Significance
Freshwaters rarely factor into global prioritization of fishery resources, partly because available spatial data on inland fisheries are coarse. Here, we develop a map of the world’s riverine fisheries and compare catches to fish diversity, ecosystem threats, and nutritional dependence. Fish species richness and ecoregional catches are positively but not causally correlated. Intensive harvests from the most biodiverse rivers raise conservation concerns, and numerous other stressors also threaten high-yield river fisheries. In human terms, poor nations depend far more on freshwater fisheries than aquaculture or marine sources. This intersection of poverty, food insecurity, biodiversity, and ecosystem threats suggests an urgent need to improve fishery management for the benefit of both humans and fishes.
Keywords: fish diversity, subsistence fishery, fishing pressure, rivers, ecosystem services
Abstract
Fisheries are an essential ecosystem service, but catches from freshwaters are often overlooked. Hundreds of millions of people around the world benefit from low-cost protein, recreation, and commerce provided by freshwater fisheries, particularly in regions where alternative sources of nutrition and employment are scarce. Here, we derive a gridded global map of riverine fisheries and assess its implications for biodiversity conservation, fishery sustainability, and food security. Catches increase with river discharge and human population density, and 90% of global catch comes from river basins with above-average stress levels. Fish richness and catches are positively but not causally correlated, revealing that fishing pressure is most intense in rivers where potential impacts on biodiversity are highest. Merging our catch analysis with nutritional and socioeconomic data, we find that freshwater fisheries provide the equivalent of all dietary animal protein for 158 million people. Poor and undernourished populations are particularly reliant on inland fisheries compared with marine or aquaculture sources. The spatial coincidence of productive freshwater fisheries and low food security highlights the critical role of rivers and lakes in providing locally sourced, low-cost protein. At the same time, intensive fishing in regions where rivers are already degraded by other stressors may undermine efforts to conserve biodiversity. This syndrome of poverty, nutritional deficiency, fishery dependence, and extrinsic threats to biodiverse river ecosystems underscores the high stakes for improving fishery management. Our enhanced spatial data on estimated catches can facilitate the inclusion of inland fisheries in environmental planning to protect both food security and species diversity.
Global strategies for environmental conservation strive to protect both biodiversity and ecosystem services (1, 2). Prioritizing among the vast number of sites in need of conservation efforts is most straightforward when biodiversity and ecosystem services show similar spatial distributions (3), enabling on-the-ground efforts to further both goals. In freshwater ecosystems, high threat levels (4, 5) create a pressing need to understand whether spatial variation in ecosystem services such as fisheries match patterns of biodiversity. However, the coarse spatial resolution of the available statistics on freshwater fisheries (6) has precluded rigorous assessment of their environmental influences, extrinsic stressors, and nutritional importance. As a result, inland catches have been overlooked in most global fishery analyses despite evidence that rivers and lakes are a vital source of local protein (7, 8) that would be difficult to replace (9, 10).
Reported worldwide catch from inland waters continues to rise due to growth of Asian and African fisheries, in sharp contrast to flat or declining harvests from oceans (11, 12). These increases are driven largely by restocking and enhancement programs; fisheries that depend on natural reproduction generally show declines (13). However, as in small-scale marine fisheries (8, 11, 14), it is likely that rivers and lakes have experienced many unrecognized local fishery collapses (15–17). Furthermore, degradation of aquatic ecosystems from habitat alteration, chemical pollution, species invasions (4), and climate change (18) continues to expand in freshwaters worldwide. Thus, targeting regions where both biodiversity and fish catches are high could help to maximize the benefits of global conservation efforts.
Ecological theory and experiments suggest that more species-rich ecosystems often show higher productivity (19), but this pattern has rarely been examined for upper trophic levels in natural systems at the global scale (20). Diversity–productivity relationships have not been tested for inland fisheries, but analyses of Pacific salmon stocks (21) and marine ecoregions (22) suggest that fish diversity can boost the predictability and magnitude of catches via a portfolio effect. Whether mechanisms such as synergistic interspecific interactions, probabilities of including inherently productive species, and statistical averaging of performance within assemblages are potent enough to engender a positive diversity–productivity relationship when comparing fish catches among disparate faunas and fishing cultures remains unknown.
There is also growing concern that underestimating the contribution of freshwater fisheries to food security leads to discounting them in resource management decisions (10, 12, 23). Even in regions where the nutritional importance of freshwater fisheries is well appreciated by local and national governments, catch statistics are often underestimates by a factor of 2 or more (8, 23) to say nothing of the employment and commerce derived from these fisheries (7, 8). Improving the spatial resolution and accuracy of freshwater fishery statistics is requisite to assessing whether catches from rivers and lakes merit greater weight in policy setting and cost–benefit analyses than they presently receive.
Here, we derive a gridded global map of estimated fish catches from the world’s rivers by using a high-resolution river flow network to downscale national statistics from the Food and Agriculture Organization of the United Nations (FAO) (11) by several orders of magnitude. This map enables us to quantify the relationship of riverine fish catches to fish diversity, ecosystem threat levels, and human nutritional needs. Together, these analyses clarify the critical role of freshwater fisheries in global efforts to both conserve biodiversity and enhance food security.
Results and Discussion
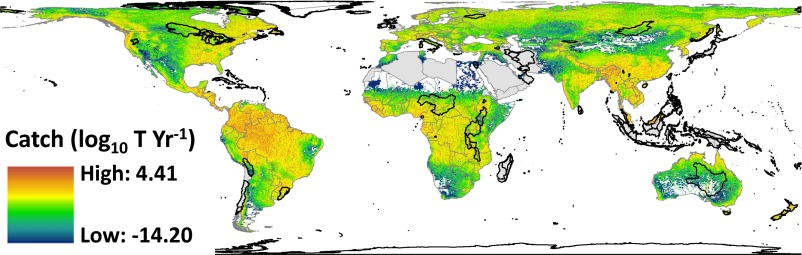
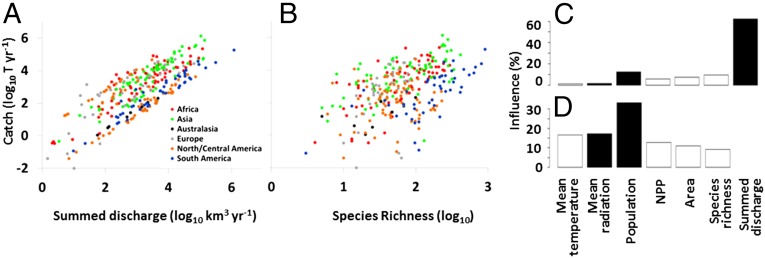
We find striking patterns of spatial variation in riverine fish catches. Our global map of estimated catches highlights the importance of fisheries from major tropical rivers, particularly those with extensive floodplains such as the Mekong, Amazon, and Niger (Fig. 1). Low catch is estimated even for large rivers in the United States and Europe, likely due to lack of reporting of recreational catches to the FAO (24). To evaluate environmental controls on fish catch, we tested the explanatory power of river discharge, ecoregional fish species richness, solar insolation, terrestrial primary productivity, air temperature, ecoregion area, and human population. Catch increases most clearly with river discharge and human population, whereas fish species richness has a small negative effect after accounting for other factors (Fig. 2). Many predictors are themselves correlated with discharge, so we also analyzed the partial dependence of catch on other variables after removing discharge effects on both (Fig. 2D). That analysis confirms the association between human population and catch, the absence of significant positive effects of fish species richness, and the minor influence of ecosystem energy. Similar results emerged from a Bayesian variable selection approach, both with and without incorporating spatial autocorrelation (Supporting Information).
Fig. 1.
