Abstract
Background
Bilirubin has anti‐oxidative and anti‐inflammatory properties, which may explain its proposed protective effects on the development of cardiometabolic disorders. Glucocorticoids affect heme oxygenase regulation in vitro, which plays a key role in bilirubin production. Effects of variations in glucocorticoid exposure on circulating bilirubin levels in humans are unknown. Here we tested whether a higher hydrocortisone replacement dose affects circulating bilirubin in hypopituitary patients.
Materials and methods
A randomized double‐blind cross‐over study (ClinicalTrials.gov, number NCT01546992) was performed in 47 patients with secondary adrenal failure [10‐week exposure to a higher hydrocortisone dose (0·4–0·6 mg/kg body weight) vs. 10 weeks of a lower hydrocortisone dose (0·2–0·3 mg/kg body weight)].
Results
Plasma total bilirubin was increased by 10% from 7 to 8 μM in response to the higher hydrocortisone dose (P = 0·033). This effect was inversely related to age (P = 0·042), but was unaffected by sex, obesity and (replacement for) other hormonal insufficiencies. The higher hydrocortisone dose also resulted in lower alkaline phosphatase (P = 0·006) and aspartate aminotransferase activities (P = 0·001).
Conclusion
Bilirubin is modestly increased in response to higher glucocorticoid exposure in humans, in conjunction with lower alkaline phosphatase and aspartate aminotransferase activities, which are supposed to represent biomarkers of a pro‐inflammatory state and enhanced liver fat accumulation.
Keywords: Alkaline phosphatase, bilirubin, hydrocortisone, secondary adrenal insufficiency, transaminases
Introduction
Bilirubin is able to scavenge peroxyl radicals, to diminish low‐density lipoprotein (LDL) oxidation, to decrease the expression of cellular adhesion molecules and to inhibit the production of pro‐inflammatory cytokines 1, 2, 3. Furthermore, circulating bilirubin levels are inversely related to the inflammation markers, high‐sensitivity C‐reactive protein and serum amyloid A, as well as to a composite measure of circulating glycosylated acute‐phase proteins 4, 5, 6. Among other mechanisms, anti‐oxidative and anti‐inflammatory properties are believed to explain the proposed protective effects of bilirubin on the development of cardiometabolic disorders 3. In line, bilirubin is inversely associated with intima media thickness, a marker of subclinical atherosclerosis 7, 8, as well as with incident cardiovascular disease 9, 10.
The heme oxygenase (HO) system is crucial for the generation of bilirubin from heme 3, 11, 12. Expression of the HO‐1 isoenzyme is inducible by a variety of factors, whereas the HO‐2 isoenzyme is considered to be expressed in a predominantly constitutive fashion 11, 12. Interestingly, the HO‐2 gene contains a functional glucocorticoid response element 11. As a result, HO‐2 transcripts and protein levels are upregulated by glucocorticoids in vitro at least in neonatal rat brain and testis 13, 14, 15. On the other hand, upregulation of HO‐1 by interleukin‐6 may be counteracted by glucocorticoids in endothelial cells 16.
Given the alleged influence of bilirubin on cardiometabolic disorders 3, it is pathophysiologically relevant to determine the contribution of glucocorticoids to bilirubin regulation, but no data are available with respect to effects of variation in glucocorticoid exposure on circulating bilirubin levels in humans. We have recently conducted a randomized cross‐over study in hypopituitary patients with secondary adrenal insufficiency (ClinicalTrials.gov, number NCT01546992) which is aimed at assessing effects of a higher compared to a lower hydrocortisone replacement dose on cognitive function, quality of life and metabolic parameters 17. The present ancillary analyses were performed to test whether the degree of hydrocortisone exposure affects circulating bilirubin levels in this patient category.
