). One possible explanation is that chronic activation of PKG signaling caused by PDE1C depletion leads to compensatory overactivation of prohypertrophic signaling, which often is antagonized by PKG signaling in PDE1C-KO myocytes. DT-2 releases PKG’s inhibition of this prohypertrophic signaling and thus could induce myocyte hypertrophy. To confirm this possibility, further studies using acute PDE1C depletion/inhibition and more specific PKG blockade will be required. Many studies have reported that PKG signaling has antihypertrophic functions in cardiac myocytes (39–41); however, because of the lack of effects of cardiomyocyte PKG depletion on cardiac myocyte hypertrophy and cardiac remodeling in certain models of cardiac hypertrophy and dysfunction, this notion remains somewhat controversial (42, 43).
The precise mechanism by which PDE1C regulates cardiac fibrosis is not clear. Our finding that medium from PDE1C-KO myocytes can partially attenuate cardiac fibroblast activation may support the notion that antifibrotic paracrine signaling occurs between cardiac myocytes and fibroblasts in PDE1C-KO hearts. This notion also could account for the differences we observed between interstitial and perivascular fibrosis; because perivascular fibroblasts are localized distally to cardiac myocytes, they are less likely than interstitial fibroblasts to be significant targets of cardiac myocyte-derived paracrine signaling. Although identifying the factor(s) responsible for this antifibrotic effect remains of great interest, other explanations for our in vivo findings regarding fibrosis do exist. For example, because cardiac myocyte loss itself can lead to the replacement of dying cardiac myocytes with fibrotic tissue (44), PDE1C depletion may attenuate fibrosis simply by preventing myocyte death. Nevertheless, the exact role of PDE1C’s regulation of the crosstalk between cardiac myocytes and fibroblasts and the underlying mechanism by which it does so remain to be characterized.
Materials and Methods
Further information is available in SI Materials and Methods.
Human Samples.
Human heart tissue was obtained from the Kaufman Center for Heart Failure Tissue Bank at Cleveland Clinic, with Institutional Review Board (IRB) approval and patient consent. Failing human heart tissue was obtained from the explanted hearts of cardiac transplant recipients, and nonfailing tissue was obtained from unmatched organ donors. All tissue was procured in the operating room after cardioplegic arrest of the heart and was transported quickly to the laboratory, where it was frozen in liquid nitrogen.
Animals.
Mice with global deletion of PDE1C were kindly provided by Haiqing Zhao, Johns Hopkins University, Baltimore, (22) and were backcrossed to C57BL/6 mice for at least nine generations. All animal procedures were performed in accordance with NIH and University of Rochester institutional guidelines.
TAC.
TAC was performed on 8- to 12-wk-old male mice as described previously (4) with modifications. Briefly, mice were anesthetized with 1.5% (vol/vol) inhaled isofluorane and placed on mechanical ventilation. Left thoracotomy was performed, and a ligature was secured around the ascending aorta between the innominate and left carotid arteries, using 6-0 silk sutures tightened around a 27-gauge needle to maintain consistent ligature tension. Sham-operated mice were given a similar procedure, without actual ligation. To assess the quality and efficacy of surgery, the stenotic pressure gradient was assessed. Only TAC-operated mice that achieved a stenotic pressure gradient greater than 100 mmHg within 4 wk of TAC were included in the study.
Hearts were removed immediately after the TAC study, weighed, and divided. Apex and base portions were flash frozen and kept for protein and RNA extraction, respectively, and center sections were fixed with 4% (wt/vol) paraformaldehyde or methanol/acetic acid solution for histology. Myocyte cell-surface area was assessed via WGA staining (Thermo Fisher), fibrosis was assessed via Masson’s trichrome staining, and apoptosis was assessed via TUNEL staining (Roche). These methods are described in greater detail in SI Materials and Methods.
Echocardiography.
Cardiac function was monitored via echocardiography in anesthetized mice using a Vevo2100 echocardiography machine equipped with a 40-MHz frequency probe (VisualSonics). LV systolic and diastolic measurements were assessed in M-mode along the short axis of the LV.
Adult Mouse Cardiac Myocytes and Cardiac Fibroblasts.
Cardiac myocytes and fibroblasts were isolated as described previously (30), with modifications. Briefly, mice were anesthetized, and the heart was removed rapidly, perfused in retrograde fashion, and digested. Cardiac myocytes were separated from fibroblasts via gravity filtration and centrifugation. Myocyte hypertrophy was assessed as described previously, with modifications (13). Myocytes were cultured with the contractility inhibitor Blebbistatin, and hypertrophy was induced via treatment with ISO (10 µM) or Ang II (100 nM) for 72 h without changing medium. Myocyte cell death or apoptosis was induced by treatment for 24–48 h with either Ang II (200 nM) or ISO (10 µM) and was assessed via Trypan blue or TUNEL staining, respectively. Experiments were repeated a minimum of three times with two or three replicates per condition in each experiment. Fibroblasts were isolated with cardiac myocytes. Fibroblast activation, after serum-starvation for 24 h, was induced via TGF-β (10 ng/mL) for 24 h.