Gridded global map of estimated riverine fish catches at 6-arcmin (∼10-km) resolution. Catch is modeled based on discharge and constrained using national statistics. Blank space represents areas with negligible overland flow, gray indicates nations that lack reliable catch data, and black outlines indicate ecoregions where catch can be estimated but dominance of lake or marine fishes required exclusion from testing environmental predictors.
Fig. 2.
Relationships of estimated riverine fish catch to river discharge (A) and fish species richness (B) across freshwater ecoregions. Hierarchical variance partitioning was used to estimate effects of each environmental factor when discharge is included in the model (C) or removed statistically (D). Bars indicate the magnitude of positive (solid) or negative (open) effects.
Steep scaling of fish catch with river discharge (Figs. 2A and Fig. S1) suggests that water abstraction and climate change are likely to have disproportionately large effects on riverine fisheries. Projected changes in global climate through 2100 are not expected to alter mean annual discharge in most major rivers, but reduced (>10%) minimum flows and increased (>4%) peak flows are expected for many intensively fished rivers such as the Yangtze, Mekong, Zambezi, and Ganges (18). These shifts in flow seasonality could strongly affect fisheries by altering spawning and feeding migrations, floodplain accessibility, and low-flow refugia (25). Elevated mean and peak water temperatures are expected in all major fishery rivers by 2100 (26), which could alter both body size and geographic range of their fish species (27).
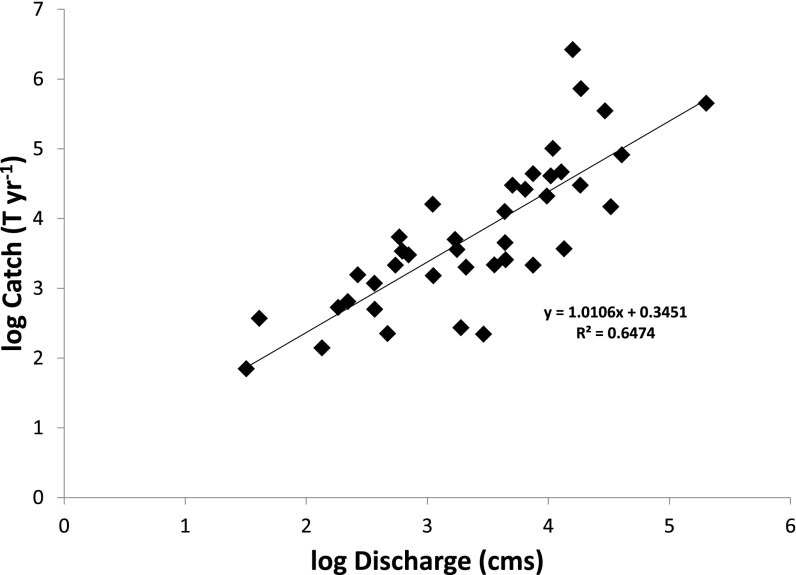
Fig. S1.
Calibration of catch distribution model. Annual catch (C) increases as a power function of mean annual discharge (Q), and both linearized and untransformed equations are indicated (R2 = 0.64). The relationship was fitted using reduced major axis regression to account for considerable uncertainty in both C and Q. Data are provided in Dataset S1.
The overall positive global correlation between ecoregional species richness and riverine catches is intriguing (Fig. 2B), even though our analyses provide no evidence that fish diversity boosts fishery productivity in a causal sense (Fig. 2 C and D). Ecological theory and small-scale experiments in aquatic systems predict positive effects of species richness on productivity at all trophic levels (19, 20, 28). Fish polycultures in stocked ponds, for example, can outperform monocultures (29), and a positive correlation between fish harvest and fish richness in large marine ecosystems was originally interpreted as evidence of a diversity–productivity relationship in fisheries (22). However, when the influence of marine fish richness was tested alongside fishing effort and environmental characteristics, its influence on fish catches was negligible (30). Our analysis indicates a similar pattern in rivers. In addition, we found no evidence that the long-term stability of riverine catches increases with fish diversity (Supporting Information), as is predicted by theory (28) and evidenced by Alaskan salmon populations (21).
Despite the lack of causality, the positive relationship between species richness and capture fisheries in the world’s rivers has profound implications for biodiversity conservation. Fishing pressure is most intense in regions where the most fish species are potentially impacted. Intensive fishing disproportionately affects large species (15, 31) and alters fundamental processes in freshwater ecosystems (32, 33). The annual removal of enormous fish biomass from rivers such as the Mekong, Ganges, and Amazon is sure to affect food web structure and fish population dynamics (15). Cascading effects on other animals, ecosystem productivity, and downstream fluxes of materials are also likely (33, 34). Moreover, the diverse capture methods used in freshwaters often lead to impacts across the full spectrum of fish sizes (15, 32, 34). Simulation models suggest that distributing fishing pressure across all body sizes may reduce extirpations and maximize catch relative to harvestable biomass (34). However, actively managing diffuse, small-scale freshwater fisheries for balanced harvest of all species would likely prove even more unwieldy than current unsuccessful mandates to avoid overharvest of favored large species.
We were surprised to find little support for ecosystem energy effects on fishery productivity in rivers, contrary to evidence from marine fisheries (30, 35) and lakes (36). In oceans and lakes, spatial patterns of primary production—and thus potential fish production—can be inferred from remote sensing of phytoplankton. No analogous ecosystem productivity metric exists for rivers. The available proxies for energy available to riverine food webs (terrestrial net primary productivity and solar insolation) vary widely among ecoregions yet have negligible statistical influence compared with discharge and human population (Fig. 2). Nutrient availability or other environmental factors may obscure the influence of energy inputs, as could the complex mixture of autochthonous and allochthonous resources that support riverine fishes (37). Given that our map of estimated fishery yields in the world’s rivers relies on catch reporting, the apparent lack of energy effects in our analysis could also reflect mediation of riverine fishing effort by regional population size (Fig. 2 C and D), cultural practices, and nutritional needs.
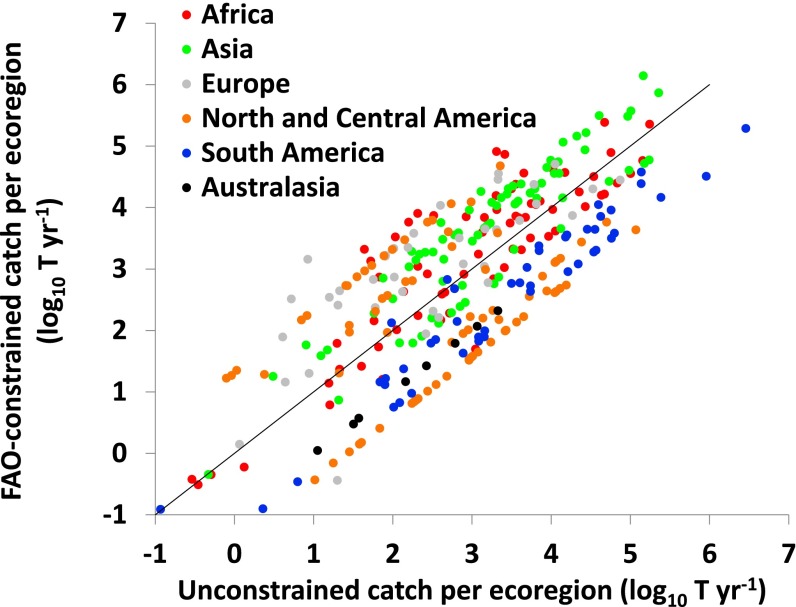
Our discharge-based model of riverine catches also enables exploratory analysis of where yields are higher or lower than expected (Fig. S2). When we calculate expected catches based on the available data from productive river fisheries (40 watersheds on five continents; Supporting Information; Dataset S1) without constraining total catch to match the FAO’s national statistics, it appears that the intensity of riverine fishery exploitation varies widely. Lower than expected catches reported in the Americas and Europe may suggest additional production capacity, and our analysis (Fig. S2) supports earlier suggestions (12, 15) that Africa and especially Asia are likely fully exploited or overexploited already. Inferring potential overharvest when reported yields exceed expectations may even be conservative given widespread underreporting of subsistence and recreational catches (8, 12, 24).
