Significance
Preeclampsia, a hypertensive disorder of pregnancy and leading cause of maternal and neonatal morbidity and mortality, is associated with defective placental implantation and vascularization. Herein, we characterized regulation and function of miR-515-5p, which belongs to the primate- and placenta-specific chromosome 19 miRNA cluster, one of the largest miRNA clusters in humans. We observed that miR-515-5p was markedly downregulated during human syncytiotrophoblast differentiation and upregulated in placentas from preeclamptic women. miR-515-5p overexpression inhibited syncytiotrophoblast differentiation. Important miR-515-5p targets were identified, including hCYP19A1/aromatase, transcription factor glial cells missing 1 and WNT receptor, frizzled 5, which share critical roles in trophoblast differentiation. Thus, miR-515-5p may serve a key role in human trophoblast differentiation and provide a marker and therapeutic target for preeclampsia.
Keywords: human placenta, differentiation, miRNAs, aromatase, Wnt signaling
Abstract
Dysregulation of human trophoblast invasion and differentiation can result in preeclampsia (PE), a hypertensive disorder of pregnancy with significant morbidity and mortality for mother and offspring. miRNA microarray analysis of RNA from human cytotrophoblasts (CytT), before and after differentiation to syncytiotrophoblast (SynT) in primary culture, revealed that members of miR-515 family—including miR-515-5p, miR-519e-5p, miR-519c-3p, and miR-518f, belonging to the primate- and placenta-specific chromosome 19 miRNA cluster (C19MC)—were significantly down-regulated upon human SynT differentiation. The proto-oncogene, c-MYC, which declines during SynT differentiation, interacted with E-boxes upstream of pri-miR-515-1 and pri-miR-515-2, encoding these mRNAs, to enhance their expression. Predicted targets of miR-515-5p, known to be critical for human SynT differentiation, including hCYP19A1/aromatase P450, glial cells missing 1 (GCM1), frizzled 5 (FZD5), WNT2, Sp1, and estrogen receptor-α (ERα) mRNA, were markedly up-regulated during SynT differentiation. Notably, overexpression of miR-515-5p in cultured primary human trophoblasts impaired SynT differentiation and specifically decreased expression of hCYP19A1, GCM1, and Fzd5, which were validated as its direct targets. Interestingly, miR-515-5p levels were significantly increased in PE placentas, whereas mRNA and protein levels of targets, hCYP19A1, GCM1, and FZD5, were significantly decreased, compared with placentas of normotensive women. Thus, miR-515-5p may serve a key role in human trophoblast differentiation; its aberrant up-regulation may contribute to the pathogenesis of PE.
Branching morphogenesis of the trophoblast epithelium and formation of a vascularized villous network are crucial for normal embryonic development and successful pregnancy. Defective placental implantation and vascularization are implicated in preeclampsia (PE), a hypertensive disorder of human pregnancy that is a leading cause of maternal and neonatal morbidity and mortality (1–3). In PE, cytotrophoblast (CytT) differentiation into invasive cells is inhibited and the placenta is relatively hypoxic. Thus, fewer arterioles are remodeled and O2 availability to the hormone-producing syncytiotrophoblast (SynT) is reduced (4). The multinucleated SynT, formed by fusion of underlying proliferative CytT, covers the chorionic villi and is bathed in maternal blood. The SynT performs essential functions to ensure growth and survival of the fetus, including transport of O2 and nutrients and synthesis of protein and steroid hormones.
Estrogen synthesis by the human placenta is increased greatly after the ninth week of gestation (5) in association with CytT invasion and enlargement of the uterine arterioles, increased blood flow, and O2 availability to floating chorionic villi (6, 7). Synthesis of estrogens from C19-steroids is catalyzed by aromatase P450 (encoded by the hCYP19A1 gene). Trophoblast stem cells and CytT do not express hCYP19A1/aromatase; however, when CytT fuse to form multinucleated SynT, aromatase is markedly induced (8, 9). Exceptionally high levels of placental aromatase likely metabolize C19-steroids produced by the human fetal adrenals, preventing their conversion to active androgens, which can masculinize the fetus. Estrogens formed by placental aromatase may enhance angiogenesis, uteroplacental blood flow, and reduce systemic vascular resistance (10–14).
In previous studies using transgenic mice and transfected human trophoblasts in primary culture, we identified an ∼300-bp region upstream of placenta-specific hCYP19A1 exon I.1 that acts as an enhancesome and is essential for placenta-specific hCYP19A1 expression. This genomic region contains response elements for estrogen receptor-α (ERα) (15), estrogen-related receptor-γ (16), Sp1 (9), and glial cells missing 1 (GCM1) (17, 18); each of these transcription factors serves a crucial role in placenta-specific hCYP19A1 expression. There is evidence to suggest that placentally derived estrogen may play an autocrine role in trophoblast differentiation (19, 20). We observed that ERα expression in human trophoblasts is up-regulated during differentiation in culture. Furthermore, estrogen acting via ERα stimulates hCYP19A1 expression (15).
To further define mechanisms that underlie human trophoblast differentiation, we have investigated the roles of miRNAs. We performed miRNA microarray analysis of total RNA from freshly isolated human CytT (0 h) and from SynT after 24 and 48 h of culture in 20% O2. Among differentially expressed miRNAs, the miR-17∼92 cluster and its paralogs were significantly down-regulated upon SynT differentiation (18). In the present study, we characterized expression and function of members of miR-515 family, also significantly down-regulated in the microarray upon SynT differentiation. The miR-515 family belongs to the placenta-specific chromosome 19 miRNA cluster (C19MC), one of the largest miRNA gene clusters in humans. Many of the C19MC-encoded miRNAs share common seed sequences and are thought to originate from a common ancestor (21). We observed that miR-515 family members, miR-515-5p, miR-519e-5p, miR-519c-3p, and miR-518f were significantly down-regulated during human SynT differentiation, whereas miR-515-5p and miR-519e-5p were significantly up-regulated in placentas from women with PE. Our studies have further revealed important targets of these miRNAs, including hCYP19A1, GCM1, and frizzled 5 (FZD5), which serve critical roles in human trophoblast differentiation.
Results
Expression of C19MC Members Is Decreased During Differentiation of Human Trophoblasts in Primary Culture.
To identify miRNAs that are differentially expressed during human trophoblast differentiation, we previously conducted miRNA microarray analysis of RNA from freshly isolated CytT before (0 h) and after 24 and 48 h of culture (18). Among the 894 mature miRNA transcripts detected in the array with signal intensities >500 at each time point, 24 were significantly (P < 0.01, Student’s t test) down-regulated and 16 were up-regulated at 24 and 48 h compared with 0 h (18). Interestingly, we found that 10 of the 24 down-regulated miRNAs belonged to the C19MC. These miRNAs included miR-519e-5p, miR-515-5p, miR-518f, miR-519c-3p, miR-515-3p, miR-520d-5p, miR-524-5p, miR-520a-5p, miR-516a-5p, and miR-518b (18).
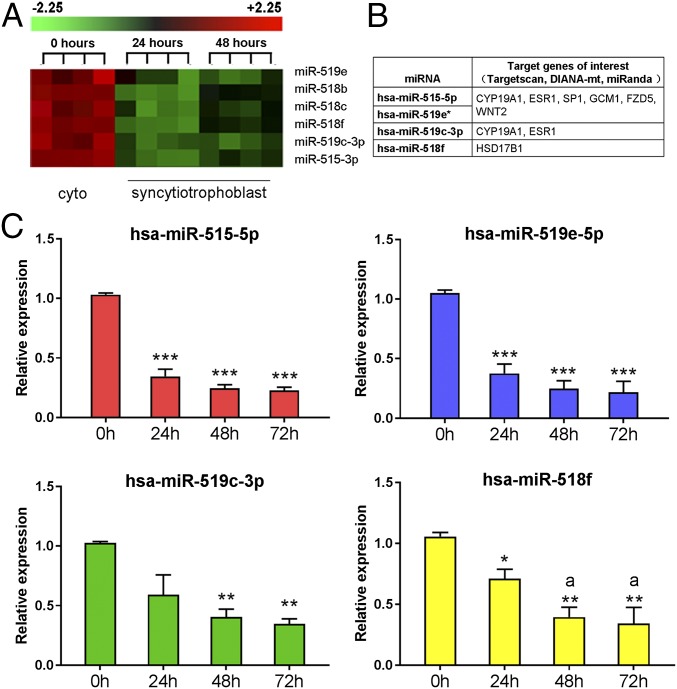
C19MC is the largest known miRNA gene cluster in humans (21, 22), encoding 46 miRNA precursors, which are then processed into 58 mature miRNAs (23). Based on their seed sequences, signal intensity values, fold-change in expression during trophoblast differentiation, and putative targets relevant to trophoblast differentiation (Fig. 1 A and B), miR-519e-5p, miR-515-5p, miR-518f, and miR-519c-3p were selected for further study using Taqman quantitative RT-PCR (qRT-PCR). Consistent with the microarray data, all four of these miRNAs were confirmed to be significantly down-regulated in primary cultures of human trophoblasts after 24, 48, and 72 h of culture, compared with the freshly isolated CytT before culture (Fig. 1C).
Fig. 1.
Members of C19MC are down-regulated in primary human trophoblasts during differentiation in culture. (A) RNA from freshly isolated CytT (Cyto, 0 h) and from SynT after 24 h and 48 h of culture was analyzed using miRNA microarray. Several members of the miR-515 family were significantly down-regulated ≥twofold at 24 and 48 h compared with 0 h. (B) Shown are predicted targets of these miRNAs known to be of importance in trophoblast differentiation. (C) To validate the results of the microarray, RNA isolated from midgestation human trophoblasts before (0 h) or after SynT differentiation (24 h and 48 h of culture in 20% O2 environment) was analyzed by Taqman qRT-PCR using U6 levels as the reference. Data are the mean ± SEM of values from three independent experiments conducted in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001 vs. 0 h; aP < 0.05 vs. 24 h.
