Abstract
Background
Skin autofluorescence (SAF) is a noninvasive marker of advanced glycation end products (AGEs). In diabetes, higher SAF levels have been positively associated with long‐term complications, cardiovascular morbidity and mortality. Because little is known about the factors that influence SAF in nondiabetic individuals, we assessed the association of clinical and lifestyle parameters with SAF as well as their interactions in a large‐scale, nondiabetic population and performed the same analysis in a type 2 diabetic subgroup.
Methods
In a cross‐sectional study in participants from the LifeLines Cohort Study, extensive clinical and biochemical phenotyping, including SAF measurement, was assessed in 9009 subjects of whom 314 (3·5%) subjects with type 2 diabetes.
Results
Mean SAF was 2·04 ± 0·44 arbitrary units (AU) in nondiabetic individuals and 2·44 ± 0·55 AU in type 2 diabetic subjects (P < 0·0001). Multivariate backward regression analysis showed that in the nondiabetic population, SAF was significantly and independently associated with age, BMI, HbA1c, creatinine clearance, genetic polymorphism in NAT2 (rs4921914), current smoking, pack‐years of smoking and coffee consumption. In the type 2 diabetic group, a similar set of factors was associated with SAF, except for coffee consumption.
Conclusions
In addition to the established literature on type 2 diabetes, we have demonstrated that SAF levels are associated with several clinical and lifestyle factors in the nondiabetic population. These parameters should be taken into consideration when using SAF as a screening or prediction tool for populations at risk for cardiovascular disease and diabetes.
Keywords: Advanced glycation end products, aging, cardiovascular disease, determinants, skin autofluorescence, type 2 diabetes
Introduction
Accumulation of advanced glycation end products (AGEs) is one of the pathophysiological mechanisms associated with ageing 1. The formation and accumulation of AGEs is increased in age‐related diseases such as diabetes 2, renal insufficiency 3 and dementia 4. AGEs are formed when proteins are chemically modified by reducing sugars 5 or by reactive carbonyl compounds 6 and represent cumulative exposure to metabolic and oxidative stress 7. Skin autofluorescence (SAF) is a marker for AGE accumulation in the body and can be assessed noninvasively with a device known as the AGE Reader.
It has been demonstrated that SAF predicts cardiovascular morbidity and mortality in diabetes and end‐stage renal failure 8, 9, 10. Furthermore, higher SAF levels have been reported to be associated with carotid artery intima media thickness 11 and peripheral artery disease 12, 13, independent of diabetes and renal failure.
SAF may be influenced by both clinical and lifestyle factors. Previous studies have shown that smokers have higher SAF levels compared to nonsmokers 9, 14 as tobacco smoke causes oxidative stress and is an exogenous source of reactive glycation products 15. Recently, caffeine consumption was found to be associated with higher skin intrinsic fluorescence (SIF) levels in type 1 diabetes 16. However, more research is needed to determine whether this association also exists in nondiabetic and type 2 diabetic subjects. From a genetic perspective, we have shown a strong association between N‐acetyltransferase 2 (NAT2) acetylator polymorphism and SAF both in subjects with type 1 and 2 diabetes and in those without diabetes 17. These findings demonstrate that genetic variation is an important modulator of SAF.
It is important to determine whether SAF is a predictor of cardiovascular morbidity in an ageing population not affected by diabetes or renal disease. However, data about factors that influence SAF in nondiabetic individuals are scarce.
Therefore, we assessed the association between several clinical and lifestyle factors and SAF, along with their interactions in a large‐scale, nondiabetic population, and performed the same analysis in a subpopulation of individuals with type 2 diabetes.
Materials and methods
Participants
Subjects included were participants from the LifeLines Cohort Study 18, a large prospective population‐based cohort study examining the interaction between genetic and environmental factors in the development of chronic diseases and healthy ageing. Between 2006 and 2013, individuals from the northern region of the Netherlands were invited to participate in the study through their general practitioner. Baseline data including physical examination and extensive questionnaires have been collected from more than 167 000 participants. Follow‐up visits are scheduled every five years to collect information on biochemical measures, lifestyle behaviour and psychological factors contributing to health and disease 19. All participants provided written informed consent before participating in the study. The study has been approved by the Medical Ethical Review Committee of the University Medical Center Groningen.
For the current analysis, we evaluated participants 18–80 years of age, from whom SAF measurements and genetic data were available. This is the same LifeLines cohort sample as in our previous study on the NAT2 polymorphism 17. We have excluded subjects with type 1 diabetes (n = 12) and with severely impaired renal function, defined as serum creatinine >140 μmol/L (n = 29). This resulted in 9009 subjects for analysis of whom 314 (3·5%) had type 2 diabetes. Of the latter subjects, 212 were already known to have diabetes and another 102 were newly diagnosed by a single fasting blood plasma glucose level (≥7·0 mmol/L) at their baseline visit at the LifeLines research site.
Skin autofluorescence
Skin autofluorescence (SAF) was assessed using the AGE Reader (DiagnOptics Technologies BV, Groningen, the Netherlands). This method has been described in detail previously 14, 20. In short, the AGE Reader illuminates a skin surface of approximately 4 cm², guarded against surrounding light, with an excitation light source whose wavelength is between 300 and 420 nm (peak intensity at ~ 370 nm). Emission light and reflected excitation light from the skin are measured with an internal spectrometer in the range 300–600 nm. Measurements were taken on the volar side of the forearm, 10 cm below the elbow, at room temperature. SAF was calculated by dividing the average emitted light intensity per nanometre in the range of 420–600 nm by the average excited light intensity per nanometre in the range 300–420 nm and multiplied by 100. SAF levels are expressed in arbitrary units and will increase or decrease per arbitrary unit (AU).
Clinical and lifestyle data
The following clinical data were collected: age, gender, body mass index (BMI), systolic and diastolic blood pressures, serum lipids, HbA1c, diabetes duration, creatinine clearance and use of medication. Participants were asked to complete an extensive questionnaire which included structured questions about smoking behaviour and coffee consumption. Subjects were classified according to smoking status at baseline as never smoker, ex‐smoker or current smoker. Coffee consumption was recorded as the number of cups of coffee per day. We were not able to distinguish between caffeinated and decaffeinated coffee consumption.