Fig. S2.
Relationship between catch estimates that were constrained by FAO national statistics vs. potential (i.e., unconstrained) catches for ecoregions listed in Dataset S2 (n = 316; gray line indicates 1:1 relationship).
Catches that greatly exceed expectations based on river discharge probably cannot be supported in the long term. Even coarse estimates of the primary productivity required to support freshwater catches reported two decades ago suggested unsustainable harvest globally (31). Recent analyses of marine fishery sustainability in terms of embodied primary productivity indicate widespread overfishing (35), but lack of systematic data on river ecosystem energetics precludes performing a similar analysis using our map of estimated catches. Instead, shifts in catch size structure and species composition in intensive fisheries of Asian and African rivers have generally been interpreted from a demographic standpoint (15, 38). Although data on fish demography and ecosystem energetics are inadequate to support rigorous analyses of the production potential of multispecies subsistence fisheries in rivers globally, models indicate that fast-growing, small-bodied species can support high yields even under intense fishing pressure (38).
Our analysis is rooted in the only comprehensive global statistics on freshwater fisheries but remains constrained by data limitations. Catch statistics are submitted voluntarily to FAO by national governments, leading to biases toward large rivers, population centers, and commercial fisheries (13, 23). Actual catch in many regions is underreported by 100–200% (8, 13, 24). Nonetheless, our catch estimates are broadly consistent with independent reports (Fig. S3), and including latitude and floodplain extent did not improve our catch downscaling model. Further progress must await better global data on freshwater fisheries, including species composition, fish size, and location data like those available for large marine fisheries. For instance, the prevalence of unidentified taxa in the FAO database precludes analyses of whether broad increases in yield with ecoregional fish richness (Fig. 2) arise from balanced harvest across more species or greater production of just a few species—a key issue for guiding effective management.
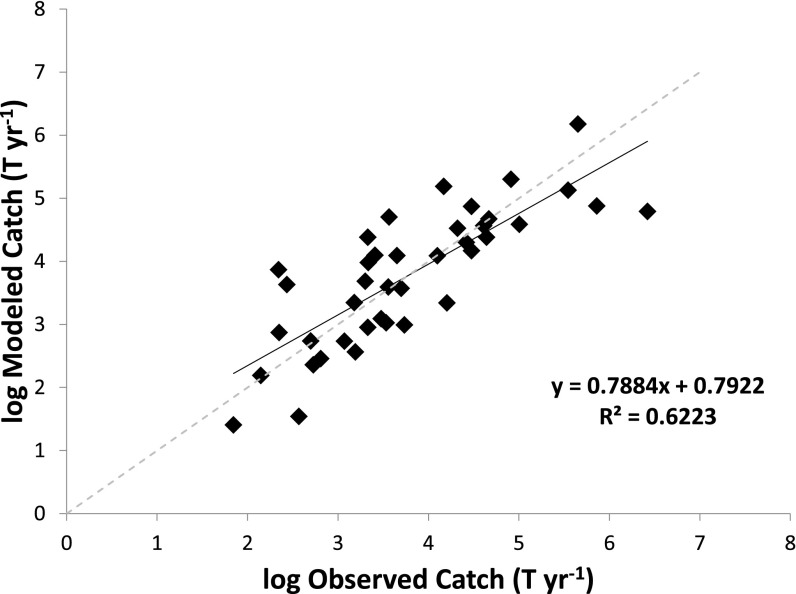
Fig. S3.
Relationship between observed and modeled catch data for river basins listed in Dataset S1 (n = 40). The dashed line indicates a 1:1 relationship; the solid line and equation indicate the fitted relationship.
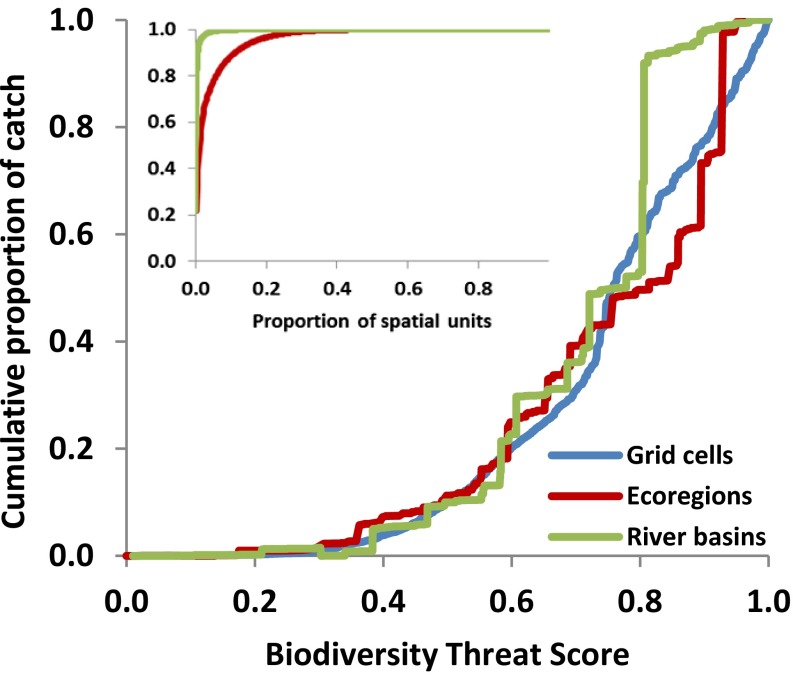
Matching our map of estimated riverine catches to an index of cumulative anthropogenic threats to river ecosystems at the same scale (ref. 4; recalculated to exclude fishing pressure as a stressor) indicates severe extrinsic stress on this ecosystem service (Fig. 3). Patterns of threat and fish catch show disconcerting parallels in eastern and southern Asia as well as western Africa, southern North America, and parts of southern Europe (Fig. S4). Worldwide, 89% of total catch is from ecoregions experiencing above-average threat, 57% of catch is from those in the upper 25% of threat, and 27% of catch is from those in the top 10% of threat. These figures are consistent whether evaluated at the grid cell, ecoregion, or river basin scale. Indeed, just 10 river basins and 20 ecoregions contribute 75% of estimated global catch (Fig. 3). Given that fishery production relies upon river ecosystem functioning, the ongoing degradation of rivers may undercut future yields.
Fig. 3.
Global riverine fish catch relative to a biodiversity threat index at three spatial scales: grid cells, ecoregions, and river basins. Lines show cumulative proportion of total catch as threat levels increase. The threat index accounts for physical, chemical, and biological alteration of rivers and their watersheds, and is modified from ref. 4 by excluding fishing pressure. The Inset illustrates the cumulative distribution of global catch across ecoregions and rivers in rank order.
