Significance
The living conditions of plant-eating mammals can be modeled by reverse-engineering the functional shapes of their teeth. Here we introduced and tested a scoring scheme for describing functional properties of large mammalian herbivore teeth to capture their relationship to environmental conditions. We found that minimum Net Primary Productivity is the environmental variable best predicted by dental traits. These findings refine and provide direct biological context to previously reported results, where traits were found to predict overall climate rather than limiting conditions. We propose that nonavailability of the preferred plant foods (e.g., during dry seasons or longer dry periods), rather than the properties of average foods consumed, is the main functional link between climate and herbivore teeth.
Keywords: herbivorous mammals, dental traits, ecometrics, Kenya, paleoecology
Abstract
A major focus in evolutionary biology is to understand how the evolution of organisms relates to changes in their physical environment. In the terrestrial realm, the interrelationships among climate, vegetation, and herbivores lie at the heart of this question. Here we introduce and test a scoring scheme for functional traits present on the worn surfaces of large mammalian herbivore teeth to capture their relationship to environmental conditions. We modeled local precipitation, temperature, primary productivity, and vegetation index as functions of dental traits of large mammal species in 13 national parks in Kenya over the past 60 y. We found that these dental traits can accurately estimate local climate and environment, even at small spatial scales within areas of relatively uniform climate (within two ecoregions), and that they predict limiting conditions better than average conditions. These findings demonstrate that the evolution of key functional properties of organisms may be more reflective of demands during recurring adverse episodes than under average conditions or during isolated severe events.
How the physical environment influences the evolution of organisms and ecosystems, through environmental forcing as well as through biological interactions, has long been a major focus in evolutionary biology. A related and possibly more tractable question is whether the average conditions encountered by the organism have greater or lesser evolutionary impact than the episodic extremes that approach the limits of its functional capability. In the terrestrial fossil record, functional relationships between traits of organisms and their environments may be examined through analysis of depositional environments (1), the sediment stable isotope record (2) the fossils themselves (3), or the changing structure of the fossil communities. Several approaches are available for the latter, including taxic methods based on the characteristics of the nearest living relatives (4) and methods based directly on functional properties of the fossils themselves (5). All of these approaches have their limitations and advantages, ranging from high cost and limited availability of data to poor resolution and the risk of circular reasoning.
The function-driven methodology, called ecometrics (5–7), uses only functional traits as proxies and describes present and past communities only in terms of the distribution of these traits. Instead of focusing on individual organisms, ecometrics deals with the functional composition of communities. This methodology assumes that trait variables are sufficiently general to accurately represent the functional relationship of extinct taxa to the environment, such as the relationship between the mean molar hypsodonty of large mammalian herbivores and precipitation (5, 8). Accepting this assumption, which is certainly reasonable, at least for later Cenozoic mammal taxa, ecometrics is expected to be invariant to how present communities map onto past communities.
Here we extend the ecometrics methodology with a dental trait scoring scheme designed to capture dental wear patterns that are directly available and reasonably reliable in fossil records. The rationale behind this dental trait scoring scheme, termed Functional Crown Types (FCT), is to capture the functional durability, structural strength, and cutting power of teeth in a robust way across different body sizes and taxonomic variety. Durability, strength, and cutting power are expected to relate to the environment owing to properties of available foods, such as the harder, dustier, and less nutritious foods found in generally arid environments, as well as those subjected to seasonal aridity (8, 9).
The set of FCT (Functional Crown Types) includes seven variables: hypsodonty (HYP), horizodonty (HOD), acute lophs (AL), obtuse (or basin-like) lophs (OL), structural fortification of cusps (SF), flat occlusal topography (OT), and coronal cementum (CM). HYP and HOD are ordinal with three possible values, whereas the others are binary (present or absent). Instructions for scoring and illustrating examples are provided in SI Appendix. Ordinated HYP is a standard feature (8), AL and OL are developed based on work of Jernvall (10), and HOD, SF, OT, and CM are introduced here.
To capture nuances in dental durability and strength (11), we introduce the concept of HOD to describe dentitions where teeth are enlarged in the horizontal rather than the vertical dimension, along with the concepts of SF and OT. SF refers to strengthening features that make the cusp more prominent in the face of wear, whereas OT, in its derived state (flat), indicates lack of any occlusal elements rising significantly above the occlusal surface, whether fortified or not. The presence of thickened CM is also recorded as a separate trait. All of these traits are designed to capture aspects of the occlusal surface that relate to the shapes of the functional surfaces, especially in how they wear and how they relate to the dynamics of the chewing power stroke and thus to the actual comminution of food.
