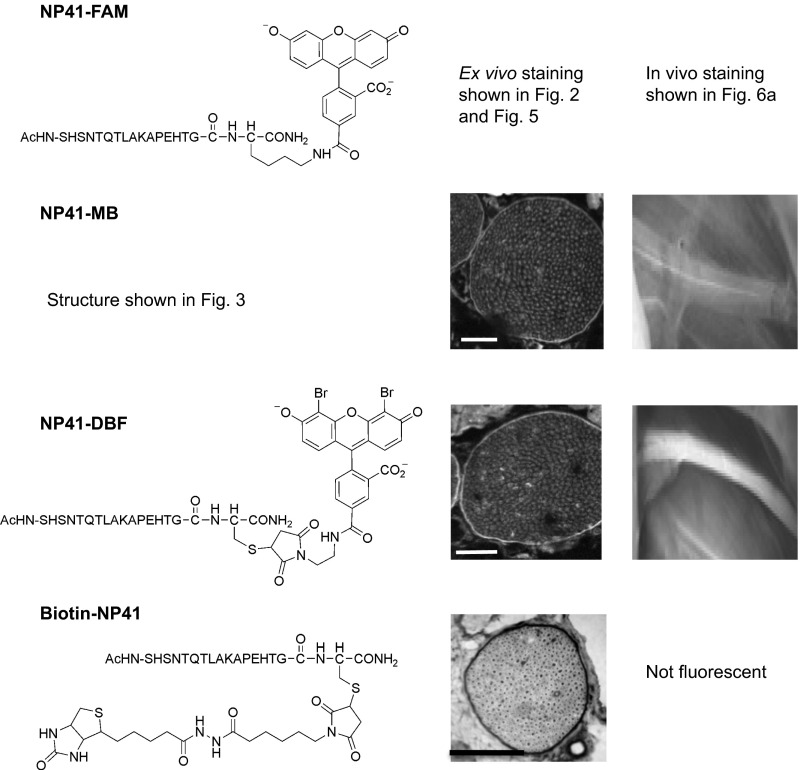
Significance
A fluorescently labeled nerve-binding peptide, NP41, holds promise to reduce surgical nerve damage and facilitate nerve repair. Clinical translation hinges on identification of binding targets to assess potential toxicity and understand the mechanism. For target identification, we developed a receptor capture method, enabling covalent tagging and identification of proteins within close proximity to a bound ligand. Extracellular matrix proteins laminin-421 and -211 were identified as NP41 binding targets, and TRICEPS-based glycoprotein capture supported laminin-421 as the primary binding target. This result explains the ability of NP41 to highlight degenerated nerve “ghosts” months after transection that were invisible to the unaided eye but contain laminins. Targeting extracellular matrix is advantageous for clinical imaging agents, likely reducing undesirable neurological effects.
Keywords: nerve highlighting, laminin, proximity-based labeling, molecular imaging, surgery with molecular navigation
Abstract
Target-blind activity-based screening of molecular libraries is often used to develop first-generation compounds, but subsequent target identification is rate-limiting to developing improved agents with higher specific affinity and lower off-target binding. A fluorescently labeled nerve-binding peptide, NP41, selected by phage display, highlights peripheral nerves in vivo. Nerve highlighting has the potential to improve surgical outcomes by facilitating intraoperative nerve identification, reducing accidental nerve transection, and facilitating repair of damaged nerves. To enable screening of molecular target-specific molecules for higher nerve contrast and to identify potential toxicities, NP41’s binding target was sought. Laminin-421 and -211 were identified by proximity-based labeling using singlet oxygen and by an adapted version of TRICEPS-based ligand-receptor capture to identify glycoprotein receptors via ligand cross-linking. In proximity labeling, photooxidation of a ligand-conjugated singlet oxygen generator is coupled to chemical labeling of locally oxidized residues. Photooxidation of methylene blue–NP41-bound nerves, followed by biotin hydrazide labeling and purification, resulted in light-induced enrichment of laminin subunits α4 and α2, nidogen 1, and decorin (FDR-adjusted P value < 10−7) and minor enrichment of laminin-γ1 and collagens I and VI. Glycoprotein receptor capture also identified laminin-α4 and -γ1. Laminins colocalized with NP41 within nerve sheath, particularly perineurium, where laminin-421 is predominant. Binding assays with phage expressing NP41 confirmed binding to purified laminin-421, laminin-211, and laminin-α4. Affinity for these extracellular matrix proteins explains the striking ability of NP41 to highlight degenerated nerve “ghosts” months posttransection that are invisible to the unaided eye but retain hollow laminin-rich tubular structures.
Molecular interactions including ligand–receptor binding are a key component of nearly every biological process. Discovering such interactions is especially challenging if they are low affinity, context-dependent, or the receptors are difficult to isolate. However, their identification improves our fundamental understanding of biological processes and enables development of synthetic ligands with clinical applications. Phage display is a powerful affinity-based molecular selection tool that has enabled the generation of peptides, proteins, and antibodies that bind to specific targets (1). Selections against complex sources, such as live cells, cell extracts, or organs, have also yielded promising results (2–4). Despite the obvious implications of these ligands as potential clinical agents and their receptors as biomarkers, few targets have been defined (5).
A variety of methods to capture native ligand–receptor interactions have used chemical or photo–cross-linking followed by mass spectrometry (MS), some requiring that ligands retain binding activity after potentially disruptive chemical treatment (6–9). In most cross-linking techniques, the ligand must reach an appropriately reactive site on the target for cross-linking to occur, while being conjugated to a potentially bulky purification tag, which can weaken specific binding. Proximity-based labeling techniques using fusion proteins have recently been developed for the discovery of new interacting or nearby proteins (10–12); however, bulky fusion proteins are likely to affect ligand binding.
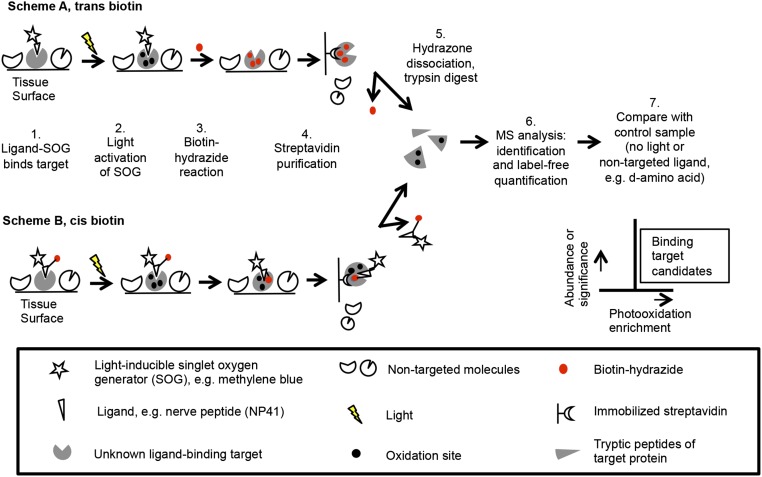
To more efficiently capture low-affinity and easily disrupted interactions, a small molecule proximity tagging method was developed using photooxidation coupled to affinity tagging (Fig. 1). A light-driven, singlet oxygen-generating molecule [SOG; e.g., methylene blue (MB), fluorescein derivative] is conjugated to a ligand. Upon binding of the ligand–SOG to tissue and exposure to light, oxidation of molecules proximal to the ligand occurs. In biological samples, singlet oxygen has an extremely short half-life, in the range of hundredths to tenths of a microsecond, during which it is estimated to diffuse within a range of tens of nanometers from its source (13, 14). Proteins are a major biological quencher of singlet oxygen, and reaction with specific amino acids forms byproducts containing ketones and aldehydes (15, 16). Such carbonyl groups are normally rare in tissues, enabling site-specific labeling of oxidized amino acids on proximal proteins. For example, tryptophan is converted into ketone-containing kynurenine or N-formylkynurenine (17–19). Biotin-conjugated hydrazides or hydrazines (BHs) have specific reactivity for ketones and aldehydes (15, 20). BHs can be reacted with tissue exogenously (Fig. 1, scheme A) or coupled directly to the ligand–SOG (Fig. 1, scheme B), allowing photooxidation and chemical conjugation to be performed simultaneously in vivo. Biotin-tagged proteins are then purified from tissue homogenates with immobilized streptavidin, dissociated via acidic hydrolysis of the hydrazone bond (21), digested with trypsin, and identified by liquid chromatography-tandem mass spectrometry (LC–MS/MS). Label-free quantification is then used to compare protein abundance relative to a control.
Fig. 1.
Schematic of photooxidation-mediated identification of ligand-proximal molecules. (Scheme A) A ligand of interest is conjugated to a SOG and applied to tissue with ligand-binding activity. Upon excitation with SOG-activating light, singlet oxygen is produced, which reacts locally and results in oxidation sites, including ketones and aldehydes. BH is applied to label these carbonyl groups. Biotinylated proteins are streptavidin-purified from tissue homogenate. The hydrazone bond is released, and proteins are trypsinized and analyzed by LC–MS/MS. Label-free quantification is used to compare relative protein amounts to a control sample using NL or a nontargeted ligand such as the d-enantiomer. (Scheme B) Ligand is conjugated to SOG, biotin, and a ketone-reactive group—for example, pyridylhydrazine—and treated on ligand-binding tissue. Tissue is exposed to light, and biotinylated proteins are purified and identified as before.
Alternatively, ligand-directed receptor capture (LRC) using TRICEPS technology has been shown to enable carbohydrate-directed tagging of receptors and subsequent identification by MS (8, 9). Mild sodium periodate (NaIO4) treatment oxidizes cis-glycol groups in carbohydrates to aldehydes, and newly generated aldehydes cross-link to a BH-containing ligand. Samples are then purified using immobilized streptavidin and released by the endoglycosidase PNGaseF.
In this report, ligand-proximal photooxidation and an adapted version of TRICEPS LRC of glycoproteins were used to identify extracellular matrix proteins laminin-421 and -211 as the binding targets of NP41. This nerve-binding peptide was previously identified by phage display and shown to fluorescently label nerves in vivo and improve surgical identification, potentially reducing iatrogenic nerve injury (2, 22, 23). This result explains NP41’s striking ability to highlight otherwise invisible, highly degenerated nerves, which are devoid of myelin and axons but contain laminin-rich extracellular matrix.
Results
To identify NP41 binding targets, affinity chromatography was chosen as the initial method of isolation. Homogenized nerve was passed over immobilized NP41 versus the control d-amino acid enantiomer, which was previously found not to bind nerves when injected into mice (2). No specific protein was selectively captured as determined by MS analysis, leading us to speculate that NP41 bound with low affinity to homogenates. Thus, to capture NP41-receptor interaction in situ, we developed a method for proximity labeling ligand-bound proteins using photooxidation and targeted BH labeling and also tested previously described glycoprotein capture using TRICEPS-based chemistry (8, 9).