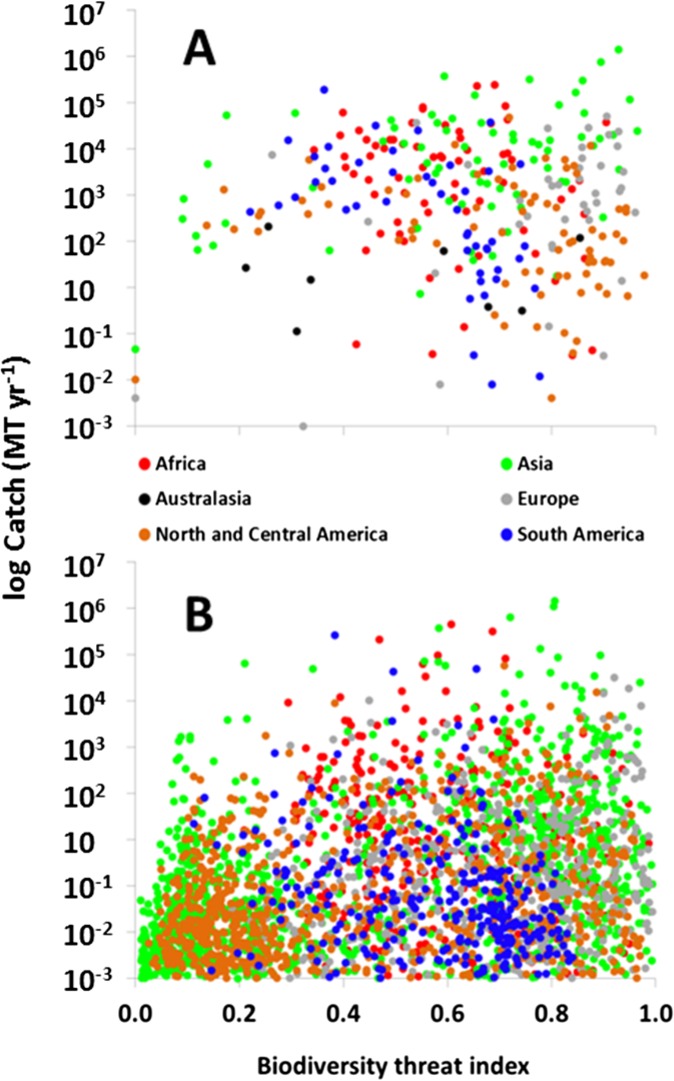
Fig. S4.
Riverine fish catch relative to a biodiversity threat index at (A) ecoregional (49) (n = 316; Dataset S2) and (B) river basin (54) (n = 4,021) scales. The threat index is modified from ref. 4 by excluding fishing pressure.
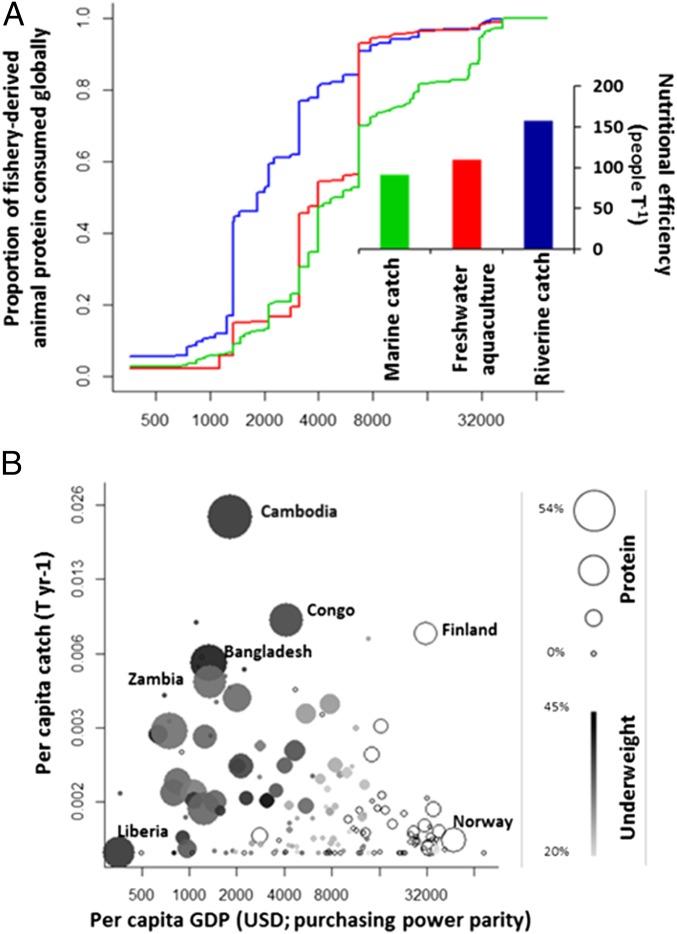
Joint analysis of fish consumption and economic status indicates that the world’s poor and malnourished rely heavily upon freshwater fisheries (Fig. 4). To account for enormous variation in diet and wealth among nations, we created an index of nutritional dependence on fisheries based on their proportional role in total animal protein consumption by the population of each country. Partitioning nutritional dependence among wild-caught freshwater, wild-caught marine, and freshwater aquaculture-derived fish in each nation indicates that wild fish from rivers and lakes provide the equivalent of the total animal protein consumption of 158 million people worldwide. Eighty-one percent of nutritional dependence on freshwater fisheries occurs in nations below global median gross domestic product (GDP) (<$4,800 purchasing power per capita annually; Fig. 4A), where alternative animal protein sources may be largely unaffordable. In contrast, a far higher proportion of nutritional dependence on aquaculture and marine fisheries arises in comparatively prosperous nations where additional sources of animal protein are more accessible, and fisheries may be valued more for recreation or export revenue than for local consumption.
Fig. 4.
Global nutritional dependence and catch rates from inland fisheries relative to national GDP per capita. (A) Dependence on fish for dietary animal protein occurs at lower GDP for freshwater fisheries than aquaculture or marine fisheries. Nutritional efficiency—the number of people whose animal protein consumption is met per ton of fish protein eaten—is highest for freshwater fish (Inset). (B) Catch per capita varies widely with GDP, as does the proportion of animal protein derived from freshwater fish (indicated by bubble size; 0–54% across 154 nations) and children <5 y of age who are underweight (indicated by bubble shading; 20–45% across 90 nations; open bubbles indicate no data). Economic and nutritional data were drawn from the FAO (11, 46) and World Bank (54).
Comparing nutritional dependence to total harvests from each fish source reveals the remarkable nutritional efficiency of inland fisheries. Each ton of inland catch supports the total annual consumption of animal protein by 157 people, representing 72% and 43% higher nutritional efficiency than marine fisheries and aquaculture, respectively (Fig. 4A). This disparity arises from the combination of low animal protein consumption and high reliance on fish in developing nations. Catches of inland fish generally decline with national GDP; aside from Finland, very high per capita catches are reported primarily in poor nations of Asia and Africa (Fig. 4B). Moreover, most nations that depend on freshwater fishing also experience high rates of malnourishment (Fig. 4B). Taking these findings together, inland fishery declines could have disastrous implications for hundreds of millions of people who have low-protein diets, are disproportionately reliant upon fish from rivers and lakes, and cannot afford alternative sources of animal protein.
The broad concordance of global spatial patterns of fish species richness, environmental stressors, and human poverty with inland fishery yields highlights the importance of conservation efforts for both human well-being and aquatic biodiversity. Our analyses suggest that the sustainability of this critical ecosystem service is jeopardized by broader environmental degradation, and that current intensive harvests impact the most species-rich rivers. Despite their critical role in food security and recreation, inland fisheries are rarely explicitly included in cost–benefit analyses of development projects in rivers (hydropower, navigation) and their watersheds (forestry, mining, urbanization), particularly in developing nations (39). The increased spatial resolution of estimated catches captured in our global map enables analyses at a wide variety of scales and for diverse purposes, removing a major barrier to accounting for the value of this ecosystem service to society. However, important limitations arise from both the underlying FAO statistics and our downscaling assumptions, hence local-scale interpretations should be avoided at present. These constraints are a reminder of the need for better inland fishery data (6, 8, 12, 13, 23)—particularly at national and watershed scales—to support management decisions. Our flexible analytical approach can readily be used to generate new maps as input data improve.
