Significance
The cbb3 cytochrome c oxidase has high affinity for oxygen and is typically used for respiration in many pathogenic bacteria in hypoxic environments. Here we show that the opportunistic human pathogen Pseudomonas aeruginosa has a capability to produce 16 different cbb3 isoforms by combinations of multiple isosubunits. Utilization of multiple isoforms with different properties contributes to the robustness of the bacterium under hypoxic growth conditions and might have practical implications for resistance to antibiotics and formations of biofilms and persister cells. Our findings indicate that the mechanism for the production of the isoforms could be possible therapeutic targets for treating chronic P. aeruginosa infection.
Keywords: cbb3, terminal oxidase, cytochrome c oxidase, respiration, Pseudomonas aeruginosa
Abstract
The ubiquitous opportunistic human pathogen Pseudomonas aeruginosa has five terminal oxidases for aerobic respiration and uses them under different growth conditions. Two of them are cbb3-type cytochrome c oxidases encoded by the gene clusters ccoN1O1Q1P1 and ccoN2O2Q2P2, which are the main terminal oxidases under high- and low-oxygen conditions, respectively. P. aeruginosa also has two orphan gene clusters, ccoN3Q3 and ccoN4Q4, encoding the core catalytic CcoN isosubunits, but the roles of these genes have not been clarified. We found that 16 active cbb3 isoforms could be produced by combinations of four CcoN, two CcoO, and two CcoP isosubunits. The CcoN3- or CcoN4-containing isoforms were produced in the WT cell membrane in response to nitrite and cyanide, respectively. The strains carrying these isoforms were more resistant to nitrite or cyanide under low-oxygen conditions. These results indicate that P. aeruginosa gains resistance to respiratory inhibitors using multiple cbb3 isoforms with different features, which are produced through exchanges of multiple core catalytic isosubunits.
The cbb3-type cytochrome c oxidase (cbb3) is a bacteria-specific terminal oxidase of the heme-copper oxidoreductase superfamily that catalyzes the four-electron reduction of molecular oxygen to water at the end of the aerobic respiratory chain (1). cbb3 has an extremely high affinity for oxygen and typically functions under low-oxygen conditions in many bacteria, including several pathogens of Helicobacter, Campylobacter, and Neisseria species (2–4). cbb3 consists of four subunits that are encoded by the ccoNOQP operon. CcoN is the core catalytic subunit, and it contains a reaction center. CcoO and CcoP are transmembrane monoheme and diheme cytochromes c, respectively (5). CcoQ is known to affect the stability of the cbb3 complex, but it is not necessarily a component of purified cbb3 (6–8).
The ubiquitously distributed opportunistic human pathogen Pseudomonas aeruginosa has two cbb3-type isoforms, cbb3-1 and cbb3-2, which are encoded by ccoN1O1Q1P1 and ccoN2O2Q2P2, respectively. It also has three low-affinity enzymes: the bo3-type quinol oxidase, cyanide-insensitive oxidase (CIO), and aa3-type cytochrome c oxidase (9–11). These terminal oxidases are differentially regulated under various growth conditions, and they contribute to the bacterium’s ability to reside in a wide variety of environmental niches. P. aeruginosa is unique in its utilization of the high-affinity cbb3 oxidases as the main terminal oxidases both under high- and low-oxygen conditions. The ccoN1O1Q1P1 cluster is constitutively expressed, whereas the ccoN2O2Q2P2 cluster is up-regulated under low-oxygen conditions or at the stationary phase (12). P. aeruginosa also possesses two orphan ccoNQ gene clusters designated ccoN3Q3 (PA1856-PA1855) and ccoN4Q4 (PA4133-PA4134) (Fig. 1A). These gene clusters cannot produce active enzymes independently, and their functions remain unknown. In this present study, we investigated the function and expression profiles of the ccoN3Q3 and ccoN4Q4 gene clusters and found that the gene products could produce active cbb3 isoforms via combinations with CcoO1 or CcoO2 and CcoP1 or CcoP2.
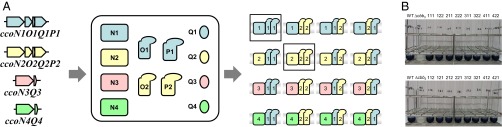
Fig. 1.
Multiple cbb3 isoforms in P. aeruginosa. (A) Sixteen isoforms of cbb3 could be produced via combinations of the isosubunits encoded by four cco gene clusters. cbb3-1 and cbb3-2 are shown in boxes. (B) Cytochrome c oxidase activity of the isoforms visualized in blue color by the Nadi assay. WT and Δcbb3 indicate PAO1 and the ccoN1O1Q1P1 ccoN2O2Q2P2 double-mutant MCb12, respectively. Triple-digit numbers indicate the combination of the CcoN, CcoO, and CcoP isosubunits. For example, 111, 212, 321, and 422 indicate CcoN1O1P1, CcoN2O1P2, CcoN3O2P1, and CcoN4O2P2, respectively. The isoforms were expressed in strain STO7 with the expression plasmids shown in Fig. S1.
Results
Production of Active cbb3 Complexes by the Orphan ccoNQ Gene Products.
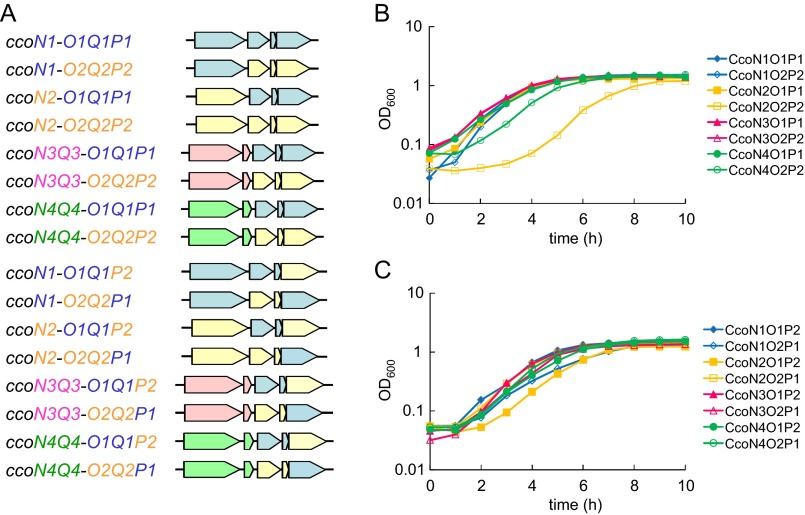
P. aeruginosa has four cco gene clusters, ccoN1O1Q1P1, ccoN2O2Q2P2, ccoN3Q3, and ccoN4Q4, which can produce four CcoN, two CcoO, two CcoP, and four CcoQ isosubunits. Because amino acid sequence identities between the isosubunits of CcoN, CcoO, and CcoP are relatively high (Table S1), we speculated that 16 isoforms of the cbb3 enzyme could be produced via different combinations of the isosubunits (Fig. 1A). To examine the possibility, we constructed 16 expression plasmids that could produce combinations of the CcoN, CcoO, and CcoP isosubunits under the control of the lac promoter (Fig. S1A). The septuple mutant strain STO7, which lacks seven terminal oxidase gene clusters, was constructed and used as a host to express one of the cbb3 isoforms as the only terminal oxidase. STO7 was unable to grow under aerobic conditions because of the lack of the active terminal oxidases. The aerobic growth of STO7 was restored via transformation with any of the expression plasmids (Fig. S1 B and C). The cytochrome c oxidase activity of the isoforms was also confirmed via the Nadi assay (Fig. 1B), which is a method used to detect cytochrome c oxidase-dependent respiration using N, N-dimethyl-p-phenylenediamine as an artificial electron donor (13). These results clearly indicate that the functional cbb3 isoforms could be produced in P. aeruginosa via any combination of the isosubunits. The isoforms could be classified to N1, N2, N3, and N4 types based on the isotypes of the core catalytic CcoN subunit.