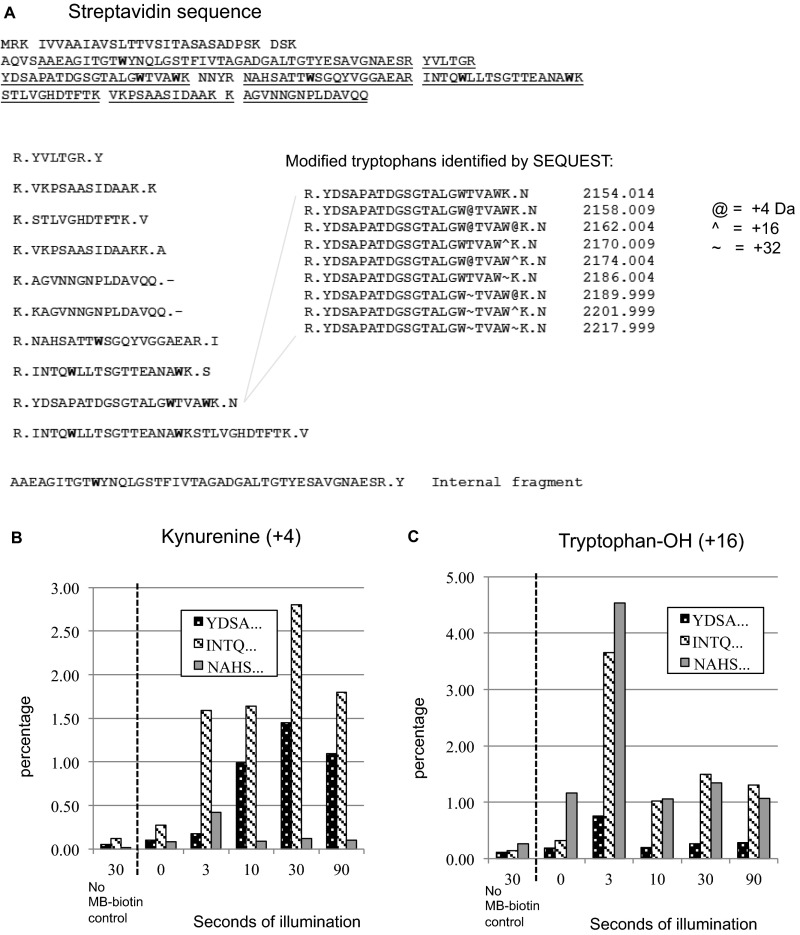
To test the concept of proximity-based oxidation of proteins through photoactivation of a ligand–SOG conjugate, ketone adducts were directly measured via MS following light exposure. Specifically, biotin–MB, used as a ligand–SOG, and streptavidin were coincubated, and then MB was photoactivated with 615/40 nm light for 0–90 s. Streptavidin was digested with trypsin and subjected to LC–MS/MS. MS1-based label-free quantification showed a light and ligand–MB-dependent increase in tryptophan oxidation in streptavidin peptides with +4 (kynurenine) and +16 (tryptophan–OH) mass changes (Fig. S1 A–C). Peak conversion occurred after brief light exposures (30 s and 3 s, respectively) with relatively lower conversion after longer exposures. This may be due to compounding reactions by singlet oxygen that result in diverse or unidentifiable products, many of which are unstable (16).
Fig. S1.
Photooxidation of MB–biotin results in tryptophan oxidation of streptavidin. (A) Primary sequence of streptavidin with observed tryptic peptides underlined; tryptophans are highlighted and modified listed with mass identified by the SEQUEST algorithm. (B) Percent of peptides containing +4 kDa tryptophan mass change (kynurenine) increases with photooxidation. The area of the modified peak is plotted as a percentage of area of unmodified peak. Peak conversion occurred after 30 s of light exposure. (C) Percent of peptides containing +16 kDa tryptophan mass change increases with photooxidation. Peak conversion occurred after 3 s of light exposure.
Imaging Biotinylation upon Ligand-Proximal Photooxidation.
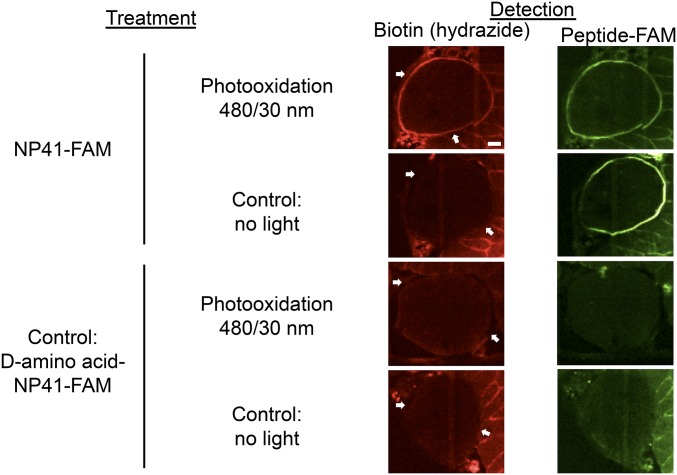
To examine whether photoactivation of a NP41–SOG conjugate would result in spatially localized BH labeling proximal to the binding site, mouse peripheral nerve sections were incubated with carboxyfluorescein (FAM)-labeled NP41 and photooxidized with 480 nm light. Controls including d-enantiomer and no light (NL) treatment were done in parallel. Oxidized sites were labeled with 50 μM BH in MES buffer, pH 5.5, and stained with streptavidin–Texas Red. High biotin staining was detected at the perineurium upon photooxidation with NP41–FAM, which correlated well with peptide binding as detected by fluorescence (Fig. 2). Neither the d-enantiomer control nor non–light-exposed tissues showed high perineurial labeling, and photobleaching of FAM occurred in the photooxidized (PO) sample, consistent with singlet oxygen destruction of the fluorophore.
Fig. 2.
Ligand-proximal photooxidation results in specific biotinylation of regions highlighted by NP41. Photooxidation of NP41–FAM-treated nerve tissue sections with 480/30 nm light induces BH labeling detected with streptavidin–Texas Red (red, Left panels), which is highest in the perineurium (white arrows) that ensheathes the myelinated Schwann cell–axon bundles. High perineurial biotin labeling is absent from both the NP41–FAM NL control and from all d-NP41 controls, both with and without photooxidation. Perineurial biotin labeling colocalizes with direct detection of peptide binding at the perineurium in the same section (green, Right panels). Perineurium was confirmed in all sections by plane light microscopy and autofluorescence when images were scaled to maximum gain. Images are at matched gain within each color and representative of at least three independent samples. (Scale bar, 50 μm.)
Proximal Photooxidation and BH Labeling Identify Laminin-α4, Laminin-α2, and Nidogen as Candidate NP41 Binding Targets.
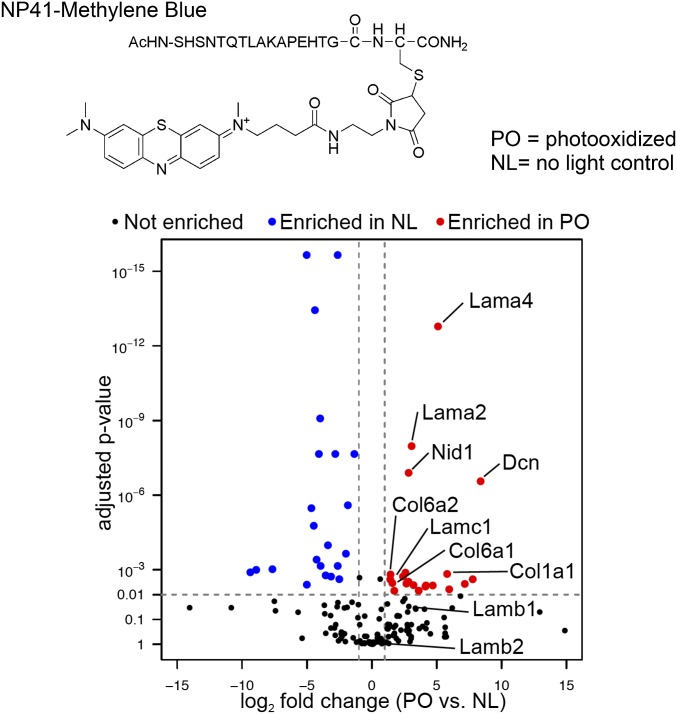
Experiments to identify NP41 binding partners by proximal photooxidation and MS were done both ex vivo on intact excised nerves and in vivo by injecting the probe into mice. For ex vivo experiments, NP41–MB (Fig. 3, Top) was used to enhance distribution of photooxidation throughout the nerve. MB has superior singlet oxygen production, and red light (640 nm) penetrates deeper into tissue than 488 nm (fluorescein excitation) light. Intact mouse sciatic nerves were treated with 50 μM NP41–MB for 2 h, rinsed, and photooxidized with 640 nm light for 10 s, 1 min, or 15 min or not exposed to light as a control (t = 0). Nerves were reacted with 50 μM BH and homogenized. Proteins were affinity-purified on avidin beads, released by acid hydrolysis, trypsinized, and identified by LC–MS/MS. Protein enrichment of PO samples compared with the NL control was determined as the fraction of the average spectral counts in PO samples relative to total spectral counts (PO/PO + NL).
Fig. 3.
