Abstract
Purpose
In order to identify challenges in pediatric pharmacoepidemiological safety studies, we assessed the characteristics of such (published) studies.
Methods
Relevant articles from inception to 2013 were retrieved from Embase and Medline. We sequentially screened titles, abstracts and full texts with independent validation. We systematically collected data regarding general information, study methods and results.
Results
Out of 4825 unique articles, 268 full texts (5.6%) were retained; 147 (54.9%) pertained to drugs rather than vaccines. Considering the 268 studies, 202 (75.4%) concerned children and adolescents (2 to 11 years) and 14 (5.3%) included preterm newborns. Most studies originated from North America (154 [57.5%]) or Europe (92 [34.3%]). Only 47 studies (17.5%) were privately funded. The majority (174 [64.9%]) were cohort studies. Out of 268 studies, 196 (73.1%) collected data retrospectively; paper medical charts were the most common data source for the exposures (85 [31.7%]) and outcomes (122 [45.5%]). Only 3 (2.0%) drug‐only studies investigated rarely used drugs. Considering all 268 studies, only 27 (10.1%) reported sample size or power calculation. Most (75 [51.0%]) drug‐only studies corrected confounding by multivariate modeling unlike stratification in 66 (55.9%) vaccine‐only studies. Considering 75 child‐only studies without any statistically significant result, 41 (54.7%) did not discuss lack of power.
Conclusions
Although the field of pediatric pharmacoepidemiology is steadily developing evaluation seldom includes neonates, is mainly focused on few drug classes and safety outcomes and concerns mainly drug use in developed countries. Small study size is a specific challenge in pediatrics. Reporting should be improved. © 2016 The Authors. Pharmacoepidemiology and Drug Safety Published by John Wiley & Sons Ltd.
Keywords: systematic review, pediatrics, ‘adverse drug reaction’, pharmacoepidemiology
Introduction
Legislation has been introduced to stimulate the conduct of clinical trials in children,1, 2, 3 leading to more evidence on efficacy of new drugs or new formulations of existing drugs in children.4 This laudable action has greatly improved the evidence for new drugs but does not impact much on the available safety data because information on rare and potentially more serious safety issues cannot be obtained from randomized clinical trials (RCTs).5, 6
Safety data can be generated more efficiently from postmarketing observational studies,7, 8 particularly relevant in children among whom the use of drugs is high and frequently off‐label but recorded in routine care records.9 The availability of large scale healthcare and claims databases provides an outstanding opportunity to perform safety studies. However, because the studies are observational, their design requires extra attention to avoid misclassification and address potential confounding. Although the field of pharmacoepidemiology has grown substantially in the last 20 years, very few researchers focus on pediatrics.
As part of the Global Research in Paediatrics—Network of Excellence (http://www.grip‐network.org/), we conducted a systematic review of the medical literature in order to assess the characteristics of pharmacoepidemiological studies evaluating the safety of drugs in children.
Methods
Search strategy
We conducted this review according to PRISMA guidelines.10 We identified relevant articles by systematically searching EMBASE.COM and MEDLINE (via OvidSP) from inception to 29 November 2013. We used the following abbreviated search strategy: “children” AND “pharmacoepidemiology” AND “comparative studies”. Details of the full search strategy are included in Appendix 1. The computer‐based searches were conducted by a biomedical information specialist (WB), and were limited to human research without language limitations. One reviewer (OO) manually searched the bibliographies of relevant articles for additional relevant studies.
Study selection
All observational studies with the main objective to quantify the association between a drug exposure(s) and the occurrence of adverse drug reaction(s) in children and adolescents (≤18 years of age) were eligible for inclusion in the review. Studies that included both children and adults were also retained. Drug exposures concerned all medicinal products including vaccines, applied either systemically or locally, and adverse drug reactions (ADRs) concerned all clinical events described as adverse outcomes to an individual (or combination of) drug(s) and/or vaccine(s).
We excluded RCTs and observational studies that evaluated drug safety signal detection in spontaneous reporting systems, compliance rates to medicinal treatments, incidence or prevalence of ADRs or other diseases within a defined population, teratogenic effects of drug exposure in pregnancy or through breast milk, medication errors, accidental and intentional poisoning, drug abuse, management of ADRs or other diseases, pharmacogenomics, pharmacoeconomics, health services utilization, environmental exposures or herbal treatments. We excluded case series, case reports, abstracts, letters, duplicate studies, preliminary publications or reviews. Only studies published in English were retained for the analysis.
All titles and abstracts were initially screened by one reviewer (OO) and full texts of potentially relevant articles were retrieved. A second reviewer (FK), blinded to the initial assessment, independently screened a sample of abstracts that comprised all abstracts retained plus a random selection of abstracts rejected by the first reviewer. Any disagreements between the two reviewers were examined by a third reviewer (G'tJ). Full texts retained through this process were independently screened by two reviewers (OO and JD), disagreements were examined by a third reviewer (CF).
Data collection
We developed a standardized form that was tested on 10 randomly selected papers, and was modified accordingly.