Statistics.
All data are presented as mean ± SEM. Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software). The unpaired Student’s t test was used for comparisons of two groups, and one-way ANOVA followed by Bonferroni’s post test was used for comparisons of multiple groups. P values <0.05 were considered significant.
SI Materials and Methods
Reagents.
IC86340 was provided by ICOS Corporation. Myristoylated PKI (14–22), butanedione monoxime (BDM), (-)-Blebbistatin, creatine monophosphate, taurine, Trypan blue, LY294002, and MK-2206 were purchased from Sigma. Rp-8-Br-PET-cGMPs and DT-2 were purchased from Biolog. The AlphaScreen phosphoprotein kit used to measure cAMP and cGMP was provided by PerkinElmer. The LDH Cytotoxicity Detection Kit used to measure cell death was purchased from Takara.
Primary antibodies for Western blotting include chicken anti-PDE1C antibody (23) and mouse anti-GAPDH (MAB374; Millipore). Secondary antibodies include anti-chicken IgY (A9046; Sigma) and anti-mouse IgY (NA931Vl GE Healthcare). For TUNEL counterstaining, sections were stained with mouse anti-sarcomeric α-actinin (A7811-111m; Sigma) and Alexa Fluor 546 goat anti-mouse IgG (A11030) purchased from Molecular Probes.
Human Samples.
Human heart tissue was obtained from the Kaufman Center for Heart Failure Tissue Bank at Cleveland Clinic, with IRB approval and patient consent. Failing human heart tissue was obtained from the explanted hearts of cardiac transplant recipients, and nonfailing tissue was obtained from unmatched organ donors. All tissue was procured in the operating room after cardioplegic arrest of the heart and was transported quickly to the laboratory, where it was frozen in liquid nitrogen.
Animals.
Global PDE1C-KO mice were originally generated and were provided by Haiqing Zhao, Johns Hopkins University, Baltimore (22). After being provided, these mice were backcrossed to C57BL/6 mice for at least nine generations to minimize genetic variation. All animal procedures were performed in accordance with NIH and University of Rochester institutional guidelines.
TAC.
Only male mice between 8 and 12 wk of age were used for TAC. Pressure overload was induced in mice by constricting the ascending aorta between the innominate and left carotid arteries. Anesthetized mice were placed on a heating pad, and respiration was stabilized by intubation and mechanical ventilation, with tidal volume at 225 µL at 130 breaths/min; anesthesia was maintained via 1.5% (vol/vol) inhaled isofluorane. In anesthetized mice, a left thoracotomy was performed in the second intercostal space, the heart was exposed, and aortic constriction was performed. For aortic constriction, silk thread was used with a 27-gauge needle to maintain tightness. For sham-operated mice, all procedures performed in TAC were also performed, except that silk thread and a needle were not actually placed on and tightened around the aorta. Vicryl-coated 6-0 sutures were used to close the chest cavity, and skin was sutured using 6-0 nylon. Then anesthesia was halted, and the mouse was allowed to recover before extubation. Aseptic technique was used throughout the study.
Echocardiography.
Cardiac function was assessed before surgery and 2, 4, 8, and 10 wk after surgery in sham- and TAC-operated mice. Echocardiography was monitored in anesthetized mice using a Vevo2100 echocardiography machine equipped with an MS-550D 40-MHz frequency probe (VisualSonics). LV systolic and diastolic measurements were assessed in M-mode along the parasternal short axis of the LV. Fractional shortening was calculated by the following formula: %FS = (LV end diastolic diameter − LV end systolic diameter)/(LV end diastolic diameter) × 100. The stenotic pressure gradient also was assessed to ensure the consistency and efficacy of TAC. Only TAC-operated mice that achieved a stenotic pressure gradient of greater than 100 mmHg within 4 wk of TAC were included in the study.
Mouse Heart Tissue Collection.
Once removed from mice, most hearts were divided into three sections. The apex and base (closest to the atria) were flash frozen in liquid nitrogen and were kept for protein and mRNA extraction, respectively. The center section was fixed with 4% (wt/vol) paraformaldehyde or methanol/acetic acid [60% methanol and 30% acetic acid in H2O (vol/vol)] for histological studies.
Cardiomyocyte Isolation.