Potential Targets of C19MC miRNAs and Their Relevance to Placental Development.
Using three miRNA-target prediction algorithms (Targetscan, DIANA-mt, miRanda), potential targets of miR-519e-5p, miR-515-5p, miR-518f, and miR-519c-3p were identified. Interestingly, several genes involved in the regulation of trophoblast differentiation and placental development were among the putative targets identified using all three databases (Fig. 1B). miR-515-5p, miR-519e-5p (referred to in Fig. 1B as hsa-miR-519e*), and miR-519c-3p are predicted to target hCYP19A1 and estrogen receptor 1 (ESR1; ERα) mRNA transcripts. Previously, we observed that hCYP19A1/aromatase was markedly induced during differentiation of human trophoblasts in primary culture (9). The estrogens formed act via ERα (which is coordinately up-regulated) in a positive feedforward loop to further up-regulate hCYP19A1 expression; this occurs via ERα binding to an estrogen response element-like sequence upstream of placenta-specific hCYP19A1 exon I.1 (15).
MiR-515-5p and miR-519e-5p also are predicted to target transcription factors Sp1 and GCM1, and the WNT ligand and its receptor, WNT2 and FZD5, respectively (Fig. 1B). The activating transcription factor Sp1 is known to mediate tissue-specific, developmental, and hormonal regulation of many genes. A GC box −233 bp upstream of hCYP19A1 exon I.1 specifically bound Sp1 and mediated Sp1 induction of hCYP19A1 promoter I.1 activity (9). GCM1 is a transcription factor critical for labyrinthine trophoblast development in mice (24) and for hCYP19A1 expression in human placental cells (17, 18). Homozygous deletion of Wnt2 in mice results in defective placental labyrinth formation (25). The Wnt receptor, Fzd5, which is predominantly expressed by SynT cells within the labyrinth of mouse placenta, is critical for normal labyrinth development and maintenance of Gcm1 expression (26). miR-518f is predicted to target HSD17B1, which metabolizes estrone to estradiol and plays an important role in placental vascular development (27).
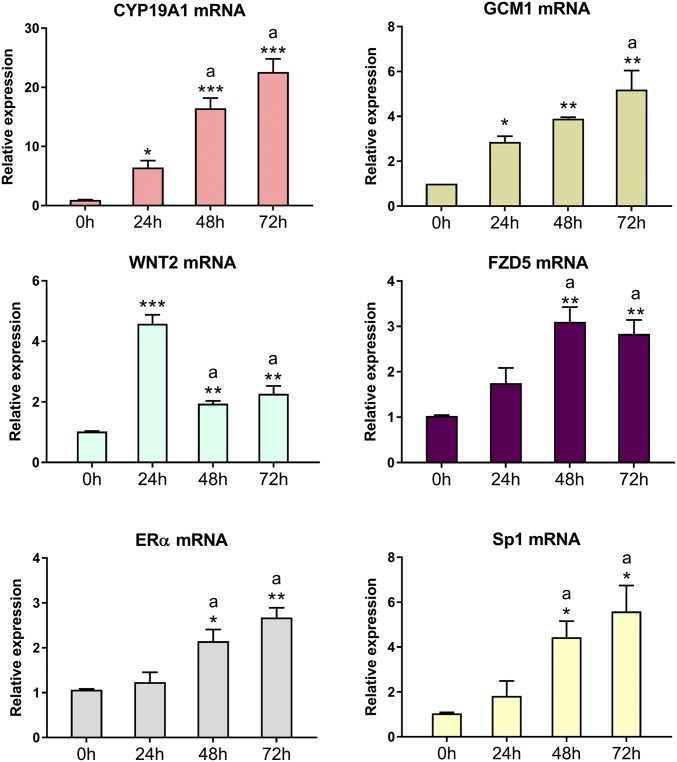
In this study, we observed that down-regulation of the selected C19MC members in human trophoblasts during differentiation in culture was associated with significant up-regulation of mRNA levels of their putative targets, hCYP19A1, GCM1, and WNT2, ERα, Sp1, and FZD5 (Fig. 2).
Fig. 2.
Expression of potential targets of the miR-515 family are up-regulated during human trophoblast differentiation. RNA from freshly isolated human trophoblasts before (0 h) or after 24 h and 48 h of culture was assessed by qRT-PCR for expression of predicted mRNA targets relevant to trophoblast differentiation. Data are the mean ± SEM of values from three independent experiments conducted in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001 vs. 0 h; aP < 0.05 vs. 24 h.
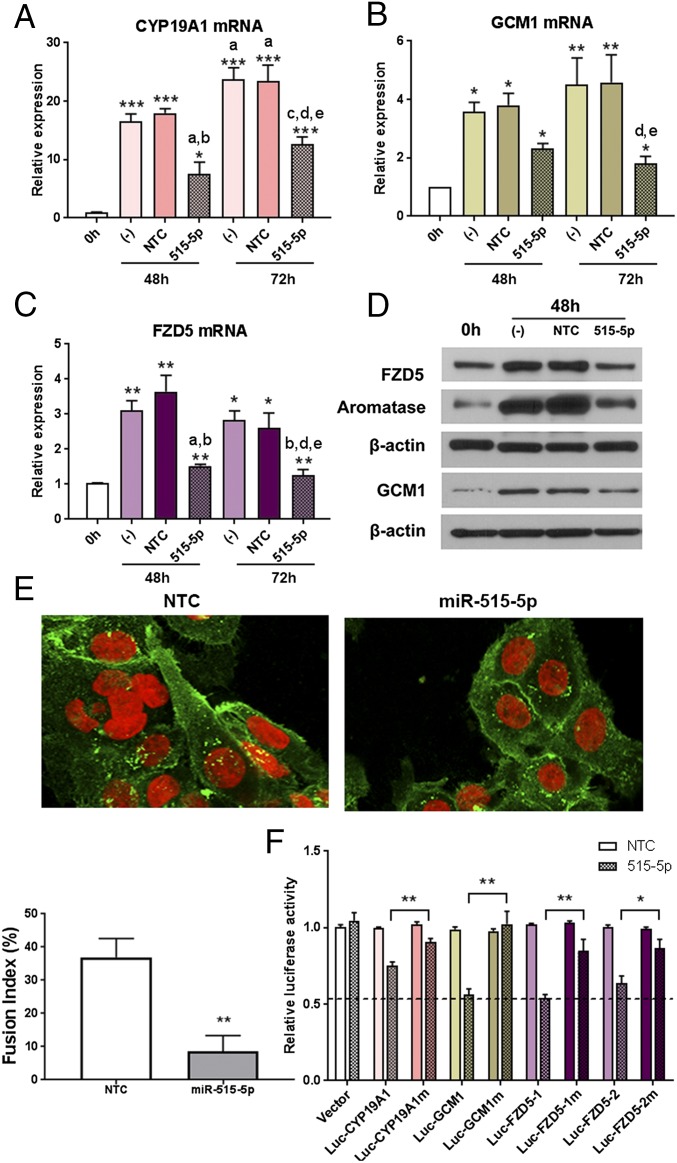
miR-515-5p Inhibits Expression of CYP19A1, GCM1, FZD5, by Complementary Base-Pairing to Their 3′UTRs in Primary Human Trophoblast.
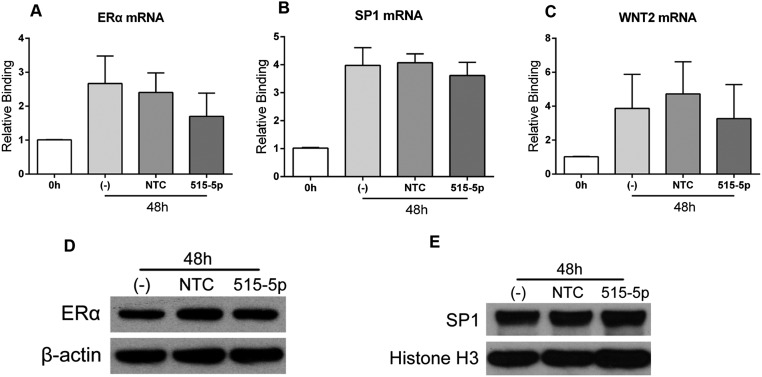
To determine which of these genes are direct targets of miR-515-5p (which shares an identical seed sequence with miR-519e-5p), we first transfected freshly isolated human primary trophoblasts with miR-515-5p mimics or nontargeting controls (NTC), and analyzed changes in their mRNA and protein expression. Parallel dishes of cells also were untransfected (−) as an additional control. As shown in Fig. 3 A–C, mRNA levels of CYP19A1, GCM1, and FZD5 were significantly decreased in trophoblasts after overexpression of miR-515-5p for 48 and 72 h, but not in cells transfected with NTC. Immunoblot analysis also indicated that expression of aromatase, GCM1, and FZD5 proteins was significantly decreased 48-h posttransfection of miR-515-5p mimics (Fig. 3D). In contrast, overexpression of miR-515-5p mimic had no effect on ERα, Wnt2, or Sp1 mRNA or ERα and Sp1 protein in the transfected cells (Fig. S1). The finding that these transcripts and proteins were unaffected under the same conditions in which miR-515-5p overexpression caused a pronounced diminution of CYP19A1, GCM1, and FZD5 mRNA and protein indicates that under the conditions used, ERα and Wnt2 do not appear to be targets of miR-515-5p.
Fig. 3.