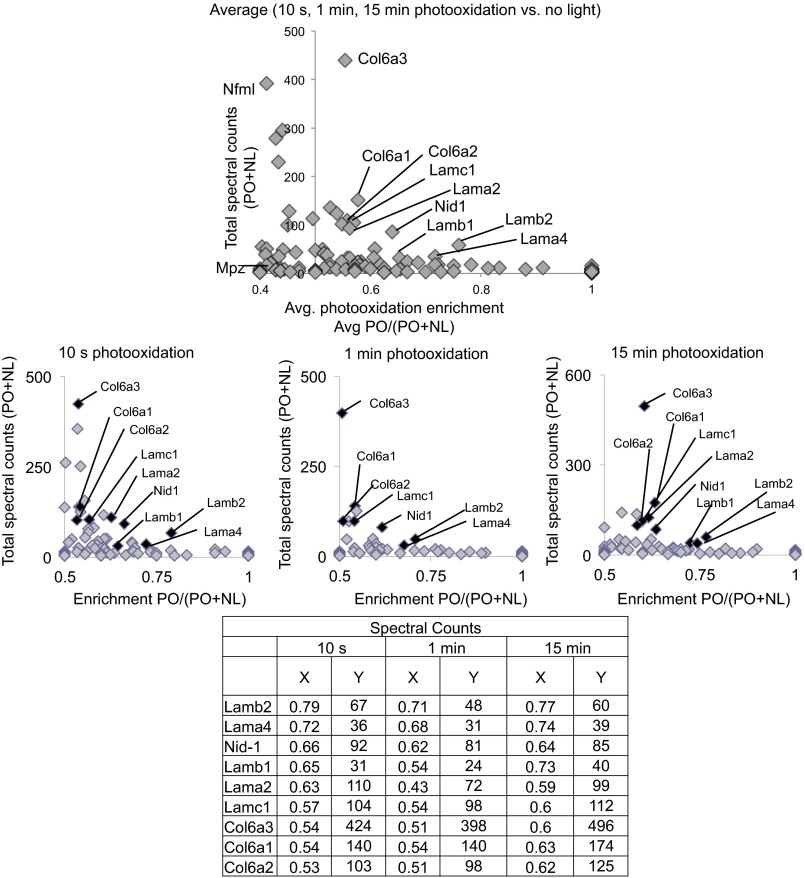
Ligand-proximal photooxidation of NP41–MB identifies binding candidates laminin, collagen, and associated proteins. (Top) Structure of NP41-MB conjugate. MB causes photooxidation at 650 nm. (Bottom) Comparison of protein abundance in the PO sample versus NL control. Shown is the label-free quantitation using relative precursor ion signal and significance testing of the PO sample (15 min) versus NL control. The most significantly enriched proteins include laminin subunits α4 (Lama4) and α2 (Lama2), nidogen 1 (Nid1), and decorin (Dcn). Proteins with less significant enrichment (P < 0.01) include collagen VI chains (Col6a1, Col6a2) and laminin-γ1 (Lamc1). Laminin-β1 (Lamb1) and -β2 (Lamb2) were not significantly enriched.
A select group of proteins including several laminins were enriched over the NL control (PO/PO + NL > 0.5) when spectral counts of different light-exposed samples were averaged or compared individually (Fig. S2). Proteins with abundant counts (>30) that showed the highest enrichment were laminin subunits α4 and β2 (0.76 and 0.72, average), which were enriched after only 10 s of photooxidation (0.79 and 0.72, at 10 s). Laminin subunits β1 (0.65, average), γ1 (0.57), and α2 (0.56); nidogen (0.64); and collagen VI chains α1, α2, and α3 (0.56, 0.57, and 0.55, respectively) were also enriched, and spectral counts were greatest after 15 min of photooxidation. Other highly abundant peripheral nerve-specific proteins, such as neurofilament light chain (0.41) and myelin protein P0 (0.41), showed no enrichment under light-exposed conditions. To normalize signal within each sample and test for significance, precursor ion abundance was used as an alternate measure of quantifying protein enrichment in light-exposed versus non–light-exposed samples. The most statistically significant enriched proteins after 15 min of light exposure were laminin-α4, -α2, and nidogen-1 (Fig. 3, Bottom; log2FC = 5.09, 3.06, and 2.83, respectively; adj. P value < 10−7). Decorin was also identified as significantly enriched (log2FC = 8.38; adj. P value < 10−7); however, it was not enriched by spectral count quantitation. Laminin-γ1 and collagen VI and I chains were among other proteins enriched with higher P values, whereas laminin β1 and β2 chains showed enrichment below the significance cutoff.
Fig. S2.
Ligand proximal photoxidation of NP41–MB enriches laminins after various photooxidation exposures. Shown are proteins identified as enriched by MS in nerve samples photooxidized with NP41–MB after light exposure (10 s, 1 min, or 15 min) versus NL control, plotted by average (Top) or individual light exposures (Middle). Spectral counts for each protein were summed [abundance (PO+NL)], and the fractions identified in the PO sample [enrichment PO/(PO+NL)] are plotted on the y and x axes, respectively. Proteins enriched in the PO compared with the NL control are shown on the right of the vertical axis at x = 0.5. Proteins with low total spectral counts (<30) were considered likely nonspecific background signal. Those identified as consistently enriched by light (x > 0.5) and with abundant spectral counts (y > 30) are highlighted in the graphs above and are identified with x and y values listed in the table. Proteins showing photooxidation-induced enrichment in multiple samples include laminin subunits, nidogen, and collagen VI subunits. Two abundant, nerve-specific proteins, neurofilament and myelin protein P0, were not enriched. Results are representative of independent replicate experiments.
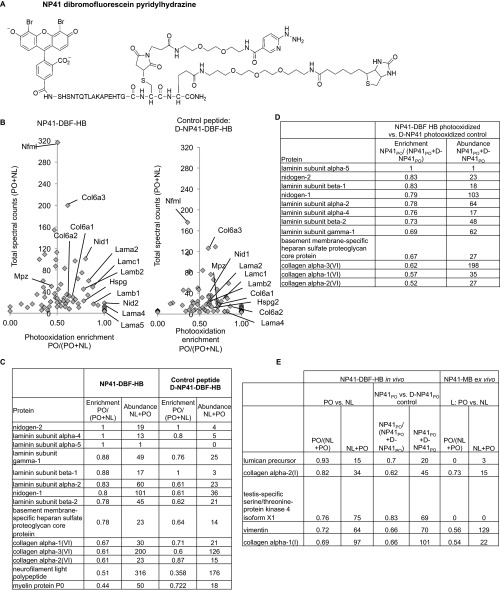
To confirm NP41’s potential interaction with these proteins in vivo, a SOG and pyridylhydrazine–biotin (HB) were directly conjugated to NP41 (Fig. 1, scheme B). Dibromofluorescein (DBF) was used as the singlet oxygen generator due to its efficient production of singlet oxygen compared with fluorescein and because it showed greater compatibility with NP41 targeting in vivo compared with MB (Fig. S3). DBF–NP41–HB (Fig. S4A) or its d-enantiomer control was injected into mice. Four hours postinjection, sciatic nerves on one side were surgically uncovered and photooxidized with 480 nm light (PO), whereas the contralateral side was kept in the dark (NL). Both sciatic nerves were dissected, immersed in MES buffer pH 5.5, and processed as previously described. Spectral counting of the PO versus the NL control revealed enrichment of nidogen-1 and -2 (1 and 0.8); laminin subunits α4 (1.0), β2 (0.78), γ1 (0.88), α2 (0.83), and β1 (0.67); heparan sulfate proteoglycan (Hspg, 0.78); and slight enrichment of collagen VI α1 (0.67), α2 (0.61), and α3 (0.61), (Fig. S4 B and C). The d-enantiomer control showed poor enrichment and lower spectral counts (Fig. S4D). An additional laminin subunit, α5, was identified as enriched with very few low spectral counts. Other enriched proteins either had ratios similar to the d-enantiomer control or showed low or no abundance in the previous MB–NP41 photooxidation experiment (Fig. S4E).
Fig. S3.
NP41 conjugated to various SOGs and biotin shows consistent perineurial localization, whereas in vivo nerve-to-muscle contrast differs between conjugates. (Left) NP41 conjugates and structures. (Middle) NP41–MB and NP41–DBF treated on nerve sections and imaged by fluorescence show perineurial staining (highlighted areas). NP41–biotin treated on tissue sections and stained by streptavidin–HRP and DAB shows strong perineurial signal (darkened areas). (Scale bar, 500 mm.) Gain levels are set individually. (Scale bar, 50 mm.) (Right) NP41 dyes, injected systemically into mice and imaged 2–4 h after, show different sciatic nerve contrast in vivo. NP41–MB showed low nerve fluorescence in vivo that was difficult to distinguish from the surrounding muscle. NP41–DBF showed higher nerve fluorescence and contrast to muscle (n = 2).
Fig. S4.
Ligand proximal photooxidation in vivo with NP41–DBF pyridylhydrazine–biotin (NP41–DBF–HB) conjugate shows enrichment of laminin, collagen, and associated proteins. (A) Structure of NP41–DBF–HB conjugate. (B) Relative quantification of spectral counts in PO sample versus NL control for each protein identified by MS. (Left) In vivo photooxidation of DBF–NP41–HB shows high enrichment of basement membrane proteins, including laminin subunits and nidogens. Collagen VI subunits showed high abundance and low enrichment, and laminin a5 showed high enrichment but low abundance. Two abundant, nerve-specific proteins, neurofilament and myelin protein P0, were not enriched. (Right) By comparison, in vivo photooxidation of a control conjugate with all D-amino acids showed low enrichment and low abundance of the same proteins. (C) Table of values of proteins identified in the graph, showing x and y values of each identified protein. (D) Relative spectral counts of a photooxidized NP41–DBF–HB conjugate compared with a photooxidized d-NP41 control conjugate, showing enrichment and total abundance of identified proteins. Laminin subunits and nidogen showed the highest target-specific enrichment with abundant spectral counts. Laminin-α5 showed high enrichment but low spectral counts, and collagen subunits showed high spectral counts but low enrichment. (E) Relative quantification of other proteins slightly enriched in the NP41–DBF–HB experiment not identified in NP41–MB as enriched and abundant. Proteins showing moderate enrichment (>0.69) upon photooxidaton of NP41–DBF–HB compared with NL control had low enrichment in comparison with the photooxidized D-amino acid control, except for testis-specific ser/thr kinase and lumican. In the MB–NP41 photooxidation experiment, the former was not identified and the latter was unenriched.
Identification of Laminin-α4 and -γ1 as Candidate NP41 Targets by TRICEPS-Based Ligand-Directed Glycoprotein Cross-Linking.
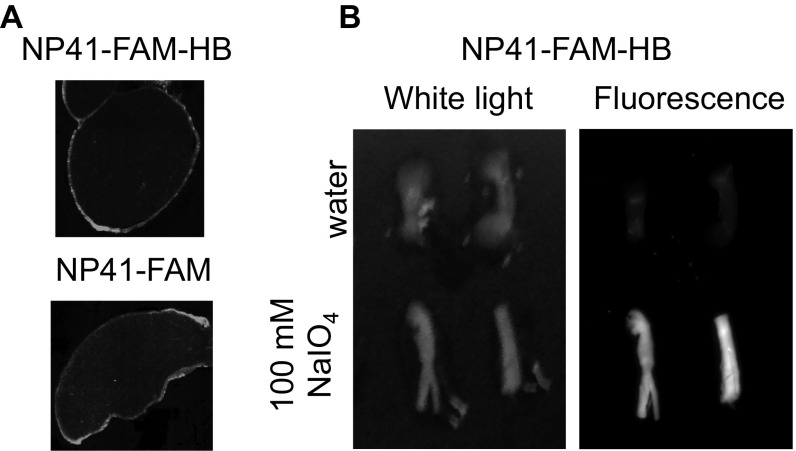
Because laminins and other extracellular matrix proteins are heavily glycosylated (24), a modified TRICEPS-based (8) procedure was used to capture in vivo targets of NP41. NP41–fluorescein–biotin hydrazine (NP41–FAM–HB; Fig. 4, Right) was first injected into mice, and excised nerves were treated with periodate to cross-link prebound peptide. Histological imaging of nerves labeled by systemic injection of NP41–FAM–HB showed similar localization to NP41–FAM (Fig. S5A). High retention of fluorescence was apparent in periodate-treated nerves and not in control-treated nerves, demonstrating hydrazine-mediated cross-linking (Fig. S5B).