Table S1.
Percentage of deduced amino acid (above the diagonal) and nucleotide (below the diagonal) sequence identities among the cco isosubunit genes for the cbb3 oxidase
| Subunits | CcoN1 | CcoN2 | CcoN3 | CcoN4 | CcoO1 | CcoO2 | CcoP1 | CcoP2 | CcoQ1 | CcoQ2 | CcoQ3 | CcoQ4 |
| CcoN1 | — | 86.3 | 83.3 | 87.4 | ||||||||
| CcoN2 | 88.1 | — | 78.5 | 83.4 | ||||||||
| CcoN3 | 83.1 | 82.3 | — | 79.9 | ||||||||
| CcoN4 | 86.7 | 86.6 | 84.0 | — | ||||||||
| CcoO1 | — | 87.6 | ||||||||||
| CcoO2 | 87.2 | — | ||||||||||
| CcoP1 | — | 71.3 | ||||||||||
| CcoP2 | 79.4 | — | ||||||||||
| CcoQ1 | — | 96.7 | 36.8 | 36.5 | ||||||||
| CcoQ2 | 95.7 | — | 34.7 | 38.5 | ||||||||
| CcoQ3 | 53.8 | 57.0 | — | 47.1 | ||||||||
| CcoQ4 | 56.1 | 55.2 | 60.9 | — |
Fig. S1.
Complementation of the terminal oxidase-null mutant with the cbb3 isoforms. (A) Plasmids used for expressing the cbb3 isoforms. The plasmids were constructed via insertion of the indicated hybrid gene clusters into the expression vector pMMB67EH. The cco genes were designed to be expressed by the lac promoter on the vector. The plasmids were used to transform the septuple mutant STO7, which lacks the cyo, cio, cox, cco1, and cco2 gene clusters encoding bo3, CIO, aa3, cbb3-1, and cbb3-2 oxidases, respectively, and ccoN3 and ccoN4. (B and C) Growth profiles of the transformants. The strains were grown aerobically in LB medium in test tubes. The data are representatives of at least three independent cultures.
Expression Profiles of the ccoN3 and ccoN4 Promoters.
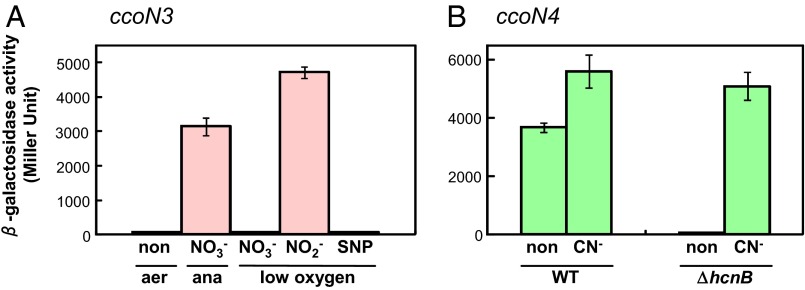
We explored the conditions under which ccoN3Q3 and ccoN4Q4 were up-regulated (Fig. 2). Previous microarray experiments illustrated that ccoN3Q3 was up-regulated under anaerobic denitrification conditions (14). Because nitrite, NO, and N2O are produced as intermediates of denitrification, ccoN3Q3 might be regulated by one of these nitrogen oxides or derived reactive nitrogen species (RNS). Activation of the ccoN3 promoter under denitrification was confirmed with a promoter assay using the lacZ transcriptional fusion fragment (Fig. 2A). The promoter was not up-regulated by nitrate or the NO-generating reagent sodium nitroprusside, but nitrite significantly activated the ccoN3 promoter under aerobic conditions. These results indicated that the induction signal for the ccoN3 promoter was nitrite.
Fig. 2.
Induction of the ccoN3 and ccoN4 promoters by nitrite and cyanide. (A) Promoter activity of ccoN3. The strain was cultured under aerobic (aer), anaerobic (ana), or 2% O2 (low-oxygen) conditions. The medium was not supplemented (non) or supplemented with 40 mM NaNO3 (NO3−), 20 mM KNO2 (NO2−), or 3 mM sodium nitroprusside (SNP). The activities were measured at exponential phase. (B) Promoter activity of ccoN4. The strain was cultured under aerobic conditions. The medium was not supplemented (non) or supplemented with 300 μM KCN (CN−). The activities were measured at early stationary phase. The promoter activity was measured as β-galactosidase activity using PAO1 (WT) or the hcnB mutant (ΔhcnB) carrying a transcriptional fusion of ccoN3 (A) or ccoN4 (B) with lacZ. Bars indicate means (error bars, SDs of at least two replicates).
ccoN4Q4 is located in a gene cluster (PA4129-4134) reported to be inducible by cyanide (15). Activation of the ccoN4 promoter by cyanide was confirmed by the lacZ assay (Fig. 2B). ccoN4 exhibited high transcriptional activity at the stationary phase because P. aeruginosa produces cyanide during this phase (16). In the hcnB KO mutant, which does not produce cyanide, the ccoN4 promoter activity was extremely low, but the activity was significantly induced by the addition of cyanide. These results demonstrated that the induction signal for the ccoN4 promoter was cyanide.
Identification of the Types of cbb3 Isoforms Expressed in the Cell Membrane.