Anthropometry
Weight was measured to the nearest 0·1 kg and height to the nearest 0·5 cm by trained technicians using calibrated measuring equipment, with participants wearing light clothing and no shoes. Body mass index (BMI) was calculated as weight divided by height‐squared (kg/m²). Systolic and diastolic blood pressures were measured every minute for 10 minutes using an automated Dinamap Monitor (GE Healthcare, Freiburg, Germany). The average of the last three readings was recorded for each blood pressure parameter.
Biochemical measures and genotyping
Blood was collected in the fasting state between 8·00 and 10·00 a.m. and transported to the LifeLines laboratory facility at room temperature or at 4 °C, depending on the sample requirements. On the day of collection, HbA1c (EDTA‐anticoagulated) was analysed using a NGSP‐certified turbidimetric inhibition immunoassay on a Cobas Integra 800 CTS analyser (Roche Diagnostics Nederland BV, Almere, the Netherlands). Serum creatinine was measured on a Roche Modular P chemistry analyser (Roche, Basel Switzerland), and creatinine clearance was calculated with the Cockcroft–Gault formula 21. Total and high‐density lipoprotein (HDL) cholesterol levels were measured using an enzymatic colorimetric method, triglycerides using a colorimetric UV method, and low‐density lipoprotein (LDL) cholesterol using an enzymatic method and also on a Roche Modular P chemistry analyser (Roche, Basel, Switzerland). Fasting blood glucose was measured using a hexokinase method.
In the analysis, we included the single nucleotide polymorphism (SNP) at the NAT2 locus (rs4921914) which was previously reported to be associated with SAF 17. The rs4921914 genotypes were imputed (imputation score 0·89) after exclusion of low‐quality samples and SNPs before imputation of the data derived from Illumina CytoSNP‐12v2 assay (Illumina, San Diego, CA, USA). Full details on genotyping platform, quality control, other filters applied to SNPs, and the imputation are described elsewhere 17.
Statistical analysis
SPSS (version 22, IBM, Armonk, NY, USA) was used for statistical analysis. Data are shown as mean ± standard deviation (SD) or median and interquartile range (IQR) in case of non‐normally distributed data. Student's t‐test or Mann–Whitney U‐test was performed to compare groups. SAF Z‐scores were calculated based on the total population to correct for age differences. Linear regression analysis were performed to determine the association between clinical and lifestyle determinants and SAF. First, a baseline model with only age was assessed. Next, the other determinants were added separately to that model to assess their individual contributions. A backward stepwise method was used including all clinical and lifestyle determinants to derive a final prediction model for SAF, including only determinants that remained significant. We assessed possible effect modification between clinical and lifestyle factors in their effect on SAF which is shown in a final interaction model including only significant determinants. To determine whether associations for SAF differed between subjects with and without diabetes, we additionally tested for the interaction between diabetes and clinical and lifestyle factors. A final prediction model for the total population was assessed including significant determinants only. P < 0·05 (two‐tailed) was considered statistically significant.
Results
Table 1 provides the clinical characteristics of the study population. Mean age of the nondiabetic population was 49 years, 10 years younger than the type 2 diabetic subgroup (P < 0·0001). SAF levels were significantly higher in the type 2 diabetic population (2·44 ± 0·55 AU) than in the nondiabetic subgroup (2·04 ± 0·44 AU) (P < 0·0001).
Table 1.
Clinical characteristics of the non‐diabetic population and type 2 diabetic group
| Parameters | Non‐diabetes | Type 2 diabetes |
|---|---|---|
| N | 8695 | 314 |
| Age (years) | 49 ± 11 | 59 ± 11** |
| Gender (male/female) n (%) | 3570 (41) / 5125 (59) | 168 (53) / 150 (47) |
| Body mass index (kg/m²) | 26·4 ± 4·2 | 30·5 ± 5·4** |
| Systolic blood pressure (mmHg) | 129 ± 16 | 137 ± 17* |
| Diastolic blood pressure (mmHg) | 75 ± 9 | 77 ± 9 |
| Total cholesterol (mmol/L) | 5·1 ± 0·9 | 4·7 ± 1·2 |
| HDL cholesterol (mmol/L) | 1·4 ± 0·4 | 1·2 ± 0·3 |
| LDL cholesterol (mmol/L) | 3·3 ± 0·9 | 2·9 ± 1·0 |
| Triglycerides (mmol/L) | 1·05 (0·8–1·5) | 1·41 (1·0–1·4) |
| Creatinine clearance (mL/min) | 113 ± 31 | 119 ± 45* |
| HbA1c (%) | 5·5 ± 0·3 | 6·8 ± 1·2** |
| HbA1c (mmol/mol) | 37 ± 3·3 | 51 ± 13 |
| Estimated diabetes duration (years) | n.a. | 6·4 (3·2–11·0) |
| Oral agents/insulin, % | n.a. | 47/15a |
| Statins, % | 6·2 | 46·2** |
| NAT2 polymorphism, n (%) | ||
| TT | 5685 (65) | 209 (67) |
| CT | 2706 (31) | 96 (30) |
| CC | 304 (4) | 9 (3) |
| Smoking status, n (%)b | ||
| Never smokers | 3508 (41) | 106 (34) |
| Ex‐smokers | 3174 (37) | 147 (48) |
| Current smokers | 1914 (22) | 56 (18) |
| Pack‐years in ex‐ and current smokers | 11 (4·6–19·0) | 18 (8·5–29·4)* |
| Coffee consumption (cups per day) | 3·8 (2·3–5·2) | 3·8 (1·9–5·5) |
| SAF (AU) | 2·04 ± 0·44 | 2·44 ± 0·55** |
SAF, skin autofluorescence; AU, arbitrary units.
Data are presented as means ± standard deviation, or median (interquartile range) and number (%). Creatinine clearance (Cockcroft–Gault formula).
Twenty‐nine subjects used oral agents + insulin.
Missing values for smoking status (n = 104).
*P < 0·001 **P < 0·0001.