The rapid rise of freshwater aquaculture could help counterbalance any future declines in freshwater capture fisheries but also creates new challenges from both food security and environmental impact perspectives (40). Relative to diffuse, low-technology harvests of riverine fishes, much modern aquaculture is a high-input, concentrated production system. The profit potential arising from globalized trade and growth efficiency of poikilotherms has fueled explosive growth of aquaculture production in the last three decades (41, 42). However, continued sectoral expansion will rely on complex supply chains, centralized production infrastructure, and extensive transportation networks, all of which are at odds with providing low-cost, locally accessible food to poor people. Moreover, the inputs of feed, energy, and clean water required to support intensive aquaculture make these operations a new source of pollution and resource demands (41, 43). Reduced water quality, species invasions, disease outbreaks, and habitat appropriation associated with aquacultural intensification (40) could depress the production of wild fishes from rivers and lakes, particularly in conjunction with the host of other stressors already impacting freshwater ecosystems (4, 5).
This study underscores the urgent need to safeguard freshwater ecosystems for the sake of both fishes and people. Diverse natural fish assemblages currently provide a local, low-input protein supply with minimal reliance on resource inputs or infrastructure. Wild-caught fish have a remarkably small environmental footprint (44, 45), and replacing them with other sources of animal protein would be ecologically and economically costly (9, 40–45). However, the same intensive harvests of fish that benefit many of the world’s poor people also threaten aquatic biodiversity by dramatically altering species abundances and food web structure in ecosystems that already face a host of extrinsic stressors. Setting harvest levels that balance food provisioning and fish conservation is admittedly complex, but our analyses suggest opportunities to prioritize sites for interventions that serve both interests. By improving fishery management and halting ecosystem degradation in the modest number of rivers where high catches and spectacular fish faunas co-occur, we can maximize the chances of maintaining both food security and biodiversity into the future.
Methods
Creating a Gridded Map of Estimated Riverine Fish Catch.
Mean annual catches of wild fishes from inland waters were calculated from the FAO FishStat database (46). Our specific query terms for downloading FishStat data included the following: “diadromous” and “freshwater” fishes, but only those captured in “inland waters.” This query explicitly excluded fish derived from aquaculture sources. Our analyses focus on 130 nations with reliable data for the 10-y period from 1999 to 2008. Our criteria for reliability include the following: (i) no more than one missing year of data, and (ii) data vary from year to year. The latter criterion excludes several nations that report exactly the same catch repeatedly, suggesting that new data are not being collected.
To focus our catch map upon riverine yields, we subtracted catches reported in the world’s great lakes (32,597 T from the Laurentian Great Lakes, 716,642 T from African Great Lakes, and 13,100 T from Lake Baikal) from the totals of their riparian nations. Contributions from smaller lakes could not be systematically separated from riverine catches, so they remained pooled with river fisheries, leading to a modest potential overestimation of catch from rivers.
To distribute catches within the boundaries of each nation, we first established an empirical power-function scaling relationship between mean annual fish catch (C) and river mean annual discharge (Q) using reduced major axis regression to fit data from 40 separate basins around the world (C = 0.3264 Q1.256; R2 = 0.64; Fig. S1). Global maps of aquatic primary production and other potential influences are not available, and pilot analyses using the Global Lakes and Wetlands Database (47) offered no evidence that wetland extent affects fishery yields. The spatial resolution of our downscaling was dictated by the 6-min grid cells of a global river hydrography (48); we applied the discharge-based scaling equation to the estimated discharge for each grid cell to estimate its relative catch. Finally, we apportioned the FAO-reported mean annual catch among the grid cells within each nation in proportion to the contribution of each cell to summed potential catch for the nation as a whole. Thus, our gridded map exactly reflects mean annual catches reported by FAO. We assume that our downscaling algorithm, which is calibrated using catch and discharge statistics from entire river basins, accurately captures the relative scaling of catch with discharge at smaller scales.
We validated our distribution algorithm by summing estimated catches from all grid cells within each of the 40 river basins used to fit the catch–discharge relationship. These estimated catches are functionally independent of the reported catches from each basin because (i) they are drawn from independent sources (national agencies vs. individual researchers) at different spatial (entire nation vs. specific basins) and temporal (10-y average vs. single-year report) scales, and (ii) the discharge–catch relationship was fitted to dozens of rivers jointly so that no single river has much influence on the equation. We found reasonable agreement between estimated and observed basin-scale catches (n = 40; R2 = 0.62; Fig. S3).
Our overall approach to downscaling national fishery data improves upon the methods we used to map fishing pressure in Vörösmarty et al. (4). Specifically, the present analysis benefits from refinements in the calibration dataset, discharge grid, and statistical modeling, all of which lead to a more robust picture of global patterns of riverine fish catches. In addition, we do not attempt to translate catch into fishing pressure in the present analysis, as in ref. 4.
Fish Species Richness Data.
We examined the relationships between biodiversity and fisheries productivity by comparing the explanatory power of fish species richness and other environmental factors in predicting catches. These analyses were carried out at the spatial scale of freshwater ecoregions, for which fish species richness has been summarized globally (49, 50). Freshwater ecoregions were originally defined using a combination of biogeographic boundaries, ecosystem characteristics, and watershed boundaries. The associated fish species data were derived from a combination of literature mining and expert workshops (49). To focus on riverine fisheries, we excluded those ecoregions dominated by lake systems. For instance, we omitted the world’s great lake ecoregions (e.g., Lake Baikal, Lake Victoria), as well as lake-defined ecoregions such as the Oregon Lakes and Cuatro Cienegas. We also discarded all island ecoregions because their freshwater fisheries data, and often hydrology and biodiversity data, are either unreliable or unrepresentative. Dataset S2 lists the 316 ecoregions included in our analyses.
Environmental Predictor Data.
In addition to fish species richness, we analyzed the following potential influences on riverine fisheries: mean annual river discharge and solar radiation summed within each ecoregion, ecoregion area, mean annual air temperature and terrestrial net primary productivity (NPP), and human population density (Table S1). We were limited to variables for which reliable spatial data were available at the global scale, but this list encompasses most major hypothesis for factors regulating fishery productivity. For instance, we expect higher fishery productivity with increasing habitat area (for which river discharge and ecoregion area serve as proxies), temperature, and ecosystem energy availability (for which radiation and terrestrial NPP serve as proxies). Fish catches are also expected to be broadly positively related to the number of people in an ecoregion, reflecting demand for fish and potential fishing effort. Environmental data were log10-transformed before analyses, with the exception of temperature and radiation.
Table S1.
Variables included in multiple regression and Bayesian model selection to predict riverine fish catch
| Predictor | Units | Dataset timeframe | Data source |
| Mean net radiation | W⋅m−2 | 2010 | earthobservatory.nasa.gov/GlobalMaps/view.php?d1=CERES_NETFLUX_M |
| Mean annual temperature | °C | 1961–1990 | https://nelson.wisc.edu/sage/data-and-models/atlas/maps.php?datasetid=37&includerelatedlinks=1&dataset=37 |
| Surface area | km2 | NA | www.feow.org |
| Population | Persons⋅km−2 | 2010 | sedac.ciesin.columbia.edu/gpw |
| Mean net primary production | kgC⋅m−2⋅y−1 | NA | https://nelson.wisc.edu/sage/data-and-models/atlas/ |
| Total species richness | Species per ecoregion | NA | www.feow.org |
Data sources are indicated, and each variable was summarized at the scale of freshwater ecoregions (49) before analysis.