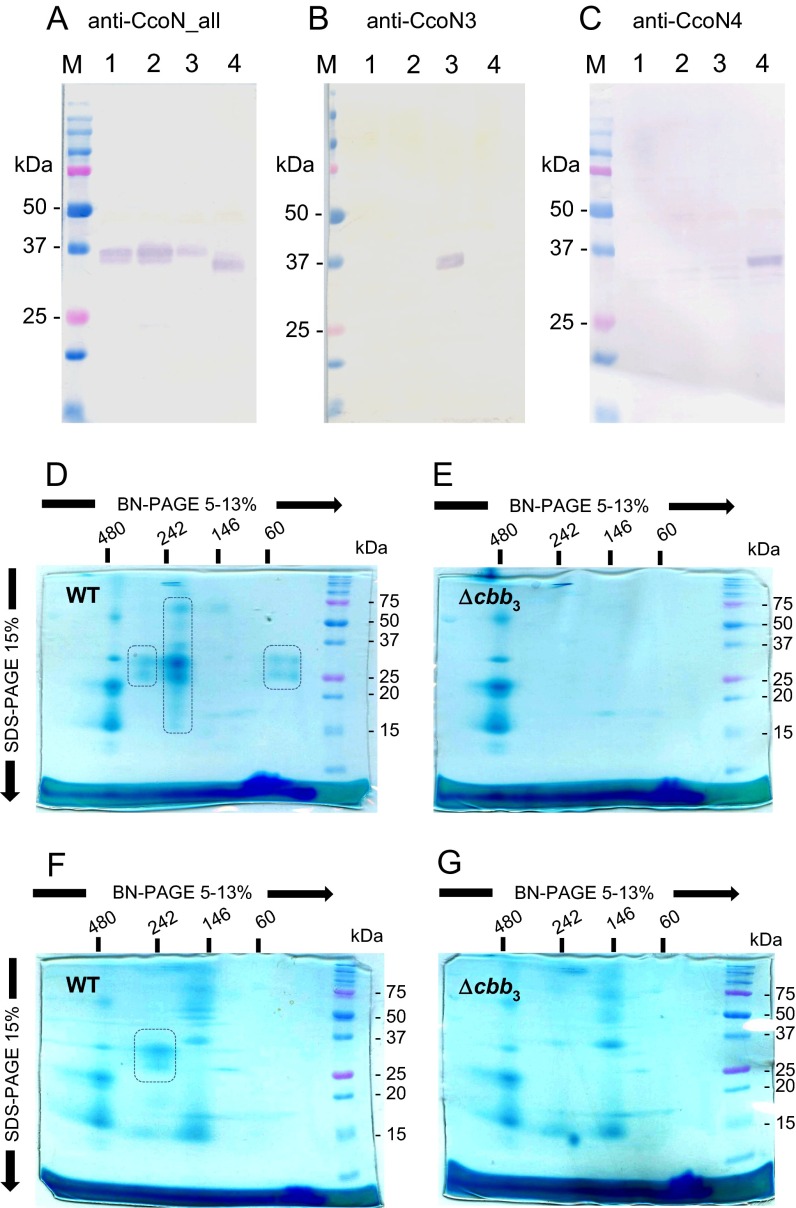
Expression of the N3- or N4-type isoforms in the cell membrane of the WT strain PAO1 was investigated by an immunological method using specific antibodies that recognize all CcoN isosubunits (anti–N-all), only CcoN3 (anti-N3), and only CcoN4 (anti-N4). The specificities of the antibodies were confirmed using the membrane fractions of the cells that expressed only one of the isoforms (Fig. S2 A–C). The CcoN isosubunits were detected at 35–37 kDa after SDS/PAGE separation, and no cross-reactivity of the anti-N3 and anti-N4 antibodies was observed. The calculated molecular weights of CcoN1, CcoN2, CcoN3, and CcoN4 were 53.1, 53.1, 53.7, and 52.3 kDa, respectively. The sizes of the detected bands on the SDS/PAGE gel were lower than the calculated values, probably because of the high hydrophobicity of CcoN isosubunits.
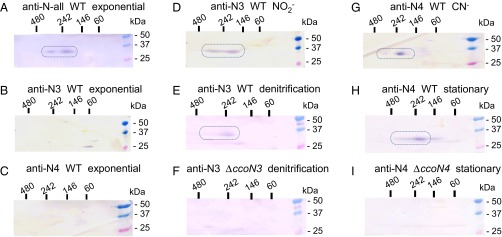
Fig. S2.
Identification of the subunits of the cbb3 oxidase after gel electrophoresis. (A–C) Detection of the CcoN isosubunits by specific antibodies. The CcoN isosubunits were detected by Western blotting after separation of the solubilized membrane fractions of the cells (3 μg) by SDS/PAGE. Antibodies that can detect four CcoN isosubunits (A), only CcoN3 (B), and only CcoN4 (C) were used. Lane M, molecular mass markers. Lanes 1, 2, 3, and 4, strain STO7 transformed with plasmids that express CcoN1O1P1, CcoN2O2P2, CcoN3O2P2, and CcoN4O2P2, respectively. (D–G) Detection of the CcoO and CcoP subunits on the 2D gels. Cytochromes c were visualized by heme staining after solubilized membrane fraction (100 μg) of PAO1 (D and F) and MDCb12 (ccoN1O1Q1P1 ccoN2O2Q2P2 double mutant) (E and G) were separated on the 2D gels (first BN/PAGE, second SDS/PAGE). The cells grown under aerobic (D and E) and anaerobic denitrification (F and G) conditions were used. The bands corresponding to CcoO and CcoP are indicated by rounded rectangles.
Two-dimensional (2D) blue native (BN)/SDS/PAGE was used to detect the CcoN isoforms that constituted the cbb3 enzyme complex in the cell membranes. BN/PAGE is a method for separating multiprotein complexes under native conditions (17). After separation of the membrane fraction of the aerobically grown WT cells by first-dimension BN/PAGE, the fraction was further separated by second-dimension SDS/PAGE, and c-type cytochromes were visualized by heme staining (18) (Fig. S2D). Cytochromes c of ∼32 and 25 kDa were detected by SDS/PAGE at ∼220 and 270 kDa and lower than 60 kDa by BN/PAGE. These bands were not detected in the membrane fraction of strain MDCb12, which is a KO mutant of the ccoN1O1Q1P1 and ccoN2O2Q2P2 genes (Fig. S2E), indicating that the 32- and 25-kDa proteins corresponded to CcoP and CcoO, respectively. When the 2D gel of the WT cell membrane was used to detect the CcoN isosubunits by Western blotting with the anti-N-all antibody, the 35-kDa proteins corresponding to the CcoN isosubunits were detected at 220 and 270 kDa by BN/PAGE (Fig. 3A). These results indicated that the 220- and 270-kDa protein complexes separated by BN/PAGE contained the CcoN, CcoO, and CcoP subunits.
Fig. 3.
Identification of the hybrid cbb3 isoforms. The CcoN isosubunits that constituted the cbb3 complexes were detected by Western blotting after solubilized membrane fractions (5–50 μg) were separated on 2D gels (horizontal, BN/PAGE; vertical, SDS/PAGE). Specific antibodies that detect all four CcoN isosubunits (A), only CcoN3 (B and D–F), and only CcoN4 (C and G–I) were used. The membrane fractions were prepared from the exponential-phase cells of PAO1 cultured aerobically in nonsupplemented LB medium (A–C) or medium supplemented with 20 mM KNO2 (D) or 300 μM KCN (G); cells of PAO1 (E) and the ccoN3 mutant (F) cultured anaerobically by denitrification; and the stationary-phase cells of PAO1 (H) and the ccoN4 mutant (I) cultured aerobically in LB medium. The bands corresponding to the CcoN isosubunits in the cbb3 complex are indicated by rounded rectangles.
The active CcoNOQP complex of Rhodobacter capsulatus was reported to be separated at 230 kDa by BN/PAGE (19). The apparent molecular weight of the CcoNOP complexes of Pseudomonas stutzeri as determined by gel filtration chromatography was ∼190 kDa (20). Thus, the protein complex separated at 220 kDa by BN/PAGE was expected to be the CcoNOP or CcoNOQP complex. The complex was probably associated with additional proteins in the 270-kDa complex. The 32- and 25-kDa cytochromes c detected at lower than 60 kDa by BN/PAGE were probably the CcoP and CcoO proteins dissociated from the cbb3 complexes (Fig. S2D). Free CcoN proteins were not detected, suggesting that CcoN was unstable without association with the CcoO and CcoP subunits. When the cells that were cultivated under the anaerobic denitrification conditions were used for the analysis, the cbb3 complex was detected at ∼240 kDa, suggesting that the CcoNOP complex was associated with other protein in the anaerobic cells (Fig. S2 F and G). From these results, the CcoN isosubunits that constituted the cbb3 isoforms via association with CcoO and CcoP were expected to appear at 220 and 270 kDa for the aerobic cells and at 240 kDa for the anaerobic cells by BN/PAGE and at 35–37 kDa by SDS/PAGE on 2D gels.