Overexpression of miR-515-5p in human trophoblasts inhibits expression of hCYP19A1, GCM1, and FZD5 mRNA and prevents fusion to form SynT. Freshly isolated human CytT were either not transfected (−) or transfected with NTC or miR-515-5p mimic. qRT-PCR was used to evaluate mRNA levels of putative targets, hCYP19A1 (A), GCM1 (B), and FZD5 (C) mRNA, 48 and 72 h posttransfection. Data are the mean ± SEM of four independent experiments conducted in triplicate. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. 0 h; aP < 0.05 vs. (−) 48 h; bP < 0.05 vs. NTC 48 h; cP < 0.05 vs. 515-5p 48 h; dP < 0.05 vs. (−) 72 h; eP < 0.05 vs. NTC 72 h. (D) Protein levels of these potential targets were analyzed 48-h posttransfection by immunoblotting. (E) Plakoglobin immunostaining (green) of trophoblasts after transfection of NTC or miR-515-5p mimic. Nuclei were stained with DAPI (blue) and pseudocolored to red for better interpretation. Shown is a representative confocal fluorescence micrograph of cells 72 h after transfection. (Magnification: 400×.) ImageJ was used to quantify aggregation and fusion of cells in 10 fields. Fusion index = (N − S)/T × 100%. N refers to the number of nuclei in the syncytia, S refers to the number of syncytia, and T refers to the total number of nuclei. (F) Sequences within the 3′UTR of CYP19A1, GCM1, or FZD5 containing one or more putative binding sites for miR-515-5p were subcloned into the psiCHECK-2 vector to create WT or mutated luciferase reporter plasmids. HEK293T cells were transfected with either an empty reporter (Vector), or recombinant reporters containing WT or mutated (m) putative miR binding sites. miR-515-5p mimic or NTC was cotransfected. Luciferase activities were measured 24-h posttransfection. Data are the mean ± SEM of values from five independent experiments, each conducted in triplicate. *P < 0.05, **P < 0.01 [mutant (m) vs. WT luciferase reporter].
Fig. S1.
Expression of ERα, Sp1, and WNT2 mRNA is not altered by miR-515-5p overexpression. Freshly isolated human CytT were either untransfected (-), or transfected with miR-515-5p mimic or NTC and mRNA levels of ERα (A), Sp1 (B), and WNT2 (C) were analyzed by qRT-PCR after 48 h of culture. In all cases, miR-515-5p overexpression caused no significant change in mRNA levels, compared with untransfected cells or cells transfected with NTC. Protein expression levels of ERα (D) and Sp1 (E), analyzed by immunoblotting, were also unaffected by miR-515-5p overexpression.
Overexpression of miR-515-5p Impairs Syncytialization of Human Trophoblasts in Primary Culture.
Because overexpression of miR-515-5p in trophoblasts inhibited expression of GCM1 and FZD5, which were reported to be critical for trophoblast fusion (26, 28, 29), we evaluated the effects of miR-515-5p overexpression on morphology of the human trophoblasts. Freshly isolated CytT were transfected with NTC or miR-515-5p mimics. After 72 h of culture, the cells were immunostained with antiplakoglobin antibody and viewed by confocal fluorescence microscopy. ImageJ was used to quantify the fusion index of SynT (26). When the cells were transfected with NTC miRNA, there was clear syncytium formation; however, when cells were transfected with miR-515-5p mimics, both the percentage and the size of syncytia were significantly reduced (Fig. 3E).
Cyp19A1, GCM1, and FZD5 Are Validated Targets of miR-515-5p.
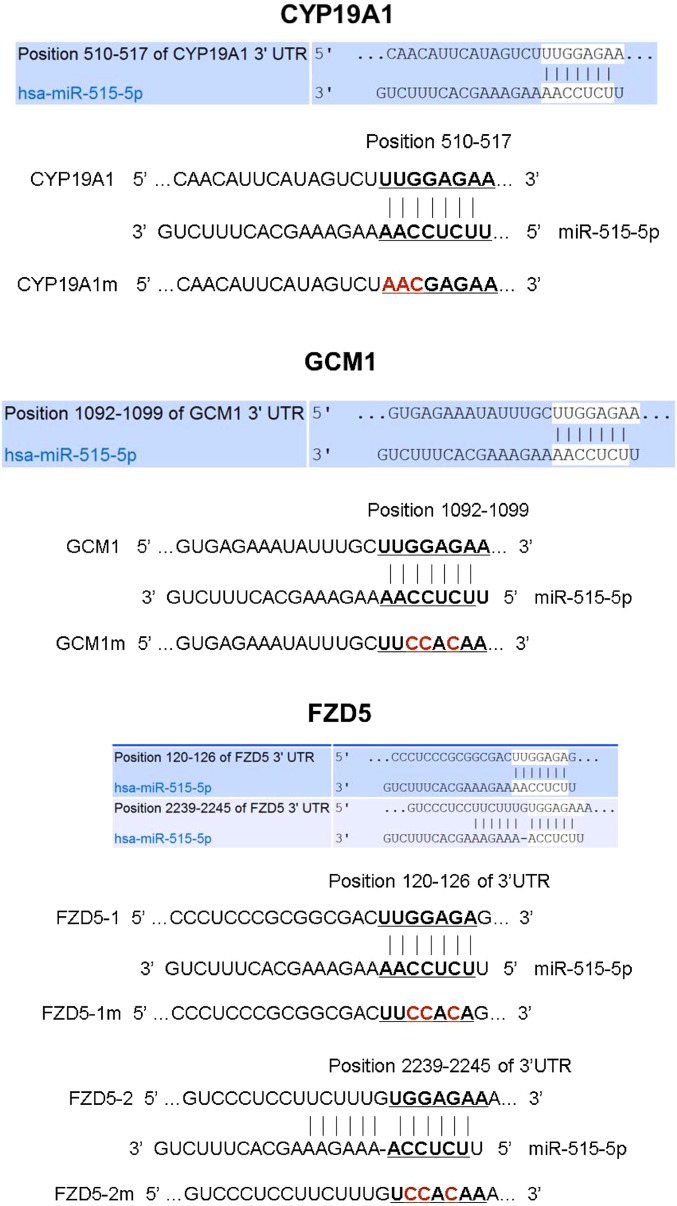
Putative binding sites for miR-515-5p within the 3′UTRs of hCYP19A1, GCM1, and FZD5 were identified using target prediction software. The 3′UTRs of hCYP19A1 and GCM1 each contain one miRNA responsive element (MRE) specific for miR-515-5p, whereas, the 3′UTR of FZD5 mRNA contains two MREs for miR-515-5p (Fig. S2). Using a dual luciferase reporter assay, HEK-293T cells were cotransfected with miR-515-5p mimics and with psiCHECK-2 reporter plasmids in which the 3′UTRs of hCYP19A1, GCM1, and FZD5 transcripts with (m) or without (WT) mutations in the MREs (Fig. S2) were subcloned downstream of luciferase. In the case of FZD5, each of the putative MREs was tested independently. For each of the WT reporter plasmids, a significant reduction in luciferase activity was evident 24-h posttransfection with miR-515 mimic, compared with cells cotransfected with NTC (Fig. 3F). However, when the putative binding sites for miR-515-5p were mutated in each of these reporters, the inhibitory effects of miR-515-5p mimics on luciferase activity were abolished (Fig. 3F). Taken together, we conclude that hCYP19A1, GCM1, and FZD5 are direct targets of miR-515-5p, and that both MREs within the FZD5 3′UTR are functional.
Fig. S2.
Sequences within the 3′UTRs of CYP19A1, GCM1, and FZD5 containing one or more putative binding sites for miR-515-5p that were subcloned into the psiCHECK-2 vector to create wild-type or mutated (m) luciferase reporter plasmids. Shown are the positions and sequences of the WT and mutated (m) putative miR-515-5p response elements within the 3′UTRs of CYP19A1, GCM1, and FZD5. The bases within the 3′UTR of each mRNA that were mutated are indicated in red.
miR-515-5p, miR-519e, and Their Targets Are Aberrantly Expressed in Placentas from PE Patients Compared with Gestation-Matched Normotensive Women.
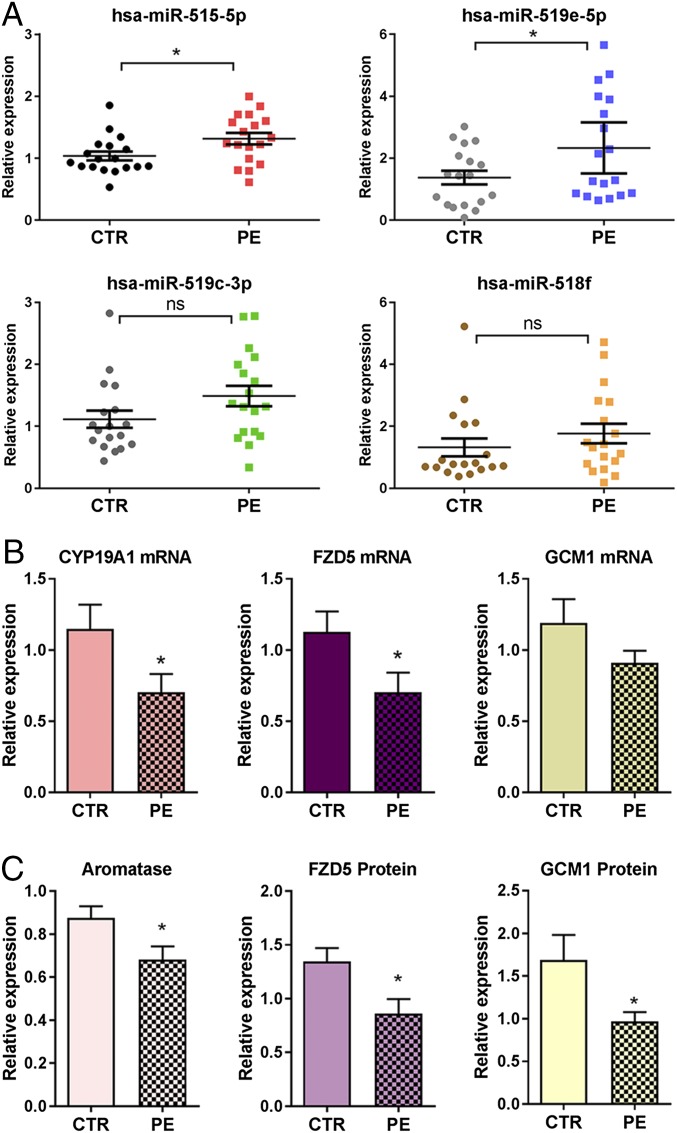
In consideration of the potential roles of miR-515-5p in human trophoblast differentiation and the impairment of trophoblast differentiation in PE, we next investigated expression of selected members of C19MC and their targets, hCYP19A1, GCM1, and FZD5, in placentas of PE and normotensive women near term. RNA and protein from term placentas of 18 PE and 18 gestation-matched normotensive women were analyzed. Although women with PE met the recently revised criteria of the American College of Obstetrics and Gynecology (ACOG) (30), 17 of 18 of the PE subjects studied also manifested proteinuria. PE and normotensive subjects were matched for gestational age at the time of delivery (38.8 vs. 39.6 wk, respectively) (Table S1). PE subjects had an average blood pressure of 157/97 mmHg (systolic/diastolic), whereas normotensive women had an average blood pressure of 124/78 mmHg (Table S1). Interestingly, we observed significantly higher levels of miR-515-5p and miR-519e-5p in placentas from PE women compared with gestation-matched normotensive women (Fig. 4A). Although miR-519c-3p and miR-518f were modestly elevated in PE subjects compared with controls, the differences were not significant (Fig. 4A). Correspondingly, CYP19A1/aromatase and FZD5 mRNA (Fig. 4B) and protein (Fig. 4C) expression were significantly decreased in PE placentas compared with those of normotensive women. GCM1 protein also was significantly down-regulated in PE placentas (Fig. 4C), although differences in mRNA expression were not significant (Fig. 4B).
