After recent setbacks, researchers hope to find approaches more attuned to the complexities of cancer biology.
The miniature craft cruises silently through a blood vessel. Sneaking through a hole in the vessel wall, it infiltrates a tumor and uses an on-board antibody key to gain entry into a cancer cell. Once inside, the sleek ship deploys its anticancer cargo to destroy the tumor. Mission accomplished.
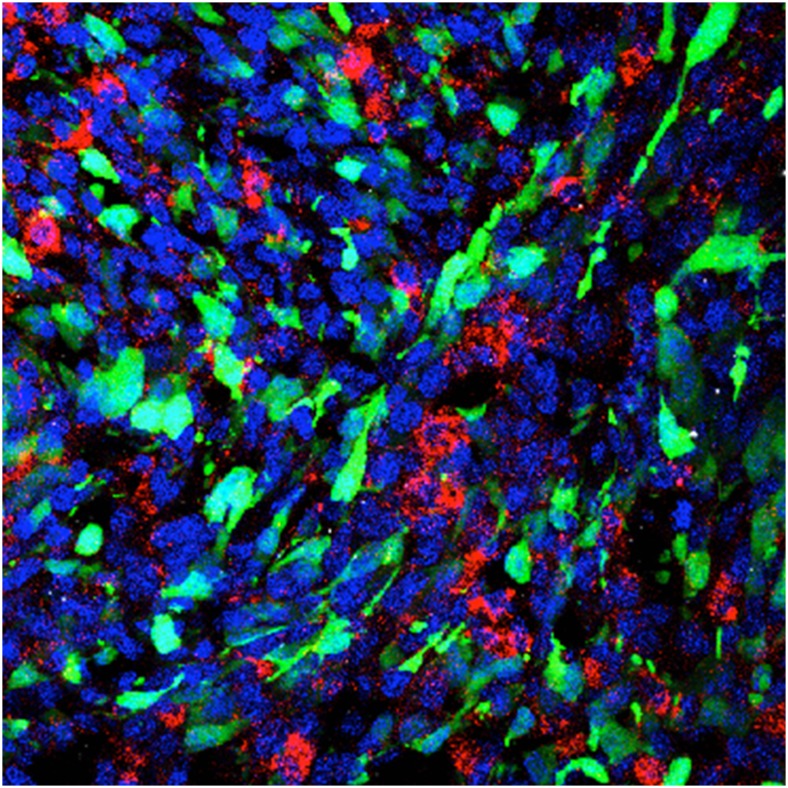
One experimental nanomedicine approach employs a rat model of glioblastoma in which nanoparticles (red), injected intracranially, are taken up by tumor cells (green). Image courtesy of Eric Song (Yale University, New Haven, CT).
This vision of nanomedicine, commonly portrayed in animations from the early 2000s, promised that nanotechnology would be cancer’s magic bullet, giving us therapies that could identify the site of disease, travel there, and wipe it out.
Omid Farokhzad, a physician and biomaterials researcher at the Brigham and Women’s Hospital in Boston, says he now feels embarrassed when he sees such videos. “Fifteen years ago, our concept was that if we put some tumor-targeting ligands on the surface of a nanoparticle, it would get into a cell,” says Farokhzad. “That is incredibly naïve.”
In principle, nanomedicine could deliver a drug (or even a combination of drugs) precisely where it’s needed in the body. This increases a drug's effectiveness while avoiding side effects caused by flooding the entire body with a conventional chemotherapy, all this by virtue of their size. One of the field’s core assumptions is that nanoparticles are small enough to leak through shoddily built blood vessels around tumors, but too big to pass through normal vasculature into healthy tissues. Based on this passive targeting principle, the field has had a handful of clinical successes that rely on bulky nanoparticle carriers to keep toxic cancer drugs out of the heart and other off-target tissues.
But at the same time, researchers want to improve the nanoparticle’s active targeting of tumor tissue, which has been much more difficult to achieve. Until recently, the messy biology of real human tumors has repulsed most attempts.
So researchers are trying a fresh approach, reengineering nanomedicine using data about how nanoparticles interact with tissues and cells. They’re also striving to understand why nanomedicine works in some patients and tumors but fails in others. While shrugging off the field’s more fantastical visions, nanomedicine researchers now hope to make more effective, targeted therapies that live up to their name.