Mice were anesthetized via i.p. injection of heparin and ketamine/midazolam and were killed via cervical dislocation. The heart was removed rapidly and placed in ice-cold perfusion buffer (120 mM NaCl, 15 mM KCl, 0.6 mM Na2HPO4, 0.6 mM KH2PO4, 1.2 mM MgSO4, 10 mM Hepes, 10 mM creatine monohydrate, 30 mM Taurine, 5.6 mM d-glucose, 4.6 mM NaHCO3, 10 mM BDM, filtered at 0.40 µm, pH 7.4). The heart was washed briefly to remove blood and excess fat, then was cannulated immediately using a blunted 20-gauge needle, and was mounted on a Langendorff apparatus (Radnoti) with a heating pump (VWR) that used gravity to drive buffer heated to 37 °C into the heart. The heart was initially perfused for 3 min using perfusion buffer, followed by calcium-free digestion buffer (perfusion buffer with 1.3 mg/mL collagenase II) for 3 min and then by digestion buffer with 28 nM CaCl2 for 6–10 min. Digestion was halted by removing the heart from the cannulating needle and placing it in 2.5 mL calcium-containing digestion buffer with 5 mL stopping buffer (perfusion buffer with 10% (vol/vol) FBS and 12.5 nM CaCl2). The atria and right ventricle were removed, and the left ventricle was dissociated using forceps in a Petri dish. Additional digestion buffer (10 mL) was added to the dissociated heart, which then was filtered through 200-µm mesh into a 50-mL conical tube. This tube was incubated for 5 min at 37 °C followed by 5 min at room temperature, allowing a pellet to form. The supernatant, which contained primarily cardiac fibroblasts, was retained in sterile conditions, if desired, or was discarded. The pellet with myocytes was resuspended in 10 mL of stopping buffer. The calcium concentration in this buffer was increased in a stepwise fashion, with 2-min intervals between each step: first to 112.5 nM, then to 512.5 nM, and finally to 1.4 µM. Myocytes were inspected visually; only isolations in which more than 60% of myocytes retained a rod-shaped morphology were used for experimentation. Myocytes were centrifuged at 1,000 × g for 2 min and were resuspended in plating medium [perfusion buffer with 2.5% (vol/vol) FBS, 2% (vol/vol) penicillin/streptomycin (P/S), and 1.4 µM CaCl2)]. Myocytes then were plated on dishes coated with 9–10 µg/mL Laminin, centrifuged at 1,000 × g for 2 min, and incubated in a cell-culture incubator for 1 h. The myocytes then were washed twice with PBS to remove dead cells and were incubated in myocyte culture medium [MEM with 0.2% (wt/vol) BSA, 10 mM Hepes, 4 mM NaHCO3 10 mM creatine, 0.5% insulin-selenium-transferrin, 10 mM BDM, pH 7.4] for 30 min to 1 h before treatment.
Cardiac Myocyte Hypertrophy in Vitro.
Adult mouse cardiac myocyte hypertrophy was analyzed as described previously, with modifications (45). Myocytes were cultured for 72 h in the presence of Blebbistatin, a myosin II inhibitor that prevents myocyte contraction and increases myocyte viability over longer culture durations (21). Myocytes were pretreated with myristoylated PKI (14–22) or DT-2 peptides for 30 min before hypertrophy was induced by treatment with either Ang II (100 nM) or ISO (10 µM). After 72 h cells were fixed in 4% (wt/vol) paraformaldehyde, and microscopic pictures were taken. The cell area was quantified using ImageJ. Each experimental condition was assessed three separate times, and 300 or more myocytes per isolation were quantified.
Trypan Blue Staining.
Twenty-four hours after treatment, isolated cardiomyocytes were centrifuged at 1,000 × g for 3 min. Then 1.5 mL of cardiomyocyte medium was removed carefully from each dish via pipetting, and 50 μL of 0.9% (wt/vol) Trypan blue solution was added to each dish and incubated for 3 min. Then 50 images of each dish were recorded using low-magnification microscopy. Trypan blue-positive and -negative cardiomyocytes were quantified in each image. Each condition was performed in triplicate for each experiment.
TUNEL Staining.
The TUNEL assay was performed using an In Situ Cell Death Detection Kit, Fluorescein (Roche) according to the manufacturer’s instructions, with modifications. In isolated cardiomyocytes, cells were fixed using a 60:30:10 mixture of methanol/acetic acid/dH2O at −20 °C for 15 min and were permeabilized using 1% (vol/vol) Triton-X 100/PBS for 1 h, followed by 0.5% (wt/vol) SDS/PBS for 30 min. At least 200 cardiomyocytes were counted per dish. Each experiment was repeated in at least four independent experiments, with two replicates per condition in each experiment.
LDH Release Assay.
The Takara LDH Cytotoxicity Kit was used according to the manufacturer’s instructions. Medium was removed from cell culture after 24 h. One hundred microliters of medium were used per reaction, in triplicate, and data were recorded using a Wallac Victor2 1420 Multilabel Counter (PerkinElmer).