Data collected from each study pertained to journal impact factor (measured in 2013), study design, study period, type of data, study population, exposure, outcome, statistical analysis and results. We used country of corresponding author as a proxy for study setting. In the absence of information regarding study design, designs were classified based on data reviewers' judgment. Case control studies included those studies that applied the nested case control design. Type of data implied primary versus secondary data (i.e. ‘large’ datasets like ‘primary care (prescription) data’, ‘outpatient (pharmacy) dispensing data’ and ‘claims data’). The age of the study population was categorized according to guidelines defined by the International Conference on Harmonization (ICH)11: newborns (0–27 days), infants and toddlers (28 days–23 months), children (2–11 years) and adolescents (12–18 years). We used the term drug to refer to small molecules as opposed to vaccines. Exposures and outcomes were classified as rare based on authors' definitions. For the sources of exposure data, inpatient dispensing data included electronic prescription data for hospitalized patients, medical charts at the clinic implied paper charts, outpatient dispensing data implied pharmacy dispensing records, and registry included those that recorded information on vaccination and drug use. To assess whether follow‐up was long enough to observe the outcomes of interest in cohort studies, we applied the following minimum time intervals from drug exposure: fever—1 day, other acute events—2 weeks, cancer and other chronic (i.e. neurological and psychiatric) events—5 years. The full data extraction form is given in Appendix 2.
Both drugs and vaccines were mapped to the World Health Organization‐Anatomical Therapeutic Chemical (WHO‐ATC) classification (second or fifth level codes). The outcomes were mapped to the main divisions of the International Classification of Diseases (ICD), ninth edition.
Two reviewers (OO and JD) independently collected data from all full text articles. Discrepancies were discussed with three senior reviewers (FK, DW and CF).
In order to check for the impact of the Best Pharmaceuticals for Children Act (BPCA) which was introduced in the US in 2002,3 we compared the number of pediatric studies published before and after its introduction. We compared pediatric studies to all the published studies (i.e. pertaining to the general population).
Data analysis
All continuous variables were described using medians (first [Q1]–third [Q3] quartiles) and categorical variables were summarized using counts and percentages. We performed hypothesis testing using the Mann–Whitney U test for continuous variables and Pearson chi‐square, Fischer's exact test or Z test for categorical variables. Analysis was performed by utilizing Statistical Package for the Social Sciences (SPSS) version 21.
Results
The search strategy yielded 4825 unique records after de‐duplication (Figure 1). After screening titles and abstracts, we retained 301 articles (inter‐reviewer concordance 90%) and after full text review, we retained 268 for analysis (inter‐reviewer concordance 92%).
Figure 1.
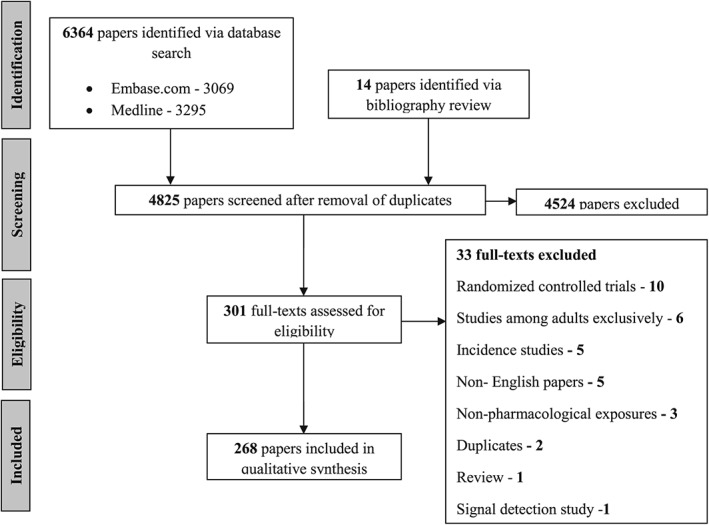
Flow chart depicting the selection of relevant papers
General characteristics of the studies
The 268 retained studies were published from 1979 to 2013. In Figure 2, we compare the 268 studies to the total number (30,098) of studies (pertaining to the general population) published during the same period. After 2002, the number of pediatric studies steadily increased, more studies (196 [73.1%]) were published during the 10‐year period from 2003 to 2013, compared to studies (72 [26.9%]) published during the preceding 24‐year period.
Figure 2.

Number of pharmacoepidemiological safety studies in children. Note: In order to retrieve all published pharmacoepidemiological safety studies that investigated the general population, we applied the same search algorithm that was utilized for studies in children except that for the former, we did not limit to the pediatric population; papers that were published in 2013 are those papers that were indexed in Embase and Medline as at 29 November
Most studies originated from North America (154 [57.5%]) or Europe (92 [34.3%]) and most studies (147 [54.9%]) assessed only drugs. Only three studies (1.1%) evaluated both drugs and vaccines, the studies investigated only children for the effect of the following drug classes (WHO‐ATC second level): ‘corticosteroids for systemic use’, ‘ántibacterials for systemic use’, ‘cough and cold preparations’ and ‘anti‐inflammatory and anti‐rheumatic products’. The investigated vaccines were ‘diphtheria–tetanus–pertussis’, ‘measles–mumps–rubella’, ‘hepatitis B virus’, ‘óral polio virus’ and ‘inactivated polio virus’.