We tested the extended ecometrics methodology on a modern-day occurrence of extant large herbivores, using mammal assemblages data from 13 Kenyan national parks spanning the past 60 y (12). We analyzed how the dental traits relate to precipitation (PREC), temperature (TEMP), Net Primary Productivity (NPP), and Normalized Difference Vegetation Index (NDVI) to better understand the functional links between the environment and herbivore teeth in Eastern Africa and in general. Our analysis aimed to identify the environmental characteristics that can be modeled with the best accuracy, along with the dental traits that are the most informative about the environment. We focused particularly on limiting conditions, because we wished to test whether low availability of preferred foods and the need to consume more demanding fallback foods, typically during unfavorable seasons, drives the evolution of functional dental traits of herbivores (5).
Results
We built regression models linking climate and environmental characteristics (one at a time) to dental trait averages of occurring species, and inspected model performance on data not used in model calibration. Estimations of local climate and environmental conditions were done for each site, i.e., the protected ecosystem of a national park or reserve. Each site was described in terms of seven variables corresponding to dental traits (i.e., HYP, HOD, AL, OL, SF, OT, and CM). Each dental variable is the average of the trait scores over all of the species occurring at that site. Dental traits are more straightforward to capture from the fossil record than are estimates of body mass or species richness, but for the purpose of scientific discussion, we also compared the performance of the models built on dental traits and models built on body mass or species counts.
How Do Dental Traits Relate to One Another and to Environmental Characteristics?
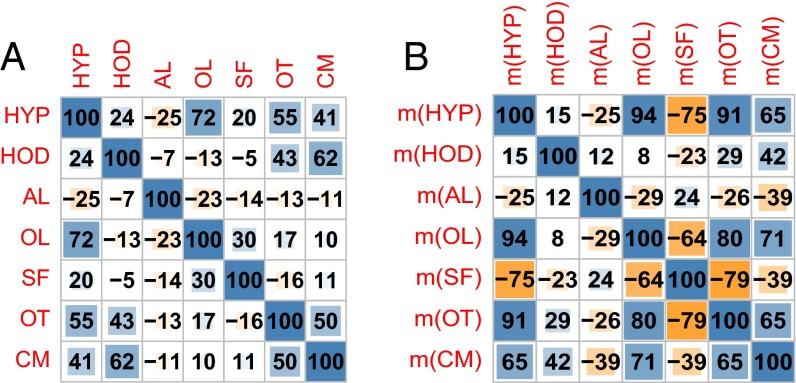
Fig. 1 depicts linear correlations between dental traits at the species level (i.e., how similar are trait values from species to species) and average values for traits at the site level (i.e., how related are the average values of traits from site to site). We can see that overall there is less correlation at the species level (Fig. 1A) than at the site level (Fig. 1B), which suggests that individual species can have rather diverse sets of traits, but when assemblages of species coming from the same place are considered, stronger relations emerge.
Fig. 1.
(A) Linear correlations among dental traits at species level (63 observations). Correlations above 0.24 or below −0.24 are statistically significant at 5% via the two-tailed t test. (B) Linear correlations among dental trait means (m) at site level (13 observations). Correlations above 0.55 or below −0.55 are statistically significant. Color-coding: blue, positive correlations; orange, negative correlations. The intensity of the color indicates the strength of the correlation.
At the site level there are strong correlations among mean HYP (hypsodonty), mean OL (obtuse lophs), flat mean OT (occlusal topography), and mean CM (cementum). This is plausible, because all of these traits capture different aspects of the same phenomenon: teeth that are more durable. Interestingly, mean SF shows a strong negative correlation with HYP at the site level (Fig. 1B), but a weak positive correlation at the species level (Fig. 1A); that is, teeth with fortified cusps tend to be moderately hypsodont and occur in humid settings with few other hypsodont species. In this dataset, SF (structural fortification) of cusps is present mainly in mesodont and hypsodont bovids, hippopotamus, and suids (except the warthog). The structurally fortified hypsodont bovids occur at sites with higher average HYP, but those sites also have more species on average, which means that in addition there are relatively more species lacking fortified cusps, making the correlation between SF and HYP negative at the site level.
This pattern suggests the hypothesis that reduncines, bovids, and hippopotamus, fresh-grass grazers with fortified cusps, act here as indicators of highly productive and species-rich wetlands that also attract herbivores lacking these traits. In our dataset, hippos and reduncines tend to occur in locations with high species counts, low elevation, low precipitation, high temperature, low apparent NPP because of low precipitation, and low NDVI owing to open water surfaces. High diversity in such a setting suggests high productivity because of supplementary surface water compensating for low rainfall, such as wetlands fed by rivers or lakes. Interested readers are referred to SI Appendix for more detailed analysis of indicator species.