Table S1.
Clinical characteristics of preeclamptic and normotensive women
| Characteristic | Normotensive (n = 18) | Preeclamptic (n = 18) | P value |
| Maternal age (y) | 25.6 ± 6.1 | 25.3 ± 7.7 | 0.89 |
| Body mass index | 28.6 ± 1.8 | 27.8 ± 1.0 | 0.69 |
| Gestational age (wk) | 39.6 ± 1.2 | 38.8 ± 1.5 | 0.08 |
| C-section ratio | 55.6% (10 of 18) | 44.4% (8 of 18) | 0.50 |
| Systolic blood pressure (mmHg) | 124 ± 8 | 157 ± 9 | <0.01 |
| Diastolic blood pressure (mmHg) | 78 ± 5 | 97 ± 4 | <0.01 |
| Infant birth weight (kg) | 3.5 ± 0.5 | 3.2 ± 0.6 | 0.12 |
| Proteinuria | None | 17 of 18 Subjects | — |
In both normotensive and preeclamptic groups, one subject was African American; all remaining subjects were Hispanic.
Fig. 4.
miR-515 family members are up-regulated in placentas from PE vs. gestation-matched normotensive women, whereas expression of miR-515-5p targets is significantly decreased. (A) RNA isolated from term placentas of PE (n = 18) and gestation-matched normotensive (CTR) women (n = 18) was analyzed for expression of several miR-515 family members using TaqMan based qRT-PCR. RNA from term placentas of PE and gestation-matched normotensive women (n = 18 per group) was analyzed for expression of CYP19A1, FZD5, and GCM1 mRNA (B) and protein (C). Relative expression of aromatase, FZD5 and GCM1 proteins was assessed by immunoblotting of normotensive (n = 18) and PE groups (n = 18). The immunoreactive bands were scanned and normalized to β-actin scanned on the same immunoblot. Values are presented as mean ± SEM. *Significantly different from CTR, P < 0.05.
c-MYC Up-Regulates Expression of miR-515-5p in Human Trophoblasts Through Binding to E-Boxes Upstream of the miR-515-1 and miR-515-2 Precursor Genes.
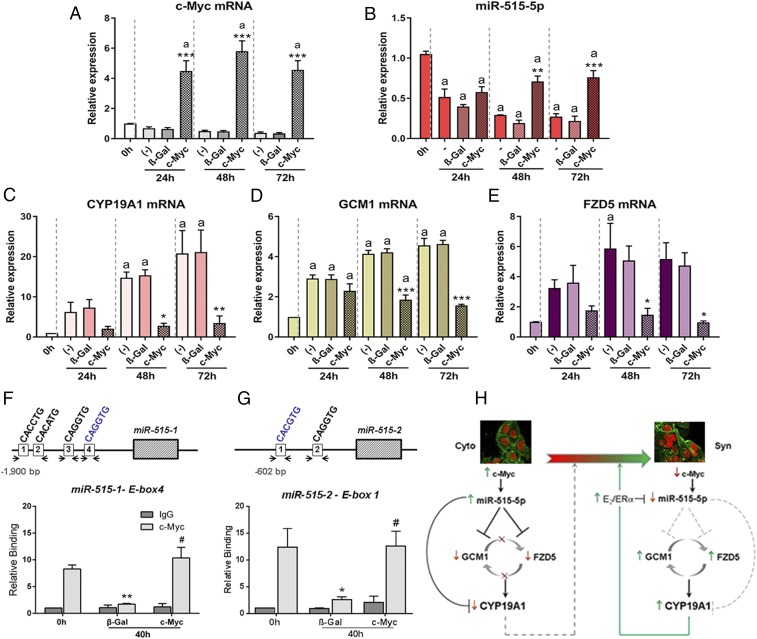
The c-MYC proto-oncogene is highly expressed in human CytT and is implicated in CytT proliferation (31). Previously, we observed that c-MYC mRNA and protein were markedly down-regulated during differentiation of human trophoblasts in primary culture (18). Moreover, c-MYC overexpression in primary human trophoblasts blocked the up-regulation of genes associated with SynT differentiation (i.e., hCYP19A1 and hCG-β) (18). Interestingly, we found that when human CytT were infected with adenoviruses expressing c-MYC and cultured for up to 72 h (Fig. 5A), levels of miR-515-5p were significantly increased at 48 and 72 h of culture compared with cells transduced with adenoviruses expressing β-Gal, as a control (Fig. 5B). Moreover, expression of miR-515-5p target genes, hCYP19A1 (Fig. 5C), GCM1 (Fig. 5D), and FZD5 (Fig. 5E), were coordinately decreased by c-MYC overexpression. Notably, miR-519e-5p was previously reported to be up-regulated in MYC-induced human lymphomas (32).
Fig. 5.
c-MYC enhances expression of miR-515-5p in CytT by binding to E-boxes upstream of the miR-515-1 and miR-515-2 genes and coordinately represses expression of its targets; endogenous c-MYC binding declines during SynT differentiation. Primary human trophoblasts were infected with recombinant adenoviruses expressing c-MYC or β-galactosidase (β-Gal) as a control and cultured for 24, 48, and 72 h. RNA was isolated from the cells and expression of c-MYC (A), miR-515-5p (B), hCYP19A1 (C), GCM1 (D), and FZD5 (E) mRNA was analyzed by qRT-PCR. n = 3 per treatment at each time point. aP < 0.01 vs. 0 h; *P < 0.05 vs. (−) and β-Gal at 48 and 72 h; **P < 0.01 vs. (−) and β-Gal at 48 and 72 h; ***P < 0.001 vs. (−) and β-Gal at 48 and 72 h. (F and G) Binding of c-MYC to genomic regions surrounding E-boxes within ∼1.9 kb upstream of the miR-515–1 precursor (F) and ∼0.6 kb upstream of the miR-515-2 precursor (G) was evaluated in freshly isolated human CytT before (0 h) and after 40 h of culture using ChIP-qPCR. Before culture, the cells were infected with recombinant adenoviruses expressing β-Gal or c-MYC. (F) Increased binding of endogenous c-MYC (relative to the IgG control) to E-box 4 of miR-515-1 was observed in freshly isolated CytT (0 h) and was markedly decreased in cells expressing β-Gal after 40 h, but remained high in cells overexpressing c-MYC. Specific binding of c-MYC to E-boxes 1–2 and E-box 3 compared with the IgG control was not detectable (Fig. S3A). (G) In the case of the miR-515-2 precursor, E-box 1 manifested increased binding of c-MYC (relative to IgG control) in freshly isolated CytT (0 h); relative binding of endogenous c-MYC markedly declined in cells transduced with β-Gal adenovirus after 40 h of culture. In contrast, in cells infected with adenoviruses expressing c-MYC, elevated binding of c-MYC to E-box 1 was detected after 40 h, consistent with induction of miR-515-5p upon c-MYC overexpression. Specific binding of c-MYC to E-box 2 over the IgG control was not detectable (Fig. S3B). Values are the mean ± SEM of data from nine replicate dishes for each treatment and at each time point. Significantly (*P < 0.05; **P < 0.01) different from 0 h. #Significantly (P < 0.05) different from c-MYC binding in cells transduced with β-Gal-expressing adenovirus at 40 h. (H) c-MYC-induced miR-515 expression in human CytT blocks differentiation to SynT by inhibiting expression of key genes in trophoblast differentiation, GCM1, FZD5, and CYP19A1.
To determine the mechanisms underlying c-MYC regulation of miR-515-5p expression, ChIP assays were performed using human trophoblasts before and after differentiation in primary culture, with or without c-MYC overexpression. MiR-515-5p is encoded by two precursors, miR-515-1 and miR-515-2, both of which are located within the C19MC. E-boxes that could serve as putative c-MYC binding sites upstream each miR-515 precursor were identified by bioinformatics analysis (PROMO 3.0.2) (Fig. 5 F and G). Freshly isolated CytT were infected with adenoviruses expressing c-MYC or β-Gal, as control. Chromatin cross-linking was performed in CytT before culture (0 h) and in cultured cells 40-h postinfection with β-Gal or c-MYC–expressing adenoviruses.