Despite the seeming circularity in using mean annual discharge both to downscale national catch and to predict catch across ecoregions, our approach ensures a strong degree of independence. Across all nations, estimated local catch becomes decoupled from local discharge because it reflects a nonlinear function of differences in relative discharge within a nation, the sum of which is constrained to equal the national catch. Environmental predictors of estimated catch are then tested at ecoregional scales by summing across thousands of grid cells, often spanning multiple nations.
Diversity–Productivity Hypothesis and Other Controls.
We used multiple statistical approaches to examine environmental controls on riverine fish catches, with special interest in testing for a diversity–productivity relationship with fish species richness. Discerning potential causality in this dataset is complicated by the fact that both fish catch and fish species richness were strongly correlated with mean annual discharge. Although coefficients from multiple regression are unbiased, their interpretation is complicated by collinearity among predictors. Because the importance of discharge as a predictor of fisheries is not in question, one approach to assessing the role of other environmental factors is to eliminate discharge effects on both the response variable (fish catch) and other predictors (e.g., fish species richness) by taking their residual variation when regressed against river discharge. Subsequently, multiple regression can be used to draw inferences about the influence of these other factors above and beyond that mediated by discharge; this is the same logic underlying partial regression coefficients and added variable plots. However, controversy remains as to whether the bias in parameter estimates from regression of residuals outweighs any shortcomings of unbiased partial regression coefficients when describing the relative influence of predictors (51, 52).
To fully assess the explanatory power of all environmental variables, we carried out three statistical approaches in parallel. First, we used multiple linear regression with all predictors in the model. Preliminary analyses using z-score standardized data in full models, and reverse stepwise regression for model selection, yielded qualitatively identical results. Second, we applied multiple linear regression to a dataset of residuals of both catch and environmental predictors against discharge (outlined above). Again, z-score standardization and stepwise variable selection yielded the same results. For both multiple-regression approaches, inferences about the relative importance of each variable were based on hierarchical variance partitioning. This method identifies robust patterns of influence by quantifying the average effect of each variable on overall explanatory power across models using all possible combinations of other variables. Our presentation of results focuses on these two multiple-regression models and associated hierarchical variance partitioning, all of which indicate concordant findings.
Third, we adopted a different methodological and philosophical approach by using Bayesian variable selection. A set of five reversible jump Markov chain Monte Carlo (53) runs was seeded with distinct starting states, and run for 150,000 iterations. The resulting models were stacked for interpretation of how many variables are required to achieve the most probable predictive models supported by the data, and which specific variables are selected. Inferences were based on posterior probabilities and associated credible intervals. To investigate potential influence of spatial autocorrelation on model results, we analyzed models with and without a spatial dependence parameter (distance between geographic midpoints of ecoregions).
Biodiversity Threat Index.
To account for extrinsic stressors that may compromise riverine fishery sustainability, we calculated a cumulative threat index that excluded fishing but accounted for flow alteration, pollution, watershed degradation, species invasions, and aquaculture. We included 22 of the 23 threat drivers originally used to calculate the biodiversity threat index presented in ref. 4, excluding fishing pressure to avoid circularity. All other procedures remain identical to those presented in ref. 4. We compared the resulting biodiversity threat index to catches at grid cell, ecoregion, and river basin (54) scales (Fig. 3).
Potential Fishery Productivity.
We also compared our potential and estimated catches at the ecoregional scale (Fig. S2). Estimated catches were derived as outlined earlier. Potential catches were calculated using the same approach, but without limiting grid cell catches to sum to the national catch reported by FAO. Thus, potential catch levels are unconstrained and reflect expectations based on the catch–discharge relationship observed in productive river fisheries.
Nutritional Dependence and Nutritional Efficiency.
To complement comparisons of the amount of fish harvested from rivers, we analyzed the relative contribution of different sources of fish protein to overall animal protein consumption in nations around the world. Our approach was to combine data for each fish source (i) on per capita consumption of fish protein (Pf) relative to total animal protein consumption per capita (Pt) in each nation (j), and multiply by national population (Nj) to quantify the equivalent number of people whose animal protein consumption is derived from fish of each type (Dij), as follows:
Summing Dij across all nations yielded an estimate of the total number of people globally whose animal protein consumption is met by each fish source, based on the fractional contribution of each type of fish to the per capita animal protein consumption. Performing these calculations at the national scale allowed us to place them in the context of GDP patterns. We compared wild-caught inland, wild-caught marine, and aquaculture (freshwater and marine combined) fisheries in terms of proportional nutritional dependence across the socioeconomic spectrum.
We were also interested in the nutritional efficiency of each source of fish protein, which we define as the equivalent number of people whose protein consumption is met per unit of fish protein consumed. We calculated nutritional efficiency globally (E) by dividing aggregate fish protein dependence by aggregate protein consumption for each source of fish (i) as follows:
This calculation weights human nutritional dependence based on disparities in total animal protein consumption, thereby accounting for the large differences among nations in dietary animal protein. If all nations have equivalent rates of total animal protein consumption, then nutritional efficiency would simply reflect aggregate consumption of each type of animal protein. However, if one source of fish is disproportionately important for animal protein consumption in all nations where total animal protein consumption is low, then that source has higher global nutritional efficiency than other types of fish that contribute primarily to diets that are high in animal protein. Nutritional efficiency is an important concept because it addresses how loss of a particular protein source would immediately affect animal protein consumption for people. Protein substitutability and replacement are beyond the scope of this analysis, but compensatory increases in consumption of other animal protein types may be unlikely in nations where per capita animal protein consumption is minimal at present.
National data on per capita consumption of protein from freshwater capture fisheries, aquaculture, all fish and seafood, and all animals were drawn from FAO statistics for the year 2009 (11, 46). Consumption of protein from marine fish was calculated by subtracting the sum of freshwater capture fishery and aquaculture protein from all fish and seafood protein for each nation. GDP and population data were drawn from World Bank statistics for the year 2010 (World Development Indicators Database; data.worldbank.org/wdi).
Additional Methods and Results
Diversity–Stability Hypothesis.
In addition to understanding controls on catch rates in the spirit of diversity–stability analysis, we also wanted to test for a benefit of fish species richness for catch stability through time. We analyzed stability during time windows of the most recent 10-, 30-, and 50-y windows between 1958 and 2008. First, we detrended the nationally reported FAO catch data using a loess smoother for the 30- and 50-y windows, and a linear smoother for the 10-y window. For each year, detrended national catches were distributed among grid cells according to the same power function described earlier (C = 0.3264Q1.256), then summed across all grid cells in each ecoregion. For the resulting time series of ecoregional catches, we calculated the SD and rate of change for the 10-, 30-, and 50-y windows.
Results of Bayesian model selection agreed closely with our inferences from multiple regression approaches. All Markov chain Monte Carlo models converged. For the models without a spatial autocorrelation term, a four-predictor model was favored (38.8% posterior probability) over models with five (29.7%), six (20.0%), or three (0.9%) predictors. Positive effects of discharge and human population were included in all models, whereas ecoregion area and fish species richness had negative effects and were almost always included (99.9% and 97.4% marginal inclusion probability, respectively). Positive effects of radiation (33.2% marginal inclusion probability) and negative effects of NPP (23.0%) and temperature (22.6%) were rarely included, and credible intervals for the slope coefficients overlapped zero for all three variables. When spatial dependence was modeled, there was strong evidence of autocorrelation (95% credible set for the spatial dependence parameter was 0.9772–0.9986, where 0 represents spatial independence and 1 represents strong spatial dependence). A three-predictor model was favored (50.12% posterior probability, compared with 25.9% for four-predictor and 6.4% for two-predictor models). The top models included positive effects of discharge (100% marginal inclusion probability) and human population (87.6%), and negative effects of ecoregion area (92.8%). Terrestrial productivity (19.2%) and radiation (15.1%) were most likely to be included in four-parameter models (14.2–16.5% marginal inclusion probability), but their slopes did not differ credibly from zero. Fish species richness was the least likely variable to be included (9.4%) when spatial autocorrelation was modeled, and had only nonsignificant negative effects.