Fig. 1B shows that HOD and AL are somewhat outside the strongly correlated, durability-related trait set (i.e., HYP, CM, SF, and OL). There is no obvious reason to expect that HOD (horizodonty) would capture anything besides the durability-related increased tooth volume in the horizontal dimension rather than the vertical dimension; the present association with temperature depends on the giant forest hog and is likely an exception to the general pattern. The presence of AL (acute lophs) is functionally related to leaf-eating and forest habitats and thus is expected to capture information related primarily not to durability, but rather to cutting capacity of teeth. The distribution of dental traits referring to durability on the one hand and cutting capacity on the other hand can be considered to represent two main axes of the environment. In the present dataset, AL is in fact a strong indicator of forest habitat. In this tropical setting, AL is also an indicator of temperature through the evapotranspirative cooling effect of tree cover (13), and also because many of our forested sites are at higher elevations than nonforested sites.
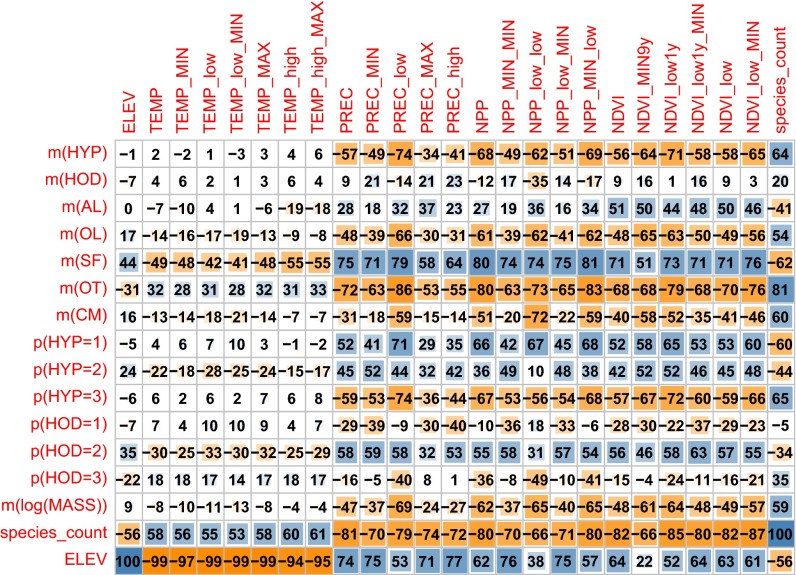
Fig. 2 depicts correlations among dental traits, environmental variables, and other characteristics of the sites. Temperature, precipitation, NPP, and NDVI are presented as averages as well as different limits and extremes (i.e., daily, monthly, and yearly minimums or maximums). In the studied domain in Eastern Africa, dental traits are only weakly informative about elevation and temperature, but quite strongly correlated with precipitation, NPP, NDVI, and species count. The sole notable correlations of temperature are with SF and OT. Somewhat weaker but still visible relations of temperature are with specific values of mesodonty and mesohorizodonty (HYP = 2; HOD = 2). Elevation and temperature are strongly correlated, but average temperature has a stronger relationship to elevation than does maximum temperature. In addition, elevation is relatively strongly correlated with species count, signaling that there are fewer large mammals at higher elevations. On the trait side, we find instead a stronger relationship with temperature maximums than with averages, particularly with SF. This suggests that species count relates more to average temperature (i.e., fewer species at cooler high elevations), but that dental traits carry complementary information that explains maximum temperatures better than averages.
Fig. 2.
Linear correlations between dental traits and environmental variables. m denotes mean, and p denotes proportion. Numbers indicate the Pearson correlation coefficients. Considering that there are 13 observations (sites), the correlation values above 0.55 or below −0.55 are statistically significant at 5% via the two-tailed t test. Table 2 provides explanations of abbreviations.
Almost all predictors correlate quite well with precipitation, NPP, and NDVI. The similar correlations for NPP and precipitation are not surprising, given that the NPP estimate is computed using precipitation as an input and is highly dependent on it. More reassuring are the correlations with NDVI, because NDVI is a direct observation of vegetation greenness and thus is independent of precipitation and temperature measurements. The NDVI does depend on climatic conditions and reflects the NPP, and thus the availability and quality of herbivores’ food. The similar patterns for NDVI and NPP that appear in Fig. 2 corroborate this assumption. Precipitation, NDVI, and NPP are also highly correlated with the number of occurring species and with elevation (Fig. 2).
Fig. 2 also highlights that among the environmental variables describing the availability of water (i.e., PREC and NPP), minimum values show stronger correlations with dental traits than do mean values. This finding suggests that functional dental trait features may better describe limiting conditions for survival in these seasonal environments compared with under average conditions. We explored this hypothesis in more detail by comparing the predictive power of regression models built on dental traits.
Which Environmental Characteristics Can Be Best Predicted from Traits of Co-Occurring Species?
We built variants of predictive models for estimating TEMP, PREC, NPP, and NDVI summarized as averages and different limits and extremes (daily, monthly, and yearly minimums or maximums), and analyze which models achieve better estimation accuracy, as measured on unseen data not used for estimating model parameters.