When lysates of exponential-phase WT cells cultured under aerobic conditions were tested on 2D gels, the anti-N-all antibody detected CcoN at the position of the cbb3 complex, but the anti-N3 and anti-N4 antibodies detected no protein, indicating that only N1- and/or N2-type isoforms were expressed under the condition (Fig. 3 A–C). CcoN3 was detected when WT cells were cultured aerobically with nitrite or anaerobically via denitrification, but not in the ccoN3-mutant cells (Fig. 3 D–F). CcoN4 was detected in the exponential-phase WT cells cultured aerobically in the presence of cyanide or in the stationary-phase WT cells, but not in the ccoN4-mutant cells (Fig. 3 G–I). These results clearly indicated that CcoN3 or CcoN4 was associated with CcoO and CcoP in the WT cells under the conditions when the ccoN3 or ccoN4 promoter was induced, respectively.
Resistance of the CcoN3- and CcoN4-Containing Isoforms to Respiratory Inhibitors.
Because nitrite and cyanide are known to inhibit cellular respiration, we examined the resistance of the isoforms to these compounds. The ccoN3 mutant displayed no difference in growth from the WT irrespective of the presence of 20 mM nitrite under aerobic [20% (vol/vol) O2] and anaerobic conditions. However, when the ambient oxygen concentration was 0.5%, the growth of the ccoN3 mutant was slightly retarded compared with that of the WT (Fig. 4A). The strains that had only the N1- or N2-type isoform exhibited a longer lag period than the strains that had only the N3-type isoforms when 20 mM nitrite was added to the medium (Fig. 4B). These results indicate that the N3-type isoforms are more resistant to nitrite or derived RNS and function under low-oxygen conditions in the presence of nitrite.
Fig. 4.
Resistance of the CcoN3- and CcoN4-containing cbb3 isoforms to nitrite and cyanide. (A) Growth profiles of PAO1 and the ccoN3 mutant in LB medium in the presence or absence of 20 mM KNO2 under low-oxygen (0.5%) conditions. (B) Growth profiles of STO7 transformed with plasmids expressing CcoN1O1P1, CcoN2O2P2, CcoN3O1P1, or CcoN3O2P2. The strains were cultured aerobically in 96-well plates. (C) Growth profiles of PAO1 and the ccoN4 mutant in LB medium in the presence or absence of 300 μM KCN under low-oxygen (0.5%) conditions. (D) Oxygen consumption activities of the membrane fraction of the cells of STO7 transformed with plasmids expressing CcoN1O1P1, CcoN2O2P2, CcoN4O1P1, or CcoN4O2P2. Ascorbate-reduced TMPD was used as an electron donor for the reaction. KCN was added at various concentrations in the reaction mixture. Values are shown as percentages of the maximal activity level in the absence of KCN. Data are representatives of at least two independent experiments.
The ccoN4 mutation had no effect on the sensitivity to cyanide under aerobic conditions because of cyanide-resistant respiration mediated by CIO. However, when the ambient oxygen concentration was 0.5%, the ccoN4 mutant exhibited sensitivity to cyanide (Fig. 4C). CIO may not be fully functional under extremely low-oxygen conditions because the affinity of CIO for oxygen is low (10). We examined the inhibitory effect of cyanide on the oxygen consumption activities of the isoforms (Fig. 4D). The 50% inhibition concentrations of CcoN1O1P1, CcoN2O2P2, CcoN4O1P1, and CcoN4O2P2 isoforms for cyanide were 0.23, 0.52, 2.0, and 5.0 μM, respectively. The values of the N4-type isoforms were one order of magnitude higher than those of the N1- or N2-type isoforms, indicating that the N4-type isoforms are involved in cyanide-resistant respiration in low-oxygen environments.
Oxygen Affinity of the cbb3 Isoforms.
We previously reported Km values of cbb3-1 and cbb3-2 using the cell membrane of quadruple mutants of P. aeruginosa (10). These mutants carried ccoN3Q3 and ccoN4Q4. Because CcoN4 was expressed at the stationary phase, the cell membranes might contain the N4-type isoforms. We determined here Km values of the isoforms for oxygen using the strains that had only one of the recombinant isoforms by a spectrophotometric method with myoglobin as an oxygen reporter (10) (Table 1). Because the recombinant isoforms were expressed from the lac promoter on the vector, the concentrations of the isoforms in the cell membranes were expected to be similar. All of the tested isoforms were found to have higher affinity for oxygen than the low-affinity enzymes aa3, bo3, and CIO (10). The N3-type isoforms had slightly lower affinity than those of the other types. The N3-type isoforms might acquire higher resistance to nitrite or RNS at the expense of affinity for oxygen.
Table 1.
Affinity of the cbb3 isoforms for oxygen
| Isoforms | Km (μM) | Vmax (nmol O2/min/mg-protein) |
| CcoN1O1P1 | 0.012 ± 0.003 | 11 ± 3 |
| CcoN1O2P2 | 0.027 ± 0.003 | 24 ± 5 |
| CcoN2O1P1 | 0.016 ± 0.006 | 16 ± 3 |
| CcoN2O2P2 | 0.022 ± 0.005 | 34 ± 3 |
| CcoN3O1P1 | 0.121 ± 0.024 | 25 ± 7 |
| CcoN3O2P2 | 0.151 ± 0.039 | 21 ± 5 |
| CcoN4O1P1 | 0.046 ± 0.015 | 15 ± 2 |
| CcoN4O2P2 | 0.035 ± 0.015 | 52 ± 13 |
Km values for oxygen were determined using the deoxygenation kinetics of oxymyoglobin. Values are presented as means ± SDs from three independent experiments. Membrane fractions of the cells of strain STO7 transformed with the plasmids expressing only one of the cbb3 isoforms were used for determination of the oxygen affinity (Fig. S1).
Discussion
Many pseudomonads have two sets of cco gene clusters encoding cbb3 oxidases of the N1 and N2 types. The isoforms corresponding to cbb3-1 and cbb3-2 of P. aeruginosa are oppositely designated as cbb3-2 and cbb3-1, respectively, in other Pseudomonas species, such as Pseudomonas putida and P. stutzeri (21, 22). Xie et al. reported that the two isoforms from P. stutzeri differed in thermal stability but had no significant difference regarding the UV-visible spectrum or enzymatic activities (22). The affinities of the N1- and N2-type isoforms for oxygen were not significantly different in P. aeruginosa or the difference was smaller than the experimental errors (Table 1). Because cbb3-1 and cbb3-2 are used as the main terminal oxidases under high- and low-oxygen conditions, respectively (12), they might have difference in the resistance and sensitivity to oxygen or ROS. The growth of the strain that had only cbb3-2 (CcoN2O2P2) exhibited a significantly prolonged lag period when it was cultured aerobically (Fig. S1B). A long lag period was also observed for the quadruple mutant strain QXCb2, which lacks the genes encoding aa3, bo3, CIO, and cbb3-1 oxidases (10). Growth retardation was not observed when the strain was cultured under low-oxygen conditions. cbb3-2, but not the other N2-type isoforms, is probably highly sensitive to oxygen or ROS. cbb3-1 must be more resistant to oxygen, and utilization of cbb3-1 under the aerobic conditions might contribute to the robustness of P. aeruginosa by suppressing the generation of ROS, because the high-affinity cbb3 oxidases scavenge submicromolar concentrations of oxygen.