Cardiac Fibroblast Culture and Activation.
Fibroblasts were isolated along with cardiac myocytes by centrifuging the supernatant remaining after the initial 10-min settling phase of cardiac myocytes at 2,000 × g for 5 min. This pellet was resuspended in DMEM + 10% (vol/vol) FBS + 2% (vol/vol) P/S. Fibroblasts were plated directly into 12-well plates for experimentation at a density of approximately at 5 × 104 cells/mL. Several hours were allowed for attachment; then cells were washed several times in PBS, and medium was replaced with DMEM containing 10% (vol/vol) FBS and 2% (vol/vol) P/S. Cells were cultured for 2 d and then were serum starved [DMEM + 2% (vol/vol) P/S] for 24 h before treatment. After serum starvation, cells were stimulated with TGF-β (10 ng/mL) for 24 h. Cells then were washed briefly with PBS and flash frozen for RNA extraction.
Interaction Between Cardiac Myocytes and Fibroblasts.
To prepare conditioned medium, cardiac myocytes were isolated from PDE1C-WT and -KO mice, plated at very high density (1.2 × 105 or more cells per 2.5-cm dish). Cells were plated in plating medium (see isolation protocol) but after 30 min were switched to 0.5 mL DMEM + 2% (vol/vol) P/S per plate. This medium was collected after 1 h, filtered through a 0.20-µm filter to remove cells and debris, and frozen at −80 °C until use. Medium was collected from at least four separate sets of cardiomyocytes and was pooled.
Cardiac fibroblasts to be treated with conditioned medium were isolated, cultured, and serum starved as described previously. Before TGF-β treatment, one-third of the fibroblast medium was replaced with conditioned medium [333 μL of conditioned medium into 666 μL of DMEM + 2% (vol/vol) P/S]. Adenosine receptor antagonists were added again to account for volume loss, and 30 min later TGF-β was added to cells. Fibroblasts were harvested after 24 h of TGF-β treatment and were flash frozen for later RNA extraction.
Measuring cAMP Levels by AlphaScreening.
AlphaScreen cAMP measurements were performed according the manufacturer’s protocol, with some modifications to optimize the protocol for cardiomyocytes. Briefly, cardiomyocytes were isolated from PDE1C-WT and -KO mice, plated in DMEM without phenol red, and cultured for 1–2 h before treatment. Myocytes were pretreated with IC86340 for 30 min before the experiment began. To begin the experiment, cardiomyocytes were treated with 100 nM Ang II. After 15 min of Ang II treatment, cardiomyocytes were lysed rapidly using a scraper in 20 µL lysis buffer per well and were stored at −80 °C until use. The screen was performed on a 96-well plate. The cAMP standard curve was prepared by serial dilution into lysis buffer in one row of plates. For samples, 5 µL lysate was used per replicate in each sample. Ten microliters of anti-cAMP acceptor beads then were added to each well of plate. Biotinylated cAMP-tracer (1 µM) and streptavidin donor beads (500 µg/mL) were mixed separately and were incubated at room temperature in darkness for 30 min. Ten microliters of biotinylated cAMP-tracer/streptavidin donor bead mix then were added to each well and incubated at room temperature in darkness for 1 h. The plate was read using the AlphaScreen/AlphaLISA protocol on the plate reader in the University of Rochester Medical Center High Throughput Core.
PDE Assay.
PDE assays were performed using the established radiolabeled nucleotide method (46). Ventricular tissue was collected from PDE1C-WT and -KO mice, flash frozen, and lysed in PDE assay lysis buffer [40 mM Tris⋅HCl (pH 7.5), 1 mM EDTA, 15 mM β-mercaptoethanol, 20% (vol/vol) glycerol, 1 mM Na3VO4, 100 nM Okadaic acid, Abcam protease inhibitor mixture]. cAMP–PDE activities were measured using 1 µM cAMP substrate and a trace of 3H-cAMP substrate in the presence of either 1 mM of EGTA (Ca2+/CaM independent PDE assay) or 4 µg/mL calmodulin and 0.8 mM CaCl2 (Ca2+/CaM-dependent PDE assay), as described previously (47). Briefly, PDE assay reactions were assembled on ice until initiation by the addition of radiolabeled nucleotide substrate. Reactions were incubated at 30 °C for 10 min and were terminated at 95 °C for 1 min. After cooling, 2.5 mg/mL snake venom (containing 5′-nucleotidase activity) was added to the reaction and incubated for 10 min at 30 °C. Assays were diluted with low-salt solution and transferred to pre-equilibrated ionic exchange resin columns. The radiolabeled nucleoside was eluted from the resin with low-salt solution and measured on a liquid scintillation counter. Total enzyme activity (percentage total minus background) was controlled in a linear range before each experiment. Specific PDE activities were defined as (total PDE activity) − (PDE activity in presence of specific inhibitor). PDE1-specific activity was measured in the presence of Ca2+/CaM; other activities were measured with EGTA. Activities were normalized to protein concentration. PDE inhibitors used include IC86340 (15 µM, PDE1); EHNA (10 µM, PDE2); milrinone (PDE3; 5 µM); RO-20-174 (PDE4; 20 µM); PF-04957325 (PDE8; 2 µM), and TP-10 (PDE10; 5 µM).