Table 1 presents a summary of performance within the four groups of climate and environmental variables, across different model inputs and different ways of quantifying limiting conditions. Based on the distribution of performance scores in this table, the following main observations can be made about how dental traits can affect climate and environmental conditions in modern ecosystems:
-
•
Our scoring scheme captures sufficient information to produce accurate estimates of local climate and environment, resolving details within areas of relatively uniform climate, such as the global ecoregions defined by the World Wildlife Fund (wwf.panda.org/about_our_earth/ecoregions/about/). Our data span two ecoregions.
-
•
Among the four climate and environmental variables, dental traits best predict NPP.
-
•
Dental traits predict specific limiting conditions consistently better than average conditions for all four climate and environmental variables. Limiting conditions are minima of PREC, NPP, and NDVI and maxima of TEMP.
-
•
Dental traits better predict regularly reoccurring limiting conditions than extremes over longer times (e.g., averages over daily extremes better than driest months, monthly extremes better than yearly or 9-y extremes).
Table 1.
Summary of model performance in the test measured via cross-validation
| Variable | Mass OLS | Teeth LARS(3) | Teeth LARS | Species OLS | All OLS |
| PREC | 0.07 | 0.38 | 0.41(2) | 0.62 | −1.46 |
| PREC_MIN | −0.11 | 0.36 | 0.36(3) | 0.41 | −0.07 |
| PREC_low | 0.36 | 0.53 | 0.53(3) | 0.55 | −0.64 |
| PREC_MAX | −0.05 | −0.01 | 0.31(6) | 0.47 | −0.51 |
| PREC_high | −0.09 | 0.24 | 0.24(3) | 0.45 | −2.07 |
| NPP | 0.28 | 0.51 | 0.54(2) | 0.59 | −1.64 |
| NPP_MIN_MIN | −0.08 | 0.40 | 0.41(1) | 0.42 | −0.06 |
| NPP_low_low | 0.38 | 0.65 | 0.65(3) | 0.33 | −0.46 |
| NPP_low_MIN | −0.05 | 0.40 | 0.44(1) | 0.43 | −0.21 |
| NPP_MIN_low | 0.33 | 0.55 | 0.55(3) | 0.57 | −0.52 |
| NDVI | 0.11 | 0.26 | 0.26(3) | 0.64 | −1.59 |
| NDVI_MIN9y | 0.13 | −0.08 | 0 | 0.28 | −1.51 |
| NDVI_low1y | 0.29 | 0.26 | 0.29(1) | 0.68 | −1.27 |
| NDVI_low1y_MIN | 0.10 | 0.20 | 0.22(4) | 0.60 | −1.68 |
| NDVI_low | 0.12 | 0.27 | 0.27(3) | 0.64 | −1.43 |
| NDVI_low_MIN | 0.23 | 0.33 | 0.35(4) | 0.72 | −0.65 |
| TEMP | −0.15 | −0.05 | 0.09(1) | 0.22 | −0.95 |
| TEMP_MIN | −0.13 | 0.03 | 0.08(1) | 0.20 | −0.72 |
| TEMP_low | −0.21 | −0.37 | 0.07(6) | 0.15 | −0.95 |
| TEMP_low_MIN | −0.19 | −0.33 | 0 | 0.14 | −0.80 |
| TEMP_MAX | −0.17 | −0.12 | 0.09(1) | 0.22 | −1.08 |
| TEMP_high | −0.11 | 0.23 | 0.23(3) | 0.26 | −0.92 |
| TEMP_high_MAX | −0.12 | 0.19 | 0.19(3) | 0.27 | −1.21 |
Mass OLS, standard regression on log(mass); Teeth LARS(3), Least Angle Regression on dental traits (three automatically selected traits) (Table 2); Teeth LARS, best-performing LARS (number in brackets indicates how many dental traits are selected); Species OLS, standard regression model on species count; All OLS, standard regression on mass and all of the dental traits. Best performances are highlighted in bold for the variables.
In addition, the following observations can be made about the ecological information contained in dental traits compared with using mass or species count:
-
•
Dental traits predict local climate and environment consistently better than does body mass. Furthermore, body mass cannot predict any temperature better than the baseline predicting a constant, whereas dental traits predict maximums of temperature with acceptable accuracy. Species count gives the most accurate predictions, but obtaining this is problematic for most fossil datasets owing to biases of fossilization, preservation, collection, and reporting.
-
•
Like dental traits, body mass better predicts limiting conditions than averages. For the NDVI, body mass captures primarily medium-term (yearly) limiting conditions, whereas dental traits capture primarily short-term (monthly) limiting conditions. This is demonstrated by mass predicting the 9-y minimum NDVI quite well and better than average NDVI, while dental traits predicting the 9-y minimum quite poorly.
-
•
Species count predicts average conditions well. For PREC and NPP, averages are predicted better than any minima or maxima.