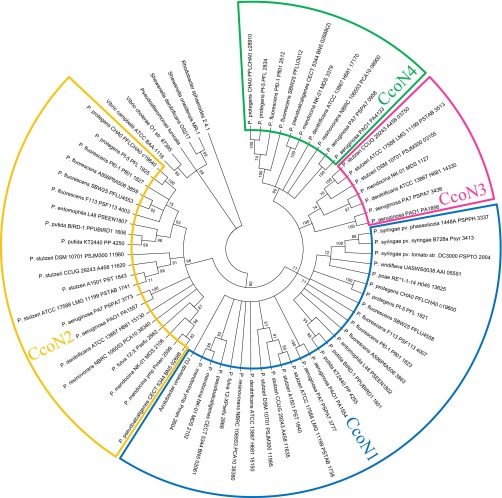
Analysis of the reported genome sequences revealed that several Pseudomonas species have one or two orphan ccoNQ gene clusters corresponding to ccoN3Q3 or ccoN4Q4 (Fig. S3). The species that carry ccoN3-type genes use denitrification genes for anaerobic respiration. Nitrite is one of the intermediates of the denitrification pathway that is temporarily accumulated during growth under denitrification (23). The N3-type enzymes, which are resistant to nitrite or derived RNS, might be operative under hypoxic conditions when aerobic respiration and denitrification occur simultaneously. The resistance to RNS might also contribute to the virulence of P. aeruginosa because macrophages of the host immune system produce NO to counteract bacterial infections.
Fig. S3.
Phylogenetic tree of CcoN from Pseudomonas species. Amino acid sequences were aligned using ClustalW. Tree topology and evolutionary distance estimations were done by the neighbor-joining method. The numbers indicated at the nodes are bootstrap values calculated from 1,000 replicates. The CcoN1-, CcoN2-, CcoN3-, and CcoN4-like sequences are indicated by blue, yellow, red, and green fan-shaped boxes, respectively. Several CcoN sequences from Rhodobacter, Shewanella, Pseudoalteromonas, and Vibrio species were used as outgroups.
P. aeruginosa produces cyanide under low-oxygen conditions (16). Cyanogenesis is believed to contribute to the virulence of P. aeruginosa by suppressing the growth of other microorganisms infected in the same niches and by killing the host cells (24). The Pseudomonas species that carry the ccoN4-type genes were found to carry hcn genes involved in the biosynthesis of cyanide (25) (Fig. S3), suggesting that the expression of the N4-type isoforms, which have higher tolerance to cyanide, is advantageous for counteracting the endogenous cyanide in the cyanogenic pseudomonads.
The respiratory system of P. aeruginosa has been uncovered to be far more complex than was previously thought. It has a capability to produce 16 different cbb3 isoforms. Although it is not clear which isoforms are present under different growth conditions, at least each ones of the N3- and N4-type isoforms were identified to be expressed in response to the respiratory inhibitors. P. aeruginosa causes life-threatening infection in the airways of patients with cystic fibrosis (CF). Because the mucus layer in the lungs of patients with CF is depleted of oxygen, the ability to survive and proliferate in hypoxic environments is important for chronic infection (14, 26). As mentioned previously, resistance to RNS and cyanide is advantageous for survival in hypoxic environments. Aerobic respiration under hypoxic environments is mediated by the high-affinity cbb3 oxidases. We revealed that P. aeruginosa acquires resistance to these respiratory inhibitors by producing multiple cbb3 isoforms with different features. The subunit switch mechanism for the production of the multiple cbb3 isoforms would be a promising therapeutic target for treating chronic P. aeruginosa infection.
Materials and Methods
Bacterial Strains, Plasmids, and Growth Conditions.
All bacterial strains, plasmids, and growth conditions used in this study are described in Table S2 and SI Materials and Methods. The primers used for PCR amplification of the DNA fragments, which were used for construction of the plasmids, are shown in Table S3.
Table S2.
Bacterial strains and plasmids used in this study
| Strains or plasmids | Relevant characteristic(s) | Source or reference |
| P. aeruginosa strains | ||
| PAO1 | PAO1 of the University of Washington (MPAO1) used as a wild-type | (27) |
| PW4739 | ΔhcnB (PA2194) from mutant library at Univ. of Washington | (27) |
| MCb12 | ΔccoN1O1Q1P1, ΔccoN2O2Q2P2 of MPAO1 | This study |
| MSccoN3 | ΔccoN3 of MPAO1 | This study |
| MSccoN4 | ΔccoN4 of MPAO1 | This study |
| PTO5 | ΔcyoABCDE, ΔcioAB, ΔcoxBA-coxC, ΔccoN1O1Q1P1, ΔccoN2O2Q2P2 of PAO1ut | (10) |
| STO7 | ΔccoN3, ΔccoN4 of PTO5 | This study |
| Plasmids | ||
| pMMB67EH | IncQ, expression vector; Apr, Cbr | (28) |
| pEX18Ap | Gene replacement vector; Apr, Cbr, oriT+, sacB+ | (29) |
| pUC18-mini-Tn7T-Gm-lacZ | mini-Tn7T, lacZ transcriptional fusion vector; Apr, Gmr | (30) |
| pMMB-N1O1Q1P1 | ccoN1O1Q1P1 on pMMB67EH | This study |
| pMMB-N1O1Q1P2 | ccoN1O1Q1P2 on pMMB67EH | This study |
| pMMB-N1O2Q2P1 | ccoN1O2Q2P1 on pMMB67EH | This study |
| pMMB-N1O2Q2P2 | ccoN1O2Q2P2 on pMMB67EH | This study |
| pMMB-N2O1Q1P1 | ccoN2O1Q1P1 on pMMB67EH | This study |
| pMMB-N2O1Q1P2 | ccoN2O1Q1P2 on pMMB67EH | This study |
| pMMB-N2O2Q2P1 | ccoN2O2Q2P1 on pMMB67EH | This study |
| pMMB-N2O2Q2P2 | ccoN2O2Q2P2 on pMMB67EH | This study |
| pMMB-N3Q3O1Q1P1 | ccoN3Q3O1Q1P1 on pMMB67EH | This study |
| pMMB-N3Q3O1Q1P2 | ccoN3Q3O1Q1P2 on pMMB67EH | This study |
| pMMB-N3Q3O2Q2P1 | ccoN3Q3O2Q2P1 on pMMB67EH | This study |
| pMMB-N3Q3O2Q2P2 | ccoN3Q3O2Q2P2 on pMMB67EH | This study |
| pMMB-N4Q4O1Q1P1 | ccoN4Q4O1Q1P1 on pMMB67EH | This study |
| pMMB-N4Q4O1Q1P2 | ccoN4Q4O1Q1P2 on pMMB67EH | This study |
| pMMB-N4Q4O2Q2P1 | ccoN4Q4O2Q2P1 on pMMB67EH | This study |
| pMMB-N4Q4O2Q2P2 | ccoN4Q4O2Q2P2 on pMMB67EH | This study |
| pEX-ΔccoN3 | Plasmid for ccoN3 mutation, a derivative of pEX18Ap | This study |
| pEX-ΔccoN4 | Plasmid for ccoN4 mutation, a derivative of pEX18Ap | This study |
| pUC-ccoN3-lacZ | mini-Tn7T; ccoN3::lacZ transcriptional fusion | This study |
| pUC-ccoN4-lacZ | mini-Tn7T; ccoN4::lacZ transcriptional fusion | This study |
Apr, ampicillin resistant; Cbr, carbenicillin resistant; Gmr, gentamicin resistant.