Western Blotting.
Western blotting was performed as described previously (13). In brief, cardiac lysates were homogenized in a modified RIPA buffer comprised of 50 mM Tris⋅HCl (pH 7.4), 1% (vol/vol) Nonidet P-40, 0.1% (wt/vol) SDS, 150 mM NaCl, 1 mM PMSF, 1 mM sodium orthovanadate, and protease inhibitor mixture (Abcam). Total protein lysates were separated using SDS/PAGE, transferred overnight to a PVDF membrane, and immunoblotted. Blocking and antibody incubations were performed in 5% (wt/vol) milk/PBS with 0.1% (vol/vol) Tween-20 (PBS-T); washes were performed with PBS-T.
RNA Isolation and Quantitative Real-Time PCR.
RNA was extracted from frozen powder of heart tissues or adherent cells using the RNeasy kit (Qiagen) according to the manufacturer’s instructions. cDNA was synthesized using a mixture of random primers and oligo(dT) primers with using either the SuperScript First Strand Synthesis Kit (Invitrogen) or the iScript cDNA synthesis kit (Bio-Rad). qPCR amplification was performed using IQ SYBR Green Supermix (Bio-Rad) according to the manufacturer’s instructions. For each reaction, 0.5 μL of cDNA was used with a 20-μL reaction volume. Each reaction was performed in duplicate. The following primer pairs were used:
Human PDE1C: 5′-GTGACTGAGCAACCATAGTGGAC-3′ (forward) and 5′-TCGCTGGACAATGTCACTCCTG-3′ (reverse)
Mouse PDE1C: 5′-TGAGAAGCCCAGGTTCAAGAG-3′ (forward) and 5′-TCGATTACAGCCGGTGGATAG-3′ (reverse)
Mouse PDE1A: 5′-TGAGCACACAGGAACAACAAAC-3′ (forward) and 5′-GTTCCGAAGATCCCTCCAGTC-3′ (reverse)
Mouse PDE2A: 5′-ACGCGCAACATTCTCTGCTTCC-3′ (forward) and 5′- TGCCACAGTAGATGGAGAAGGC-3′ (reverse)
Mouse PDE3A: 5′-ATACCTGCTCGGACTCTGAGGA-3′ (forward) and 5′- TGGCAGAGGTGGTAGTTGTCCA-3′ (reverse)
Mouse PDE4D: 5′-CACCAGCACTTAGAGGAGAAGAG-3′ (forward) and 5′-CTCTGCGTTCTCAAGGCAAAGG-3′ (reverse)
Mouse PDE8A: 5′-CCAAAGCGGTTTCCTCCAGAAC-3′ (forward) and 5′-GGACTGTTTTCCTGGGCAGCAT-3′ (reverse)
Mouse atrial natriuretic peptide (ANP): 5′-CAAGAACCTGCTAGACCACC-3′ (forward) and 5′-AGCTGTTGCAGCCTAGTCC-3′ (reverse)
Mouse brain natriuretic peptide (BNP): 5′-CCAGAGACAGCTCTTGAAGG-3′ (forward) and 5′-TCCGATCCGGTCTATCTTG-3′ (reverse)
Mouse β-myosin heavy chain (β-MHC): 5′-ACTGTCAACACTAAGAGGGTCA-3′ (forward) and 5′-TTGGATGATTTGATTTCCAGGG-3′ (reverse)
Mouse α-smooth muscle actin: 5′- GCTTCGCTGGTGATGATGCTC-3′ (forward) and 5′-AGTTGGTGATGATGCCGTGTTC-3′ (reverse)
Human GAPDH: 5′-TTGACTCCGACCTTCACCTTCC-3′ (forward) and 5′-CGCTCTCTGCTCCTCCTGTTC-3′ (reverse)
Mouse GAPDH: 5′-TCAAGAAGGTGGTGAAGCAG-3′ (forward) and 5′-TGGGAGTTGcTGTTGAAGTC-3′ (reverse)
Mouse 18S rRNA: 5′-GCTTAATTTGACTCAACACGGGA-3′ (forward) and 5′-AGCTATCAATCTGTCAATCCTGTC-3′ (reverse)
Histological and Immunohistochemical Analysis.