-
•
Overall, dental traits are informative about short-term reoccurring limiting conditions; body mass, about medium-term limiting conditions; and the number of species, about long-term averages.
We find that Least Angle Regression (LARS) models, which look like normal regression equations but are obtained with a different model-fitting process, perform notably better than Ordinary Least Squares (OLS). LARS iteratively selects which dental traits to include in the model, whereas OLS uses all of the input traits. Our results confirm a previous expectation that OLS would generalize poorly because of too many candidate predictors in relation to the low number of data points.
Complementarity and Predictive Contribution of the Dental Traits.
Table 2 presents the regression models with three most informative dental traits resulting from LARS fitted on all the 13 Kenyan national park sites studied. We investigated which dental traits carry the most predictive power about each group of climate and environmental variables.
Table 2.
Models fitted on all data via LARS with three best dental traits, and their accuracy
| Prediction target | Regression coefficients | Accuracy | Description of the prediction target | ||||||||
| Intercept | HYP | HOD | AL | OL | SF | OT | CM | R2 fit | R2* test | ||
| PREC | −1,765 | 2,156 | 3,969 | −2,441 | 0.68 | 0.38 | Annual precipitation | ||||
| PREC_MIN | −1,688 | 1,481 | 2,325 | −762 | 0.66 | 0.36 | Driest month over year | ||||
| PREC_low | 966 | 1,456 | −2,444 | −310 | 0.76 | 0.53 | Driest place over year | ||||
| PREC_MAX | 1,486 | 775 | 3,741 | −245 | 0.27 | −0.01 | Wettest month over year | ||||
| PREC_high | −3,561 | 3,553 | 5,339 | −1,300 | 0.56 | 0.24 | Wettest place over year | ||||
| NPP | 1,179 | 2,907 | −2,331 | −15 | 0.71 | 0.51 | Average annual NPP | ||||
| NPP_MIN_MIN | −2,447 | 2,049 | 4,112 | −876 | 0.66 | 0.40 | Coldest and driest month over year | ||||
| NPP_low_low | 972 | 25 | 2,639 | −3,495 | 0.77 | 0.65 | Coldest days with driest place | ||||
| NPP_low_MIN | −2,265 | 1,896 | 4,071 | −914 | 0.67 | 0.40 | Coldest days with driest month | ||||
| NPP_MIN_low | 1,162 | 2,307 | −2,041 | −667 | 0.74 | 0.55 | Coldest month driest place over year | ||||
| NDVI | 0.337 | 1.429 | 0.879 | −0.374 | 0.59 | 0.26 | Average NDVI | ||||
| NDVI_MIN9y | 0.410 | 0.789 | −0.098 | −0.479 | 0.50 | −0.08 | Global minimum over 9 y | ||||
| NDVI_low1y | 0.419 | 0.721 | 0.396 | −0.869 | 0.66 | 0.26 | Average over yearly minimums | ||||
| NDVI_low1y_MIN | 0.314 | 0.846 | 0.88 | −0.378 | 0.51 | 0.20 | Minimum over yearly averages | ||||
| NDVI_low | 0.347 | 1.281 | 0.818 | −0.447 | 0.59 | 0.27 | Average over monthly minimums | ||||
| NDVI_low_MIN | 0.300 | 0.986 | 0.887 | −0.63 | 0.67 | 0.33 | Minimum over monthly minimums | ||||
| TEMP | 43.9 | −23.0 | −53.5 | 13.1 | 0.57 | −0.05 | Average temperature | ||||
| TEMP_MIN | 44.3 | −24.7 | −56.4 | 11.6 | 0.60 | 0.04 | Coldest month over year | ||||
| TEMP_low | 28.6 | −13.6 | −35.7 | 7.2 | 0.37 | −0.37 | Average over coldest days of months | ||||
| TEMP_low_MIN | 28.3 | −13.4 | −37.8 | 2.8 | 0.37 | −0.33 | Minimum over coldest days of months | ||||
| TEMP_MAX | 44.7 | −22.5 | −52.3 | 13.7 | 0.54 | −0.12 | Hottest month over year | ||||
| TEMP_high | 55.2 | −16.8 | −20.7 | −67.9 | 0.62 | 0.23 | Average over hottest days of months | ||||
| TEMP_high_MAX | 54.0 | −8.9 | −18.0 | −62.9 | 0.57 | 0.19 | Maximum over hottest days of months | ||||
HYP means mean(HYP), and so on. R2 fit indicates goodness of fit (model calibration accuracy), and R2* test indicates predictive power (accuracy measured via cross-validation). Bold indicates best performing model in each group of environmental and climate variables.