Table S3.
Primers used in this study
| Primer name | Sequence (5′→3′) | Restriction site |
| ccoN1-1 | tccctaaaagagctcattaaccgtggaacc | SacI |
| ccoN1-2 | gtggttctagatttacgtcgggctcctcag | XbaI |
| ccoN2-1 | agccagagctcataaccgtggatggaagcc | SacI |
| ccoN2-2 | tatctagagtttcatgctcggctcctcaggcg | XbaI |
| ccoN3-1 | gcgagctcccatgaacgatacggatagcag | SacI |
| ccoN3-2 | atctctagatgatggagtcggttcaggcgagg | XbaI |
| ccoN4-1 | tcgcgagctcttcatttctttcgggactgtaca | SacI |
| ccoN4-2 | cgagctctagaagataaagccctgaagcgactgg | XbaI |
| ccoOQP1-1 | gatctctagaggagcccgacgtaaatga | XbaI |
| ccoOQP1-2 | cgtggtcgacggagcgcttattcggcgc | SalI |
| ccoOQP2-1 | gatctctagaggagccgagcatgaaac | XbaI |
| ccoOQP2-2 | gtgggtcgacgggtcagttgctgccctg | SalI |
| ccoQ-F | cgacgaagcgacgatgctgc | |
| ccoQ-R | gttactcctagaagcttgctcg | |
| 1856A | cgccgaattcctgcagcgcctggtccag | EcoRI |
| 1856B | gcgcgagctcggcggcgccaccttcgcc | SacI |
| 1856C | cgatgagctcccccaggcccatgccgag | SacI |
| 1856D | ggcttctagaagatcgcccacaaatacc | SalI |
| 4133A | ctggggtacctgccgatcaaggcctgc | KpnI |
| 4133B | tgggtctagaccagttgcgaggcgatc | XbaI |
| 4133C | ttcgtctagacgctggtggccagccatc | XbaI |
| 4133D | cggcaagcttccgatggcgctggtgatg | HindIII |
| ccoN3-A | tgatgaaaagcttgtcggtggtcggcat | HindIII |
| ccoN3-B | cgtcgtagtggtacccgtccggatcttt | KpnI |
| ccoN4-A | agatcgagcgactgaagcttatgaccag | HindIII |
| ccoN4-B | caggtaccgtcgttgctttgttcatctg | KpnI |
Determination of Enzymatic Activities and Biochemical Parameters.
Cytochrome c oxidase activity was visualized using the Nadi assay (13). Cells of overnight cultures were suspended in 1 mL 20 mM Tris⋅HCl (pH 7.5). After adding 200 μL of a 1:1 mixture of 35 mM α-naphthol in ethanol and 30 mM N,N-dimethyl-p-phenylenediamine monohydrochloride to the cell suspension, the development of blue color by the formation of indophenol blue was observed. The reaction mixtures were incubated for 5 min at room temperature. Oxygen consumption activity was determined amperometrically using an Apollo 4000 free radical analyzer equipped with a 2-mm ISO-OXY-2 O2 electrode (WPI) according to the procedures described previously (10). The method for determination of the Km values for oxygen is described in SI Materials and Methods.
Western Blotting and Heme Staining.
Preparation of solubilized membrane fractions and gel electrophoresis are described in SI Materials and Methods. After separation of the solubilized membrane fraction by SDS/PAGE or 2D BN/SDS/PAGE, proteins were transferred to PVDF membranes (Sequi-Blot PVDF membrane; Bio-Rad) using a semidry blotting system (HorizBlot AE-6677P; ATTO). Polyclonal anti–N-all, anti-N3, and anti-N4 antibodies were raised against synthetic oligopeptides in rabbits. The sequences of the anti–N-all, anti-N3, and anti-N4 oligopeptides were C + MWRAVNEDGTLTYS, C + TLRSAQDQRQPVLA, and C + RTVRQARPEGIL, respectively. The oligopeptides and antisera were prepared by Eurofins Genomics. Goat anti-rabbit IgG, pAb HRP conjugate (Enzo Life Science) was used as the secondary antibody. The desired proteins were detected using a POD Immunostain Set (Wako). Cytochromes c in the 2D gels were visualized by heme-linked peroxidase staining with 3,3′,5,5′-tetramethylbenzidine as previously described (18).
SI Materials and Methods
Bacterial Strains and Growth Conditions.
The bacterial strains and plasmids used in this work are described in Table S2. P. aeruginosa PAO1 from the University of Washington (MPAO1) was used as the WT strain. The hcnB mutant strain PW4739 was obtained from the transposon mutant library at the University of Washington (27). Strain MCb12 was constructed by deleting ccoN1O1Q1P1 and ccoN2O2Q2P2 from MPAO1, according to a previously described method (10). Strains MSccoN3 and MSccoN4 were constructed by deleting ccoN3 and ccoN4, respectively. A septuple terminal oxidase-null mutant strain STO7 was constructed by deleting ccoN3 and ccoN4 from the quintuple mutant strain PTO5, which lacks the cox, cio, cyo, cco1, and cco2 gene clusters (10). Plasmids pEX-ΔccoN3 and pEX-ΔccoN4 were used to delete ccoN3 and ccoN4, respectively, according to a previously described method (10). The strains were cultivated in LB medium at 37 °C. For maintenance of the expression plasmids, 200 μg/mL carbenicillin was added to the medium. For expression of the recombinant cbb3 isoforms, 0.5 mM isopropyl β-d-thiogalactopyranoside (IPTG) was added to the medium. Test tubes with gas-permeable plugs or 96-well plates were used for aerobic cultivation. Test tubes sealed with butyl rubber stoppers were used for anaerobic cultivation and the gas phase was replaced with argon. The medium was supplemented with 40 mM NaNO3 for anaerobic cultivation. For cultivation under low-oxygen conditions, a gas mixture consisting of 0.5% O2 and 99.5% (vol/vol) N2 was continuously supplied through a needle to the test tubes sealed with butyl rubber stoppers. Cell growth was monitored by measuring the optical density of the culture at 600 nm using a mini photo 518R (TAITEC) for the test tube cultures or at 590 nm using Emax precision microplate reader (Molecular Devices) for the 96-well plate cultures.
Construction of Plasmids.
For expression of the cbb3 isoforms in STO7, 16 plasmids were constructed by insertion of the hybrid gene clusters shown in Fig. S1 into pMMB67EH (28). Because CcoQ is predicted to be involved in the stability of cbb3, ccoQ3, and ccoQ4 were included in the plasmids together with ccoQ1 or ccoQ2 for expression of the CcoN3- and CcoN4-containing isoforms, respectively.