Univariate associations with SAF
The univariate associations between clinical and lifestyle determinants and SAF showed that in the nondiabetic population, age, male gender, BMI, HbA1c, total cholesterol, LDL cholesterol, triglycerides, current smoking, ex‐smoking, pack‐years of smoking and coffee consumption were positively associated with SAF (Table S1).
Negative associations were found for creatinine clearance and the fast acetylator allele of NAT2. In the type 2 diabetic group, age, HbA1c, current smoking, pack‐years of smoking and coffee consumption were positively associated with SAF. Creatinine clearance, total cholesterol, LDL cholesterol and the fast acetylator allele of NAT2 were negatively associated with SAF (Table S1).
Multivariate associations with SAF
A baseline model including age explained 28·5% of the variance in SAF in the nondiabetic population (Table 2). Pack‐years of smoking (4·0%), current smoking (3·7%), coffee consumption (3·6%) and NAT2 polymorphism (2·1%) had the highest additional contribution. In the type 2 diabetic population, 23·8% of the variance in SAF could be explained by age. Current smoking had the highest additional contribution (8·9%) to the baseline model, followed by pack‐years of smoking (4·4%) and NAT2 polymorphism (2·7%).
Table 2.
Multivariate linear regression model for skin autofluorescence (SAF) in the non‐diabetic population and type 2 diabetic group
| Determinants | Coefficient β | SE | P Value | R 2 (%) |
|---|---|---|---|---|
| Non‐diabetes (N = 8695) | ||||
| Baseline model | ||||
| Age | 0·021 | 3·6 × 10 −4 | 1·0 × 10 −200 | 28·5 |
| Clinical and lifestyle parameters | ΔR 2 (%) | |||
| Male gender | 0·053 | 0·008 | 4·1 × 10 −11 | 0·4 |
| Body mass index | 0·004 | 0·001 | 1·5 × 10 −4 | 0·1 |
| HbA1c | 0·082 | 0·014 | 3·2 × 10 −9 | 0·3 |
| Creatinine clearance (mL/min) | 2·0 × 10 −4 | 3·1 × 10 −4 | 3·2 × 10 −8 | 0·3 |
| Total cholesterol | 0·001 | 0·004 | 0·893 | 0·0 |
| LDL cholesterol | 0·004 | 0·005 | 0·396 | 0·0 |
| HDL cholesterol | −0·084 | 0·010 | 6·7 × 10 −16 | 0·5 |
| Triglycerides | 0·030 | 0·005 | 2·5 × 10 −9 | 0·3 |
| NAT2 polymorphism (CC vs. CT and TT) | −0·114 | 0·007 | 1·2 × 10 −57 | 2·1 |
| Current smoking vs. never smoking | 0·205 | 0·009 | 5·4 × 10 −101 | 3·7 |
| Ex‐smoking vs. never smoking | −0·029 | 0·009 | 0·001 | 0·1 |
| Pack‐years | 0·007 | 4·6 × 10 −4 | 4·2 × 10 −56 | 4·0 |
| Coffee consumption (cups per day) | 0·076 | 0·002 | 3·6 × 10 −108 | 3·6 |
| Type 2 diabetes (N = 314) | ||||
| Baseline model | ||||
| Age | 0·025 | 0·003 | 3·8 × 10 −20 | 23·8 |
| Clinical and lifestyle parameters | ΔR 2 (%) | |||
| Male gender | 0·151 | 0·054 | 0·005 | 1·9 |
| Body mass index | 0·003 | 0·005 | 0·589 | 0·1 |
| HbA1c | 0·064 | 0·023 | 0·006 | 1·8 |
| Creatinine clearance (mL/min) | 0·005 | 0·002 | 0·011 | 1·6 |
| Total cholesterol | −0·063 | 0·023 | 0·007 | 1·8 |
| LDL cholesterol | −0·072 | 0·026 | 0·006 | 1·6 |
| HDL cholesterol | −0·250 | 0·086 | 0·004 | 1·8 |
| Triglycerides | 0·036 | 0·021 | 0·087 | 0·5 |
| Estimated diabetes duration | 0·005 | 0·004 | 0·246 | 0·6 |
| NAT2 polymorphism (CC vs. CT and TT) | −0·167 | 0·050 | 0·001 | 2·7 |
| Current smoking vs. never smoking | 0·436 | 0·069 | 7·0 × 10 −10 | 8·9 |
| Ex‐smoking vs. never smoking | −0·032 | 0·056 | 0·570 | 0·1 |
| Pack‐years | 0·006 | 0·002 | 0·001 | 4·4 |
| Coffee consumption (cups per day) | 0·029 | 0·012 | 0·018 | 1·6 |
NAT2, N‐acetyltransferase 2; SE, standard error; R 2: explained variance in SAF (%); ΔR 2: additional explained variance of clinical and lifestyle parameters on top of baseline model.
In the nondiabetic population, the multivariate regression model showed that age, BMI, HbA1c, creatinine clearance, NAT2 polymorphism, current smoking, pack‐years of smoking and coffee consumption were independent predictors of SAF explaining 33·8% of the variance in SAF (Table 3). In the type 2 diabetic population, a similar set of predictors excluding coffee consumption explained 46·8% of the variance in SAF.
Table 3.
Prediction model for skin autofluorescence (SAF) in the non‐diabetic population and the type 2 diabetic group
| Predictors | Coefficient β | SE | P Value | R 2 (%) |
|---|---|---|---|---|
| Non‐diabetes (n = 8695) | ||||
| Age | 0·018 | 0·001 | 1·3 × 10 −116 | 33·8 |
| Body mass index | 0·006 | 0·002 | 0·001 | |
| HbA1c | 0·061 | 0·019 | 0·001 | |
| Creatinine clearance (mL/min) | −0·001 | 2·8 × 10 −4 | 7·9 × 10 −7 | |
| NAT2 polymorphism (CC vs. CT and TT) | −0·119 | 0·009 | 1·0 × 10−35 | |
| Current smoking vs. never smoking | 0·115 | 0·013 | 9·6 × 10 −20 | |
| Pack‐years | 0·004 | 4·9 × 10 −4 | 7·0 × 10 −20 | |
| Coffee consumption (cups per day) | 0·032 | 0·002 | 3·0 × 10 −40 | |
| Type 2 diabetes (N = 314) | ||||
| Age | 0·017 | 0·006 | 0·004 | 46·8 |
| Body mass index | 0·020 | 0·010 | 0·039 | |
| HbA1c | 0·112 | 0·035 | 0·002 | |
| Creatinine clearance (mL/min) | −0·005 | 0·002 | 0·001 | |
| NAT2 polymorphism (CC vs. CT and TT) | −0·210 | 0·069 | 0·003 | |
| Current smoking vs. never smoking | 0·327 | 0·101 | 0·002 | |
| Pack‐years | 0·005 | 0·002 | 0·018 | |
NAT2, N‐acetyltransferase 2; SE, standard error; R 2: explained variance in SAF (%).