In Table 2, SF and OT are included in almost all of the models and appear to be the most informative traits about the environment. Interestingly, HYP is almost never used. Recall from Fig. 1 that HYP is strongly correlated with SF, OT and OL, as well as CM across sites. LARS selects predictors in such a way that they are the most informative but also not redundant. Thus, a lack of HYP in the models does not mean that HYP as such is not informative, but rather means that the relevant information is already captured by other predictors that perhaps carry a stronger signal in this case, making HYP redundant.
The coefficients of OT for predicting water availability (precipitation, NPP, and NDVI) are negative and intuitive; the more occlusally flat teeth, found primarily in grazing forms, the less rain is expected. The coefficients of SF of cusps for PREC, NPP, and NDVI are positive; the more emphasis on cusps, the more rain. For predicting temperature, the coefficients of SF and OT are reversed: the flatter the teeth, the higher the temperature, and the more emphasis on cusps, the lower the temperature. Assuming that high temperatures are associated with dry environments, this directly relates to our observations regarding water availability. In a tropical setting where cooling by evapotranspiration is a major factor in local temperature (13), this is a reasonable finding. Possibly a more important factor is the association of low temperature with high altitudes and higher precipitation in this dataset and in tropical regions in general. Paleo-altitude is notoriously difficult to assess, and it is conceivable that ecometric estimates of temperature could help estimate elevation in the past.
Overall, different sets of dental traits appear to be the most informative about different groups of environmental variables. Whereas lophs almost never occur in the PREC and NPP equations, they play important roles in the NDVI and TEMP estimation. In the case of NDVI, AL variables are strongly present, in addition to SF and OT. AL comes with a positive coefficient; the higher the proportion of species with AL, the higher the NDVI. This is expected from the functional association of AL with leaf-eating. In our dataset, four species have an AL of 1: Diceros bicornis and three primates, two of which are present only in one site each; only Colobus guereza is present more widely. D. bicornis and C. guereza have almost opposite occurrence patterns, effectively turning AL into a proxy for species count; the higher the AL, the fewer species present. This may be generally true as a reflection of the high number of ungulate species in the grasslands relative to the forests.
For predicting temperature, OL appears to be the most informative in addition to SF and OT. In our dataset, obtuse lophs are found in 12 out of 63 species and OL therefore has a strong relationship with HYP and number of species (Figs. 1B and 2). The relationship is similar to that with the other environmental variables; OL captures the number of species, but also must capture characteristic information for temperature because it comes consistently into the TEMP equations. This is probably because in our study area, open habitats are warmer than closed habitats, as discussed above. In the cases where models predict temperatures reasonably well (maximum reoccurring temperature), both loph features—forest-related AL (cool) and savanna-related OL (hot)—are used in the same equations. This is a biologically plausible explanation for the temperature sensitivity of dental traits; the underlying cause is related to the biophysics of the environment rather than to food or climate as such.
Discussion and Conclusions
Our analysis shows that productivity-related environmental characteristics of 13 tropical sites in Kenya can be reproduced with reasonable accuracy using only dental morphology of their larger herbivore fauna. This finding reinforces previous suggestions that environment and dental morphology are closely related and suggests that the extremes of environmental conditions are part of the shaping mechanism for dental characteristics.
Among all of the considered climatic variables, limiting productivity (NPP_low_low, representing productivity of the driest place at the coldest times in each park) is the one best predictable from dental traits. These findings add considerable biological emphasis to previously reported findings, where HYP was found to successfully predict mean rainfall (6, 8), despite being considered primarily a proxy for diet and habitat (14). Our results indicate that preferred diet or habitat might not be as important for dental traits as the occasional restricted availability of these resources (e.g., during dry seasons or longer dry periods). We propose that this is the strongest functional link between climate and herbivore teeth.
The results suggest that the FCT dental trait scoring scheme makes it possible to resolve differences in local climate and environment at small spatial scales. SF and OT, the morphology known as plagiolophodonty, stand out as the most powerful predictors of climate and productivity. In addition, different dental traits add valuable information for predicting different environmental variables; HOD and CM relate to precipitation, whereas AL and OL relate to highest temperatures. It is encouraging that the dental traits reveal relationships that are either biologically intuitive or readily explainable as peculiar to this dataset. It is especially satisfactory that the seemingly unlikely relationship between dental traits and temperature can be readily explained through these variables’ association with the physics of altitude (low temperatures) and forest cover (evapotranspiration). On a slightly more speculative line, this temperature signal could be turned into a proxy for either tree cover or altitude, given appropriate additional information. Similarly, the fact that dental traits appear to distinguish between wet and dry grasslands is highly promising and will, if verified, offer a valuable complement to existing proxies for grasslands and grazing.