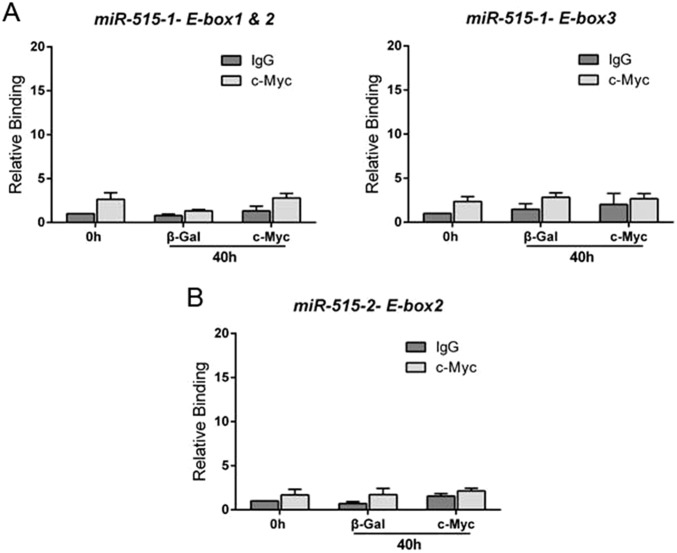
Binding of c-MYC to genomic regions containing four E-boxes ∼1.9 kb upstream of the miR-515-1 precursor (Fig. 5F) and two E-boxes ∼0.6 kb upstream of the miR-515-2 precursor (Fig. 5G) were evaluated by ChIP-qPCR. As shown in Fig. 5F, elevated binding activity of endogenous c-MYC (relative to the IgG control) to E-box 4 was observed in freshly isolated CytT, whereas, relative binding of c-MYC was markedly decreased in cells expressing β-Gal after 40 h, but remained high in cells infected with recombinant adenoviruses containing a c-MYC expression vector. Specific binding of c-MYC to E-boxes 1–3 over the IgG control was not detectable (Fig. S3A).
Fig. S3.
c-Myc does not bind specifically to E-boxes 1–3 upstream of miR-515-1 or to E-box 2 upstream of miR-515-2. Binding of c-Myc to genomic regions surrounding E-boxes 1 and 2 and E-box 3 upstream of the miR-515-1 precursor (A) and to E-box 2 upstream of the miR-515-2 precursor (B) was evaluated in freshly isolated human CytT before (0 h) and after 40 h of culture using ChIP-qPCR. Before culture, the cells were infected with recombinant adenovirus expressing β-Gal or c-Myc. In contrast to what was observed for miR-515-1 E-box 4 and miR-51-2 E-box 1 (Fig. 5), binding of c-Myc to E-boxes 1–2 and 3 upstream the miR-515-1 gene (A) and to E-box 2 upstream the miR-515-2 gene (B) was not significantly different from the IgG control at each time point and treatment.
In the case of the miR-515-2 precursor, E-box 1, which contains the canonical consensus sequence for c-MYC binding (CACGTC), manifested specific binding of endogenous c-MYC (relative to IgG control) in freshly isolated CytT (0 h); relative binding of endogenous c-MYC dramatically declined in cells transduced with β-Gal adenovirus after 40 h of culture (Fig. 5G). In contrast, in cells infected with adenoviruses expressing c-MYC, increased binding of c-MYC to E-box 1 was detected at the 40-h time point (Fig. 5G), consistent with induction of miR-515-5p with c-MYC overexpression (Fig. 5B). Specific binding of c-MYC to E-box 2 of miR-515-2 over the IgG control was not detectable (Fig. S3B). These collective findings suggest that expression of miR-515-5p is induced by increased expression of c-MYC in CytT and its binding to E-box 4 of miR-515-1 and E-box 1 of miR-515-2. Thus, during differentiation of CytT to SynT, down-regulation of miR-515-5p is associated with a decline in c-MYC expression (18) and its recruitment to E-boxes in the genomic regions upstream of the miR-515 precursors.
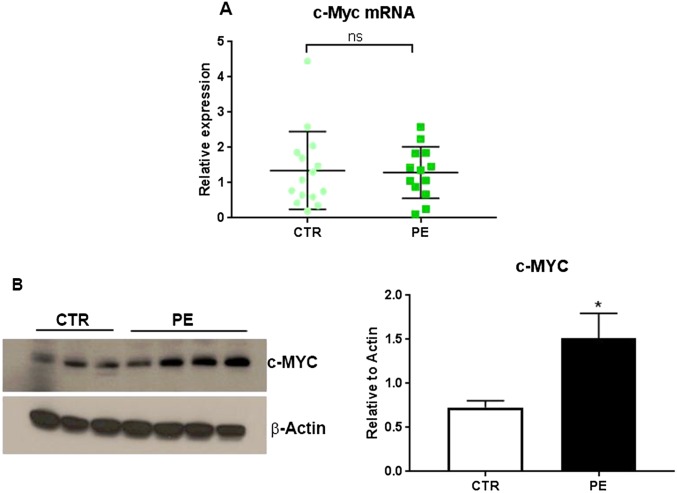
These findings raise the question of whether c-Myc is up-regulated in placentas of PE vs. normotensive women. Interestingly, we observed that, although c-Myc mRNA levels were similar in placentas of PE vs. normotensive subjects, protein levels were significantly elevated in PE placentas (Fig. S4). These findings suggest that aberrant elevation of c-Myc expression in PE subjects may occur at the posttranslational level.
Fig. S4.
cMyc protein is significantly increased in placentas of PE subjects, compared with normotensive subjects, whereas, c-Myc mRNA levels are unaltered. c-Myc mRNA [n = 13 PE; n = 15 normotensive (CTR) subjects] and protein levels (PE n = 8; CTR n = 6) were analyzed in placental tissues from PE and normotensive term subjects. Although c-Myc mRNA was similar in placentas of PE vs. normotensive subjects (A), protein levels were significantly elevated in PE placentas (B) (*P < 0.05). These findings suggest that the decline in c-Myc expression in normotensive subjects may be posttranslationally regulated during pregnancy, whereas in PE this decline does not occur.
Discussion
In this study, we made the striking observation that selected members of the C19MC were markedly down-regulated in midgestation human trophoblasts during differentiation to SynT in primary culture. These included members of the miR-515 family (33), miR-515-5p, miR-519e-5p, miR-519c-3p, and miR-518f. Using luciferase reporter assays, we found that miR-515-5p and miR-519e-5p, which share the same seed sequence, directly targeted hCYP19A1, GCM1, and FZD5, which were significantly induced in concert with the decline of these miRNAs and with SynT differentiation. Importantly, miR-515-5p and miR-519e-5p were significantly up-regulated in placentas of women with PE, compared with gestational age-matched normotensive subjects, whereas their targets were significantly decreased. Although WNT2, ERα, and Sp1 are predicted targets of these miRNAs and were significantly up-regulated during SynT differentiation, we were unable to establish that they are direct targets. On the other hand, ERα mRNA was reported to be directly targeted by members of the miR-17∼92 cluster, miR-18a and miR-19a, in human neuroblastoma cells (34). These miRNAs were previously found to be markedly down-regulated during differentiation of human trophoblasts in culture (18) as ERα expression increased (15).
The C19MC, which is primate-specific, largely placenta-specific, and expressed from the paternally inherited chromosome, spans ∼100 kb of genomic DNA and encodes 46 pri-miRNA genes that are processed into 58 mature miRNAs (21, 23). C19MC is also highly expressed in human embryonic stem cells and has been linked to cell proliferation and stemness (35); expression declines upon stem cell differentiation (36, 37). Furthermore, C19MC expression is up-regulated in a number of cancers and has been linked to tumor invasion and metastasis (22, 38, 39). Exosomes secreted by primary human trophoblasts were found to be enriched in miRNAs encoded within the C19MC (40). Secretion of these exosomal miRNAs has been suggested to play a role in fetoplacental–maternal communication (41) and to confer viral resistance (42). Exposure of nonplacental cells to trophoblast-derived, C19MC-enriched exosomes induced autophagy, which may promote degradation of foreign pathogens (43).
Our present findings, that miR-515-5p directly targeted GCM1 and FZD5, is of great interest. Gcm1 knockout mice die at midgestation because of a complete block of chorionic villous branching and absence of a functional placental labyrinth containing syncytial cells, analogous to the human SynT (24, 44). GCM1 induces expression of Syncytin 1, a gene involved in trophoblast fusion (45), and regulates expression of placental growth factor (46). hCYP19A1 promoter I.1 contains a GCM1 response element required for promoter activity (17). We observed that GCM1 serves an important role in regulating expression of hCYP19A1 and hCGβ during human trophoblast differentiation (18). Studies using gene-targeted mice further indicated a crucial role of Wnt/β-catenin signaling pathway components in chorion branchpoint initiation, labyrinth development, and in SynT fusion. These studies include knockout of the Wnt receptor Fzd5 (26), as well as R-spondin3 (47) and Bcl9l (29), potentiators of Wnt-β-catenin signaling. Gcm1 up-regulates Fzd5 at sites in the basal chorion where branching is initiated. In turn, Fzd5/β-catenin maintains Gcm1 expression in a positive feedback loop (26). Interestingly, Gcm1 may promote branching by stimulating epithelial-to-mesenchymal transition in chorionic trophoblasts, by causing decreased expression of tight junction proteins, zona dens-1 and claudins-4 and -7 (26).
To elucidate mechanisms for down-regulation of miR-515-5p in human trophoblasts during differentiation, we considered the role of c-MYC, which is highly expressed in CytT and declines during SynT differentiation (18). c-MYC, a basic helix–loop–helix zipper transcription factor, is believed to regulate an estimated 15% of genes in the human genome via binding to E-boxes within target promoters (48). We previously observed that c-MYC activated expression of the miR-17∼92 cluster and its paralogs (18), which we found to be significantly down-regulated during SynT differentiation, in association with the fall in c-MYC expression and its binding to their promoters (18). We observed that miR-17∼92 and paralogous members, miR-19b and miR-106a, directly targeted hCYP19A1 mRNA, whereas miR-19b also targeted GCM1. Overexpression of these miRNAs impaired SynT differentiation (18). Several members of the miR-17∼92 (miR-17, -19b, -20a) and miR-106a∼363 (miR-19b, -20b) clusters are known to target ERα (ESR1) (49), which we previously found to be up-regulated during human SynT differentiation and essential for induction of hCYP191A promoter I.1 activity (15). Placentas from PE women had higher levels of miR-106a and -19b and lower aromatase and GCM1 (18).