The effect of coffee consumption on SAF
In the nondiabetic population, coffee consumption was significantly and dose‐dependently associated with higher SAF Z‐scores (P < 0·001), whereas for the type 2 diabetic population a nonsignificant trend was found (P = 0·104) (Fig. 1). The association between coffee consumption and SAF was modified by NAT2 polymorphism (P = 0·001) (Table S2). Among subjects having a TT genotype, a mean intake of one cup of coffee per day was associated with 0·052 AU increase in SAF (0·032 AU and 0·029 AU for CT, respectively, CC genotype) (Table S3). In the type 2 diabetic population, a mean intake of one cup of coffee per day was associated with 0·049 AU increase among subjects with a TT genotype. The associations for CT genotype (0·016 AU) and CC genotype (0·005 AU) were not significant (P = 0·380 and P = 0·948, respectively).
Figure 1.
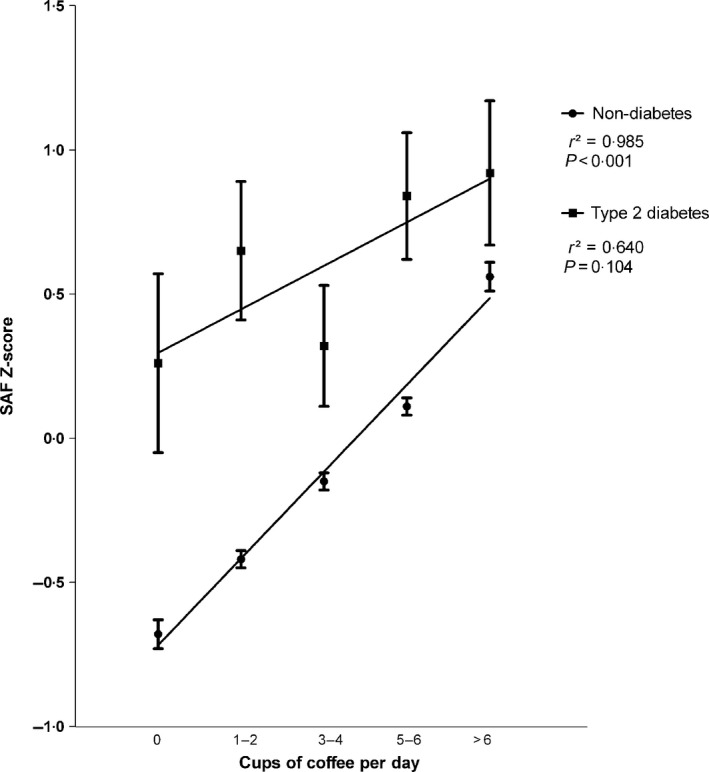
The effect of coffee consumption on SAF in the non‐diabetic population and type 2 diabetic group. Dots (type 2 diabetes) and squares (non‐diabetes) show mean SAF Z‐scores ± SEM, r 2 correlation coefficient. Sample size per category: non‐diabetes, 0 cups of coffee per day (n = 563), type 2 diabetes, 0 cups of coffee per day (n = 11); non‐diabetes, 1–2 cups of coffee per day (n = 1491), type 2 diabetes, 1–2 cups of coffee per day (n = 43); non‐diabetes, 3–4 cups of coffee per day (n = 3044), type 2 diabetes, 3–4 cups of coffee per day (n = 79); non‐diabetes, 5–6 cups of coffee per day (n = 2263), type 2 diabetes, 5–6 cups of coffee per day (n = 63); non‐diabetes, >6 cups of coffee per day (n = 1061), type 2 diabetes, >6 cups of coffee per day (n = 35). SAF, skin autofluorescence.
Smoking and SAF
Figure 2 shows the age‐adjusted SAF Z‐scores for different smoking groups. Within each smoking group, subjects from the nondiabetic population had significantly lower SAF Z‐scores compared to subjects with type 2 diabetes (never smokers, P < 0·05; ex‐smokers, P < 0·0001; current smokers, P < 0·0001). In both groups, current and ex‐smokers had higher SAF Z‐scores compared to never smokers (P < 0·05 – P < 0·0001).
Figure 2.
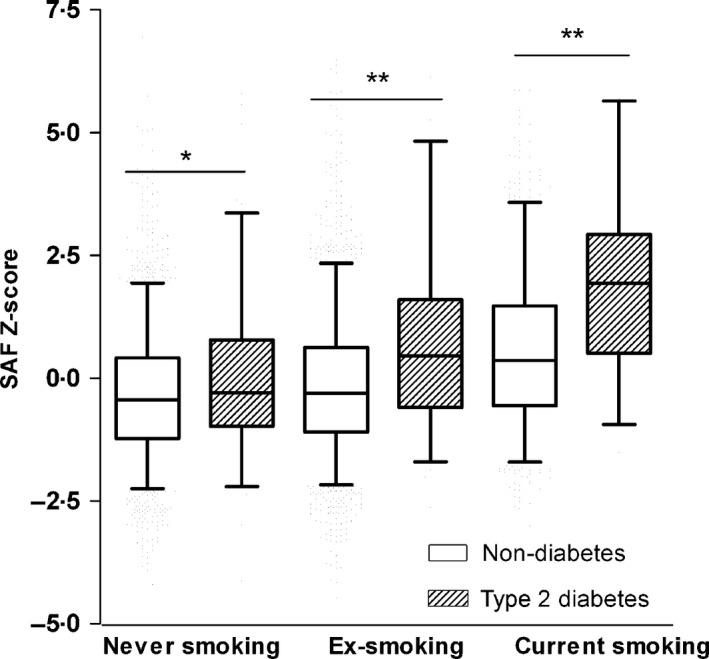
Age‐adjusted SAF Z‐scores stratified for smoking status in the non‐diabetic population and type 2 diabetic group. Boxes show mean, minimum and maximum SAF Z‐scores, whiskers represent the 5th and 95th percentile. Sample size per category: non‐diabetes, never smoker (n = 3516), type 2 diabetes, never smoker (n = 106); non‐diabetes, ex‐smoker (n = 3186), type 2 diabetes, ex‐smoker (n = 150); non‐diabetes, current smoker (n = 1919), type 2 diabetes, current smoker (n = 57). SAF, skin autofluorescence. *P < 0·05, **P < 0·0001.