Kenya’s protected areas are relics of long-gone undisturbed ecosystems, and indeed large portions of the original habitats now are heavily farmed (Naivasha, Shompole, and Aberdares), ranched (Masaai Mara, and Samburu), logged (Kakamega), or settled (Nairobi and Naivasha) (15). Human impacts have a strong potential to alter the geographic distributions of species, either by displacement or by attraction toward humans in areas where food and water are plentiful. The fact that our models nevertheless successfully predict local differences in climate among our sites suggests that our method has considerable robustness and predictive power for fossil assemblages undisturbed by human ecological impact.
Although we would expect good performance in small geographic scales with reasonably accurate records of species occurrence, our model’s predictive power here is somewhat limited by the small number of sites in our dataset. The fact that different dental traits consistently come into play with different environmental variables in biologically intuitive ways shows that these traits also capture underlying information about environmental conditions. Climatic minima and maxima are better predicted from dental traits than are averages, and out of the limiting conditions, regularly reoccurring limits are better predicted than rarely occurring extremes. Species counts also successfully predict climate, but the property of identifying limiting factors is effectively restricted to dental traits. An interesting question for further exploration is whether this difference is generally true of functional traits versus species diversity.
The fact that we were able to successfully discern relatively minor differences in climate and productivity within 13 national parks and reserves in Kenya indicates that our method has considerable power for paleoecological resolution. Our models use only data that can be computed from fossil assemblages, where species counts are often unreliable, and thus this methodology holds great promise for investigating past environmental boundary conditions for fossil localities.
Our finding that dental traits predict recurrent minima and maxima better than averages supports the view that dental traits reflect primarily limiting or extreme environmental conditions and the need for herbivores to process structurally demanding fallback foods, rather than the requirements of preferred foods under average or optimal conditions. These findings suggest that the functional dental traits, especially those associated with the harsh end of the environmental spectrum, are shaped by evolution to adapt to the long-term frequency (and perhaps severity) of climatically adverse conditions. Along with general biophysical relationships between foods and habitats, this explains how a fundamentally dietary signal is transformed into a climatic one. Our results further imply that the long-term evolution of these dental traits was due more to selection imposed by recurring extremes than by average conditions.
Materials and Methods
We used environmental and species occurrence data covering the last 60 y from 13 protected areas (sites) of national parks in Kenya. Presence-absence of species with body masses, as well as site elevations, have been published previously (12). Updated presence-absence data were provided by A.B.T. Body mass data were obtained from Pantheria (16) and the MOM database (17). We considered only the orders Proboscidea, Primates, Perissodactyla, and Artiodactyla (8). Dental traits were newly developed and scored (by M.F.) for this study according to our FCT scheme, described in detail in SI Appendix. HYP values were obtained from Liu et al. (18) and updated by M.F. TEMP and PREC data were obtained from WorldClim (19) and represent interpolated and averaged observations for 1950–2000, roughly the same period as the mammal occurrence data (1950–2012). NPP was computed from TEMP and PREC data using the classic formula of Leith et al. (20), as cited by Liu et al. (18). Data for NDVI, which indicates the amount of live green vegetation, were downloaded from the National Aeronautics and Space Administration’s Earth Observations dataset (neo.sci.gsfc.nasa.gov/view.php?datasetId=MOD13A2_M_NDVI) covering 2001–2009 for every 16 d. Averages, maximums, and minimums for TEMP, PREC, NPP, and NDVI were computed over different time spans, as well as spatially, to capture short- and longer-term limiting conditions for productivity, i.e., the availability of high-quality plant foods (SI Appendix).
Our computational methodology builds on work of Liu et al. (18), in which an ecometric regression model was developed for estimating NPP using dental functional traits (i.e., molar height and the number of longitudinal lophs). The models were fitted using LARS (21), which is suited for high-dimensional data relative to the number of observations with correlated predictors. We used LARS with dental traits as inputs to predict each environmental variable separately. The resulting models look like normal linear regression equations, but the procedure for obtaining these equations is special. For comparison, we report the standard OLS regression performance on log-mass alone, on species count alone, as well as on the seven dental traits plus log-mass. We used the leave-one-out cross-validation procedure to analyze the generalization accuracy of the models. We used the normalized coefficient of determination (R2*) (22) to assess the predictive performance (SI Appendix). The data and the code for reproducing the computational experiments reported in this study are available online (https://github.com/zliobaite/paper-Kenya-parks). In addition, all datasets are provided in tables in SI Appendix.
Supplementary Material
Acknowledgments
Aleksis Karme and Juha Saarinen helped develop the FCT scheme, and Nikos Solounias helped to form the new Greek terms. A.B.T. and A.K.B. thank S. Kate Lyons and members of the National Museum of Natural History Ecological Reading Group for help with data compilation for the Kenyan national parks and reserves. The research leading to these results was funded in part by the Academy of Finland (Expected Climate Change and Options for European Silviculture project). Funding for data compilation by A.B.T., as published previously (12), was provided by the Evolution of Terrestrial Ecosystems Program and the Natural History Research Experiences program at the National Museum of Natural History, Smithsonian Institution. M.F. was the recipient of a research award from the Alexander von Humboldt Foundation. This is Evolution of Terrestrial Ecosystems Program (ETE) publication 344. It is also and equally a contribution from the Valio Armas Korvenkontio Unit of Dental Anatomy in Relation to Evolutionary Theory.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1609409113/-/DCSupplemental.