Mixed Results
This year, nanomedicine suffered a setback that seems to have intensified the introspective mood of nanotechnologists. In May, one of the leading nanomedicine companies, BIND Therapeutics of Cambridge, Massachusetts, declared bankruptcy after disappointing clinical trial results and poorly performing stock increased pressure on the company to repay debts. Cofounded by Farokhzad and prominent Massachusetts Institute of Technology (MIT) chemical engineer and serial entrepreneur Robert Langer, BIND had received a lot of attention. Observers lauded its aim of using sophisticated chemical engineering and computer-automated design techniques to make polymer nanocarriers that would actively target cancer cells with tumor-specific binding molecules on their surfaces.
Nanomedicine researchers had high hopes for BIND’s strategy, and were disappointed when it fell short. More consternation followed with the publication of a critical meta-analysis of the field in April (1). University of Toronto nanotechnologist Warren Chan and colleagues reviewed more than 100 nanomedicine papers from the past 10 years, and found that an average of just 0.7% of any nanoparticle dose—whether actively targeted or not—gets into tumors. “I was surprised it was that low,” says Chan. “When you think ‘nano’ you think ‘targeted’—it’s a psychological assumption.”
The review has had its fair share of criticism, with some arguing that it unfairly lumps together data on many different tumors, drugs, and nanoparticles. And although one percent or so doesn’t sound like much, “that level of accumulation can actually be useful if it happens in the right cells,” says Sangeeta Bhatia, a biomedical engineer at MIT.
Still, others have welcomed Chan’s full-throated call for convincing demonstrations that nanoparticles can significantly improve targeted delivery of drugs, and make a marked difference in clinical outcomes for patients. Chan’s results “have been presented as a very dark, negative thing, but we have to be realistic and move on,” says Shanta Dhar, a biomedical engineer at the University of Miami Medical School.
There has been good news for nanomedicine this year as well. In June, for example, Celator, a subsidiary of Jazz Pharmaceuticals of Dublin, Ireland, announced positive results of a phase 3 nanomedicine trial in acute myeloid leukemia. The company’s combination of two cancer drugs carried in a liposome nanoparticle modestly improved outcomes. Because overall survival for patients with this disease is poor, even a small improvement is noteworthy, says Piotr Grodzinski, director of the Office of Cancer Nanotechnology Research at the National Cancer Institute in Bethesda, Maryland.
Grodzinski thinks that nanomedicine could make a bigger impact by focusing on diseases that lack conventional therapy options. “The field needs to establish some niche applications where therapies or diagnostics are almost nonexistent,” he says.
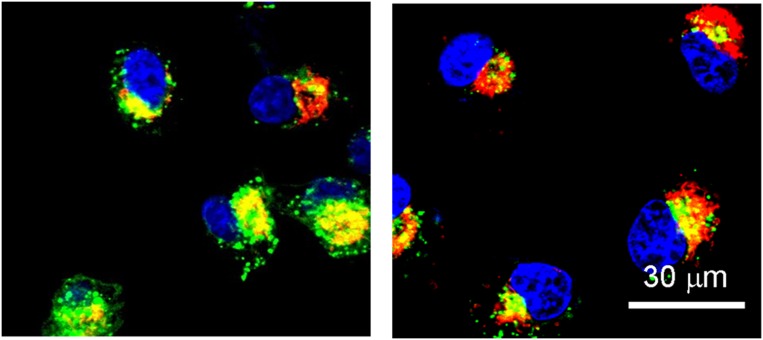
These confocal laser scanning microscope images show nanoparticles (red) releasing a payload of gene-silencing RNA into cervical cancer cells. Image courtesy of Xia-Ding Xu (Harvard Medical School, Boston).
Going Places
Yale University biomedical engineer Mark Saltzman believes glioblastoma is just such a disease. This brain cancer has dismal survival figures, and most people die within a year of diagnosis. Saltzman hopes to design a nanomedicine that targets cancer stem cells, which can linger deep in the brain after surgery or conventional chemotherapy, and eventually restart a tumor. Getting a drug deep into the brain is critical for treating glioblastoma.
However, delivering drugs into the brain is difficult. The cells lining the brain’s blood vessels are connected by much tighter junctions than in the rest of the circulatory system, creating a highly policed entryway called the blood–brain barrier. It lets in glucose and other essential supplies, but tends to keeps out toxins and nanoparticles. The brain is also adept at flushing out drugs once they get in.
The stalwart blood–brain barrier spurred Saltzman to abandon the usual approach of injecting nanoparticles into the bloodstream and hoping they get to the right place. Instead, the Yale researcher aims to infuse nanocarriers behind the barrier, directly into the interstitial fluid that bathes the brain. Then, a process called convection-enhanced delivery would carry the drug deeper. The method involves applying positive pressure when infusing the drug, which moves the liquid surrounding the brain and sets up a convection current.