Fig. 4.
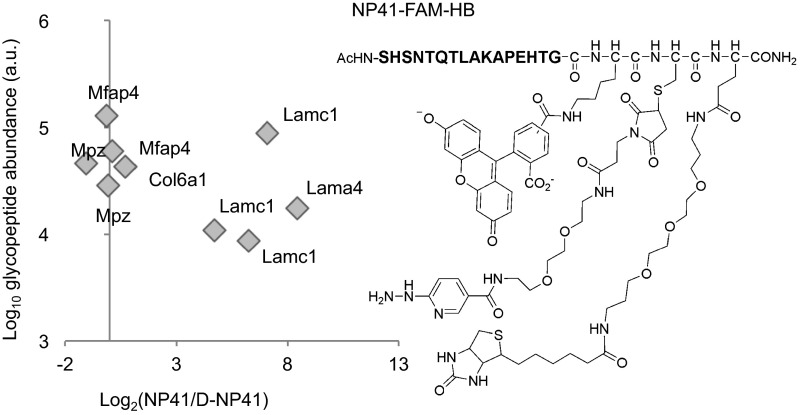
Ligand-based glycoprotein receptor capture identifies laminin subunits α4 and γ1 as NP41 binding partner candidates. (Right) Structure of NP41–FAM–HB conjugate. Fluorescein allows for visualization of the peptide, pyridylhydrazine cross-links the peptide to glycans after tissue is oxidized with sodium periodate, and biotin is used to isolate the peptide target from nerve homogenates. (Left) Glycoprotein receptor capture with NP41--FAM--HB versus all d-amino acid control conjugate identifies formerly glycosylated peptides laminin-γ1 (Lamc1) and -α4 (Lama4) peptides.
Fig. S5.
NP41–FAM–HB localizes to perineurium and cross-links to the nerve after treatment with periodate. (A) Nerves from NP41–FAM–HB- or NP41–FAM-treated mice show similar fluorescence localization with high perineurial staining. (B) Fluorescence is retained in nerves from animals injected with NP41–FAM–HB after periodate glycan oxidation and extensive washout but not in control nonperiodate-treated nerves, indicating glycan-mediated cross-linking.
For target identification, NP41–FAM–HB or a d-enantiomer control was injected into mice, and nerves were excised before periodate application, then processed and analyzed as previously described (8). Quantification of individual peptides containing formerly glycosylated asparagine residues (N[115]-X-S/T motif) revealed laminin-γ1 and -α4 as enriched in the NP41-treated sample over the control (Fig. 4, Left). Specifically enriched peptides included laminin-γ1 peptides TAN[115]ETSAEAYNLLLR, RIPAIN[115]R, and IASAVQKN[115]ATSTKADAER and laminin-α4 peptide HVTDMN[115]STIHLLR.
Confirmation of Ligand–Target Colocalization and NP41–Laminin Binding.
Laminins are trimers of α, β, and γ subunits that complex with nidogen, collagen IV, and Hspg to form basement membranes (25). Collagen VI is a trimer of α1–3 chains and forms microfibrils, collagen I forms fibrils, and decorin is a small proteoglycan that bridges the two (26, 27). Laminin-α4β2γ1 (laminin-421), laminin-α5β2γ1 (laminin-521), laminin-α2β1γ1 (laminin-211), and collagen VI have been previously shown to localize in the peripheral nerve (28, 29).
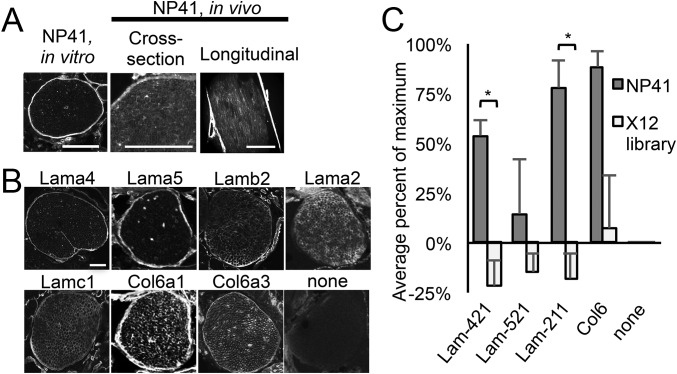
To directly compare the histological localization of NP41 in vitro and in vivo with candidate binding targets in nerve, FAM- or Cy5-conjugated NP41 was topically applied to mouse facial nerve sections or injected into mice before dissection and imaging. Perineurial staining was striking both in vitro (topically applied) and in vivo (systemically injected), whereas endoneurial basement membrane labeling was more prevalent in in vivo- than in in vitro-labeled nerves (Fig. 5A). For comparison, analogous mouse facial nerve sections were treated with antibodies to laminin-α4, -α5, -α2, -β2, and -γ1 and collagen VI α1 and α3 and fluorescent secondary antibody. Although laminin-α5 did not show abundant enrichment by ligand-proximal photooxidation and was not identified by glycoprotein capture, it is known to form a complex with laminin subunits β2 and γ1 in sciatic nerves, making it a candidate of interest (28). Anti–laminin-α4 and -α5 showed mostly perineurial staining, whereas anti–laminin-α2 labeled only endoneurial basement membranes, and anti–laminin-β2 and -γ1 and collagen VI chains highlighted both the perineurium and endoneurium (Fig. 5B). Nidogen and Hspg, also identified as enriched by ligand-proximal photooxidation, are ubiquitously expressed basement membrane proteins known to complex with most laminin isoforms (25) and may be photooxidized due to their proximity to the nerve-specific target or pulled down in complex with laminins.
Fig. 5.
NP41 colocalizes with neural laminins and collagen and binds to purified laminin-421 and -211. (A) NP41–FAM treated on facial nerve sections showed typical bright perineurial staining with little endoneurial staining (Left), whereas systemically injected NP41, imaged in sciatic nerves in cross-section (NP41-FAM, Middle) and longitudinally (NP41-Cy5, Right), shows both high perineurial staining and endoneurial basement membrane. (Scale bar, 500 μm; Left scale bar, 100 μm.) (B) Immunofluorescence of laminin and collagen subunits on mouse facial nerve tissue sections has similar localization to NP41 binding. Perineurium and endoneurial basement membranes were stained by antibodies to laminin and collagen chains. Background staining of no primary antibody control (none) was negligible. (C) ELISA of NP41-expressing phage shows significantly greater binding to purified laminin (Lam)-421 and -211 than control phage expressing a library of random 12-amino acid peptides (X12) but not to Lam-521 or collagen VI (Col6). Shown is the average signal of four independent experiments each performed in triplicate. Values were background-subtracted for binding to control uncoated wells and expressed as a percent of the maximum signal in each experiment. Error bars represent SEM. *P value < 0.01. Student’s t test, P value = 0.0039 (Lam-421), 0.25 (Lam-521), 0.0026 (Lam-211), and 0.071 (Col6).
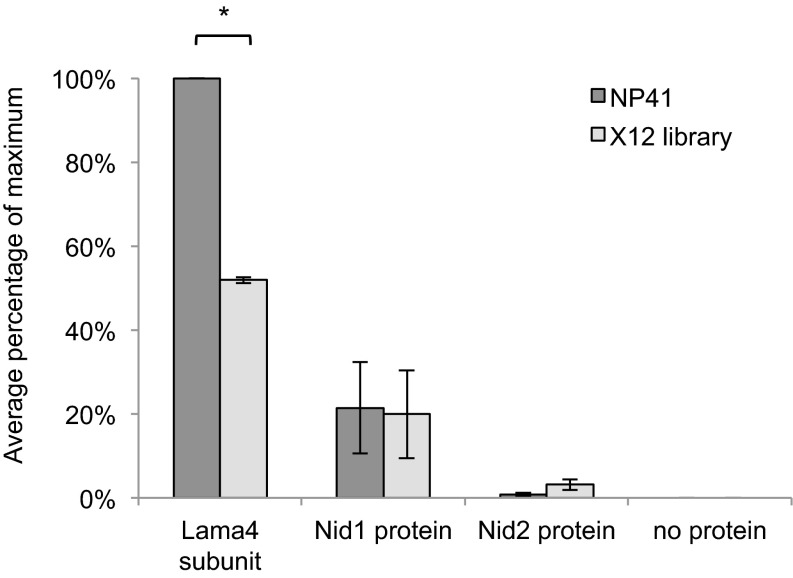
To test NP41–laminin binding, multivalent NP41-expressing M13 phage, from which NP41 was originally discovered (2), or control phage expressing a library of random peptides (X12) was added to plates coated with purified recombinant protein; laminin-421, -521, and -211; or collagen VI and detected with an anti-M13 antibody labeled with horseradish peroxidase. NP41–phage showed significantly greater binding than X12-phage to laminin-421 and -211 (P < 0.01, Student’s t test) but not to collagen VI (P = 0.07) or laminin-521 (P = 0.25; Fig. 5C), neither of which showed great enrichment with the MS techniques. This led us to believe that binding occurred through the α4 domain. To control for the possibility that purified laminins contained nidogen, which was identified in photooxidation experiments but not in carbohydrate-directed LRC and could be the source of NP41 affinity, the assay was repeated on plates coated with laminin-α4, nidogen-1, or nidogen-2. NP41–phage showed significantly higher binding to purified laminin-α4 subunit than did X12 control phage (P < 0.01) and no binding to either nidogen-1 or -2 (Fig. S6).
Fig. S6.
NP41 binds to purified laminin-α4 subunit. ELISA of NP41-expressing phage shows significantly greater binding to purified laminin-α4 subunit (recombinant mouse isoform) than phage expressing a library of random 12-amino acid peptides (*P < 0.01). Binding to nidogen-1 (Nid1, human recombinant isoform) and nidogen 2 (Nid2, mouse recombinant isoform) was not significantly greater than the control. The mean signal, background-subtracted from no protein control, is graphed as a percentage of the maximum signal in two independent experiments, each performed in triplicate. Error bars represent SD. Paired two-tailed t test, P = 0.007 Lama4, P = 0.13 Nid1, and P = 0.16 Nid 2.
Confirmation of Laminin Colocalization with NP41 in Nerve Degeneration Model.