Furthermore, SAF levels increased with the number of pack‐years smoked, indicating a dose‐dependent effect (Fig. S1).
Clinical and lifestyle interactions
Next, we evaluated effect modification for clinical and lifestyle determinants in their effect on SAF. A significant interaction between current smoking and age (P < 0·0001) was observed showing that one‐year increase in age was associated with an additional 0·002 AU increase in SAF, which implies a 12% increase in age dependency (data not shown). The final interaction model for the nondiabetic population, including significant determinants only, is presented in Table S2.
Finally, we assessed whether associations with SAF differed between subjects with and without diabetes. Effect modification was observed for type 2 diabetes and HbA1c (P < 0·0001) (data not shown) with HbA1c having a larger effect on SAF in subjects with type 2 diabetes compared to individuals without diabetes. Also, a significant interaction between current smoking and type 2 diabetes (P = 0·04) in their association with SAF was found (data not shown). Current smoking had a larger effect on SAF in subjects with type 2 diabetes compared to nondiabetic individuals which was already demonstrated in Table 2 and Fig. 2. The final prediction model for SAF for the total population is given in Table S4.
Discussion
This is the first study to report an integrated analysis of both lifestyle and clinical factors that influence SAF in a large‐scale nondiabetic population as well as in a subpopulation with type 2 diabetes. We have shown that SAF is significantly and independently associated with age, BMI, HbA1c, creatinine clearance, NAT2 polymorphism, current smoking, pack‐years of smoking and coffee consumption explaining 33·8% of the variance in SAF. In the type 2 diabetic population, SAF was associated with the same factors with the exception of coffee consumption and explained 46·8% of the variance in SAF.
Coffee consumption was dose‐dependently associated with higher SAF levels both in the nondiabetic population and in subgroup of type 2 diabetic individuals. Recently, Eny et. al. reported a positive association between caffeine consumption and SIF in type 1 diabetes 16. However, a previous Dutch study examining the association between dietary habits and SAF found no association between coffee consumption and SAF among 147 elderly subjects 22. The average amount of coffee consumed daily was comparable to our study (mean 3·4 cups compared to a median of 3·8 cups); but, the subjects in our study were on average 10 years younger. Another – more likely – explanation for the different findings might be that their study was underpowered due to the small sample size.
Factors that could explain elevated SAF levels in coffee consumers may be fluorescent substances in coffee (fluorophores) or indirectly as a consequence of the roasting process of coffee beans, which can be considered as a Maillard reaction, leading to the formation of browning products such as melanoidins 23. Also, it might be that coffee consumers are more likely to smoke which would result in a positive association between coffee consumption and SAF. Nevertheless, we found that both determinants were independently associated with SAF in the multivariate model, whereas no effect modification was observed.
Coffee is one of the most consumed beverages around the world, and many studies have examined its association with health and disease. Moderate amounts of coffee consumption have been reported to be protective against cardiovascular disease 24 and type 2 diabetes 25, 26. In addition, a recent study has demonstrated that coffee consumption was associated with a lower risk of overall mortality 27. Coffee is a major source of the phenolic antioxidant chlorogenic acid, and its daily intake from coffee consumption is estimated to be 0·5–1 g 28. Part of the beneficial effects of coffee consumption might be attributed to chlorogenic acid which reduces oxidative stress and inhibits hydrolysis of glucose‐6‐phosphatase, leading to lower plasma glucose concentrations 29. Interestingly, chlorogenic acid has been reported to inhibit AGE formation in vitro 30, 31. During the roasting process of coffee beans however, a significant amount of chlorogenic acid is lost 32. Hereby, the inhibitory effect of chlorogenic acid on AGE formation might be attenuated. The roasting process of coffee beans further leads to profound changes in the chemical composition of coffee brew, including the formation of melanoidins as the end products of the Maillard reaction 23. This may explain how coffee consumption, as an exogenous source of AGE can contribute to increased SAF levels. Overall, the present study shows that coffee consumption is associated with higher SAF levels. When using SAF to predict cardiovascular events, this may lead to overestimation of true risk in those with high coffee consumption.
Previously, we have shown that N‐acetyltransferase 2 (NAT2) acetylator polymorphism was significantly associated with SAF 17. NAT2 is a drug‐metabolizing enzyme for which certain gene polymorphisms have been associated with increased risk of several cancers 33. Interestingly, in the present study, effect modification was observed for NAT2 polymorphism and coffee consumption in their effect on SAF.
The effect of coffee consumption on SAF was strongest for individuals with the slow acetylator genotype and weakest for individuals with the fast acetylator genotype. Previous studies have shown that NAT2 is involved in the metabolic pathway of caffeine 34, 35. As caffeine has fluorescent properties 36, it may be that the association between NAT2 and SAF was influenced by fluorescence of caffeine present in the skin. However, both NAT2 polymorphism and coffee consumption were also independently associated with SAF when analysed together.
Our results showing significantly higher SAF levels in current smokers compared to never smokers are in agreement with earlier studies performed in type 2 diabetes 9, 14. In addition, a higher number of pack‐years was associated with higher SAF levels, which was also found in a study among patients with chronic obstructive pulmonary disease 37.