Patients and methods
Participants and study design
Reporting of the study conforms to CONSORT and the broader EQUATOR guidelines 18. This randomized double‐blind cross‐over study was performed in a university hospital setting in Groningen, the Netherlands. The study has been registered with ClinicalTrials.gov, number NCT01546992, and has been approved by the medical ethics committee of the University Medical Center Groningen, the Netherlands. All participants provided written informed consent. The rationale and design of the study have been provided in detail elsewhere 17. Figure S1 shows eligibility, inclusion and follow‐up of the participants. In brief, hypopituitary patients with established secondary adrenal insufficiency (based on internationally accepted criteria) were eligible for this randomized double‐blind cross‐over study. They were recruited from the outpatient endocrinology clinic of the University Medical Center, Groningen, the Netherlands. The following inclusion criteria were applied: treatment for pituitary disorder (surgery and/or radiotherapy) at least 1 year before study entry, stable replacement therapy for other pituitary hormone deficiencies for at least 6 months (thyroid hormone deficiency, growth hormone deficiency, testosterone/estradiol deficiency, diabetes insipidus), age between 18 and 75 years and body weight between 50 and 100 kg 17. Main exclusion criteria were major cognitive impairment, drug abuse, current psychiatric disorders, shift work, malignancy, previous Cushing's disease, a history of frequent episodes of hypocortisolism, medically treated diabetes possibly leading to hypoglycaemia (insulin, sulfonylurea) and anti‐epileptic drugs 17. Subjects experiencing a hospital admission during the study were also excluded.
Before entry in the study, all patients who were treated with cortisone acetate were switched to hydrocortisone in a bioequivalent dose during a 4‐week run‐in period 17. The hydrocortisone dose administered during the run‐in period averaged 25 mg (0·31 mg/kg body weight) per day. The participants were then randomized to a lower hydrocortisone dose during 10 weeks followed by a higher hydrocortisone dose during another 10‐week period or vice versa. The lower hydrocortisone dose was 0·20–0·30 mg hydrocortisone per kg body weight per day divided into three doses, taken before breakfast, before lunch and before dinner. A double dose, also divided into three doses and taken at the same time points, was administered during the higher hydrocortisone dose period. Hydrocortisone administration was performed in a double‐blind fashion using 5‐mg tablets in the lower and 10‐mg tablets in the higher hydrocortisone replacement period. In case of intercurrent illnesses, doubling or tripling the hydrocortisone dose was allowed for 7 days maximally (+10% of the cumulative study dose). Compliance to hydrocortisone tablet intake was verified as described 17. Sixty‐three patients were initially randomized of whom 47 completed the study. Reasons for withdrawal were described elsewhere 17. All these 47 subjects were included in the present analysis. Twenty‐two patients received the lower hydrocortisone dose first, whereas 25 participants were first given the higher hydrocortisone dose. Detailed clinical characteristics of the participants, including medical history, cause of secondary adrenal insufficiency and other hormone replacement therapies, have been provided elsewhere 17.
Venous blood was obtained after an overnight fast at 8 a.m.; that is, 1 h after the morning hydrocortisone dose was taken. Twenty‐four‐hour urine collections were obtained 1 day before the scheduled visits at the end of the lower and higher hydrocortisone periods. Body mass index was calculated as body weight (in kg) divided by length (in m) squared.
Laboratory analyses
Plasma and serum samples were prepared by centrifugation at 4 °C. The samples were then stored at −80 °C until analysis.
Total bilirubin was measured in heparinized plasma by a colorimetric assay (Roche, Mannheim, Germany). The detection limit was 1·7 μM. The intra‐assay coefficient of variation (CV) was < 2% at the lower normal range. In healthy subjects, bilirubin is most abundantly present in plasma in its unconjugated form 19. In a validation experiment (n = 80), a strong correlation between total bilirubin and unconjugated bilirubin (Spearman's r = 0·92, P < 0·001), as well as between total bilirubin and conjugated direct bilirubin (Spearman's r = 0·82, P < 0·001) was observed. For this study, we only used total bilirubin in agreement with other reports 5, 6, 8, 9, 10.
Plasma alkaline phosphatase (ALP), alanine transferase (ALT), aspartate aminotransferase (AST) and gamma‐glutamyltransferase (GGT) were routinely measured on a Roche Modular platform. All tests were performed according to the International Federation of Clinical Chemistry recommendations. ALT and AST were measured with pyridoxal phosphate activation. The intra‐assay coefficients of variation (CVs) were < 2·5%.
Serum cortisol was measured by electrochemiluminescence immunoassay (Roche Modular Systems, Mannheim, Germany; intra‐assay CV < 2%). Urinary free cortisol was analysed by automated online solid phase extraction in combination with liquid chromatography tandem–mass spectrometry (XLC‐MS/MS) (intra‐assay CV < 2·5%).