The parallel results from all three statistical approaches suggest a robust pattern of environmental control on riverine fish catches. River habitat (reflected by discharge) is the single most important predictor, and human population also has consistent positive effects. Ecoregion area and fish species richness had marginal negative effects or no effects depending on the statistical approach, and ecosystem energy (reflected by temperature, radiation, and terrestrial NPP) had no significant effect. Thus, we can confidently reject the hypothesis that fish catches increase with the fish species richness of an ecoregion.
Diversity–Stability Hypothesis.
No relationship between catch stability (as either SD or rate of change) and fish species richness was evident across ecoregions for any time frame. The same was true when we did not detrend the catch data. Thus, our analysis provides no evidence for a diversity–stability relationship in riverine fisheries based on the available data. However, we consider this to be a weak test of the stabilizing effects of species richness because temporal variation in catch for a given nation is likely to be partly attributable to inconsistent reporting. Thus, any conclusion about lack of benefits of fish species richness for stabilizing riverine fish catches remains tentative.
Potential Fishery Yields.
As a metric of potential fishery yields, we used the catch–discharge relationship to estimate gridded catches globally without constraining them to sum to the FAO’s mean reported catch for each nation. This potential fishery yield was then compared against the FAO-constrained estimated catches at the ecoregional scale. This comparison suggests that most of the world’s riverine fisheries are mostly or fully exploited. However, individual ecoregions appear to range from highly overexploited to underexploited (Fig. S2). In general, reported catches from temperate latitudes and Latin America are lower than their apparent potential, whereas tropical Asia and Africa are exploited at or beyond expectations. The high rates of exploitation in Asia and Africa are probably sustained by restocking and species propagation, as well as reflecting cultural traditions of harvesting and consuming smaller fishes. These disparities parallel previous inferences from national statistics (9), but our data allow quantitative analysis at a much finer scale.
Considerable uncertainty remains regarding the estimation of fishery potential, and applying our catch–discharge relationship in this way has not been validated. Nonetheless, the approach offers a simple and transparent means of calculating an approximate potential yields. Thus, we use the disparity between estimate FAO-constrained catch estimate and potential yield as a reasonable basis to assess the place of each ecoregion along the exploitation spectrum.
Nutritional Dependence and Nutritional Efficiency.
We find that the world’s waters provided the equivalent of all animal protein consumed by 158, 371, and 743 million people worldwide through freshwater capture fisheries, aquaculture (primarily freshwater), and marine capture fisheries in 2009. Collectively, these figures represent 18.7% of global human population in 2009 (6.8 billion people) (54), which is substantially higher (+12.7%) than the widely cited figure that fish account for 16.6% of total animal protein intake by humans worldwide (11). The 12.7% higher importance of fish in fulfilling nutritional dependence on animal protein compared with aggregate animal protein consumption reflects the fact that fish are disproportionately important in the diet of people who have minimal access to animal protein in general. Moreover, our analyses reveal that protein dependence upon freshwater fish occurs primarily in lower GPD nations than for aquaculture and marine capture fisheries (Fig. 4A). By extension, the nutritional efficiency of freshwater fisheries exceeds that of aquaculture or marine capture fisheries (Fig. 4A). Note that these calculations do not account for underreporting of capture fisheries, or for the rapid rise in aquaculture production since 2009 (11, 43). Both factors would dramatically increase the apparent importance of fish protein relative to other sources of animal protein in diets of people around the world.
Supplementary Material
Acknowledgments
We thank J. D. Allan, C. Hawkins, and the P.B.M. Laboratory group for advice; G. Klever for data entry; and M. Liermann, N. Keuler, and Z. Zheng for statistical assistance. The manuscript was improved by feedback from the editor and two reviewers. Fish richness data were provided by the World Wildlife Fund and The Nature Conservancy. Funding was provided by the University of Wisconsin, Packard Fellowship, and National Science Foundation Grant DEB-1115025. This study is a product of the bioDISCOVERY project of Future Earth.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1521540113/-/DCSupplemental.
References
- 1.Palmer MA, Febria CM. Ecology. The heartbeat of ecosystems. Science. 2012;336(6087):1393–1394. doi: 10.1126/science.1223250. [DOI] [PubMed] [Google Scholar]
- 2.Tittensor DP, et al. A mid-term analysis of progress toward international biodiversity targets. Science. 2014;346(6206):241–244. doi: 10.1126/science.1257484. [DOI] [PubMed] [Google Scholar]
- 3.Larsen FW, Londoño-Murcia MC, Turner WR. Global priorities for conservation of threatened species, carbon storage, and freshwater services: Scope for synergy? Conserv Lett. 2011;4(5):355–363. [Google Scholar]
- 4.Vörösmarty CJ, et al. Global threats to human water security and river biodiversity. Nature. 2010;467(7315):555–561. doi: 10.1038/nature09440. [DOI] [PubMed] [Google Scholar]
- 5.Strayer DL, Dudgeon D. Freshwater biodiversity conservation: Recent progress and future challenges. J N Am Benthol Soc. 2010;29(1):344–358. [Google Scholar]
- 6.De Graaf G, Bartley D, Jorgensen J, Marmulla G. The scale of inland fisheries, can we do better? Alternative approaches for assessment. Fish Manag Ecol. 2015;22(1):64–70. [Google Scholar]
- 7.Dugan P, Delaporte A, Andrew N, O'Keefe M, Welcomme R. 2010 Blue Harvest: Inland Fisheries as an Ecosystem Service. The WorldFish Center Working Papers (United Nations Environment Programme; UNON, Publishing Services Section, Nairobi). Available at www.unep.org/pdf/Blue_Harvest.pdf. Accessed June 1, 2011.
- 8.FAO World Fish Center 2008 Small-Scale Capture Fisheries—A Global Overview with Emphasis on Developing Countries. Available at pubs.iclarm.net/resource_centre/Big_Numbers_Project_Preliminary_Report.pdf. Accessed June 1, 2011.
- 9.Orr S, Pittock J, Chapagain A, Dumaresq D. Dams on the Mekong River: Lost fish protein and the implications for land and water resources. Glob Environ Change. 2012;22(4):925–932. [Google Scholar]
- 10.Beard TD, Jr, et al. Ecosystem approach to inland fisheries: Research needs and implementation strategies. Biol Lett. 2011;7(4):481–483. doi: 10.1098/rsbl.2011.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FAO . State of the World Fisheries and Aquaculture. FAO; Rome: 2014. [Google Scholar]
- 12.Welcomme RL. An overview of global catch statistics for inland fish. ICES J Mar Sci. 2011;68(8):1751–1756. [Google Scholar]
- 13.Welcomme RL. 2011. Review of the State of the World Fishery Resources: Inland Fisheries (FAO, Rome), FAO Fisheries and Aquaculture Circular No. 942, Rev. 2.