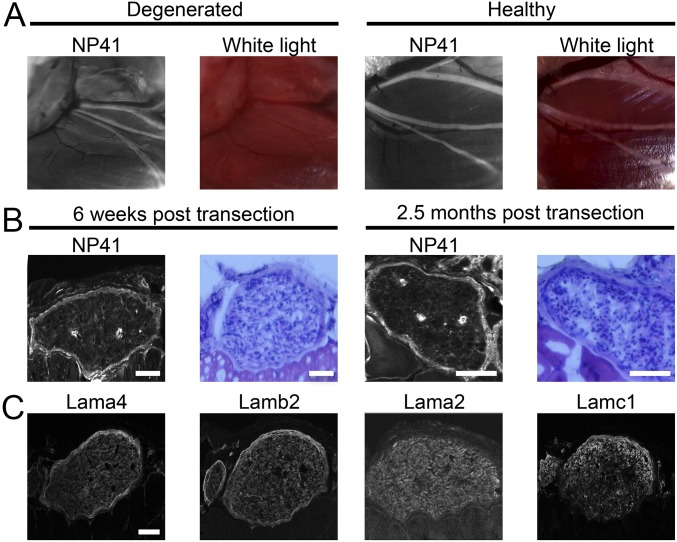
Following nerve injury and subsequent Wallerian degeneration, the tubular space previously occupied by the axon within the nerve becomes infiltrated with immune cells. Many components are cleared, such as myelin and neurofilament, however the extracellular matrix structure and component proteins are retained or up-regulated (30, 31). Because NP41 showed evidence of binding to structural targets, NP41 colocalization with laminin expression was examined in a degenerated nerve model (22, 23). NP41–FAM was injected into mice in which the main branch of the facial nerve had been surgically transected 3 mo prior and allowed to degenerate. Consistent with NP41 binding of extracellular matrix, fluorescence imaging showed NP41–FAM labeling of degenerated nerves in vivo that were not detectable by white light reflectance (Fig. 6A). To verify NP41 binding histologically, cryosections of degenerated facial nerve within surrounding tissues at 6 wk and 2.5 mo posttransection were treated with NP41–FAM, stained with hematoxylin and eosin (H&E), or immunostained for subunits of laminin-421 and -211. In sections, NP41 labeling showed consistent perineurial staining in highly cellular, demyelinated nerves (Fig. 6B). Immunostaining similarly revealed the retention of laminin-421 and -211, which colocalized at the perineurium and endoneurial basement membranes (Fig. 6C).
Fig. 6.
Nerve peptide highlights chronically degenerated nerve ghosts due to conserved laminin structure. (A) NP41-FAM labeling in vivo visualized by fluorescence is retained in degenerated facial nerve (Left), which is invisible by white light reflectance (Middle Left), whereas healthy facial nerve (Middle Right) shows both NP41-FAM fluorescence and is visible by white light imaging (Right) due to the white appearance of intact myelinated nerve bundle. (B) NP41 staining on sections of degenerated nerve shows continued peptide binding despite extensive Wallerian degeneration. Sections of degenerated facial nerve 6 wk (Left and Middle Left) and 2.5 mo posttransection (Middle Right and Right) treated with NP41–FAM (Left and Middle Right) retain perineurial fluorescence. H&E staining (Middle Left and Right) shows that transected nerves are highly cellular (dark blue) with few myelinated sheaths (pink round structures), indicating extensive degeneration. Gain is set individually. (Scale bar, 500 μm.) (C) Immunostaining of laminin subunits shows retention in degenerated nerve basement membranes similar to NP41. Immunostaining on serial sections of mouse facial nerves, 6 wk posttransection, shows continued perineurial and endoneurial basement membrane staining of laminin-α4 (Lama4), laminin-β2 (Lamb2), laminin-α2 (Lama2), and laminin-γ1 (Lamc1). Gain is set individually. (Scale bar, 500 μm for all images.)
Discussion
Fluorescent highlighting of peripheral nerves with molecules like NP41-dye conjugates offers the potential to improve a myriad of surgical procedures by reducing morbidity caused by inadvertent nerve injury. However, characterization of the molecular basis of nerve targeting, which hinges on the identification of the binding target, is necessary for clinical translation. Optimization of NP41 binding to laminins may result in improved peptides, thus facilitating enhanced nerve visualization and repair and preventing nerve damage. Toward this goal, laminin-421 and -211 have here been identified as likely binding targets of NP41 using a ligand-proximal photooxidation assay. An adapted version of previously reported TRICEPS-based LRC also identified laminin-421 chains, suggesting that it is a principle target in vivo. This conclusion was supported by prominent perineurial colocalization of NP41 and laminin subunits α4, β2, and γ1 in healthy and degenerated nerve. The ability of phage displaying NP41 peptide to bind purified laminin-421 as well as laminin-211 supported interaction with both proteins. We speculate that targeting is primarily mediated through α4 subunit as evidenced by striking staining of the perineurium, significant NP41 binding to laminin-421 and α4 laminin subunit, and the lack of significant binding to laminin-521.
Laminins and collagens form extracellular matrix in the majority of tissues but are diverse in their composition of subunit isoforms. Although laminin-421 has tissue-selective expression, it is not neural-exclusive, as it is expressed in other tissues including blood vessels (28). Having been developed by phage display selection for binding intact nerves, it is logical that NP41 targets extracellular matrix and perineurial laminin given their high abundance, prominent exterior location, and the likely low tissue penetration of large phage particles (between 100 and 1,000 nm long). Targeting extracellular matrix proteins is likely beneficial for clinical imaging agents due to the high accessibility of these proteins from circulation and because adverse side effects may be less likely than for molecules that bind proteins within nerve axons or myelin sheaths and potentially alter nerve conduction. NP41 has been criticized for highlighting degenerated as well as live nerves (32), but we regard this as an advantage because it aids surgical repair of degenerated nerves (22, 23), whereas motor nerve function is amenable to assessment by other means.
Proximity-based labeling offers advantages over alternative approaches for fairly weak interactions because it does not necessitate retention of ligand binding during chemical treatment. Most current cross-linking methods require a specific reactive group to exist in a ligand-accessible location on the target macromolecule. This limitation is eliminated with proximal photooxidation and exogenously added BH, in which labeling potentially includes both direct ligand-binding targets as well as closely interacting proteins. In cis-labeling, hydrazine reaction is spatially limited by its tether length to the ligand, likely resulting in tagging of the closest reactive site, presumably within the direct binding target. In addition, photo–cross-linkers such as azides and diazirines are limited to UV wavelengths and can only be photolyzed once (7), whereas photooxidation is compatible with a multitude of SOGs, ranging from UV to infrared, and can undergo multiple cycles, limited only by bleaching. Disadvantages include the limited knowledge of SOG hydrazide-reactive modifications on amino acids, glycans, and lipids and potential loss of specificity due to singlet oxygen’s diffusion range.
Other proximity labeling techniques for MS, such as ascorbate peroxidase coupled to biotin–phenol labeling (APEX) and promiscuous biotin ligase (BioID), use bulky enzyme conjugates requiring biological expression rather than chemical conjugation (10, 11). For the identification of intracellular interactions by photooxidation and BH labeling, genetically encodable SOGs could be substituted, such as miniSOG, FlAsH, or ReAsH (33, 34). MiniSOG's use in proximity labeling for mass spectrometry-based identification of interacting partners has been explored using biotin-thiol to form disulfide bonds with cysteine residues on nearby proteins in the presence of singlet oxygen (12). MiniSOG was also recently demonstrated to facilitate the measurement of distances of proteins within complexes in live cells by singlet oxygen triplet energy transfer of a fluorescent singlet oxygen detector on two putative interacting proteins (35). Furthermore, ligand-proximal photooxidation techniques may be modifiable with alternatives to BH such as antibody pull-down of proteins containing methionine sulfoxide or N-formylkynurenine (36, 37). Future studies will address if molecules with higher affinity toward human laminin subunits α4, α5, or α2 can generate greater clinically useful nerve contrast. Alternatively, natural biomolecules known to target laminins, for instance ML-LBP21 or phenolic glycolipid (PGL-1), of the peripheral nerve-homing pathogen Mycobacterium leprae (38, 39) could be tested.
Materials and Methods
All experiments on mice were performed under protocols approved by the University of California, San Diego Institutional Animal Care and Use Committee. SKH1 mice (Charles River Laboratories) or wild-type albino C57BL6 mice (Jackson Laboratories) were used for all tissues. Synthesis and chemical conjugation of peptides and synthesis of DBF are described in SI Materials and Methods.
Ligand-Proximal Photooxidation on Tissue Sections for Imaging.
Facial nerve and surrounding muscle and fasciae were embedded in Optimal Cutting Temperature (OCT) and frozen on dry ice. Sections 10 μm thick were cut on a cryostat (Leica) and dried on glass slides. Tissues were preblocked with 50 μM nicotinic hydrazide in MES buffer, pH 5.5, for 30 min. NP41–FAM at 375 μM or d-amino acid control in 0.5× Hank’s Buffered Saline Solution (HBSS) was added for 20 min. Slides were washed in PBS for at least 5 min and then exposed for 15 min to 480 nm excitation light (30 nm bandwidth, 0.07 W/cm2) on a solar simulator, wheras control slides were kept dark. Slides were stained with streptavidin–Texas Red for 1 h, washed in PBS, and imaged on a confocal microscope (Zeiss) with 488 nm excitation and 505 nm LP emission (<1 s exposure) to image FAM–peptide and 532 nm excitation and 560–675 nm emission to image Texas Red. Zeiss Zen and Adobe Photoshop software were used to make composite tiled images.
Ligand-Proximal Photooxidation with NP41-MB on Whole Nerves and LC–MS/MS.
Sciatic nerves were submerged in 50 μM NP41–MB in PBS for 2 h. After washing in PBS, nerves were exposed to 640 nm light for 10 s, 1 min, or 15 min or kept dark as a control. Nerves were transferred to 50 mM MES buffer, pH 5.5, with 50 μM BH for 30 min and then homogenized in PBS using a motorized tissue grinder. Sample preparation, LC–MS/MS, and data analysis are described in SI Materials and Methods.
TRICEPS-Based NP41–FAM–HB LRC for MS.
Mice were injected with 200 nmol of FAM–NP41–HB or its d-enantiomer control into the tail vein, and four sciatic nerves each were dissected after 1.5 h. Nerves were minced and resuspended in 0.8 mL 50 mM ammonium bicarbonate (Sigma-Aldrich). Sample preparation, LC–MS/MS, and data analysis are described in SI Materials and Methods.
Immunofluorescence.