Histological staining was performed as described previously (14, 30). Mouse hearts were excised, washed, fixed for 48 h in either 10% (vol/vol) buffered formalin or 60% methanol/30% acetic acid/10% (vol/vol) H2O, and then were paraffin embedded and transversely sectioned (5 μm). For analysis of the cell surface area, sections were deparaffinized and subjected to heat-induced epitope retrieval (HIER) in a 10-mM citrate buffer, followed by 200 mM glycine/PBS (pH 7.4) for 3 h to quench autofluorescence. Sections then were stained with FITC-conjugated WGA (Sigma-Aldrich) at 10 μg/mL for 1 h at room temperature to visualize cell membranes. WGA-stained myocyte CSAs were calculated using NIH ImageJ software and were averaged from 200 or more myocytes per section for each animal.
Cardiac fibrosis was assessed using Masson’s trichrome stain. Briefly, slides were deparaffinized and fixed in Bouin’s fixative containing saturated picric acid, glacial acetic acid, and formalin for 1 h at 56 °C. Slides were rinsed under running water before staining with Weigert’s iron hematoxylin for 10 min and Biebrich scarlet-acid fuchsin solution for 10 min and were differentiated in phosphomolybdic-phosphotungstic acid solution for 10 min followed by staining in aniline blue solution for 5 min. Sections were rinsed briefly in distilled water and 1% (vol/vol) glacial acetic acid solution, followed by dehydration in alcohol and xylene before mounting. Fifteen to twenty randomly chosen images were recorded from each ventricle at 100× total magnification. Image-Pro software (Media Cybernetics) was used to quantify fibrosis by calculating the perivascular fibrotic, interstitial fibrotic, and nonfibrotic surface area in each image and then summarizing these parameters over each heart.
To analyze apoptotic myocytes in the heart, autofluorescence was quenched by treating 10-µm paraffin-embedded sections with 200 mM glycine/PBS for 3 h before performing TUNEL staining (Roche) following the manufacturer’s protocol and using Proteinase K treatment. Sections were counterstained for α-actinin to distinguish cardiac myocytes from other types of cells. Apoptotic myocytes were quantified in several low-magnification fields of view. For each animal, 2,000–3,500 myocytes were counted.
Statistics.
All analysis was conduced using GraphPad Prism (GraphPad), and all data are presented as mean ± SEM. For comparisons between two groups, the unpaired Student’s t test was used. For most multiple group comparisons, one-way ANOVA (as appropriate) followed by Bonferroni’s post test was used. P values <0.05 were considered significant.
Acknowledgments
This work was supported by NIH Grants HL111291 and HL088400 (to C.Y.) and HL120919 and R01 HL133761 (to E.M.S.); American Heart Association (AHA) Grant-in-Aid 12GRNT12080014 (to C.Y.); AHA Predoctoral Fellowship 12PRE12050664 (to W.E.K.); and National Heart, Lung, and Blood Institute Grant T32 5T32HL007937 (to W.E.K.). W.E.K. is the recipient of the Howard Hughes Medical Institution Med Into Grad Fellowship in Cardiovascular Science at the Aab Cardiovascular Research Institute, University of Rochester.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1607728113/-/DCSupplemental.
References
- 1.Xu J, Kochanek KD, Murphy SL, Tejada-Vera B. Deaths: Final data for 2007. Natl Vital Stat Rep. 2010;58(19):1–19. [PubMed] [Google Scholar]
- 2.Frey N, Olson EN. Cardiac hypertrophy: The good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 3.Communal C, Singh K, Sawyer DB, Colucci WS. Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis : Role of a pertussis toxin-sensitive G protein. Circulation. 1999;100(22):2210–2212. doi: 10.1161/01.cir.100.22.2210. [DOI] [PubMed] [Google Scholar]
- 4.Ding B, et al. Functional role of phosphodiesterase 3 in cardiomyocyte apoptosis: Implication in heart failure. Circulation. 2005;111(19):2469–2476. doi: 10.1161/01.CIR.0000165128.39715.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Headrick JP, Ashton KJ, Rose’meyer RB, Peart JN. Cardiovascular adenosine receptors: Expression, actions and interactions. Pharmacol Ther. 2013;140(1):92–111. doi: 10.1016/j.pharmthera.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Pierre S, Eschenhagen T, Geisslinger G, Scholich K. Capturing adenylyl cyclases as potential drug targets. Nat Rev Drug Discov. 2009;8(4):321–335. doi: 10.1038/nrd2827. [DOI] [PubMed] [Google Scholar]
- 7.Lee DI, Kass DA. Phosphodiesterases and cyclic GMP regulation in heart muscle. Physiology (Bethesda) 2012;27(4):248–258. doi: 10.1152/physiol.00011.2012. [DOI] [PubMed] [Google Scholar]