Theoretically, many years of smoking – and thus exposure to long‐term oxidative stress – may contribute either directly or indirectly to increased accumulation of AGEs throughout life. As smoking enhances the risk for diabetes‐related cardiovascular complications 38, it might be that the larger effect of smoking on SAF in type 2 diabetic subjects translates into higher cardiovascular risk of smoking in diabetes. Future follow‐up studies are needed to confirm this hypothesis.
As expected, age was significantly and independently associated with SAF in both the nondiabetic population and type 2 diabetic subgroup. In general, mean SAF was significantly higher in the type 2 diabetic subgroup compared to the nondiabetic population, but it should be emphasized that the former group was on average 10 years older. Ageing has been thought to be a key factor in nonenzymatic glycation of proteins 1, 5 which has been confirmed in several studies using the AGE Reader, all showing a linear relationship between increasing age and higher SAF 14, 20.
The pathway of endogenous AGE formation might be different between subjects with and without diabetes. In diabetes, AGE formation is based on a combination of chronic hyperglycaemia and oxidative stress 6, 7 as well as through lipid‐derived intermediates, resulting in advanced lipoxidation end products (ALEs) 39. In addition, as serum AGEs are cleared by the kidney 3, changes in renal function may also influence AGE accumulation 40. In subjects without diabetes however, ageing is thought to be the most important factor contributing to endogenous AGE formation 1, 5, as can be deduced from our results.
HbA1c, a measure of semi‐recent glycaemic status, was significantly associated with higher SAF levels, with a larger effect among type 2 diabetic individuals. A study among type 2 diabetic individuals concluded that SAF was poorly predicted by HbA1c level 41. In type 1 diabetes, SAF was associated with long‐term mean HbA1c, but not with most recent HbA1c 42. Another study among Japanese type 1 diabetes patients showed that SAF significantly correlated with HbA1c over the past 15 years which probably is a better measure of long‐term glycaemic load 43. An earlier study showed that Hb‐AGE was a better indicator for long‐term blood glucose control compared to HbA1c 44. In clinical practice, HbA1c represents the average glycaemic control over the last 5–6 weeks 44. HbA1c levels in diabetes can fluctuate over short‐time periods 45, which makes it plausible that the association with SAF, reflecting a much longer‐term metabolic memory (~ 15 years) 46, is inconsistent. In nondiabetic subjects, HbA1c levels are expected to show fewer fluctuations which could explain a more consistent relationship with SAF in the population without diabetes.
Our study has some strengths and limitations. First, the large majority of our study population were individuals without diabetes making this SAF study unique. Moreover, due to the large number of participants, we were able to perform analysis for different smoking statuses and the variety in the amount of coffee consumption typically found in the general population. Because clinical data were obtained at the same time of the SAF measurement, the associations found are highly reliable. A limitation of our study is a potential misclassification of some individuals with regard to their smoking status as we cannot rule out misreporting of smoking habits or history. Secondly, diagnoses of type 2 diabetes were made by a single fasting plasma glucose only. Unfortunately, we were not able to include in our analysis the use of other caffeine‐rich beverages, such as tea or soft drinks, or caffeine from foods.
In addition to the established literature in type 2 diabetes, we have demonstrated that SAF is influenced by clinical and lifestyle factors, including smoking and coffee consumption in a large‐scale nondiabetic population. These parameters need to be taken into consideration when using SAF as a screening or prediction tool for populations at risk for cardiovascular disease and diabetes.
Authors’ contributions
RPW, MMvdK and BHRW conceived and designed the study. RPW and BHRW performed the statistical analysis and analysed the data. RPW, BHRW, MMvdK, HLL, JVO, RG, SNS and ADP interpreted the data. RPW drafted the manuscript. All authors intellectually contributed to the manuscript, helped drafting the manuscript and have read and approved the final version.
Conflict of interests
RG is founder and shareholder of DiagnOptics BV, Groningen, the Netherlands, manufacturing autofluorescence readers (http://www.diagnoptics.com/) which have been used in this study. All other authors declare that they have no competing interests.
Funding
This research received no specific grant from any funding agency in the public, commercial or not‐for‐profit sectors.
Address
Department of Endocrinology, University of Groningen, University Medical Center Groningen, Groningen, 9700 RB, The Netherlands (R. P. van Waateringe, S. N. Slagter, M. M. van der Klauw, J. V. van Vliet‐Ostaptchouk, R. Graaff, H. L. Lutgers, B. H. R. Wolffenbuttel); Program in Genetics and Genome Biology, Hospital for Sick Children, Toronto, Ontario, M5G 0A4, Canada (A. D. Paterson).
Supporting information
Figure S1. The association between skin autofluorescence (SAF) and pack‐years in the non‐diabetic population and type 2 diabetic group.
Table S1. Univariate linear regression model for skin autofluorescence (SAF) in the non‐diabetic population and type 2 diabetic group.
Table S2. Interaction model for skin autofluorescence (SAF) including clinical‐lifestyle interactions in the non‐diabetic population (N = 8695).
Table S3. Univariate linear regression model for skin autofluorescence (SAF) and coffee consumption stratified by NAT2 genotype in the non‐diabetic population and type 2 diabetic group
Table S4. Final prediction model for skin autofluorescence (SAF) in the total population (N = 9009).
Acknowledgements
This work was supported by Netherlands Consortium for Healthy Ageing (NCHA) and Biobank Standardisation and Harmonization for Research Excellence in the European Union (Bio‐SHaRE‐EU). Bioresource research impact factor is BRIF4568. The manuscript is based on data from the LifeLines Cohort Study. LifeLines adheres to standards for open data availability. The data catalogue of LifeLines is publicly accessible on www.lifelines.net. All international researchers can apply for data at the LifeLines research office (LLscience@umcg.nl). The LifeLines system allows access for reproducibility of the study results. We thank Sally Hill for providing scientific medical writing services.
Eur J Clin Invest 2016; 46 (5): 481–490
Prior presentations: Parts of this study were presented in the abstract form at the American Diabetes Association (ADA), June 2014, San Francisco (USA), and at the European Association for the Study of Diabetes (EASD), September 2014, Vienna (Austria).