Histological imaging of NP41 in vivo binding was performed as previously described (2). Immunostaining was performed with rabbit antisera to mouse laminin-α4, -β2, and -γ1 (kindly provided by T. Sasaki, Oita University, Oita, Japan), -α5 (kindly provided by J. Miner, Washington University, St. Louis), and collagen VI α1 and α3 and rat antibody to laminin-α2 (clone 4H8-2, Santa Cruz Biotechnology). Additional information is provided in SI Materials and Methods.
Phage Binding ELISA.
Plates (96-well) were coated with purified recombinant human laminin-421, -211, and -521 (Biolamina); collagen VI (Abcam); or no protein as a control at 10 μg/mL in sodium bicarbonate (7.5%), pH 9.5, at 4 °C overnight and then blocked with BSA. M13 phage expressing NP41 or random 12-amino acid peptides on pIII (Ph.D.12, New England Biolabs) were added for 1 h, washed in PBS, detected by anti-M13–HRP (GE Healthcare) and TMB 1-Step Ultra colorimetric substrate (Thermo Fisher), and measured on a Tecan plate reader at 650 nm.
Imaging NP41 of Healthy and Degenerated Nerves in Vivo.
Facial nerves were transected as previously described (23). Three months after main branch transection, distal facial nerve branches were imaged, as was a healthy facial nerve control, in 6-mo-old female SKH1 mice. Mice were anesthetized with an i.p. injection of ketamine (150 mg/kg) and xylazine (10 mg/kg). We injected 20 nmol/g FAM–NP41 through the tail vein, and facial nerves were exposed and imaged after 2.5 h washout using a customized Olympus fluorescence dissecting microscope with white light reflectance and with fluorescence light with 450–490 nm excitation and 500–550 nm emission.
SI Materials and Methods
MS of Photooxidation with Streptavidin–MB.
We combined 10 µM streptavidin in PBS with MB–biotin (AttoTec) at 14 µM or PBS as a control. Samples were photooxidized for 0, 3, 10, 30, and 90 s on a solar simulator with 615/40 nm light. A concentration of 0.4% Rapigest and TNE buffer (50 mM Tris·HCl pH 8, 100 mM NaCl, 1 mM EDTA) was added to samples, and solutions were trypsin-digested overnight at 37 °C. LC–MS/MS was performed as described in Ligand-Proximal Photooxidation, LC–MS/MS, except MS database contained sequences only for streptavidin, avidin, and trypsin. The areas of the modified and unmodified peaks were manually integrated.
Imaging of Peptide Binding on Tissue Sections.
NP41 conjugated to MB, DBF, or biotin was topically applied to frozen, unfixed mouse facial nerve sections (10 mm) at 375 mM in 0.5× HBSS for 20 min and then washed in PBS. For imaging biotin–NP41, sections were incubated with an avidin-conjugated peroxidase (ABC Reagent, Vector Labs) and stained for DAB precipitation. Slides were imaged with white light microscopy. Confocal microscopy was used to image NP41–MB (640 nm excitation, 670 nm emission) and DBF–NP41 (488 nm excitation, 520 nm emission; Nikon).
Imaging NP41–SOGs in Vivo.
Adult SKH1 mice (Charles River Laboratories) or wild-type albino C57BL6 mice (Jackson Laboratories) were used for all experiments, and injections were done i.v. through the tail vein.
We injected 300 nmol NP41–DBF and imaged it after 3 h of washout. We injected 150 nmol eosin–NP41 and imaged it 2 h postinjection. We injected 200 nmol MB-NP41 and imaged it 2.3 h postinjection. For imaging, mice were anesthetized with an i.p. injection of 100 µL of a 1:1 mixture of ketamine HCl (100 mg/mL) and midazolam (5 mg/mL), and sciatic nerves were exposed and imaged with Zeiss Lumar.
Ligand-Proximal Photooxidation, LC–MS/MS.
Homogenates were solubilized in four volumes of freshly prepared buffer containing 15 mM Na2HPO4, pH 7, 150 mM NaCl, 1 mM EDTA, 0.2 mM AEBSF, 10 mg/mL aprotinin, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 1 mM DTT. Solution was centrifuged for 5 min at 930 × g and decanted to monomeric avidin agarose beads (Pierce Ultralink) and incubated on ice for 1 h with mild stirring. Supernatant was removed and discarded, and beads were washed three times on ice.
The hydrazone bond was released with two additions of 200 mL 8 M urea Tris buffer, pH 7, followed by 8 M urea 0.6% trifluoroacetic acid (TFA), pH 3, then 50 mM triethylamine, each time transferring the eluted protein to the top of a Millipore Ultracon 0.5 mL concentrator tube. The Filter-Aided Sample Prep (FASP) method was used to remove detergent. Reduction of disulfide bonds during FASP used 5 mM DTT (Sigma-Aldrich) followed by 12 mM iodoacetamide (Sigma-Aldrich). The buffer was exchanged to 100 mM ammonium acetate before overnight trypsin (Promega, 1 μg) digestion at 37 °C. The digest was acidified with 0.4% TFA, and then C18 reverse-phase solid-phase extraction was used to prepare samples of ∼20 μL for LC–MS (TopTip, Glygen Corp.).
Two 3-h LC–MS runs were done for each of the four samples (Orbitrap XL, Thermo Fisher). In each LC–MS run, an 8 μL sample was injected. Peptide samples were separated by reverse-phase chromatography on a HPLCy (HPLC) column (100 μm inner diameter, Polymicro Technologies fused silica) that was packed in-house with a 7-cm stationary phase (Zorbax SB-C18, 80 Å, 5 μm, Agilent) and connected to an Agilent 1100 HPLC and autosampler with a flow rate of 200 µL/min and a flow splitter (microTee, Upchurch). The HPLC was coupled to a LTQ-Orbitrap XL mass spectrometer (Thermo Scientific) equipped with a nanoelectrospray ion source (Thermo Scientific). Peptides were loaded onto the column with 100% buffer A (100% H2O, 0.1% formic acid) and eluted at approximately 200 nL/min over a 148-min linear gradient from 2% to 30% buffer B (10% H2O, 90% acetonitrile, 0.1% formic acid) [all concentration percentages expressed as (vol/vol)]. After the gradient, the column was washed with 100% buffer B and reequilibrated with buffer A. Mass spectra were acquired in a data-dependent manner, with an automatic switch between MS and MS/MS scans. High-resolution MS scans were acquired in the Orbitrap (60,000 FWHM, target value 106) to monitor peptide ions in the mass range of 400–1,600 m/z, followed by collision-induced dissociation (CID) MS/MS scans in the ion trap (minimum signal threshold, 1,000; target value, 105; isolation width, 3 m/z) of the 10 most intense precursor ions. To avoid multiple scans of dominant ions, the precursor ion masses of scanned ions were dynamically excluded from MS/MS analysis for 52 s. Singly charged ions and ions with unassigned charge states were excluded from MS/MS fragmentation.
The mass spectra data were searched with Sequest (Bioworks version 3.3.1 software, Thermo Fisher) using up to three modifications per peptide—that is, +15.9949 Da for methionine and tryptophan and also +3.9949 and +31.9898 Da for tryptophan—to detect kynurenine reverted from the protein–hydrazone linkage. Probability values were calculated by the software, and a filter of P ≤ 0.05 was applied to the peptide spectrum matches (PSMs) for inclusion as a hit. Proteins with fewer than four hits or PSMs were not included in the results shown in the final ratio plots. The database was UniProtKB/Swiss-Prot release number 2015_02 of Mus musculus. Spectral counts for peptide–protein matches were tabulated using Matlab software (Mathworks). The output from the Bioworks Search Results File (.srf) was exported as an Excel comma separated value (.csv) spreadsheet.
The spreadsheet data, for each LC–MS run, were imported into Matlab. A Matlab script was written in-house to collate the listed number of spectral counts for each protein observed in each run. In Excel, the spectral count hits for each condition were totaled—light versus NL or l-peptide versus D-peptide control.
To check the peptide probability calculation, the data were searched against a reversed database and listed with the same P ≤ 0.05 filter as above. The number of False Positive Hits was summed for all of the proteins. This result was divided into the total number of hits for the search of the forward database, multiplied by 100, to give a false discovery rate (FDR) at the peptide level of 4.5%, in agreement with the filter that was set. The probability value P is an inexact estimate for FDR, and higher values of P (for MS/MS spectra from peptides already identified in multiple datasets) were accepted to reduce variability in the ratio of PO/(PO + NL).
Alternate Data Analysis of Ligand-Proximal Photooxidation LC–MS/MS Measurements.
LC–MS/MS raw files were converted to the open mzML format with msconvert. Proteowizard (40) mzML files were searched by the Comet search engine (41) against UniProtKB/Swiss-Prot protein database version 57.15 of M. musculus concatenated with the sequences of common contaminants. Search parameters for the peptide identification included a precursor mass tolerance of 25 ppm, a minimum of one tryptic terminus, and a maximum of two internal trypsin cleavage sites. Cysteine carbamidomethylation (+57.021 Da) was set as a static amino acid modification, and methionine and tryptophan oxidation (+15.995 Da), tryptophan oxidation to kynurenin (+3.995 Da), and tryptophan oxidation to formylkynurenine (+31.989 Da) were set as differential modifications. The PeptideProphet and ProteinProphet tools of the Trans-Proteomic Pipeline (TPP version 4.5) (42) were used for probability scoring of peptides and proteins, and identifications were filtered to an FDR of ≤1%. Identified peptides were quantified by label-free quantification using the Progenesis (Nonlinear Dynamics) software package. Data normalization, imputation for missing values, and statistical testing were carried out using the statistical package MSstats (version 2.1.5) (43) within the R environment.
In Vivo Photooxidation and Labeling with Intramolecular Pyridylhydrazine and Biotin for MS Identification.
SKH1 was tail vein-injected with 450 nmol NP41–DBF–HB or the d-amino acid peptide. After 4 h of washout, mice were anesthetized with an i.p. injection of 100 mL of a 1:1 mixture of ketamine HCl (100 mg/mL) and midazolam (5 mg/mL), sciatic nerves were exposed, and one side was photooxidized with 480 nm (30 nm bandwidth, 0.07 W/cm2) of light on a solar simulator for 15 min, whereas the other was kept dark. Nerves were harvested from killed animals and reacted in 50 mM Mes buffer, pH 5.5. Tissue was processed as described previously for LC–MS/MS analysis.
TRICEPS-Based NP41–FAM–HB Imaging.
Sciatic nerves from mice injected with NP41–FAM–HB were exposed to either 100 mM sodium periodate (NaIO4) for 1 h at 25 °C or water and then washed overnight at 4 °C before imaging FAM fluorescence as described in Materials and Methods.
TRICEPS-Based LRC, Sample Preparation, LC–MS/MS, and Analysis.