Statistical analysis
Sample size calculation and information regarding the random allocation sequence and its implementation have been provided elsewhere 17. Data are presented as mean ± SD or as median (interquartile range). Because of skewed distribution total bilirubin, ALP, ALT, AST, GGT and urinary free cortisol excretion were natural log‐transformed to achieve approximately normal distributions. Changes are presented as mean (95% confidence intervals) for parametrically distributed variables and as median (interquartile range) for nonparametrically distributed variables. The effect of higher vs. lower hydrocortisone dose on the various variables was compared by Student's t‐tests. The univariate relationships of changes in bilirubin with continuous and dichotomous baseline variables were tested using Pearson's correlation coefficients and unpaired Student's t‐tests, respectively. To check for period and treatment by period interaction effects (carry‐over effect), the procedure developed by Altman was used 20.
Results
Twenty‐nine men and 18 women completed the study. At entry, mean age at entry was 52 ± 14 years, and mean body mass index was 27·0 ± 4·0 kg/m2. Thyroid hormone was diagnosed in 92% of patients and replaced in all; growth hormone deficiency was diagnosed in 66% and replaced in 68%; testosterone deficiency was diagnosed 79% of men and replaced in all; eight women were premenopausal in whom estradiol deficiency was diagnosed in 50% and treated in all (postmenopausal estradiol deficiency not replaced); and diabetes insipidus was diagnosed in 19% and replaced in all. Baseline clinical characteristics were provided elsewhere 17. The various hormonal substitution regimens were left unchanged during the 4‐week run‐in period, as well as during the 10‐week lower and higher hydrocortisone replacement periods. A doubling of the hydrocortisone dose as stress‐related dose adjustment was reported 159 times on the low dose (1·6% of the total dose administrations) and 146 times on the high dose (1·5% of the total dose administrations).
Doubling of the hydrocortisone dose elicited an increase in serum cortisol, as well as in urinary free cortisol excretion (Table 1). Body mass index was slightly increased with the higher hydrocortisone dose, but plasma glucose was unchanged (Table 1). On average, total bilirubin was increased by 10% during the higher dose of hydrocortisone, whereas serum ALP and AST activity were slightly decreased (Table 1; Fig. 1). ALT and GGT activity did not significantly change. In univariate analysis, the increase in total bilirubin was inversely related to age (r = −0·298, P = 0·042), but was related neither to sex, BMI, the use of thyroid hormone, the use of sex steroids, growth hormone deficiency, diabetes insipidus and plasma glucose (P > 0·29 for all), nor to the sequence of the lower and higher hydrocortisone doses (P = 0·35). No period or treatment by period interaction effects was found for all the outcome measures (all P > 0·05).
Table 1.
Body mass index, estimates of cortisol metabolism, plasma glucose, plasma total bilirubin, alkaline phosphatase activity, transaminase activities and γ‐glutamyltransferase (GGT) activity during randomized periods of lower and higher hydrocortisone replacement doses in 47 hypopituitary patients with secondary adrenal insufficiency
| Lower dose | Higher dose | Change | P‐value | |
|---|---|---|---|---|
| Hydrocortisone dose (mg/day) | 17·93 ± 2·23 | 35·85 ± 4·46 | 17·93 (17·29–18·56) | < 0·001 |
| Body mass index (kg/m2) | 27·0 ± 4·0 | 27·2 ± 4·1 | 0·2 (0·0–0·3) | 0·026 |
| Serum cortisol (1 h after intake; nM) | 643 ± 241 | 918 ± 245 | 275 (206–345) | < 0·001 |
| Urinary free cortisol (nmol/24 h) | 78 (46–111) | 274 (199–408) | 207 (131–314) | < 0·001 |
| Fasting plasma glucose (mM) | 5·24 ± 0·9 | 5·21 ± 0·8 | −0·03 (−0·18–0·12) | 0·70 |
| Plasma total bilirubin (μM) | 7 (5–12) | 8 (6–11) | 1 (−1–2) | 0·033 |
| Plasma ALP activity (U/L) | 60 (48–73) | 55 (47–73) | −2 (−9–2) | 0·006 |
| Plasma AST activity (U/L) | 26 (22–31) | 22 (20–28) | −3 (−7–0) | 0·001 |
| Plasma ALT activity (U/L) | 21 (16–33) | 19 (15–29) | −1 (−7–3) | 0·085 |
| Plasma GGT activity (U/L) | 23 (16–33) | 23 (18–34) | 1 (−2–4) | 0·30 |
Data in mean ± SD or median (interquartile range). Changes are given in mean with 95% confidence intervals (CI) for parametrically distributed data or as median (interquartile range) for nonparametrically distributed data. ALP, alkaline phosphatase activity; ALT, alanine transferase activity; AST, aspartate aminotransferase activity; GGT, γ‐glutamyltransferase activity.