References
- 1. Dyer DG, Dunn JA, Thorpe SR, Bailie KE, Lyons TJ, McCance DR et al Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J Clin Invest 1993;91:2463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brownlee M. Lilly Lecture 1993. Glycation and diabetic complications. Diabetes 1994;43:836–41. [DOI] [PubMed] [Google Scholar]
- 3. Busch M, Franke S, Ruster C, Wolf G. Advanced glycation end‐products and the kidney. Eur J Clin Invest 2010;40:742–55. [DOI] [PubMed] [Google Scholar]
- 4. Srikanth V, Maczurek A, Phan T, Steele M, Westcott B, Juskiw D et al Advanced glycation endproducts and their receptor RAGE in Alzheimer's disease. Neurobiol Aging 2011;32:763–77. [DOI] [PubMed] [Google Scholar]
- 5. Monnier VM. Nonenzymatic glycosylation, the Maillard reaction and the aging process. J Gerontol 1990;45:B105–11. [DOI] [PubMed] [Google Scholar]
- 6. Miyata T, van Ypersele de Strihou C, Kurokawa K, Baynes JW. Alterations in nonenzymatic biochemistry in uremia: origin and significance of “carbonyl stress” in long‐term uremic complications. Kidney Int 1999;55:389–99. [DOI] [PubMed] [Google Scholar]
- 7. Baynes JW, Thorpe SR. Glycoxidation and lipoxidation in atherogenesis. Free Radic Biol Med 2000;28:1708–16. [DOI] [PubMed] [Google Scholar]
- 8. Meerwaldt R, Hartog JW, Graaff R, Huisman RJ, Links TP, den Hollander NC et al Skin autofluorescence, a measure of cumulative metabolic stress and advanced glycation end products, predicts mortality in hemodialysis patients. J Am Soc Nephrol 2005;16:3687–93. [DOI] [PubMed] [Google Scholar]
- 9. Lutgers HL, Graaff R, Links TP, Ubink‐Veltmaat LJ, Bilo HJ, Gans RO et al Skin autofluorescence as a noninvasive marker of vascular damage in patients with type 2 diabetes. Diabetes Care 2006;29:2654–9. [DOI] [PubMed] [Google Scholar]
- 10. Gerrits EG, Lutgers HL, Kleefstra N, Graaff R, Groenier KH, Smit AJ et al Skin autofluorescence: a tool to identify type 2 diabetic patients at risk for developing microvascular complications. Diabetes Care 2008;31:517–21. [DOI] [PubMed] [Google Scholar]
- 11. Lutgers HL, Graaff R, de Vries R, Smit AJ, Dullaart RP. Carotid artery intima media thickness associates with skin autofluoresence in non‐diabetic subjects without clinically manifest cardiovascular disease. Eur J Clin Invest 2010;40:812–17. [DOI] [PubMed] [Google Scholar]
- 12. de Vos LC, Noordzij MJ, Mulder DJ, Smit AJ, Lutgers HL, Dullaart RP et al Skin autofluorescence as a measure of advanced glycation end products deposition is elevated in peripheral artery disease. Arterioscler Thromb Vasc Biol 2013;33:131–8. [DOI] [PubMed] [Google Scholar]
- 13. de Vos LC, Mulder DJ, Smit AJ, Dullaart RP, Kleefstra N, Lijfering WM et al Skin autofluorescence is associated with 5‐year mortality and cardiovascular events in patients with peripheral artery disease. Arterioscler Thromb Vasc Biol 2014;34:933–8. [DOI] [PubMed] [Google Scholar]
- 14. Koetsier M, Lutgers HL, de Jonge C, Links TP, Smit AJ, Graaff R. Reference values of skin autofluorescence. Diabetes Technol Ther 2010;12:399–403. [DOI] [PubMed] [Google Scholar]
- 15. Cerami C, Founds H, Nicholl I, Mitsuhashi T, Giordano D, Vanpatten S et al Tobacco smoke is a source of toxic reactive glycation products. Proc Natl Acad Sci U S A 1997;94:13915–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eny KM, Orchard TJ, Grace MR, Maynard J, Grant DM, Costacou T et al Caffeine Consumption Contributes to Skin Intrinsic Fluorescence in Type 1 Diabetes. Diabetes Technol Ther 2015;17:726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eny KM, Lutgers HL, Maynard J, Klein BE, Lee KE, Atzmon G et al GWAS identifies an NAT2 acetylator status tag single nucleotide polymorphism to be a major locus for skin fluorescence. Diabetologia 2014;57:1623–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stolk RP, Rosmalen JG, Postma DS, de Boer RA, Navis G, Slaets JP et al Universal risk factors for multifactorial diseases: LifeLines: a three‐generation population‐based study. Eur J Epidemiol 2008;23:67–74. [DOI] [PubMed] [Google Scholar]
- 19. Scholtens S, Smidt N, Swertz MA, Bakker SJ, Dotinga A, Vonk JM et al Cohort Profile: LifeLines, a three‐generation cohort study and biobank. Int J Epidemiol 2015;44:1172–80. [DOI] [PubMed] [Google Scholar]
- 20. Meerwaldt R, Graaff R, Oomen PH, Links TP, Jager JJ, Alderson NL et al Simple non‐invasive assessment of advanced glycation endproduct accumulation. Diabetologia 2004;47:1324–30. [DOI] [PubMed] [Google Scholar]
- 21. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16:31–41. [DOI] [PubMed] [Google Scholar]
- 22. Jochemsen B, Mulder G, van DJ, Volmer M, Graaff R, Smit A. Relation between food and drinking habits, and skin autofluorescence and intima media thickness in subjects at high cardiovascular risk. J Food Nutr Res 2009;48:51–8. [Google Scholar]
- 23. Bekedam EK, Loots MJ, Schols HA, Van Boekel MA, Smit G. Roasting effects on formation mechanisms of coffee brew melanoidins. J Agric Food Chem 2008;56:7138–45. [DOI] [PubMed] [Google Scholar]
- 24. Ding M, Bhupathiraju SN, Satija A, van Dam RM, Hu FB. Long‐term coffee consumption and risk of cardiovascular disease: a systematic review and a dose‐response meta‐analysis of prospective cohort studies. Circulation 2014;129:643–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Dam RM, Feskens EJ. Coffee consumption and risk of type 2 diabetes mellitus. Lancet 2002;360:1477–8. [DOI] [PubMed] [Google Scholar]