- 8.Rao YJ, Xi L. Pivotal effects of phosphodiesterase inhibitors on myocyte contractility and viability in normal and ischemic hearts. Acta Pharmacol Sin. 2009;30(1):1–24. doi: 10.1038/aps.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehnart SE, et al. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell. 2005;123(1):25–35. doi: 10.1016/j.cell.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takimoto E, et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11(2):214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 11.Lee DI, et al. Phosphodiesterase 9A controls nitric-oxide-independent cGMP and hypertrophic heart disease. Nature. 2015;519(7544):472–476. doi: 10.1038/nature14332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandeput F, et al. Cyclic nucleotide phosphodiesterase PDE1C1 in human cardiac myocytes. J Biol Chem. 2007;282(45):32749–32757. doi: 10.1074/jbc.M703173200. [DOI] [PubMed] [Google Scholar]
- 13.Miller CL, et al. Role of Ca2+/calmodulin-stimulated cyclic nucleotide phosphodiesterase 1 in mediating cardiomyocyte hypertrophy. Circ Res. 2009;105(10):956–964. doi: 10.1161/CIRCRESAHA.109.198515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller CL, et al. Cyclic nucleotide phosphodiesterase 1A: A key regulator of cardiac fibroblast activation and extracellular matrix remodeling in the heart. Basic Res Cardiol. 2011;106(6):1023–1039. doi: 10.1007/s00395-011-0228-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, et al. MAGNet consortium RNA-Seq identifies novel myocardial gene expression signatures of heart failure. Genomics. 2015;105(2):83–89. doi: 10.1016/j.ygeno.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan C, et al. Molecular cloning and characterization of a calmodulin-dependent phosphodiesterase enriched in olfactory sensory neurons. Proc Natl Acad Sci USA. 1995;92(21):9677–9681. doi: 10.1073/pnas.92.21.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5(3):247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54(1):146–151. doi: 10.2337/diabetes.54.1.146. [DOI] [PubMed] [Google Scholar]
- 19.Solenkova NV, Solodushko V, Cohen MV, Downey JM. Endogenous adenosine protects preconditioned heart during early minutes of reperfusion by activating Akt. Am J Physiol Heart Circ Physiol. 2006;290(1):H441–H449. doi: 10.1152/ajpheart.00589.2005. [DOI] [PubMed] [Google Scholar]
- 20.Morrison RR, Tan XL, Ledent C, Mustafa SJ, Hofmann PA. Targeted deletion of A2A adenosine receptors attenuates the protective effects of myocardial postconditioning. Am J Physiol Heart Circ Physiol. 2007;293(4):H2523–H2529. doi: 10.1152/ajpheart.00612.2007. [DOI] [PubMed] [Google Scholar]
- 21.Kabaeva Z, Zhao M, Michele DE. Blebbistatin extends culture life of adult mouse cardiac myocytes and allows efficient and stable transgene expression. Am J Physiol Heart Circ Physiol. 2008;294(4):H1667–H1674. doi: 10.1152/ajpheart.01144.2007. [DOI] [PubMed] [Google Scholar]
- 22.Cygnar KD, Zhao H. Phosphodiesterase 1C is dispensable for rapid response termination of olfactory sensory neurons. Nat Neurosci. 2009;12(4):454–462. doi: 10.1038/nn.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai Y, et al. Role of cAMP-phosphodiesterase 1C signaling in regulating growth factor receptor stability, vascular smooth muscle cell growth, migration, and neointimal hyperplasia. Circ Res. 2015;116(7):1120–1132. doi: 10.1161/CIRCRESAHA.116.304408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sassi Y, et al. Cardiac myocyte-secreted cAMP exerts paracrine action via adenosine receptor activation. J Clin Invest. 2014;124(12):5385–5397. doi: 10.1172/JCI74349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anonymous Deal watch: Intra-Cellular Therapies and Takeda to develop PDE1 inhibitors for schizophrenia. Nat Rev Drug Discov. 2011;10(5):329. doi: 10.1038/nrd3438. [DOI] [PubMed] [Google Scholar]
- 26.Hamad EA, et al. Cardioprotection of controlled and cardiac-specific over-expression of A(2A)-adenosine receptor in the pressure overload. PLoS One. 2012;7(7):e39919. doi: 10.1371/journal.pone.0039919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wakeno M, et al. Long-term stimulation of adenosine A2b receptors begun after myocardial infarction prevents cardiac remodeling in rats. Circulation. 2006;114(18):1923–1932. doi: 10.1161/CIRCULATIONAHA.106.630087. [DOI] [PubMed] [Google Scholar]
- 28.Chang G, et al. Cardioprotective effects of exenatide against oxidative stress-induced injury. Int J Mol Med. 2013;32(5):1011–1020. doi: 10.3892/ijmm.2013.1475. [DOI] [PubMed] [Google Scholar]