Figure 1.
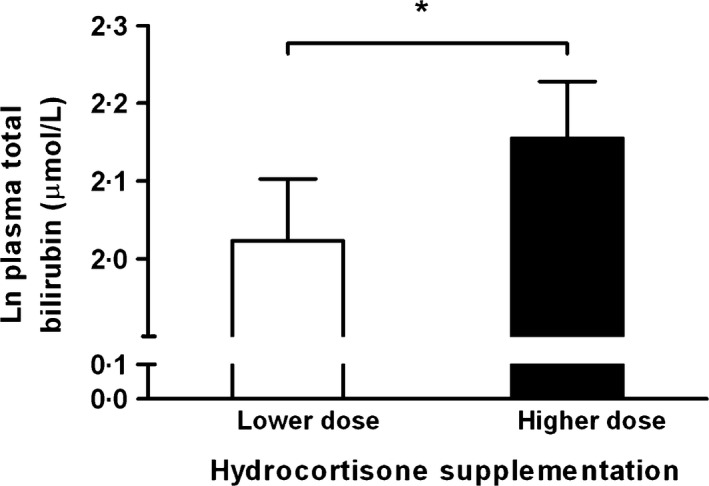
Plasma total bilirubin in response to higher vs. lower hydrocortisone replacement doses. Natural log‐transformed (Ln) values of plasma bilirubin are shown. Bars indicate mean ± SE values. *P = 0·033.
Discussion
This randomized double‐blind cross‐over study demonstrates to our knowledge for the first time that a higher hydrocortisone replacement regimen increases circulating bilirubin levels in hypopituitary subjects with secondary adrenal insufficiency. This effect appeared to be less outspoken in older individuals, but was unrelated to sex, obesity, fasting glucose and (replacement for) other hormonal deficiencies. Moreover, we could rule out confounding due to carry‐over effects of the higher and the lower hydrocortisone replacement doses. The present findings are, therefore, consistent with the notion that variations in glucocorticoid exposure affect bilirubin metabolism in humans.
In view of the supposition that bilirubin levels reflect overall status of HO activity 21, our results consent with the hypothesis that glucocorticoids stimulate HO expression in humans. While this possibility agrees with previous results in rodent models 13, 14, 15, it is obvious that the current findings do not allow to discern between differential effects of hydrocortisone on specific HO isoforms nor in specific tissues. Furthermore, it should be noted that bilirubin levels are also determined by biliary elimination via uridine diphosphate glucuronosyltransferase 1A1, which conjugates bilirubin and enables bilirubin elimination 3. This enzyme is induced by glucocorticoids in HepG2 cells 22, 23. Although an opposing contribution to the glucocorticoid increasing effects on circulating bilirubin via uridine diphosphate glucuronosyltransferase 1A1 stimulation cannot be excluded, short‐time glucocorticoid administration does not affect biliary bilirubin output in humans 24.
Doubling the hydrocortisone replacement dose expectedly increased serum cortisol and urinary free cortisol excretion, and may have resulted in mild hypercortisolism in some individuals 25. The increase in serum bilirubin in response to the higher hydrocortisone replacement regimen averaged 10%. While this previously unreported bilirubin response may have pathophysiological relevance, its clinical significance is uncertain. Based on large prospective cohort studies and meta‐analysis, the risk of incident cardiovascular disease has been estimated to be about 18% lower per doubling of the bilirubin level 9, 10. In this context, the impact of the observed bilirubin increase on cardiovascular risk is likely to be small. Of note, we also documented a drop in ALP activity in response to a higher hydrocortisone replacement dose. As ALP is regarded as an inflammatory biomarker 26, 27, this finding may point to less chronic low‐grade inflammation consequent to modestly higher glucocorticoid exposure 28. However, a limitation of our study is that we did not measure other inflammation markers such as high‐sensitivity C‐reactive protein.
It is pertinent that overt hypercortisolism coincides with hepatic fat accumulation 29, 30. However, plasma AST activity was also modestly decreased after the higher hydrocortisone replacement dose. Considering that this enzyme may provide a biomarker of liver fat accumulation 31, 32, this finding would be consistent with some beneficial effect on nonalcoholic fatty liver disease (NAFLD) development, putatively consequent to higher bilirubin levels 33. In addition, it seems possible that the increasing effect of a higher hydrocortisone dose on plasma bilirubin could provide a potentially relevant additional mechanism which contributes to the anti‐inflammatory role commonly ascribed to glucocorticoids 28.