- 26. Tuomilehto J, Hu G, Bidel S, Lindstrom J, Jousilahti P. Coffee consumption and risk of type 2 diabetes mellitus among middle‐aged Finnish men and women. JAMA 2004;291:1213–19. [DOI] [PubMed] [Google Scholar]
- 27. Ding M, Satija A, Bhupathiraju SN, Hu Y, Sun Q, Han J et al Association of coffee consumption with total and cause‐specific mortality in three large prospective cohorts. Circulation 2015;132:2305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Clifford MN. Chlorogenic acid and other cinnamates—nature, occurence, dietary burden, absorption and metabolism. J Sci Food Agric 2000;8:1033–43. [Google Scholar]
- 29. Arion WJ, Canfield WK, Ramos FC, Schindler PW, Burger HJ, Hemmerle H et al Chlorogenic acid and hydroxynitrobenzaldehyde: new inhibitors of hepatic glucose 6‐phosphatase. Arch Biochem Biophys 1997;339:315–22. [DOI] [PubMed] [Google Scholar]
- 30. Kim J, Jeong IH, Kim CS, Lee YM, Kim JM, Kim JS. Chlorogenic acid inhibits the formation of advanced glycation end products and associated protein cross‐linking. Arch Pharm Res 2011;34:495–500. [DOI] [PubMed] [Google Scholar]
- 31. Fernandez‐Gomez B, Ullate M, Picariello G, Ferranti P, Mesa MD, del Castillo MD. New knowledge on the antiglycoxidative mechanism of chlorogenic acid. Food Funct 2015;6:2081–90. [DOI] [PubMed] [Google Scholar]
- 32. Moon JK, Yoo HS, Shibamoto T. Role of roasting conditions in the level of chlorogenic acid content in coffee beans: correlation with coffee acidity. J Agric Food Chem 2009;57:5365–9. [DOI] [PubMed] [Google Scholar]
- 33. Hein DW, Doll MA, Fretland AJ, Leff MA, Webb SJ, Xiao GH et al Molecular genetics and epidemiology of the NAT1 and NAT2 acetylation polymorphisms. Cancer Epidemiol Biomarkers Prev 2000;9:29–42. [PubMed] [Google Scholar]
- 34. Grant DM, Tang BK, Kalow W. A simple test for acetylator phenotype using caffeine. Br J Clin Pharmacol 1984;17:459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Butler MA, Lang NP, Young JF, Caporaso NE, Vineis P, Hayes RB et al Determination of CYP1A2 and NAT2 phenotypes in human populations by analysis of caffeine urinary metabolites. Pharmacogenetics 1992;2:116–27. [DOI] [PubMed] [Google Scholar]
- 36. Karim MM, Jeon CW, Lee HS, Alam SM, Lee SH, Choi JH et al Simultaneous determination of acetylsalicylic acid and caffeine in pharmaceutical formulation by first derivative synchronous fluorimetric method. J Fluoresc 2006;16:713–21. [DOI] [PubMed] [Google Scholar]
- 37. Hoonhorst SJ, Lo Tam Loi AT, Hartman JE, Telenga ED, van den Berge M, Koenderman L et al Advanced glycation end products in the skin are enhanced in COPD. Metabolism 2014;63:1149–56. [DOI] [PubMed] [Google Scholar]
- 38. Haire‐Joshu D, Glasgow RE, Tibbs TL. Smoking and diabetes. Diabetes Care 1999;22:1887–98. [DOI] [PubMed] [Google Scholar]
- 39. Thorpe SR, Baynes JW. Maillard reaction products in tissue proteins: new products and new perspectives. Amino Acids 2003;25:275–81. [DOI] [PubMed] [Google Scholar]
- 40. Makita Z, Yanagisawa K, Kuwajima S, Yoshioka N, Atsumi T, Hasunuma Y et al Advanced glycation endproducts and diabetic nephropathy. J Diabetes Complications 1995;9:265–8. [DOI] [PubMed] [Google Scholar]
- 41. Gerrits EG, Lutgers HL, Kleefstra N, Groenier KH, Smit AJ, Gans RO et al Skin advanced glycation end product accumulation is poorly reflected by glycemic control in type 2 diabetic patients (ZODIAC‐9). J Diabetes Sci Technol 2008;2:572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aroda VR, Conway BN, Fernandez SJ, Matter NI, Maynard JD, Orchard TJ et al Cross‐sectional evaluation of noninvasively detected skin intrinsic fluorescence and mean hemoglobin a1c in type 1 diabetes. Diabetes Technol Ther 2013;15:117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sugisawa E, Miura J, Iwamoto Y, Uchigata Y. Skin autofluorescence reflects integration of past long‐term glycemic control in patients with type 1 diabetes. Diabetes Care 2013;36:2339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wolffenbuttel BH, Giordano D, Founds HW, Bucala R. Long‐term assessment of glucose control by haemoglobin‐AGE measurement. Lancet 1996;347:513–15. [DOI] [PubMed] [Google Scholar]
- 45. Kilpatrick ES. The rise and fall of HbA(1c) as a risk marker for diabetes complications. Diabetologia 2012;55:2089–91. [DOI] [PubMed] [Google Scholar]
- 46. Verzijl N, DeGroot J, Thorpe SR, Bank RA, Shaw JN, Lyons TJ et al Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem 2000;275:39027–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The association between skin autofluorescence (SAF) and pack‐years in the non‐diabetic population and type 2 diabetic group.
Table S1. Univariate linear regression model for skin autofluorescence (SAF) in the non‐diabetic population and type 2 diabetic group.
Table S2. Interaction model for skin autofluorescence (SAF) including clinical‐lifestyle interactions in the non‐diabetic population (N = 8695).
Table S3. Univariate linear regression model for skin autofluorescence (SAF) and coffee consumption stratified by NAT2 genotype in the non‐diabetic population and type 2 diabetic group
Table S4. Final prediction model for skin autofluorescence (SAF) in the total population (N = 9009).