Others had shown that this principle works with liposome nanoparticles, and that the particles were big enough to get stuck inside the brain. But liposomes do not excel at long-term drug release, so Saltzman turned to nanoparticles made of a polymer called poly(lactic-coglycolic acid) (PLGA) that is already used in sutures and other medical implants. He had to make the PLGA nanoparticles smaller than in previous nanomedicine formulations so they could fit through the interstitial spaces of the brain, which are as narrow as tens of nanometers in tumors.
In 2013, the Yale group reported that PLGA nanoparticles about 70 nanometers wide were small enough to move around in the brain, yet still capable of carrying and releasing drugs (2). The researchers screened a library of anticancer compounds for effectiveness against glioblastoma cancer stem cells, and combined promising candidates with the carrier. In mice, this brain-targeted, glioblastoma stem cell-specific therapy shrank tumors derived from human brain cancer stem cells.
Mouse brains are much smaller than human ones, though. In people, glioblastomas spread out to locations up to two centimeters from the initial site, which means the tiny nanoparticles have a lot of ground to cover. Tests in pig brains have confirmed that convection-enhanced delivery carries their nanodrugs into volumes over 1,000 cubic millimeters. The Yale group is now working on an application to run a clinical trial at the university’s medical center.
One appeal of this delivery vehicle is that it could be a platform for any sort of drug that needs to get deep
“Nobody's infused nanoparticles into the human brain in the presence of a tumor before, so there is a lot to learn.”
—Mark Saltzman
into the brain and be released in a controlled fashion over time, at which PLGA nanoparticles excel. If the brain-specialized drug carrier shows clinical promise, scientists could try using it to deliver new anticancer compounds and other neurological drugs. “Nobody’s infused nanoparticles into the human brain in the presence of a tumor before, so there is a lot to learn,” says Saltzman. “But we’re confident it works in animals.”
Sweating the Small Stuff
Everyone working in nanomedicine should apply this sort of back-to-basics approach, says Chan. He argues that the nanomedicine community should focus on making sure their results are repeatable, and perform fundamental studies of how nanoparticles actually work inside the human body. “If you don’t know what an alternator does and you try to build a car, it’s not going to work,” he says. “If you understand how a system works, you can engineer your system.”
The fundamental challenges are many. Take, for example, the near-universal problem of nanoparticle-clogging proteins. “Anytime you introduce a nanoparticle to the bloodstream, a lot of proteins bind weakly to the particle and form a cloud around it,” says Kim Hamad-Schifferli, a biological engineer at MIT. “It’s impossible to make a particle that doesn’t form a protein corona.” These protein clouds can cover the tumor-binding molecules that nanoengineers have painstakingly designed and placed on the surface of their drug carriers.
Because there’s no good way to prevent the corona from forming, engineers are figuring out how to use it to their advantage. Farokhzad thinks that gaining control of the protein coating could be key: the proteins on a nanoparticle are part of what determine how long it can circulate, how the immune system reacts to it, and where it goes. Often, a coated nanoparticle is either excreted through the kidneys or taken up by immune cells called macrophages and marched to the liver, lungs, or spleen. But these pathways are not inevitable, and Farokhzad is working on systematic studies of how the size of a nanoparticle affects the chemistry of the protein corona. It’s possible the right nanoparticle could attract a protein corona that instructs the body to send it to a particular tissue, or to a tumor. And Hamad-Schifferli has shown in vitro that the protein coating can improve the function of a nanoparticle that can switch blood clotting on and off (3).
Chan is already working on translating detailed studies of nanoparticle fates to people. “We’re trying to design for people but we can’t test on them,” says Chan. So his laboratory has started a collaboration with the University of Toronto’s medical center to work on the next best thing: patient samples. Resected liver tumors, for example, can be perfused with nourishing fluid and kept alive long enough to study nanoparticle paths through these tissues.
Projects like these are logistically difficult for most biomaterials researchers to do on their own, but Chan believes they could easily be incorporated into clinical trials. By monitoring patients for clinical outcomes, and correlating them with information about how an investigational nanodrug moves through a particular patient’s tumor biopsy, companies could learn why a drug works, or doesn’t work. That knowledge can then feed back into future nanoparticle designs. This strategy promises to reveal fundamental information about the realities of human tumors, and a growing number of researchers are now pursuing this approach.