Several other methodological issues and limitations of our study need to be discussed. First, our trial was performed in hypopituitary patients with established secondary adrenal insufficiency to rule out confounding due to coexisting mineralocorticoid deficiency – in particular on cognitive parameters – as much as possible. Consequently, extrapolation to other patient categories is limited. Second, our study was not designed to establish glucocorticoid dose‐ and time‐dependent effects on circulating bilirubin. However, the present comparison of a lower and higher hydrocortisone replacement regimen puts the changes in bilirubin in a physiological perspective as much as possible. Additionally, it seems plausible that 10‐week administration periods are sufficient to establish a steady state of altered glucocorticoid exposure to various metabolic processes. We believe the same holds true for a 4‐week run‐in period in particular given the fact that the total daily hydrocortisone dose during the run‐in period was lower than that during the higher hydrocortisone dose replacement period. Third, all participants received adequate replacement treatment for other pituitary hormone deficiencies if deemed clinically necessary. Replacement doses with thyroid hormone, growth hormone, sex steroids and desmopressin were left unchanged during the study. This approach was chosen to limit coexisting effects of other hormonal factors on bilirubin metabolism 34, 35.
In conclusion, circulating bilirubin is modestly increased in response to higher glucocorticoid exposure. Our findings support the hypothesis that glucocorticoids contribute to bilirubin metabolism in humans.
Conflict of interest statement
The study is investigator‐driven, and the authors do not have any financial or other conflict of interests to declare.
Contributors
Werumeus Buning, van Beek and Dullaart had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The manuscript was completely drafted by Werumeus Buning, van Beek and Dullaart. Brummelman, Wolffenbuttel, van Beek and Dullaart conceptualized and designed the study. Werumeus Buning, Kootstra‐Ros, Brummelman, van den Berg, van der Klauw, Wolffenbuttel, van Beek and Dullaart collected, analysed and interpreted the data. Werumeus Buning, van Beek and Dullaart drafted the manuscript. Werumeus Buning, Koostra‐Ros, Brummelman, van den Berg, van der Klauw, Wolffenbuttel, van Beek and Dullaart critical revised the manuscript for important intellectual content. Werumeus Buning and Dullaart statistically analysed the data. Van Beek supervised the study.
Address
Department of Endocrinology, University of Groningen, University Medical Center Groningen, P.O. Box 30.001, Groningen, 9700 RB, The Netherlands (J. Werumeus Buning, P. Brummelman, P. Brummelman, G. van den Berg, M. van der Klauw, B. H. R. Wolffenbuttel, A. P. van Beek, R. P.F. Dullaart); Laboratory Center, University of Groningen, University Medical Center Groningen, P.O. Box 30.001, Groningen, 9700 RB, The Netherlands (J. E. Kootstra‐Ros).
Supporting information
Figure S1. Eligibility, inclusion and follow‐up of the participants.
Acknowledgements
The expert technical assistance of Dr. A. Muller Kobold, PhD, Dr. W. de Jong, PhD and Prof. I Kema, PhD, Laboratory Center, University Medical Center Groningen, the Netherlands, in measurement of cortisol and glucocorticoid metabolites is greatly appreciated. The Pharmacy Department, University Medical Center Groningen, the Netherlands, performed the randomization procedure and provided study medication.