In the present study, we observed that c-MYC overexpression significantly increased miR-515-5p. This observation was associated with a significant decline in hCYP191A, GCM1, and FZD5 mRNA levels. miR-515-5p is encoded by two pri-miRs within C19MC, pri-miR-515-1 and pri-miR-515-2. Using ChIP-qPCR, we found that endogenous c-MYC bound specifically to E-boxes upstream of pri-miR-515-1 and pri-miR-515-2 in freshly isolated CytT; binding declined in trophoblasts during SynT differentiation. Thus, increased expression of c-MYC in CytT may prevent SynT differentiation, in part, by up-regulating miRNAs in both the C19MC and miR-17∼92 clusters, which target important regulators of branching morphogenesis and cell fusion.
In conclusion, our studies have uncovered an inhibitory role of miR-515-5p, a miRNA within the primate-specific C19MC, in human trophoblast differentiation (Fig. 5H). We observed that miR-515-5p, which was expressed at relatively high levels in CytT, isolated from midgestation human placenta, serves a critical role through its action to inhibit expression of a number of key genes involved in human trophoblast differentiation. Early in the first trimester when the placenta is poorly vascularized and relatively hypoxic, c-MYC expression is enhanced, resulting in increased transcription of the miR-515-1 and miR-515-2 precursors. Increased levels of miR-515-5p were found to directly target both FZD5 and GCM1, which are essential for SynT differentiation (26).
After ∼10 wk of gestation in the human, increased CytT invasion of the spiral arteries results in increased placental perfusion and enhanced O2 tension (Fig. 5H). This process is associated with suppression of c-MYC and decreased expression of the miR-515-1 and miR-515-2 precursors, and allows up-regulation of GCM1 and FZD5, which exist in a positive feedforward loop. In turn, Fzd5/β-catenin maintain elevated Gcm1 expression (26). GCM1 promotes trophoblast fusion, in part via its action to enhance expression of Syncytin 1 (45). GCM1 also regulates expression of placental growth factor (46), as well as hCYP19A1/aromatase (17, 18), which contribute to SynT differentiation. Notably, increased E2 has been observed to act via ERα to inhibit miR-515-5p expression (50). Thus, the up-regulation of E2/ERα signaling further suppresses miR-515-5p expression to promote human SynT differentiation. We suggest that aberrant regulation of this signaling pathway interferes with normal induction of trophoblast differentiation, and may contribute to the pathogenesis of preeclampsia.
Materials and Methods
Patients and Tissue Samples.
This study was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center. Informed consent was obtained from all participants. Classification of PE vs. normotensive subjects were in accord with recently revised ACOG guidelines (30). Patient selection criteria and tissue processing are described in SI Materials and Methods.
Primary Culture of Human Trophoblast Cells.
Primary human CytT from midgestation placenta were isolated as described previously (9, 15) and detailed in SI Materials and Methods.
qRT-PCR.
Total RNA was extracted from tissues or cultured cells using a miRNeasy mini kit (Qiagen). MicroRNA expression was quantified using a TaqMan MicroRNA assay (Applied Biosystems), as previously described (18) and detailed in SI Materials and Methods. Sequences of primer pairs are presented in Table S2.
Table S2.
Primers sets used in this study
| Gene | Sequence (5′→ 3′) |
| CYP19A1 | Forward: 5′-ACGGAAGGTCCTGTGCTCG-3′ |
| Reverse: 5′-GTATCGGGTTCAGCATTTCCA-3′ | |
| GCM1 | Forward: 5′-CTGAAGGGGAGCACAGAGAC-3′ |
| Reverse: 5′-TAGAGCTTCATGGGGTCCAC-3′ | |
| FZD5 | Forward: 5′-TGTGGGATGAAGTGGATGAA-3′ |
| Reverse: 5′-AGGGACAGCAGAGGTGAGAA-3′ | |
| ESR1 | Forward: 5′-AGAGAAGTATTCAAGGACATAACGAC-3′ |
| Reverse: 5′-TCTTCCTCCTGTTTTTATCAATGG-3′ | |
| WNT2 | Forward: 5′-AGAATGCCAGCACCAGTTCCG-3′ |
| Reverse: 5′-CCTCCAGAGATAATCGCCCGTT-3′ | |
| SP1 | Forward: 5′-GTTTCCTTGGGGCAGACCAG-3′ |
| Reverse: 5′-TCCTTCCTCTCCACCTGCTG-3′ | |
| c-Myc | Forward: 5′-GGACGACGAGACCTTCATCAA-3′ |
| Reverse: 5′-CCAGCTTCTCTGAGACGAGCTT-3′ | |
| h36B4 | Forward: 5′-TGCATCAGTACCCCATTCTATCA-3′ |
| Reverse: 5′-AAGGTGTAATCCGTCTCCACAGA-3′ |
Immunoblot Analysis.
Immunoblot analysis of putative miR-515-5p targets was carried out as described in SI Materials and Methods.
Transfection of miRNA Mimics.
For overexpression of miR-515-5p, freshly isolated CytT were transfected with either 20 nM miR-515-5p mimics (Applied Biosystems) or nontargeting control (Applied Biosystems) using the HiPerfect transfection reagent (Qiagen), according to the manufacturer’s instructions. After 24 h, medium was changed to DMEM/F12 containing 2% (vol/vol) FBS. The cells were harvested for further analysis after 48-h posttransfection.
Luciferase Reporter Assay.
Putative miR-515-5p targets (CYP19A1, GCM1, FZD5) known to be involved in the regulation of trophoblast differentiation were validated using luciferase reporter assays as detailed in SI Materials and Methods.
Adenovirus Overexpression.
Adenovirus-mediated overexpression of CMV-c-MYC (Vector Biolabs) vs. CMV-β-Gal (51), as control, in human primary trophoblasts was performed as previously described (18) and detailed in SI Materials and Methods.
ChIP-qPCR.
E-boxes corresponding to putative c-MYC responsive elements in the genomic regions upstream of miR-515-1 and miR-515-2 precursors were identified by an online bioinformatics analysis program (PROMO 3.0). Human trophoblasts were transduced either with recombinant adenoviruses expressing c-MYC or β-Gal, as described above. After 40 h of culture, ChIP-qPCR assays were performed to analyze binding of c-MYC to each of the E-boxes using the Magna ChIP A kit (Millipore) according to the manufacturer’s instructions and as detailed in SI Materials and Methods.
Morphological Analysis.
CytT transfected with 20 nM miR-515-5p mimics or nontargeting control were cultured in DMEM/F12 containing 2% (vol/vol) FBS for 72 h. Cells were immunostained for plakoglobin, with second antibody conjugated to Alexa Fluor 488. Nuclei were counterstained with DAPI and images were captured on a Zeiss confocal microscope (400× magnification), as detailed in SI Materials and Methods. ImageJ was used to quantify the fusion index of cells in 10 fields. Fusion index = (N − S)/T × 100%. N refers to the number of nuclei in the syncytia, S refers to the number of syncytia, and T refers to the total number of nuclei. Therefore, the more syncytia, the fewer the number of nuclei per syncytia and the lower the number of fusion events.
Data Analysis.
All statistical analyses were performed using GraphPad Prism 6.04. Values are expressed as means ± SEM. Differences between two groups were analyzed by Student’s t test. One-way ANOVA, followed by Newman–Keuls post hoc test or nonparametric Mann–Whitney test was used for multiple comparisons. Statistical significance was defined as P < 0.05. For each assay, data were collected from at least three independent cell preparations performed in triplicate.
SI Materials and Methods
Patients and Tissue Samples.
This study was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center. Informed consent was obtained from all participants. PE was defined in our subjects as the onset of hypertension after 20 wk of gestation with systolic blood pressure ≥ 140 mmHg and diastolic blood pressure ≥ 90 mmHg taken in two separate measurements at least 4–6 h apart, accompanied with proteinuria (≥300 mg in a 24-h urine collection or a random urine dipstick reading of ≥1+). For the PE group, 17 of 18 subjects with new onset hypertension had proteinuria. The one subject without proteinuria had new onset hypertension with low platelets and raised liver enzymes. Notably, all criteria met the new ACOG guidelines published in 2013 (30), which do not require proteinuria, as long as a number of other criteria are met. However, 17 of the 18 subjects also had proteinuria. PE was considered severe if the patient’s blood pressure was >160/110 mmHg or in the setting of platelets < 100,000/μL, aspartate transaminase or alanine transaminase greater than twice normal, creatine > 1.1 mg/dL, headache, visual disturbances, right upper quadrant pain, or pulmonary edema. Term placental tissues from PE subjects and gestational age-matched normotensive women were obtained from Parkland Memorial Hospital, Dallas, TX. After delivery, the placental samples were collected, minced, washed with cold sterile PBS and stored in RNAlater (Qiagen) immediately. The tissues were snap-frozen in liquid nitrogen and kept at –80 °C for further study. In total, 18 PE women and 18 gestationally matched normotensive controls were enrolled in this study.
Primary Culture of Human Trophoblast Cells.
Primary human CytT from midgestation placentas were isolated as described previously (9, 15). Briefly, midtrimester human placentas were obtained from Advanced Bioscience Resources in accordance with the Donors Anatomical Gift Act of the State of Texas. The placental tissues were rinsed with HBSS (pH 7.4) and finely minced. After repeated digestion with 0.125% trypsin (Difco) and 0.02% DNase I (Sigma-Aldrich) in HBSS at 37 °C, the collected cells were suspended in DMEM (Life Technologies) and layered over Percoll gradients (70–5%). After centrifugation at 1,200 × g for 30 min, the cells in the middle layer (density of 1.045–1.062 g/mL) were collected and resuspended in DMEM containing 10% (vol/vol) FBS and 1.2% (vol/vol) antibiotic/antimycotic solution (Life Technologies). The cells were plated at a density of 2 × 106 cells per 35-mm dish and cultured in 95% air–5% CO2 at 37 °C overnight. The medium was then changed to DMEM supplemented with 2% (vol/vol) FBS and replaced daily.