The hybrid gene clusters were constructed via combinations of the upstream SacI-XbaI cassettes carrying ccoN1, ccoN2, ccoN3Q3, or ccoN4Q4 and the downstream XbaI-SalI cassettes carrying ccoO1Q1P1, ccoO2Q2P2, ccoO1Q1P2, or ccoO2Q2P1. The upstream gene cassettes contain 22-, 25-, 2-, or 24-bp sequence upstream from the initiation codon of ccoN1, ccoN2, ccoN3, or ccoN4, respectively, but do not carry the natural promoters of the cco gene clusters. The ccoN1, ccoN2, ccoN3Q3, ccoN4Q4, ccoO1Q1P1, and ccoO2Q2P2 cassettes were PCR amplified from the chromosomal DNA of PAO1 with primer sets ccoN1-1/ccoN1-2, ccoN2-1/ccoN2-2, ccoN3-1/ccoN3-2, ccoN4-1/ccoN4-2, ccoOQP1-1/ccoOQP1-2, and ccoOQP2-1/ccoOQP2-2, respectively. For construction of the ccoO1Q1P2 cassette, the ccoO1Q1 and ccoP2 fragments were PCR-amplified from plasmids carrying ccoO1Q1P1 and ccoO2Q2P2, respectively, with primer sets ccoOQP1-1/ccoQ-R and ccoQ-F/ccoOQP2-2, respectively. Using the mixture of the amplified fragments as templates, the ccoO1Q1P2 fragment was amplified via a second PCR with primer sets ccoOQP1-1/ccoOQP2-2. The ccoO2Q2P1 cassette was constructed in the same manner using primer sets ccoOQP2-1/ccoQ-R and ccoQ-F/ccoOQP1-2 for the first PCR and ccoOQP2-1/ccoOQP1-2 for the second PCR. The primers used for the PCR reactions are shown in Table S3.
pEX-ΔccoN3 and pEX-ΔccoN4 were used to delete ccoN3 and ccoN4, respectively. To construct pEX-ΔccoN3, 1.2-kb SalI-SacI and 1.1-kb SacI-EcoRI fragments containing the upstream and downstream flanking regions of ccoN3, respectively, were amplified by PCR from the chromosomal DNA of PAO1 using primer sets 1856C/1856D and 1856A/1856B, respectively, and then tandemly inserted into the respective sites of the suicide vector pEX18Ap (29). pEX-ΔccoN4 was constructed in the same manner using 1.1-kb KpnI-XbaI and 1.0-kb XbaI-HindIII fragments amplified with primer sets 4133A/4133B and 4133C/4133D, respectively.
pUC-ccoN3-lacZ and pUC-ccoN4-lacZ were derivatives of pUC18-mini-Tn7T-Gm-lacZ (30) and used for to assay the transcriptional activities of ccoN3 and ccoN4, respectively. The 0.73- and 0.71-kb DNA fragments containing the promoter regions of ccoN3 and ccoN4 were amplified by PCR from the chromosomal DNA of PAO1 with primer sets ccoN3-A/ccoN3-B and ccoN4-A/ccoN4-B, respectively. The amplified fragments were digested with HindIII and KpnI and inserted to the respective sites of pUC18-mini-Tn7T-Gm-lacZ.
Promoter Assay.
The ccoN3::lacZ and ccoN4::lacZ transcriptional fusion fragments were inserted into the chromosome of PAO1 or PW4739 at the attTn7 site located downstream of glmS by using a Tn7-based method (30) with pUC-ccoN3-lacZ and pUC-ccoN4-lacZ. A β-galactosidase assay was performed using a standard protocol (12).
Preparation of Solubilized Membrane Fractions and Gel Electrophoresis.
P. aeruginosa strains were cultured in 200 mL LB medium in Erlenmeyer flasks for aerobic conditions or in vials for anaerobic conditions. When necessary, the medium was supplemented with 20 mM KNO2 or 300 μM KCN. For anaerobic cultivation, the medium was supplemented with 40 mM NaNO3, and the air in the vial was replaced with argon. Cells were pelleted by centrifugation at 6000 × g, washed twice with 20 mM Tris⋅HCl (pH 7.5), and stored at −80 °C until use. The cell pellet was resuspended in 20 mM Tris⋅HCl (pH 7.5) containing 5 mM MgCl2 and 0.5 M NaCl. DNase at 0.01 mg/mL (final concentration) was added to the cell suspension. The cells were then disrupted by passing the suspension twice through a French Pressure cell at 16,000 lb/in2. Unbroken cells were removed by centrifugation at 6,000 × g. The membrane fraction was collected by centrifugation at 100,000 × g for 1 h at 4 °C and resuspended in 20 mM Tris⋅HCl (pH 7.5) containing 0.5 mM EDTA and 10% (wt/vol) glycerol to give a protein concentration between 1 and 3 mg/mL The membrane fraction was solubilized by the addition of n-dodecyl-β-d-maltoside (DDM) to a final concentration of 2% (wt/vol) and incubation for 2 h. The solubilized membrane fraction was concentrated, and the buffer was exchanged to 20 mM Tris⋅HCl (pH 7.5) containing 0.02% DDM and 0.5 mM EDTA using a 100,000 NMWL Amicon Ultra-15 centrifugal filter device (Millipore). The protein concentration was determined by the Bradford method using a protein assay kit (Bio-Rad) with BSA as the standard.
Two-dimensional BN/SDS/PAGE was conducted according to a previously described method (17). A gradient gel of 5–13% (wt/vol) acrylamide was used for the first-dimension BN/PAGE. A 15% (wt/vol) SDS-polyacrylamide gel was used for the second-dimension SDS/PAGE. NativeMark Unstained Protein Standard (Life Technologies) was used as a size marker for BN/PAGE.
Determination of Oxygen Affinity.
Determination of the Km values of the isoforms for oxygen using deoxygenation kinetics of oxymyoglobin was performed according to the method described previously (10, 31–33). Membrane fractions were prepared as described above except that 20 mM potassium phosphate buffer (pH 7.0) containing 1 mM EDTA was used for washing and resuspension of the cells. Equine heart myoglobin (Sigma-Aldrich) was reduced with dithionite and desalted by passage through a PD-10 column (GE Healthcare). The concentration of myoglobin was determined by the spectrum of alkaline pyridine hemochrome using an extinction coefficient value for pyridine hemochrome of 33.9 mM−1 cm−1 at 556 nm. The reaction was performed in a sealed cuvette containing 5 mL degassed reaction mixture, which consisted of 20 mM potassium phosphate (pH 7.0), 1 mM EDTA, 15 mM d,l-malate, and 19.3 mM oxymyoglobin. After addition of the membrane fraction sample (20–70 μL), the deoxygenation of oxymyoglobin was continuously monitored at 581 and 563 nm, using a U-2910 spectrophotometer (Hitachi). The concentration of oxygen in the reaction mixture and the oxygen-consumption rate at several time points were calculated according to the method described by Bergersen and Turner (31). The value for the dissociation constant used in the calculations was 0.786 for oxymyoglobin. Eadie–Hofstee plots were used for the determination of Km and Vmax for oxygen.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas “Oxygen Biology: A new criterion for integrated understanding of life” (15H01393; to H.A.) of The Ministry of Education, Culture, Sports, Science and Technology, Japan.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1613308113/-/DCSupplemental.