References
- 1.Behrensmeyer AK, Hook RW. Paleoenvironmental contexts and taphonomic modes. In: Behrensmeyer AK, et al., editors. Terrestrial Ecosystems Through Time. Univ Chicago Press; Chicago: 1992. pp. 15–136. [Google Scholar]
- 2.Levin NE, Brown FH, Behrensmeyer AK, Bobe R, Cerling TE. Paleosol carbonates from the Omo Group: Isotopic records of local and regional environmental change in East Africa. Palaeogeogr Palaeoclimatol Palaeoecol. 2011;307:75–89. [Google Scholar]
- 3.Cerling TE, et al. Stable isotope-based diet reconstructions of Turkana Basin hominins. Proc Natl Acad Sci USA. 2013;110(26):10501–10506. doi: 10.1073/pnas.1222568110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosbrugger V. Nearest-living-relative method. In: Gornitz V, editor. Encyclopedia of Paleoclimatology and Ancient Environments. Springer; Dordrecht, The Netherlands: 2009. pp. 607–609. [Google Scholar]
- 5.Fortelius M, et al. Evolution of Neogene mammals in Eurasia: Environmental forcing and biotic interactions. Annu Rev Earth Planet Sci. 2014;42:579–604. [Google Scholar]
- 6.Eronen J, et al. Precipitation and large herbivorous mammals, part II: Application to fossil data. Evol Ecol Res. 2010;12:235–248. [Google Scholar]
- 7.Polly PD, et al. History matters: Ecometrics and integrative climate change biology. Proc Biol Sci. 2011;278(1709):1131–1140. doi: 10.1098/rspb.2010.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fortelius M, et al. Fossil mammals resolve regional patterns of Eurasian climate change during 20 million years. Evol Ecol Res. 2002;4:1005–1016. [Google Scholar]
- 9.Fortelius M. Ungulate cheek teeth: Developmental, functional and evolutionary interrelations. Acta Zool Fenn. 1985;180:1–76. [Google Scholar]
- 10.Jernvall J. Mammalian molar cusp patterns: Developmental mechanisms of diversity. Acta Zool Fenn. 1995;198:1–61. [Google Scholar]
- 11.Janis CM, Fortelius M. On the means whereby mammals achieve increased functional durability of their dentitions, with special reference to limiting factors. Biol Rev Camb Philos Soc. 1988;63(2):197–230. doi: 10.1111/j.1469-185x.1988.tb00630.x. [DOI] [PubMed] [Google Scholar]
- 12.Tóth AB, Lyons SK, Behrensmeyer AK. Mammals of Kenya’s protected areas from 1888 to 2013. Ecology. 2014;95(6):1711. [Google Scholar]
- 13.Bonan GB. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science. 2008;320(5882):1444–1449. doi: 10.1126/science.1155121. [DOI] [PubMed] [Google Scholar]
- 14.Damuth J, Janis CM. On the relationship between hypsodonty and feeding ecology in ungulate mammals, and its utility in palaeoecology. Biol Rev Camb Philos Soc. 2011;86(3):733–758. doi: 10.1111/j.1469-185X.2011.00176.x. [DOI] [PubMed] [Google Scholar]
- 15.Tóth AB, Lyons SK, Behrensmeyer AK. A century of change in Kenya’s mammal communities: Increased richness and decreased uniqueness in six protected areas. PLoS One. 2014;9(4):e93092. doi: 10.1371/journal.pone.0093092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones KE, et al. PanTHERIA: A species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology. 2009;90:2648. [Google Scholar]
- 17.Smith FA, et al. Body mass of late Quaternary mammals. Ecology. 2003;84:3402. [Google Scholar]
- 18.Liu L, et al. Dental functional traits of mammals resolve productivity in terrestrial ecosystems past and present. Proc Roy Soc B. 2012;279(1739):2793–2799. doi: 10.1098/rspb.2012.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25:1965–1978. [Google Scholar]
- 20.Leith H. Modeling the primary productivity of the world. In: Leith H, Whittaker RH, editors. Primary Productivity of the Biosphere. Springer; Dordrecht, The Netherlands: 1972. pp. 237–263. [Google Scholar]
- 21.Efron B, Hastie T, Johnstone I, Tibshirani R. Least Angle Regression. Ann Stat. 2004;32(2):407–499. [Google Scholar]
- 22.Žliobaitė I, Tatti N. 2016. A note on adjusting R2 for using with cross-validation. arXiv 1605.01703.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.