Morphological Analysis of Trophoblast Fusion.
CytT transfected with 20 nM miR-515-5p mimics or NTC were plated onto coverslips and cultured in DMEM/F12 containing 2% (vol/vol) FBS for 72 h. The cells were rinsed in PBS, fixed in 4% (vol/vol) paraformaldehyde, and blocked with 3% (vol/vol) BSA in 1× PBS for 30 min at room temperature. Cell fusion was assessed by immunostaining using a primary antibody to plakoglobin (BD Biosciences; 1:250, clone 15) overnight at 4 °C. The coverslips were washed three times with 1× PBS for 5 min each and incubated with a secondary antibody conjugated to Alexa Fluor 488 (1:500) for 1 h at room temperature. The coverslips were then washed three times with 1× PBS for 5 min each and mounted on glass slides in mounting medium containing DAPI (Fluoroshield, Sigma Aldrich). Images were captured using a Zeiss confocal microscope at 400× magnification. Images were processed using ImageJ software, where the DAPI (blue) stained nuclei were pseudocolored to red for better interpretation. Number of syncytia were also counted using ImageJ. Ten individual fields were analyzed for quantification of the fusion index. Fusion index = (N − S)/T × 100%. N refers to the number of nuclei in the syncytia, S refers to the number of syncytia, and T refers to the total number of nuclei. Therefore, the more syncytia, the fewer the number of nuclei per syncytia and the lower the number of fusion events.
qRT-PCR.
Total RNA was extracted from tissues or cultured cells using a miRNeasy mini kit (Qiagen). MicroRNA expression was quantified using a TaqMan MicroRNA assay (Applied Biosystems) with U6 snRNA as an endogenous control, as previously described (18). Amplification was performed at 50 °C for 2 min, 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, and 60 °C for 1 min. For mRNA analysis, cDNA was synthesized using iScript cDNA synthesis kit (Bio-Rad Laboratories) and the expression of specific transcripts was measured using a Bio-Rad CFX384 PCR detection system with Power SYBR green PCR master mix (Applied Biosystems). h36B4 was used as an internal control. The sequences of primer pairs are presented in Table S2. The cycling conditions for mRNA were 95 °C for 3 min, 40 cycles at 95 °C for 10 s, and 60 °C for 30 s. The 2−∆∆Ct method was used to calculate relative expression of mRNAs and miRNAs of interest.
Immunoblot Analysis.
Proteins were extracted from tissues or cultured cells using RIPA buffer (Thermo Scientific). Protein concentration was determined by the Pierce bicinchoninic acid (BCA) assay kit (Thermo Scientific). Equivalent amounts of proteins were resolved by electrophoresis on 4–12% Bis-Tris gels (Invitrogen) and transferred to Hybond-P membranes (Amersham Biosciences). Membranes were then incubated overnight at 4 °C with the following primary antibodies: rabbit anti-aromatase (A7981, 1:1,000; Sigma-Aldrich); rabbit anti-GCM1 (ARP36863_P050, 1:1,000; Aviva Systems Biology); rabbit anti-FZD5 (06-756, 1:1,000; Millipore); rabbit anti-β-actin (ab8227, 1:8,000; Abcam). Signals of HRP-conjugated secondary antibodies (anti-rabbit IgG, 1:10,000; GE Healthcare) were detected with SuperSignal West Pico chemiluminescent substrate or SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific).
Luciferase Reporter Assay.
Comparative analysis of three target prediction programs (Targetscan, DIANA-mt, miRanda) was used to identify the potential targets of miR-515-5p. Among these, three putative targets (CYP19A1, GCM1, FZD5) known to be involved in the regulation of trophoblast differentiation were further validated by luciferase reporter assay. Sequences within 3′UTR of CYP19A1, GCM1, or FZD5 containing a putative binding site for miR-515-5p were amplified from human genomic DNA using PrimeSTAR HS DNA polymerase (Takara). The amplicons were double-digested using restriction endonucleases XhoI and NotI (New England Biolabs) and subcloned into the psiCHECK-2 vector (Progema) to create WT luciferase reporter plasmids. Mutations in the putative binding sites for miR-515-5p in each recombinant plasmid were generated using a QuikChange II site-directed mutagenesis kit (Agilent Technologies). The sequences of all constructs were confirmed by DNA sequencing. HEK 293T cells maintained in DMEM/F12 with 10% (vol/vol) FBS were transfected with either an empty psiCHECK-2 luciferase plasmid, or recombinant plasmids containing the WT or mutated 3′UTRs of hCYP19A1, GCM1, or FZD5. miR-515-5p mimics or NTC were cotransfected using Lipofectamine 2000 reagents (Invitrogen). Twenty-four hours after transfection, cells were harvested and luciferase activities were measured using a Dual-Luciferase Reporter Assay System (Progema).
Adenovirus Overexpression.
Adenovirus-mediated overexpression of CMV-c-MYC (Vector Biolabs) vs. CMV-β-Gal (50), as control, in human primary trophoblasts was performed as previously described (18). Briefly, freshly isolated CytT were infected with the recombinant adenoviruses at a multiplicity of infection of 10. After 4–6 h, the medium was removed and replaced with DMEM/F12 containing 2% (vol/vol) FBS. Cells were harvested after 24 h, 48 h and 72 h of culture for RNA and protein analysis.
ChIP-qPCR.
miR-515 precursors are encoded by two genes, miR-515-1 and miR-515-2. E-boxes corresponding to putative c-Myc responsive elements in the genomic regions upstream of miR-515-1 and miR-515-2 precursors were identified by an online bioinformatics analysis program (PROMO 3.0). Human trophoblasts were transduced either with recombinant adenoviruses expressing c-Myc or β-Gal, as described above. After 40 h of culture, ChIP assays were performed to analyze binding of c-Myc to each of the E-boxes using the Magna ChIP A kit (Millipore) according to the manufacturer’s instructions. c-Myc antibody (Cell Signaling Technology) was used to immunoprecipitate the cross-linked chromatin; nonimmune rabbit IgG (sc-2027, Santa Cruz Biotechnology) was used as a control. Quantitative real-time PCR was performed to assess the fold-enrichment of c-Myc at the putative Myc-responsive elements before and after culture, with or without c-Myc overexpression. SYBR Green PCR master mix (Applied Biosystems) and a Bio-Rad CFX384 real-time PCR system were used for the qPCR detection. Signals were normalized to input samples and expressed relative to control IgG. Data from three independent experiments were analyzed.
Acknowledgments
The authors thank Ms. Jo Smith for her assistance in isolating human trophoblast cells. This work was supported by NIH Grant 5-R01-DK031206.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1607849113/-/DCSupplemental.