References
- 1.Ekici S, Pawlik G, Lohmeyer E, Koch HG, Daldal F. Biogenesis of cbb3-type cytochrome c oxidase in Rhodobacter capsulatus. Biochim Biophys Acta. 2012;1817(6):898–910. doi: 10.1016/j.bbabio.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagata K, Tsukita S, Tamura T, Sone N. A cb-type cytochrome-c oxidase terminates the respiratory chain in Helicobacter pylori. Microbiology. 1996;142(Pt 7):1757–1763. doi: 10.1099/13500872-142-7-1757. [DOI] [PubMed] [Google Scholar]
- 3.Jackson RJ, et al. Oxygen reactivity of both respiratory oxidases in Campylobacter jejuni: The cydAB genes encode a cyanide-resistant, low-affinity oxidase that is not of the cytochrome bd type. J Bacteriol. 2007;189(5):1604–1615. doi: 10.1128/JB.00897-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, et al. Organization of the electron transfer chain to oxygen in the obligate human pathogen Neisseria gonorrhoeae: Roles for cytochromes c4 and c5, but not cytochrome c2, in oxygen reduction. J Bacteriol. 2010;192(9):2395–2406. doi: 10.1128/JB.00002-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buschmann S, et al. The structure of cbb3 cytochrome oxidase provides insights into proton pumping. Science. 2010;329(5989):327–330. doi: 10.1126/science.1187303. [DOI] [PubMed] [Google Scholar]
- 6.Zufferey R, Preisig O, Hennecke H, Thöny-Meyer L. Assembly and function of the cytochrome cbb3 oxidase subunits in Bradyrhizobium japonicum. J Biol Chem. 1996;271(15):9114–9119. doi: 10.1074/jbc.271.15.9114. [DOI] [PubMed] [Google Scholar]
- 7.Oh JI, Kaplan S. Oxygen adaptation. The role of the CcoQ subunit of the cbb3 cytochrome c oxidase of Rhodobacter sphaeroides 2.4.1. J Biol Chem. 2002;277(18):16220–16228. doi: 10.1074/jbc.M200198200. [DOI] [PubMed] [Google Scholar]
- 8.Peters A, Kulajta C, Pawlik G, Daldal F, Koch H-G. Stability of the cbb3-type cytochrome oxidase requires specific CcoQ-CcoP interactions. J Bacteriol. 2008;190(16):5576–5586. doi: 10.1128/JB.00534-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arai H. Regulation and function of versatile aerobic and anaerobic respiratory metabolism in Pseudomonas aeruginosa. Front Microbiol. 2011;2:103. doi: 10.3389/fmicb.2011.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arai H, et al. Enzymatic characterization and in vivo function of five terminal oxidases in Pseudomonas aeruginosa. J Bacteriol. 2014;196(24):4206–4215. doi: 10.1128/JB.02176-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stover CK, et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406(6799):959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 12.Kawakami T, Kuroki M, Ishii M, Igarashi Y, Arai H. Differential expression of multiple terminal oxidases for aerobic respiration in Pseudomonas aeruginosa. Environ Microbiol. 2010;12(6):1399–1412. doi: 10.1111/j.1462-2920.2009.02109.x. [DOI] [PubMed] [Google Scholar]
- 13.Marrs B, Gest H. Genetic mutations affecting the respiratory electron-transport system of the photosynthetic bacterium Rhodopseudomonas capsulata. J Bacteriol. 1973;114(3):1045–1051. doi: 10.1128/jb.114.3.1045-1051.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarez-Ortega C, Harwood CS. Responses of Pseudomonas aeruginosa to low oxygen indicate that growth in the cystic fibrosis lung is by aerobic respiration. Mol Microbiol. 2007;65(1):153–165. doi: 10.1111/j.1365-2958.2007.05772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frangipani E, Pérez-Martínez I, Williams HD, Cherbuin G, Haas D. A novel cyanide-inducible gene cluster helps protect Pseudomonas aeruginosa from cyanide. Environ Microbiol Rep. 2014;6(1):28–34. doi: 10.1111/1758-2229.12105. [DOI] [PubMed] [Google Scholar]
- 16.Pessi G, Haas D. Transcriptional control of the hydrogen cyanide biosynthetic genes hcnABC by the anaerobic regulator ANR and the quorum-sensing regulators LasR and RhlR in Pseudomonas aeruginosa. J Bacteriol. 2000;182(24):6940–6949. doi: 10.1128/jb.182.24.6940-6949.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schägger H. Blue-native gels to isolate protein complexes from mitochondria. Methods Cell Biol. 2001;65:231–244. doi: 10.1016/s0091-679x(01)65014-3. [DOI] [PubMed] [Google Scholar]
- 18.Thomas PE, Ryan D, Levin W. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal Biochem. 1976;75(1):168–176. doi: 10.1016/0003-2697(76)90067-1. [DOI] [PubMed] [Google Scholar]
- 19.Kulajta C, Thumfart JO, Haid S, Daldal F, Koch HG. Multi-step assembly pathway of the cbb3-type cytochrome c oxidase complex. J Mol Biol. 2006;355(5):989–1004. doi: 10.1016/j.jmb.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 20.Urbani A, Gemeinhardt S, Warne A, Saraste M. Properties of the detergent solubilised cytochrome c oxidase (cytochrome cbb3) purified from Pseudomonas stutzeri. FEBS Lett. 2001;508(1):29–35. doi: 10.1016/s0014-5793(01)03006-x. [DOI] [PubMed] [Google Scholar]
- 21.Ugidos A, Morales G, Rial E, Williams HD, Rojo F. The coordinate regulation of multiple terminal oxidases by the Pseudomonas putida ANR global regulator. Environ Microbiol. 2008;10(7):1690–1702. doi: 10.1111/j.1462-2920.2008.01586.x. [DOI] [PubMed] [Google Scholar]
- 22.Xie H, Buschmann S, Langer JD, Ludwig B, Michel H. Biochemical and biophysical characterization of the two isoforms of cbb3-type cytochrome c oxidase from Pseudomonas stutzeri. J Bacteriol. 2014;196(2):472–482. doi: 10.1128/JB.01072-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zumft WG. Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev. 1997;61(4):533–616. doi: 10.1128/mmbr.61.4.533-616.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson RD, Roddam LF, Bettiol S, Sanderson K, Reid DW. Biosignificance of bacterial cyanogenesis in the CF lung. J Cyst Fibros. 2010;9(3):158–164. doi: 10.1016/j.jcf.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Laville J, et al. Characterization of the hcnABC gene cluster encoding hydrogen cyanide synthase and anaerobic regulation by ANR in the strictly aerobic biocontrol agent Pseudomonas fluorescens CHA0. J Bacteriol. 1998;180(12):3187–3196. doi: 10.1128/jb.180.12.3187-3196.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Worlitzsch D, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002;109(3):317–325. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobs MA, et al. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2003;100(24):14339–14344. doi: 10.1073/pnas.2036282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fürste JP, et al. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48(1):119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- 29.Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: Application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene. 1998;212(1):77–86. doi: 10.1016/s0378-1119(98)00130-9. [DOI] [PubMed] [Google Scholar]
- 30.Choi KH, Schweizer HP. mini-Tn7 insertion in bacteria with single attTn7 sites: Example Pseudomonas aeruginosa. Nat Protoc. 2006;1(1):153–161. doi: 10.1038/nprot.2006.24. [DOI] [PubMed] [Google Scholar]
- 31.Bergersen FJ, Turner GL. Systems utilizing oxygenated leghemoglobin and myoglobin as sources of free dissolved O2 at low concentrations for experiments with bacteria. Anal Biochem. 1979;96(1):165–174. doi: 10.1016/0003-2697(79)90569-4. [DOI] [PubMed] [Google Scholar]
- 32.Bergersen FJ, Turner GL. Properties of terminal oxidase systems of bacteroids from root-nodules of soybean and cowpea and of N2-fixing bacteria grown in continuous culture. J Gen Microbiol. 1980;118(1):235–252. [Google Scholar]
- 33.D’Mello R, Hill S, Poole RK. Determination of the oxygen affinities of terminal oxidases in Azotobacter vinelandii using the deoxygenation of oxyleghemoglobin and oxymyoglobin - cytochrome bd is a low-affinity oxidase. Microbiology. 1994;140(6):1395–1402. [Google Scholar]