Watch Closely
If the goal is to improve clinical outcomes immediately, though, there may be an easier way: using imaging methods to assess how receptive a patient will be before giving them a nanomedicine. Personalized medicine is a central doctrine of just about every other aspect of cancer research, and it’s increasingly being applied to nanomedicine as well. No breast cancer patient is put on Herceptin unless her tumor has the receptor it binds to; nor should a patient be put on a nanomedicine if her tumor won’t let it in.
Merrimack Pharmaceuticals of Cambridge, Massachusetts, is using a Food and Drug Administration-approved MRI agent called ferumoxytol, which contains iron oxide nanoparticles, as a sort of reconnaissance operative to reveal whether a tumor will let in nanoparticles. If the ferumoxytol gets into the tumor, the company reasons, the therapeutic nanoparticle probably will too. At the American Association for Cancer Research annual meeting in San Diego in 2014, the company presented results from a pilot trial of 12 patients with advanced tumors. Tumors that accumulated the imaging nanoparticle also tended to take up their nanotherapy MM-398, a liposome carrying the cancer drug irinotecan. The company now hopes to prove the predictive effect of its imaging screen in two phase 1 clinical trials: one for patients with advanced breast cancer and other solid tumors, and another for patients whose tumors are metastatic, or cannot be surgically removed.
Meanwhile, Kazunori Kataoka at the University of Tokyo is using imaging to better understand passive targeting. His goal is to design nanoparticles that are suited to particular types of tumors and different kinds of tumor blood vessel dynamics.
Kataoka has previously developed four nanomedicines that are now in clinical trials being run by Chiba, Japan-based nanomedicine maker NanoCarrier and Nippon Kayaku, a Tokyo-based multinational chemical and drug company (4). One of these drugs, which is in phase 3 clinical trials in pancreatic cancer, responds to the relatively acidic environment of the tumor to trigger drug release.
In a study published this February, Kataoka focused instead on how nanoparticles of different sizes get into pancreatic tumors, and what happens when they do (5). Pancreatic cancer is particularly difficult to treat, and the tumors are dense, making tough going for nanoparticles. His team imaged pancreatic tumors and their vasculature every 10 minutes for 10 hours after dosing mice with different-sized calcium phosphate nanoparticles. These nanoparticles don’t carry any drugs, but they are visible under microscopy in living animals.
The researchers found that small particles of 30 nanometers or so can leak into pancreatic tumors, but larger particles only enter during random “nanoeruptions” of blood. “The mechanism is not so simple as people believe,” says Kataoka. And whereas the smaller particles can migrate into the tumor’s dense tissue, larger particles—even when they enter through an eruption—get stuck at the periphery. However, the researchers did not see the nanoeruptions in colorectal and ovarian tumor vasculature, the blood vessels of which are naturally leakier and let in larger nanoparticles. This kind of information should inform the selection of nanocarriers, smaller ones for pancreatic cancer, larger ones for other tumors, says Kataoka. In the right patient and the right tumor, the ideal nanomedicine can be “like a spaceship carrying tiny fighters,” he says.
This new wave of mechanistic studies, says Grodzinski, will help tailor nanoparticles to the tumor, the tissue, and the patient. Gone are the notions of sleek and precise nano-sized silver bullets that effortlessly home in on tumor targets. But by focusing on the basics, researchers are optimistic they can design tailored nanotherapies that come close to their science-fiction visions.
References
- 1.Wilhelm S, et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mat. 2016;1:1–12. [Google Scholar]
- 2.Zhou J, et al. Highly penetrative, drug-loaded nanocarriers improve treatment of glioblastoma. Proc Natl Acad Sci USA. 2013;110(29):11751–11756. doi: 10.1073/pnas.1304504110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Puig H, Cifuentes Rius A, Flemister D, Baxamusa SH, Hamad-Schifferli K. Selective light-triggered release of DNA from gold nanorods switches blood clotting on and off. PLoS One. 2013;8(7):e68511. doi: 10.1371/journal.pone.0068511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabral H, Kataoka K. Progress of drug-loaded polymeric micelles into clinical studies. J Control Release. 2014;190:465–476. doi: 10.1016/j.jconrel.2014.06.042. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto Y, et al. Vascular bursts enhance permeability of tumour blood vessels and improve nanoparticle delivery. Nat Nanotechnol. 2016;11(6):533–538. doi: 10.1038/nnano.2015.342. [DOI] [PubMed] [Google Scholar]