- 14.Costello C, et al. Status and solutions for the world’s unassessed fisheries. Science. 2012;338(6106):517–520. doi: 10.1126/science.1223389. [DOI] [PubMed] [Google Scholar]
- 15.Allan JD, et al. Overfishing of inland waters. Bioscience. 2006;55(12):1041–1051. [Google Scholar]
- 16.Post JR, et al. Canada’s recreational fisheries: The invisible collapse? Fisheries (Bethesda, Md) 2002;27(1):6–17. [Google Scholar]
- 17.Humphries P, Winemiller KO. Historical impacts on river fauna, shifting baselines, and challenges for restoration. Bioscience. 2009;59(8):673–684. [Google Scholar]
- 18.van Vliet MTH, et al. Global river discharge and water temperature under climate change. Glob Environ Change. 2013;23(2):450–464. [Google Scholar]
- 19.Duffy JE, et al. The functional role of biodiversity in ecosystems: Incorporating trophic complexity. Ecol Lett. 2007;10(6):522–538. doi: 10.1111/j.1461-0248.2007.01037.x. [DOI] [PubMed] [Google Scholar]
- 20.Cardinale BJ, et al. Biodiversity loss and its impact on humanity. Nature. 2012;486(7401):59–67. doi: 10.1038/nature11148. [DOI] [PubMed] [Google Scholar]
- 21.Schindler DE, et al. Population diversity and the portfolio effect in an exploited species. Nature. 2010;465(7298):609–612. doi: 10.1038/nature09060. [DOI] [PubMed] [Google Scholar]
- 22.Worm B, et al. Impacts of biodiversity loss on ocean ecosystem services. Science. 2006;314(5800):787–790. doi: 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
- 23.Bartley DM, De Graaf GJ, Valbo‐Jørgensen J, Marmulla G. Inland capture fisheries: Status and data issues. Fish Manag Ecol. 2015;22(1):71–77. [Google Scholar]
- 24.Arlinghaus R, Cooke SJ. Recreational fisheries: Socio-economic importance, conservation issues and management challenges. In: Dickson B, Hutton J, Adams B, editors. Recreational Hunting, Conservation and Rural Livelihoods: Science and Practice. Wiley-Blackwell; Chichester, UK: 2009. pp. 39–58. [Google Scholar]
- 25.Welcomme RL. Fisheries Ecology of Floodplain Rivers. Longman; London: 1979. [Google Scholar]
- 26.van Vliet MTH, Ludwig F, Kabat P. Global streamflow and thermal habitats of freshwater fishes under climate change. Clim Change. 2013;121(4):739–754. [Google Scholar]
- 27.Cheung WWL, et al. Shrinking of fishes exacerbates impacts of global ocean changes on marine ecosystems. Nat Clim Chang. 2013;3:254–258. [Google Scholar]
- 28.Hooper DU, et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol Monogr. 2005;75(1):3–35. [Google Scholar]
- 29.Milstein A. Ecological aspects of fish species interactions in polyculture ponds. Hydrobiologia. 1992;231(3):177–186. [Google Scholar]
- 30.Chassot E, et al. Global marine primary production constrains fisheries catches. Ecol Lett. 2010;13(4):495–505. doi: 10.1111/j.1461-0248.2010.01443.x. [DOI] [PubMed] [Google Scholar]
- 31.Pauly D, Christensen V, Dalsgaard J, Froese R, Torres F., Jr Fishing down marine food webs. Science. 1998;279(5352):860–863. doi: 10.1126/science.279.5352.860. [DOI] [PubMed] [Google Scholar]
- 32.McIntyre PB, Jones LE, Flecker AS, Vanni MJ. Fish extinctions alter nutrient recycling in tropical freshwaters. Proc Natl Acad Sci USA. 2007;104(11):4461–4466. doi: 10.1073/pnas.0608148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Estes JA, et al. Trophic downgrading of planet Earth. Science. 2011;333(6040):301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- 34.Garcia SM, et al. Conservation. Reconsidering the consequences of selective fisheries. Science. 2012;335(6072):1045–1047. doi: 10.1126/science.1214594. [DOI] [PubMed] [Google Scholar]
- 35.Swartz W, Sala E, Tracey S, Watson R, Pauly D. The spatial expansion and ecological footprint of fisheries (1950 to present) PLoS One. 2010;5(12):e15143. doi: 10.1371/journal.pone.0015143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deines AM, Bunnell DB, Rogers MW, Beard TD, Taylor WW. A review of the global relationship among freshwater fish, autotrophic activity, and regional climate. Rev Fish Biol Fish. 2015;25(2):323–336. [Google Scholar]
- 37.Baxter CV, Fausch KR, Saunders WC. Tangled webs: Reciprocal flows of invertebrate prey link streams and riparian zones. Freshw Biol. 2005;50(2):201–220. [Google Scholar]
- 38.McCann KS, et al. Food webs and the sustainability of indiscriminate fisheries. Can J Fish Aquat Ecol. 2016;73(4):656–665. [Google Scholar]
- 39.Ziv G, Baran E, Nam S, Rodríguez-Iturbe I, Levin SA. Trading-off fish biodiversity, food security, and hydropower in the Mekong River Basin. Proc Natl Acad Sci USA. 2012;109(15):5609–5614. doi: 10.1073/pnas.1201423109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klinger D, Naylor R. Searching for solutions in aquaculture: Charting a sustainable course. Annu Rev Environ Resour. 2012;37:247–276. [Google Scholar]
- 41.Naylor RL, et al. Feeding aquaculture in an era of finite resources. Proc Natl Acad Sci USA. 2009;106(36):15103–15110. doi: 10.1073/pnas.0905235106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bostock J, et al. Aquaculture: Global status and trends. Philos Trans R Soc Lond B Biol Sci. 2010;365(1554):2897–2912. doi: 10.1098/rstb.2010.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao L, et al. Global food supply. China’s aquaculture and the world’s wild fisheries. Science. 2015;347(6218):133–135. doi: 10.1126/science.1260149. [DOI] [PubMed] [Google Scholar]
- 44.Rawitscher M, Mayer J. Nutritional outputs and energy inputs in seafoods. Science. 1977;198(4314):261–264. doi: 10.1126/science.561995. [DOI] [PubMed] [Google Scholar]
- 45.Gephart JA, Pace ML, D’Odorico P. Freshwater savings from marine protein consumption. Environ Res Lett. 2014;9(1):014005. [Google Scholar]
- 46.FAO 2013 FishStatJ—Software for Fishery Statistical Time Series. Available at www.fao.org/fishery/statistics/software/fishstatj/en. Accessed February 15, 2014.
- 47.Lehner B, Döll P. Development and validation of a global database of lakes, reservoirs and wetlands. J Hydrol (Amst) 2004;296:1–22. [Google Scholar]
- 48.Wisser D, Fekete BM, Vörösmarty CJ, Schumann AH. Reconstructing 20th century global hydrography: A contribution to the Global Terrestrial Network–Hydrology (GTN-H) Hydrol Earth Syst Sci. 2010;14:1–24. [Google Scholar]
- 49.Abell R, et al. Freshwater ecoregions of the world: A new map of biogeographic units for freshwater biodiversity conservation. Bioscience. 2008;58(5):403–414. [Google Scholar]
- 50.Abell R, et al. Concordance of freshwater and terrestrial biodiversity. Conserv Lett. 2011;4(2):127–136. [Google Scholar]
- 51.Freckleton RP. On the misuse of residuals in ecology: Regression of residuals vs. multiple regression. J Appl Ecol. 2002;71(3):542–545. [Google Scholar]
- 52.Smith AC, Koper N, Francis CM, Fahrig L. Confronting collinearity: Comparing method for disentangling the effects of habitat loss and fragmentation. Landsc Ecol. 2009;24:1271–1285. [Google Scholar]
- 53.Green PJ. Reversible jump Markov chain Monte Carlo computation and Bayesian model determination. Biometrika. 1995;82(4):711–732. [Google Scholar]
- 54.Lehner B, Grill G. Global river hydrography and network routing: Baseline data and new approaches to study the world’s large river systems. Hydrol Processes. 2013;27(15):2171–2186. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.