Eur J Clin Invest 2016; 46 (5): 475–480
References
- 1. Neuzil J, Stocker R. Free and albumin‐bound bilirubin are efficient co‐antioxidants for alpha‐tocopherol, inhibiting plasma and low density lipoprotein lipid peroxidation. J Biol Chem 1994;269:16712–9. [PubMed] [Google Scholar]
- 2. Mazzone GL, Rigato I, Ostrow JD, Bossi F, Bortoluzzi A, Sukowati CH et al Bilirubin inhibits the TNFalpha‐related induction of three endothelial adhesion molecules. Biochem Biophys Res Commun 2009;386:338–44. [DOI] [PubMed] [Google Scholar]
- 3. Wagner KH, Wallner M, Mölzer C, Gazzin S, Bulmer AC, Tiribelli C et al Looking to the horizon: the role of bilirubin in the development and prevention of age‐related chronic diseases. Clin Sci (Lond) 2015;129:1–25. [DOI] [PubMed] [Google Scholar]
- 4. Hwang HJ, Lee SW, Kim SH. Relationship between bilirubin and C‐reactive protein. Clin Chem Lab Med 2011;49:1823–8. [DOI] [PubMed] [Google Scholar]
- 5. Deetman PE, Bakker SJ, Dullaart RPF. High sensitive C‐reactive protein and serum amyloid A are inversely related to serum bilirubin: effect‐modification by metabolic syndrome. Cardiovasc Diabetol 2013;12:166. doi:10.1186/1475‐2840‐12‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dullaart RPF, Gruppen EG, Connelly MA, Lefrandt JD. A pro‐inflammatory glycoprotein biomarker is associated with lower bilirubin in metabolic syndrome. Clin Biochem 2015;48:1045–7. [DOI] [PubMed] [Google Scholar]
- 7. Vítek L, Novotný L, Sperl M, Holaj R, Spácil J. The inverse association of elevated serum bilirubin levels with subclinical carotid atherosclerosis. Cerebrovasc Dis 2006;21:408–14. [DOI] [PubMed] [Google Scholar]
- 8. Dullaart RPF, Kappelle PJ, de Vries R. Lower carotid intima media thickness is predicted by higher serum bilirubin in both non‐diabetic and Type 2 diabetic subjects. Clin Chim Acta 2012;414:161–5. [DOI] [PubMed] [Google Scholar]
- 9. Horsfall LJ, Nazareth I, Petersen I. Cardiovascular events as a function of serum bilirubin levels in a large, statin‐treated cohort. Circulation 2012;126:2556–64. [DOI] [PubMed] [Google Scholar]
- 10. Kunutsor SK, Bakker SJ, Gansevoort RT, Chowdhury R, Dullaart RPF. Circulating total bilirubin and risk of incident cardiovascular disease in the general population. Arterioscler Thromb Vasc Biol 2015;35:716–24. [DOI] [PubMed] [Google Scholar]
- 11. Muñoz‐Sánchez J, Chánez‐Cárdenas ME. A review on hemeoxygenase‐2: focus on cellular protection and oxygen response. Oxid Med Cell Longev 2014;604981. doi: 10.1155/2014/604981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abraham NG, Junge JM, Drummond GS. Translational significance of heme oxygenase in obesity and metabolic syndrome. Trends Pharmacol Sci 2015. pii: S0165‐6147(15)00202‐3. doi: 10.1016/j.tips.2015.09.003. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maines MD, Eke BC, Zhao X. Corticosterone promotes increased heme oxygenase‐2 protein and transcript expression in the newborn rat brain. Brain Res 1996;722:83–94. [DOI] [PubMed] [Google Scholar]
- 14. Raju VS, McCoubrey WK Jr, Maines MD. Regulation of heme oxygenase‐2 by glucocorticoids in neonatal rat brain: characterization of a functional glucocorticoid response element. Biochim Biophys Acta 1997;1351:89–104. [DOI] [PubMed] [Google Scholar]
- 15. Liu N, Wang X, McCoubrey WK, Maines MD. Developmentally regulated expression of two transcripts for heme oxygenase‐2 with a first exon unique to rat testis: control by corticosterone of the oxygenase protein expression. Gene 2000;241:175–83. [DOI] [PubMed] [Google Scholar]
- 16. Lavrovsky Y, Drummond GS, Abraham NG. Downregulation of the human heme oxygenase gene by glucocorticoids and identification of 56b regulatory elements. Biochem Biophys Res Commun 1996;218:759–65. [DOI] [PubMed] [Google Scholar]
- 17. Werumeus Buning J, Brummelman P, Koerts J, Dullaart RPF, van den Berg G, van der Klauw MM et al The effects of two different doses of hydrocortisone on cognition in patients with secondary adrenal insufficiency–results from a randomized controlled trial. Psychoneuroendocrinology 2015;55:36–47. [DOI] [PubMed] [Google Scholar]