- 29.Ding B, et al. A positive feedback loop of phosphodiesterase 3 (PDE3) and inducible cAMP early repressor (ICER) leads to cardiomyocyte apoptosis. Proc Natl Acad Sci USA. 2005;102(41):14771–14776. doi: 10.1073/pnas.0506489102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oikawa M, et al. Cyclic nucleotide phosphodiesterase 3A1 protects the heart against ischemia-reperfusion injury. J Mol Cell Cardiol. 2013;64:11–19. doi: 10.1016/j.yjmcc.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Gregorio G, et al. The p85 regulatory subunit of PI3K mediates TSH-cAMP-PKA growth and survival signals. Oncogene. 2007;26(14):2039–2047. doi: 10.1038/sj.onc.1210011. [DOI] [PubMed] [Google Scholar]
- 32.Di Zazzo E, et al. The p85 regulatory subunit of PI3K mediates cAMP-PKA and insulin biological effects on MCF-7 cell growth and motility. ScientificWorldJournal. 2014;2014:565839. doi: 10.1155/2014/565839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leroy J, et al. Phosphodiesterase 4B in the cardiac L-type Ca²⁺ channel complex regulates Ca²⁺ current and protects against ventricular arrhythmias in mice. J Clin Invest. 2011;121(7):2651–2661. doi: 10.1172/JCI44747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai Y, et al. Cyclic nucleotide phosphodiesterase 1 regulates lysosome-dependent type I collagen protein degradation in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2011;31(3):616–623. doi: 10.1161/ATVBAHA.110.212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han P, Werber J, Surana M, Fleischer N, Michaeli T. The calcium/calmodulin-dependent phosphodiesterase PDE1C down-regulates glucose-induced insulin secretion. J Biol Chem. 1999;274(32):22337–22344. doi: 10.1074/jbc.274.32.22337. [DOI] [PubMed] [Google Scholar]
- 36.Wu X, Eder P, Chang B, Molkentin JD. TRPC channels are necessary mediators of pathologic cardiac hypertrophy. Proc Natl Acad Sci USA. 2010;107(15):7000–7005. doi: 10.1073/pnas.1001825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onohara N, et al. TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J. 2006;25(22):5305–5316. doi: 10.1038/sj.emboj.7601417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ilatovskaya DV, et al. Angiotensin II has acute effects on TRPC6 channels in podocytes of freshly isolated glomeruli. Kidney Int. 2014;86(3):506–514. doi: 10.1038/ki.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fiedler B, et al. Inhibition of calcineurin-NFAT hypertrophy signaling by cGMP-dependent protein kinase type I in cardiac myocytes. Proc Natl Acad Sci USA. 2002;99(17):11363–11368. doi: 10.1073/pnas.162100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frantz S, et al. Stress-dependent dilated cardiomyopathy in mice with cardiomyocyte-restricted inactivation of cyclic GMP-dependent protein kinase I. Eur Heart J. 2013;34(16):1233–1244. doi: 10.1093/eurheartj/ehr445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blanton RM, et al. Protein kinase g iα inhibits pressure overload-induced cardiac remodeling and is required for the cardioprotective effect of sildenafil in vivo. J Am Heart Assoc. 2012;1(5):e003731. doi: 10.1161/JAHA.112.003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lukowski R, et al. Cardiac hypertrophy is not amplified by deletion of cGMP-dependent protein kinase I in cardiomyocytes. Proc Natl Acad Sci USA. 2010;107(12):5646–5651. doi: 10.1073/pnas.1001360107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patrucco E, et al. Roles of cGMP-dependent protein kinase I (cGKI) and PDE5 in the regulation of Ang II-induced cardiac hypertrophy and fibrosis. Proc Natl Acad Sci USA. 2014;111(35):12925–12929. doi: 10.1073/pnas.1414364111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piek A, de Boer RA, Silljé HH. The fibrosis-cell death axis in heart failure. Heart Fail Rev. 2016;21(2):199–211. doi: 10.1007/s10741-016-9536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murray DR, et al. β2 adrenergic activation induces the expression of IL-18 binding protein, a potent inhibitor of isoproterenol induced cardiomyocyte hypertrophy in vitro and myocardial hypertrophy in vivo. J Mol Cell Cardiol. 2012;52(1):206–218. doi: 10.1016/j.yjmcc.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beavo JA, Hardman JG, Sutherland EW. Hydrolysis of cyclic guanosine and adenosine 3′,5′-monophosphates by rat and bovine tissues. J Biol Chem. 1970;245(21):5649–5655. [PubMed] [Google Scholar]
- 47.Kim D, et al. Upregulation of phosphodiesterase 1A1 expression is associated with the development of nitrate tolerance. Circulation. 2001;104(19):2338–2343. doi: 10.1161/hc4401.098432. [DOI] [PubMed] [Google Scholar]