Nerves were dissociated by indirect sonication (100% amplitude, 0.8 cycle, 60 s) in a VialTweeter (Hielscher) in 2% (wt/vol) RapiGest surfactant (Waters). Proteins were reduced with 5 mM TCEP (Pierce) at 20 °C for 30 min and alkylated with 10 mM iodoacetamide (Fluka) at 20 °C for 30 min. Trypsin (Sigma-Aldrich) was added at a 1:20 ratio to protein and incubated at 37 °C overnight. Samples were heated to 96 °C for 10 min to inactivate proteases and cleared by centrifugation (13,000 × g for 10 min). Affinity purification was performed by addition of 80 μL washed Streptavidin Plus UltraLink Resin (Pierce) and incubated for 2 h on a slow rotator at 4 °C. Beads were washed extensively in Mobicols (Boca Scientific) connected to a Vac-Man Laboratory Vacuum Manifold (Promega) with four separate buffers: 5 M NaCl; followed by 100 mM NaCl, 100 mM glycerol, 50 mM Tris, and 1% Triton-X 100; followed by 100 mM NaHCO3 pH 11; followed by 50 mM ammonium bicarbonate. Washed beads were incubated with 400 μL 50 mM ammonium bicarbonate containing 2 μL PNGase F (New England Biolabs) in an end-over-end shaker overnight at 37 °C. Peptides were eluted by spinning of the Mobicol and subsequent addition of another 500 μL of 50 mM ammonium bicarbonate. Combined eluates were acidified with 40 μL 10% formic acid and subjected to C18 purification using 3–30 μg UltraMicroSpin Columns (The Nest Group) according to the manufacturer’s instructions.
Peptide samples were separated by reversed-phase chromatography on a high-performance liquid chromatography column (75 μm inner diameter; New Objective) that was packed in-house with a 10-cm stationary phase (Magic C18AQ, 200 Å, 3 μm, Michrom Bioresources) and connected to a nano-flow EASY-nLC II Liquid Chromatograph (Thermo Scientific).
The nLC system was coupled to an LTQ-Orbitrap XL mass spectrometer (Thermo Scientific) equipped with a nanoelectrospray ion source (Thermo Scientific). Peptides were loaded onto the column with 95% buffer A (98% water, 2% acetonitrile, 0.1% formic acid) and eluted with 300 nL/min over a 40 min linear gradient from 5% to 35% buffer B (2% water, 98% acetonitrile, 0.1% formic acid) [all concentration percentages expressed as (vol/vol)]. After the gradient, the column was washed with 95% buffer B and reequilibrated with buffer A. Mass spectra were acquired in a data-dependent manner, with an automatic switch between MS and MS/MS scans. High-resolution MS scans were acquired in the Orbitrap (60,000 FWHM, target value 106) to monitor peptide ions in the mass range of 350–1650 m/z, followed by CID MS/MS scans in the ion trap (minimum signal threshold, 150; target value, 10,000; isolation width, 2 m/z) of the five most intense precursor ions. To avoid multiple scans of dominant ions, the precursor ion masses of scanned ions were dynamically excluded from MS/MS analysis for 15 s. Singly charged ions and ions with unassigned charge states were excluded from MS/MS fragmentation.
Raw data were converted to the open mzXML format with ReAdW (version 4.3.1). mzXML files were searched by the SEQUEST search engine against UniProtKB/Swiss-Prot protein database version 57.15 of M. musculus concatenated with the sequences of common contaminants. Search parameters for the peptide identification included a precursor mass tolerance of 0.05 Da, a minimum of one tryptic terminus, and a maximum of two internal trypsin cleavage sites. Cysteine carbamidomethylation (+57.021 Da) was set as a static amino acid modification, and methionine oxidation (+15.995 Da) and asparagine deamidation (+0.9840 Da) were set as differential modifications. The PeptideProphet and ProteinProphet tools of the TPP (version 4.5) were used for probability scoring of peptides and proteins, and identifications were filtered to an FDR of ≤1%. For the analysis of NP41-captured glycopeptides, peptide features were filtered for the presence of the N[115]-X-S/T motif introduced by PNGaseF cleavage (wherein N[115] stands for a deamidated asparagine residue, X for any amino acid except proline, and S/T for serine or threonine). Peptides and proteins were quantified by label-free quantification using the Progenesis (Nonlinear Dynamics) software package.
Immunofluorescence.
Healthy or degenerated facial nerves (6 wk or 2.5 mo posttransection) in surrounding muscle were fixed in 4% paraformaldehyde in PBS, blocked with 5% normal goat serum (Invitrogen), and stained with the primary antibodies listed in Materials and Methods. Signal was detected with AlexaFluor488 anti-rabbit and -rat antibodies (Invitrogen) and imaged with Nikon confocal fluorescence microscope. Serial sections stained with H&E were imaged with white light microscopy.
Phage-Binding ELISA.
The 96-well plates were coated with purified recombinant mouse laminin-α4 subunit, human nidogen-1, mouse nidogen-2, or no protein as a control at 10 µg/mL in 2 M sodium bicarbonate, pH 9.5. ELISA was performed as described in Materials and Methods. Multiple measurements were taken every 5 min over the course of HRP development, each normalized by background subtraction and expressed as a percentage relative to the maximum signal. All measurements except at time 0 were averaged.
Peptide Synthesis and Labeling.
All NP41 peptides (acetyl-SHSNTQTLAKAPEHTGC-amide) were labeled on the C terminus cysteine by maleimide derivatives of MB (AttoTec), DBF, eosin (Life Technologies), or biotin (Sigma-Aldrich). NP41–FAM was prepared as previously described. Peptides were purified to >95% purity using C-18 reverse-phase HPLCy (HPLC) with a 20–50% acetonitrile gradient in 0.05% TFA and confirmed by MS [all concentration percentages expressed as (vol/vol)].
5,6-carboxy-4′,5′-DBF.
To a stirred suspension of 5,6-FAM (376 mg, 1 mmol) in 80% aq. HOAc (5 mL) at room temperature, bromine (102 mL, 2 mmol) was added dropwise. The solid dissolved, and after stirring overnight, the crude product was collected by filtration, washed with water, and dried in vacuo over P2O5 (Yield, 0.5 g). LC–MS revealed the desired product (56% by absorbance at 254 nm) with unreacted starting material (1%) and mono- (5.5%), tri- (15.6%), and tetrabrominated (21.7%) products and was used without further purification [ES-MS(M+H+), 534.8; calculated, 534.9].
5,6-carboxy-4′,5′-DBF N-hydroxysuccinimide ester (DBF-NHS).
Crude 5,6-carboxy-4’,5′-DBF (200 mg, 0.37 mmol) was dissolved in anhydrous THF (10 mL) under N2, and NHS (52 mg, 0.45 mmol) was added followed by diisopropylcarbodiimide (DIPC; 59 µL, 0.38 mmol). After 4 h of stirring at room temperature, LC–MS indicated an incomplete reaction, so an additional 25 µL of DIPC was added. After overnight incubation, the reaction mix was evaporated, dissolved in dry DMSO (1 mL), filtered, and purified by prep HPLC eluting with a gradient of 10–90% acetonitrile–water–0.05% TFA for 20 min. The collected fractions were pooled and immediately frozen and lyophilized to give a red solid (ES–MS(M+H+), 631.9; calculated, 632.9).
l-NP41–DBF–biotin–pyridylhydrazine.
NH2–SHSNTQTLAKAPEHTGCE(biotinyl-PEG)–CONH2 was synthesized by standard Fmoc chemistry on NovaSyn TGR resin using Fmoc-glu(biotinyl-PEG)–OH (Novabiochem). The washed and dried resin-bound peptide (160 mg, 25 µmol nominal) was suspended in dry DMF (1 mL), and DBF–NHS ester (15 mg, 24 µmol) was added with 4-methylmorpholine (50 µL, 0.45 mmol). After stirring overnight at room temperature, the product was collected by filtration, washed with DMF (3 × 5 mL) and methanol (3 × 5 mL), and dried in vacuo. Following cleavage for 2 h with 2 mL of freshly prepared cleavage mixture (2.5% water, 2.5% thioanisole, 2.5% TIPS, 2.5% EDT, 90% TFA) and removal of the resin by filtration, the peptide was precipitated with cold diethyl ether-hexane (1:1 vol/vol) and collected by centrifugation. The product was purified by prep reverse-phase HPLC using a gradient of acetonitrile–water with 0.05% TFA to yield 11.1 mg l- and 11.8 mg d-peptides [ES–MS(M+3H+), 952.6; calculated, 952.6; (M+4H+), 714.8; calculated, 714.7].
The peptide was dissolved in 50% ACN–water–0.1% TFA (0.5 mL, ∼8 mM) and treated with maleimide–peg2–pyridylhydrazine acetone hydrazone (70 µL, 100 mM solution in DMSO) and K.Mops (100 µL, 0.1 M in water pH 7.2). After 1 h at room temperature, acetic acid (100 µL) and acetone (50 µL) were added and kept overnight at 4 °C. The product was purified by prep HPLC to yield 11.5 mg [ES–MS(M+3H+), 1,110.7; calculated, 1110.4; (M+4H+), 833.4; calculated, 833.1].
d-NP41–FAM–biotin–pyridylhydrazine.
NH2–shsntqtlakapehtgcE(biotinyl-PEG)–CONH2 was synthesized using the procedure for l-NP41–DBF–biotin–pyridylhydrazine [ES–MS(M+3H+), 1,110.9; calculated, 1,110.4; (M+4H+), 833.4; calculated, 833.1].
NP41–FAM–biotin–pyridylhydrazine.
NH2–SHSNTQTLAKAPEHTGK(FAM)CE(biotinyl-PEG)–CONH2 was synthesized by standard Fmoc chemistry on NovaSyn TGR resin using Fmoc-glu(biotinyl-PEG)–OH (Novabiochem) and Fmoc-lys(5,6-FAM) (Anaspec). The washed and dried resin-bound peptide (25 µmol nominal) was suspended in dry DMF (2 mL), and N-acetoxysuccinimde (100 mg, 0.64 mmol) was added with 4-methylmorpholine (100 µL, 0.91 mmol). The procedure from l-NP41–DBF–Biotin–pyridylhydrazine was then followed [ES–MS(M+3H+), 1,114.7; calculated, 1,114.7; (M+4H+), 836.6; calculated, 836.3]. Partial loss of acetone occurs to the free hydrazine [ES–MS(M+3H+), 1,101.2; calculated, 1,101.3; (M+4H+), 826.4; calculated, 826.3].