- 18. Simera I, Moher D, Hoey J, Schulz KF, Altman DGA. A catalogue of reporting guidelines for health research. Eur J Clin Invest 2010;40:14–22. [DOI] [PubMed] [Google Scholar]
- 19. Tisdale WA, Klatskin G, Kinsella ED. The significance of the direct‐reacting fraction of serum bilirubin in hemolytic jaundice. Am J Med 1959;26:214–27. [DOI] [PubMed] [Google Scholar]
- 20. Altman DG. Practical Statistics for Medical Research. London: Chapman and Hall, CRC Press; 1991. [Google Scholar]
- 21. McCarty MF. Serum bilirubin may serve as a marker for increased heme oxygenase activity and inducibility in tissues ‐ A rationale for the versatile health protection associated with elevated plasma bilirubin. Med Hypotheses 2013;81:607–10. [DOI] [PubMed] [Google Scholar]
- 22. Kanou M, Usui T, Ueyama H, Sato H, Ohkubo I, Mizutani T. Stimulation of transcriptional expression of human UDP‐glucuronosyltransferase 1A1 by dexamethasone. Mol Biol Rep 2004;31:151–8. [DOI] [PubMed] [Google Scholar]
- 23. Usui T, Kuno T, Mizutani T. Induction of human UDP‐glucuronosyltransferase 1A1 by cortisol‐GR. Mol Biol Rep 2006;33:91–6. [DOI] [PubMed] [Google Scholar]
- 24. Shay H, Sun DC. Possible effect of hydrocortisone on bilirubin excretion by the liver. N Engl J Med 1957;257:62–5. [DOI] [PubMed] [Google Scholar]
- 25. Kraan GP, Dullaart RP, Pratt JJ, Wolthers BG, Drayer NM, De Bruin R. The daily cortisol production reinvestigated in healthy men. The serum and urinary cortisol production rates are not significantly different. J Clin Endocrinol Metab 1998;83:1247–52. [DOI] [PubMed] [Google Scholar]
- 26. Webber M, Krishnan A, Thomas NG, Cheung BM. Association between serum alkaline phosphatase and C‐reactive protein in the United States National Health and Nutrition Examination Survey 2005‐2006. Clin Chem Lab Med 2010;48:167–73. [DOI] [PubMed] [Google Scholar]
- 27. Kunutsor SK, Bakker SJ, Kootstra‐Ros JE, Gansevoort RT, Gregson J, Dullaart RPF. Serum alkaline phosphatase and risk of incident cardiovascular disease: interrelationship with high sensitivity C‐reactive protein. PLoS One 2015;7:e0132822. doi: 10.1371/journal.pone.0132822. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. De Bosscher K, Beck IM, Ratman D, Berghe WV, Libert C. Activation of the glucocorticoid receptor in acute inflammation: the SEDIGRAM concept. Trends Pharmacol Sci 2016;37:4–16. [DOI] [PubMed] [Google Scholar]
- 29. Rockall AG, Sohaib SA, Evans D, Kaltsas G, Isidori AM, Monson JP et al Hepatic steatosis in Cushing's syndrome: a radiological assessment using computed tomography. Eur J Endocrinol 2003;149:543–8. [DOI] [PubMed] [Google Scholar]
- 30. Woods CP, Hazlehurst JM, Tomlinson JW. Glucocorticoids and non‐alcoholic fatty liver disease. J Steroid Biochem Mol Biol 2015;154:94–103. [DOI] [PubMed] [Google Scholar]
- 31. Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol 2003;98:960–7. [DOI] [PubMed] [Google Scholar]
- 32. Yu AS, Keeffe EB. Elevated AST or ALT to nonalcoholic fatty liver disease: accurate predictor of disease prevalence? Am J Gastroenterol 2003;98:955–6. [DOI] [PubMed] [Google Scholar]
- 33. Chang Y, Ryu S, Zhang Y, Son HJ, Kim JY, Cho J et al A cohort study of serum bilirubin levels and incident non‐alcoholic fatty liver disease in middle aged Korean workers. PLoS One 2012;7:e37241. doi:10.1371/journal.pone.0037241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guéraud F, Daveloose D, Vezin H, Viret J, Paris A. In vivo modification of the UDP‐glucuronosyltransferase functional state in rat liver following hypophysectomy and partial or complete hormonal restoration. J Biochem 2003;134:641–53. [DOI] [PubMed] [Google Scholar]
- 35. Deetman PE, Bakker SJ, Kwakernaak AJ, Navis G, Dullaart RPF, PREVEND Study Group . The relationship of the anti‐oxidant bilirubin with free thyroxine is modified by insulin resistance in euthyroid subjects. PLoS One 2014;3:e90886. doi: 10.1371/journal.pone.0090886. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Eligibility, inclusion and follow‐up of the participants.