References
- 1.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308(5728):1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 2.Ness RB, Sibai BM. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. Am J Obstet Gynecol. 2006;195(1):40–49. doi: 10.1016/j.ajog.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 3.Pineles BL, et al. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. Am J Obstet Gynecol. 2007;196(3):261.e1–261.e6. doi: 10.1016/j.ajog.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Lunell NO, Nylund LE, Lewander R, Sarby B. Uteroplacental blood flow in pre-eclampsia measurements with indium-113m and a computer-linked gamma camera. Clin Exp Hypertens B. 1982;1(1):105–117. doi: 10.3109/10641958209037184. [DOI] [PubMed] [Google Scholar]
- 5.Everett RB, MacDonald PC. Endocrinology of the placenta. Annu Rev Med. 1979;30:473–488. doi: 10.1146/annurev.me.30.020179.002353. [DOI] [PubMed] [Google Scholar]
- 6.Genbacev O, Joslin R, Damsky CH, Polliotti BM, Fisher SJ. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J Clin Invest. 1996;97(2):540–550. doi: 10.1172/JCI118447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang B, Kamat A, Mendelson CR. Hypoxia prevents induction of aromatase expression in human trophoblast cells in culture: potential inhibitory role of the hypoxia-inducible transcription factor Mash-2 (mammalian achaete-scute homologous protein-2) Mol Endocrinol. 2000;14(10):1661–1673. doi: 10.1210/mend.14.10.0539. [DOI] [PubMed] [Google Scholar]
- 8.Fournet-Dulguerov N, et al. Immunohistochemical localization of aromatase cytochrome P-450 and estradiol dehydrogenase in the syncytiotrophoblast of the human placenta. J Clin Endocrinol Metab. 1987;65(4):757–764. doi: 10.1210/jcem-65-4-757. [DOI] [PubMed] [Google Scholar]
- 9.Kamat A, Alcorn JL, Kunczt C, Mendelson CR. Characterization of the regulatory regions of the human aromatase (P450arom) gene involved in placenta-specific expression. Mol Endocrinol. 1998;12(11):1764–1777. doi: 10.1210/mend.12.11.0190. [DOI] [PubMed] [Google Scholar]
- 10.Rosenfeld CR, Morriss FH, Jr, Battaglia FC, Makowski EL, Meschia G. Effect of estradiol-17β on blood flow to reproductive and nonreproductive tissues in pregnant ewes. Am J Obstet Gynecol. 1976;124(6):618–629. doi: 10.1016/0002-9378(76)90064-8. [DOI] [PubMed] [Google Scholar]
- 11.Jobe SO, et al. Estradiol-17β and its cytochrome P450- and catechol-O-methyltransferase-derived metabolites stimulate proliferation in uterine artery endothelial cells: Role of estrogen receptor-α versus estrogen receptor-β. Hypertension. 2010;55(4):1005–1011. doi: 10.1161/HYPERTENSIONAHA.109.146399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanasaki K, et al. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature. 2008;453(7198):1117–1121. doi: 10.1038/nature06951. [DOI] [PubMed] [Google Scholar]
- 13.Lee SB, et al. Preeclampsia: 2-methoxyestradiol induces cytotrophoblast invasion and vascular development specifically under hypoxic conditions. Am J Pathol. 2010;176(2):710–720. doi: 10.2353/ajpath.2010.090513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albrecht ED, Pepe GJ. Estrogen regulation of placental angiogenesis and fetal ovarian development during primate pregnancy. Int J Dev Biol. 2010;54(2-3):397–408. doi: 10.1387/ijdb.082758ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar P, Kamat A, Mendelson CR. Estrogen receptor α (ERalpha) mediates stimulatory effects of estrogen on aromatase (CYP19) gene expression in human placenta. Mol Endocrinol. 2009;23(6):784–793. doi: 10.1210/me.2008-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar P, Mendelson CR. Estrogen-related receptor γ (ERRgamma) mediates oxygen-dependent induction of aromatase (CYP19) gene expression during human trophoblast differentiation. Mol Endocrinol. 2011;25(9):1513–1526. doi: 10.1210/me.2011-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamada K, Ogawa H, Honda S, Harada N, Okazaki T. A GCM motif protein is involved in placenta-specific expression of human aromatase gene. J Biol Chem. 1999;274(45):32279–32286. doi: 10.1074/jbc.274.45.32279. [DOI] [PubMed] [Google Scholar]
- 18.Kumar P, Luo Y, Tudela C, Alexander JM, Mendelson CR. The c-Myc-regulated microRNA-17∼92 (miR-17∼92) and miR-106a∼363 clusters target hCYP19A1 and hGCM1 to inhibit human trophoblast differentiation. Mol Cell Biol. 2013;33(9):1782–1796. doi: 10.1128/MCB.01228-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petraglia F, Florio P, Nappi C, Genazzani AR. Peptide signaling in human placenta and membranes: Autocrine, paracrine, and endocrine mechanisms. Endocr Rev. 1996;17(2):156–186. doi: 10.1210/edrv-17-2-156. [DOI] [PubMed] [Google Scholar]
- 20.Cronier L, Guibourdenche J, Niger C, Malassiné A. Oestradiol stimulates morphological and functional differentiation of human villous cytotrophoblast. Placenta. 1999;20(8):669–676. doi: 10.1053/plac.1999.0423. [DOI] [PubMed] [Google Scholar]
- 21.Morales-Prieto DM, Ospina-Prieto S, Chaiwangyen W, Schoenleben M, Markert UR. Pregnancy-associated miRNA-clusters. J Reprod Immunol. 2013;97(1):51–61. doi: 10.1016/j.jri.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Flor I, Bullerdiek J. The dark side of a success story: MicroRNAs of the C19MC cluster in human tumours. J Pathol. 2012;227(3):270–274. doi: 10.1002/path.4014. [DOI] [PubMed] [Google Scholar]
- 23.Bentwich I, et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet. 2005;37(7):766–770. doi: 10.1038/ng1590. [DOI] [PubMed] [Google Scholar]
- 24.Anson-Cartwright L, et al. The glial cells missing-1 protein is essential for branching morphogenesis in the chorioallantoic placenta. Nat Genet. 2000;25(3):311–314. doi: 10.1038/77076. [DOI] [PubMed] [Google Scholar]
- 25.Monkley SJ, Delaney SJ, Pennisi DJ, Christiansen JH, Wainwright BJ. Targeted disruption of the Wnt2 gene results in placentation defects. Development. 1996;122(11):3343–3353. doi: 10.1242/dev.122.11.3343. [DOI] [PubMed] [Google Scholar]
- 26.Lu J, et al. A positive feedback loop involving Gcm1 and Fzd5 directs chorionic branching morphogenesis in the placenta. PLoS Biol. 2013;11(4):e1001536. doi: 10.1371/journal.pbio.1001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishibashi O, et al. Hydroxysteroid (17-β) dehydrogenase 1 is dysregulated by miR-210 and miR-518c that are aberrantly expressed in preeclamptic placentas: A novel marker for predicting preeclampsia. Hypertension. 2012;59(2):265–273. doi: 10.1161/HYPERTENSIONAHA.111.180232. [DOI] [PubMed] [Google Scholar]
- 28.Baczyk D, et al. Glial cell missing-1 transcription factor is required for the differentiation of the human trophoblast. Cell Death Differ. 2009;16(5):719–727. doi: 10.1038/cdd.2009.1. [DOI] [PubMed] [Google Scholar]
- 29.Matsuura K, et al. Identification of a link between Wnt/β-catenin signalling and the cell fusion pathway. Nat Commun. 2011;2:548. doi: 10.1038/ncomms1551. [DOI] [PubMed] [Google Scholar]
- 30.American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 31.Rydnert J, Pfeifer-Ohlsson S, Goustin AS, Ohlsson R. Temporal and spatial pattern of cellular myc oncogene expression during human placental development. Placenta. 1987;8(4):339–345. doi: 10.1016/0143-4004(87)90061-0. [DOI] [PubMed] [Google Scholar]
- 32.Sander S, et al. MYC stimulates EZH2 expression by repression of its negative regulator miR-26a. Blood. 2008;112(10):4202–4212. doi: 10.1182/blood-2008-03-147645. [DOI] [PubMed] [Google Scholar]
- 33.Kamanu TK, Radovanovic A, Archer JA, Bajic VB. Exploration of miRNA families for hypotheses generation. Sci Rep. 2013;3:2940. doi: 10.1038/srep02940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lovén J, et al. MYCN-regulated microRNAs repress estrogen receptor-α (ESR1) expression and neuronal differentiation in human neuroblastoma. Proc Natl Acad Sci USA. 2010;107(4):1553–1558. doi: 10.1073/pnas.0913517107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laurent LC, et al. Comprehensive microRNA profiling reveals a unique human embryonic stem cell signature dominated by a single seed sequence. Stem Cells. 2008;26(6):1506–1516. doi: 10.1634/stemcells.2007-1081. [DOI] [PubMed] [Google Scholar]
- 36.Bar M, et al. MicroRNA discovery and profiling in human embryonic stem cells by deep sequencing of small RNA libraries. Stem Cells. 2008;26(10):2496–2505. doi: 10.1634/stemcells.2008-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ren J, Jin P, Wang E, Marincola FM, Stroncek DF. MicroRNA and gene expression patterns in the differentiation of human embryonic stem cells. J Transl Med. 2009;7:20. doi: 10.1186/1479-5876-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fornari F, et al. In hepatocellular carcinoma miR-519d is up-regulated by p53 and DNA hypomethylation and targets CDKN1A/p21, PTEN, AKT3 and TIMP2. J Pathol. 2012;227(3):275–285. doi: 10.1002/path.3995. [DOI] [PubMed] [Google Scholar]
- 39.Huang Q, et al. The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat Cell Biol. 2008;10(2):202–210. doi: 10.1038/ncb1681. [DOI] [PubMed] [Google Scholar]
- 40.Luo SS, et al. Human villous trophoblasts express and secrete placenta-specific microRNAs into maternal circulation via exosomes. Biol Reprod. 2009;81(4):717–729. doi: 10.1095/biolreprod.108.075481. [DOI] [PubMed] [Google Scholar]
- 41.Mouillet JF, Ouyang Y, Bayer A, Coyne CB, Sadovsky Y. The role of trophoblastic microRNAs in placental viral infection. Int J Dev Biol. 2014;58(2-4):281–289. doi: 10.1387/ijdb.130349ys. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delorme-Axford E, et al. Human placental trophoblasts confer viral resistance to recipient cells. Proc Natl Acad Sci USA. 2013;110(29):12048–12053. doi: 10.1073/pnas.1304718110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delorme-Axford E, Bayer A, Sadovsky Y, Coyne CB. Autophagy as a mechanism of antiviral defense at the maternal-fetal interface. Autophagy. 2013;9(12):2173–2174. doi: 10.4161/auto.26558. [DOI] [PubMed] [Google Scholar]
- 44.Schreiber J, et al. Placental failure in mice lacking the mammalian homolog of glial cells missing, GCMa. Mol Cell Biol. 2000;20(7):2466–2474. doi: 10.1128/mcb.20.7.2466-2474.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu C, et al. GCMa regulates the syncytin-mediated trophoblastic fusion. J Biol Chem. 2002;277(51):50062–50068. doi: 10.1074/jbc.M209316200. [DOI] [PubMed] [Google Scholar]
- 46.Chang M, et al. Glial cell missing 1 regulates placental growth factor (PGF) gene transcription in human trophoblast. Biol Reprod. 2008;78(5):841–851. doi: 10.1095/biolreprod.107.065599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aoki M, et al. R-spondin3 is required for mouse placental development. Dev Biol. 2007;301(1):218–226. doi: 10.1016/j.ydbio.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 48.Sabò A, Amati B. Genome recognition by MYC. Cold Spring Harb Perspect Med. 2014;4(2):a014191. doi: 10.1101/cshperspect.a014191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castellano L, et al. The estrogen receptor-α-induced microRNA signature regulates itself and its transcriptional response. Proc Natl Acad Sci USA. 2009;106(37):15732–15737. doi: 10.1073/pnas.0906947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pinho FG, et al. Downregulation of microRNA-515-5p by the estrogen receptor modulates sphingosine kinase 1 and breast cancer cell proliferation. Cancer Res. 2013;73(19):5936–5948. doi: 10.1158/0008-5472.CAN-13-0158. [DOI] [PubMed] [Google Scholar]
- 51.Alcorn JL, et al. Genomic elements involved in transcriptional regulation of the rabbit surfactant protein-A gene. Mol Endocrinol. 1993;7(8):1072–1085. doi: 10.1210/mend.7.8.8232306. [DOI] [PubMed] [Google Scholar]