Acknowledgments
In memory of Roger Y. Tsien. We thank John Ngo for providing suggestions, Paul Steinbach for support, Takako Sasaki and Jeffrey Miner for providing antibodies, and members of the R.Y.T. laboratory for helpful discussions and comments. This work was supported by NIH/National Cancer Institute (NCI) Grant 5R01CA158448 and NIH/National Institute of Neurological Disorders and Stroke (NINDS) Grant 2RO1NS027177 (to R.Y.T.), NIH/National Institute of Biomedical Imaging and Bioengineering (NIBIB) Grant 1R01EB014929 (to Q.T.N.), and grant support from the Swiss National Science Foundation (31003A_160259 to B.W.) and the Special Opportunity Project HDL-X from the Swiss Initiative in Systems Biology SystemsX.ch (to B.W.).
Footnotes
The authors declare no conflict of interest.
2Deceased August 24, 2016.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1611642113/-/DCSupplemental.
References
- 1.Smith GP, Petrenko VA. Phage display. Chem Rev. 1997;97(2):391–410. doi: 10.1021/cr960065d. [DOI] [PubMed] [Google Scholar]
- 2.Whitney MA, et al. Fluorescent peptides highlight peripheral nerves during surgery in mice. Nat Biotechnol. 2011;29(4):352–356. doi: 10.1038/nbt.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasqualini R, Ruoslahti E. Organ targeting in vivo using phage display peptide libraries. Nature. 1996;380(6572):364–366. doi: 10.1038/380364a0. [DOI] [PubMed] [Google Scholar]
- 4.Brown KC. New approaches for cell-specific targeting: Identification of cell-selective peptides from combinatorial libraries. Curr Opin Chem Biol. 2000;4(1):16–21. doi: 10.1016/s1367-5931(99)00045-9. [DOI] [PubMed] [Google Scholar]
- 5.Weissleder R. Molecular Imaging: Principles and Practice. People’s Medical Pub. House–USA; Shelton, CT: 2010. p. xxii, 135. [Google Scholar]
- 6.Sinz A. Investigation of protein-protein interactions in living cells by chemical crosslinking and mass spectrometry. Anal Bioanal Chem. 2010;397(8):3433–3440. doi: 10.1007/s00216-009-3405-5. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka Y, Bond MR, Kohler JJ. Photocrosslinkers illuminate interactions in living cells. Mol Biosyst. 2008;4(6):473–480. doi: 10.1039/b803218a. [DOI] [PubMed] [Google Scholar]
- 8.Frei AP, et al. Direct identification of ligand-receptor interactions on living cells and tissues. Nat Biotechnol. 2012;30(10):997–1001. doi: 10.1038/nbt.2354. [DOI] [PubMed] [Google Scholar]
- 9.Frei AP, Moest H, Novy K, Wollscheid B. Ligand-based receptor identification on living cells and tissues using TRICEPS. Nat Protoc. 2013;8(7):1321–1336. doi: 10.1038/nprot.2013.072. [DOI] [PubMed] [Google Scholar]
- 10.Rhee HW, et al. Proteomic mapping of mitochondria in living cells via spatially restricted enzymatic tagging. Science. 2013;339(6125):1328–1331. doi: 10.1126/science.1230593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roux KJ, Kim DI, Raida M, Burke B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J Cell Biol. 2012;196(6):801–810. doi: 10.1083/jcb.201112098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.To TL, et al. Photoactivatable protein labeling by singlet oxygen mediated reactions. Bioorg Med Chem Lett. 2016;26(14):3359–3363. doi: 10.1016/j.bmcl.2016.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moan J, Berg K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem Photobiol. 1991;53(4):549–553. doi: 10.1111/j.1751-1097.1991.tb03669.x. [DOI] [PubMed] [Google Scholar]
- 14.Baker A, Kanofsky JR. Quenching of singlet oxygen by biomolecules from L1210 leukemia cells. Photochem Photobiol. 1992;55(4):523–528. doi: 10.1111/j.1751-1097.1992.tb04273.x. [DOI] [PubMed] [Google Scholar]
- 15.Silvester JA, Timmins GS, Davies MJ. Protein hydroperoxides and carbonyl groups generated by porphyrin-induced photo-oxidation of bovine serum albumin. Arch Biochem Biophys. 1998;350(2):249–258. doi: 10.1006/abbi.1997.0495. [DOI] [PubMed] [Google Scholar]
- 16.Davies MJ. Reactive species formed on proteins exposed to singlet oxygen. Photochem Photobiol Sci. 2004;3(1):17–25. doi: 10.1039/b307576c. [DOI] [PubMed] [Google Scholar]
- 17.Nilsson R, Merkel PB, Kearns DR. Unambiguous evidence for the participation of singlet oxygen ( 1 ) in photodynamic oxidation of amino acids. Photochem Photobiol. 1972;16(2):117–124. doi: 10.1111/j.1751-1097.1972.tb07343.x. [DOI] [PubMed] [Google Scholar]
- 18.Kim J, et al. Oxidative modification of cytochrome c by singlet oxygen. Free Radic Biol Med. 2008;44(9):1700–1711. doi: 10.1016/j.freeradbiomed.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies MJ. Singlet oxygen-mediated damage to proteins and its consequences. Biochem Biophys Res Commun. 2003;305(3):761–770. doi: 10.1016/s0006-291x(03)00817-9. [DOI] [PubMed] [Google Scholar]
- 20.Hensley K. Detection of protein carbonyls by means of biotin hydrazide-streptavidin affinity methods. Methods Mol Biol. 2009;536:457–462. doi: 10.1007/978-1-59745-542-8_46. [DOI] [PubMed] [Google Scholar]
- 21.Kalia J, Raines RT. Hydrolytic stability of hydrazones and oximes. Angew Chem Int Ed Engl. 2008;47(39):7523–7526. doi: 10.1002/anie.200802651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu AP, et al. Improved facial nerve identification with novel fluorescently labeled probe. Laryngoscope. 2011;121(4):805–810. doi: 10.1002/lary.21411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hussain T, et al. Fluorescently labeled peptide increases identification of degenerated facial nerve branches during surgery and improves functional outcome. PLoS One. 2015;10(3):e0119600. doi: 10.1371/journal.pone.0119600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Timpl R, et al. Laminin--A glycoprotein from basement membranes. J Biol Chem. 1979;254(19):9933–9937. [PubMed] [Google Scholar]
- 25.Paulsson M, et al. Laminin-nidogen complex. Extraction with chelating agents and structural characterization. Eur J Biochem. 1987;166(1):11–19. doi: 10.1111/j.1432-1033.1987.tb13476.x. [DOI] [PubMed] [Google Scholar]
- 26.Chen P, Cescon M, Megighian A, Bonaldo P. Collagen VI regulates peripheral nerve myelination and function. FASEB J. 2014;28(3):1145–1156. doi: 10.1096/fj.13-239533. [DOI] [PubMed] [Google Scholar]
- 27.Nareyeck G, et al. Differential interactions of decorin and decorin mutants with type I and type VI collagens. Eur J Biochem. 2004;271(16):3389–3398. doi: 10.1111/j.1432-1033.2004.04273.x. [DOI] [PubMed] [Google Scholar]
- 28.Patton BL, Miner JH, Chiu AY, Sanes JR. Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. J Cell Biol. 1997;139(6):1507–1521. doi: 10.1083/jcb.139.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von der Mark H, Aumailley M, Wick G, Fleischmajer R, Timpl R. Immunochemistry, genuine size and tissue localization of collagen VI. Eur J Biochem. 1984;142(3):493–502. doi: 10.1111/j.1432-1033.1984.tb08313.x. [DOI] [PubMed] [Google Scholar]
- 30.Salonen V, Peltonen J, Röyttä M, Virtanen I. Laminin in traumatized peripheral nerve: Basement membrane changes during degeneration and regeneration. J Neurocytol. 1987;16(5):713–720. doi: 10.1007/BF01637662. [DOI] [PubMed] [Google Scholar]
- 31.Wallquist W, et al. Laminin chains in rat and human peripheral nerve: Distribution and regulation during development and after axonal injury. J Comp Neurol. 2002;454(3):284–293. doi: 10.1002/cne.10434. [DOI] [PubMed] [Google Scholar]
- 32.Massaad CA, et al. Fluorescently-tagged anti-ganglioside antibody selectively identifies peripheral nerve in living animals. Sci Rep. 2015;5:15766. doi: 10.1038/srep15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shu X, et al. A genetically encoded tag for correlated light and electron microscopy of intact cells, tissues, and organisms. PLoS Biol. 2011;9(4):e1001041. doi: 10.1371/journal.pbio.1001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tour O, Meijer RM, Zacharias DA, Adams SR, Tsien RY. Genetically targeted chromophore-assisted light inactivation. Nat Biotechnol. 2003;21(12):1505–1508. doi: 10.1038/nbt914. [DOI] [PubMed] [Google Scholar]
- 35.To TL, Fadul MJ, Shu X. Singlet oxygen triplet energy transfer-based imaging technology for mapping protein-protein proximity in intact cells. Nat Commun. 2014;5:4072. doi: 10.1038/ncomms5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oien DB, et al. Detection of oxidized methionine in selected proteins, cellular extracts and blood serums by novel anti-methionine sulfoxide antibodies. Arch Biochem Biophys. 2009;485(1):35–40. doi: 10.1016/j.abb.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ehrenshaft M, et al. Immunological detection of N-formylkynurenine in oxidized proteins. Free Radic Biol Med. 2009;46(9):1260–1266. doi: 10.1016/j.freeradbiomed.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimoji Y, Ng V, Matsumura K, Fischetti VA, Rambukkana A. A 21-kDa surface protein of Mycobacterium leprae binds peripheral nerve laminin-2 and mediates Schwann cell invasion. Proc Natl Acad Sci USA. 1999;96(17):9857–9862. doi: 10.1073/pnas.96.17.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ng V, et al. Role of the cell wall phenolic glycolipid-1 in the peripheral nerve predilection of Mycobacterium leprae. Cell. 2000;103(3):511–524. doi: 10.1016/s0092-8674(00)00142-2. [DOI] [PubMed] [Google Scholar]
- 40.Kessner D, Chambers M, Burke R, Agus D, Mallick P. ProteoWizard: open source software for rapid proteomics tools development. Bioinformatics. 2008;24(21):2534–2536. doi: 10.1093/bioinformatics/btn323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eng JK, Jahan TA, Hoopmann MR. Comet: an open-source MS/MS sequence database search tool. Proteomics. 2013;13(1):22–24. doi: 10.1002/pmic.201200439. [DOI] [PubMed] [Google Scholar]
- 42.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Analytical chemistry. 2002;74(20):5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 43.Choi M, et al. MSstats: an R package for statistical analysis of quantitative mass spectrometry-based proteomic experiments. Bioinformatics. 2014;30(17):2524–2526. doi: 10.1093/bioinformatics/btu305. [DOI] [PubMed] [Google Scholar]