Considering 268 studies, 183 (68.3%) included only children, the remainder studied both children and adults. Studies on drug safety evaluation included most frequently children aged 2–11 years while vaccine safety studies were most frequently conducted in infants and toddlers (Table 1). Only 14 studies (5.0%) included preterm newborns.
Table 1.
General characteristics for 268 pharmacoepidemiological studies that evaluated the safety of drugs and vaccines in children (≤18 years)
| Total (268) | Type of exposure investigated | |||
|---|---|---|---|---|
| Only drugs (147) | Only vaccines (118) | |||
| Number(%) or median(Q1–Q3) | Number(%) or median(Q1–Q3) | Number(%) or median(Q1–Q3) | p‐Value | |
| Continent of the corresponding author | 0.90 | |||
| North America | 154 (57.5) | 83 (56.5) | 70 (59.3) | |
| Europe | 92 (34.3) | 52 (35.4) | 39 (33.1) | |
| Asia | 12 (4.5) | 7 (4.8) | 4 (3.4) | |
| Others | 10 (3.7) | 5 (3.4) | 5 (4.2) | |
| Type of journal | 0.07 | |||
| Pediatric specialty | 88 (32.8) | 45 (30.6) | 42 (35.6) | |
| Pharmacology/pharmacoepidemiology | 30 (11.2) | 21 (14.3) | 7 (5.9) | |
| General medical* | 39 (14.6) | 17 (11.6) | 22 (18.6) | |
| Others† | 111 (41.4) | 64 (43.5) | 47 (39.8) | |
| 2013 two‐year journal impact factor | 3.8 (3.1–5.3) | 3.7 (2.6–5.5) | 4.6 (3.6–5.6) | 0.01 |
| Missing data | 18 (6.7) | 14 (9.5) | 4 (3.4) | |
| Funding sources | <0.01 | |||
| Public | 96 (35.8) | 38 (25.9) | 55 (46.6) | |
| Private | 47 (17.5) | 25 (17.0) | 22 (18.6) | |
| Public and private | 26 (9.7) | 14 (9.5) | 12 (10.2) | |
| No funding | 7 (2.6) | 5 (3.4) | 2 (1.7) | |
| Funding not reported | 92 (34.3) | 65 (44.2) | 27 (22.9) | |
| Study period, years | 3.7 (1.7–8.9) | 4.6 (1.7–9.0) | 3.2 (1.7–7.0) | 0.31 |
| Missing data | 14 (5.2) | 11 (4.1) | 3 (1.1) | |
| Study population ‡ | ||||
| Age at inclusion | ||||
| Minimum age, years | 0.1 (0–2.0) | 0.1 (0–3.0) | 0.2 (0–1.0) | 0.51 |
| Maximum age, years | 16.5 (2.0–21.0) | 18.0 (13.5–63.5) | 5.0 (1.5–17.0) | <0.01 |
| Preterm newborns | <0.01 | |||
| Exclusively | 9 (3.3) | 9 (6.1) | 0 | |
| Partially | 5 (1.9) | 4 (2.7) | 1 (0.8) | |
| No | 254 (94.8) | 134 (91.2) | 117 (99.2) | |
| Term newborns (0–27 days) | 106 (39.5) | 61 (41.5) | 44 (37.3) | 0.49 |
| Infants/toddlers (28 days–23 months) | 168 (62.9) | 80 (54.4) | 86 (72.9) | <0.01 |
| Children (2–11 years) | 202 (75.4) | 117 (80.0) | 82 (69.5) | 0.05 |
| Adolescents (12–18 years) | 157 (58.9) | 110 (74.8) | 45 (38.1) | <0.01 |
| Mixed (adults and children) | 85 (31.7) | 67 (45.6) | 18 (15.3) | <0.01 |
| WHO‐ATC level of investigated exposure (reported name) § | <0.01 | |||
| Fifth level (specific compound) | 203 (75.7) | 87 (59.2) | 116 (98.3) | |
| Second,third or fourth level (class) | 51 (19.0) | 51 (34.7) | 0 | |
| Both | 14 (5.2) | 9 (6.1) | 2 (1.7) | |
| Number of specific compounds (WHO‐ATC fifth level) that were investigated ¶ | 0.14 | |||
| 1 | 137 (63.1) | 54 (56.3) | 81 (68.6) | |
| 2 | 35 (16.1) | 20 (20.8) | 15 (12.7) | |
| ≥3 | 45 (20.7) | 22 (22.9) | 22 (18.6) | |
| Number of drug/vaccine classes (WHO‐ATC second, third or fourth level) that were investigated ∥ | 0.27 | |||
| 1 | 48 (73.8) | 45 (75.0) | 1 (50.0) | |
| 2 | 8 (12.3) | 7 (11.7) | 1 (50.0) | |
| ≥3 | 9 (13.8) | 8 (13.3) | 0 | |
Note: Missing data is presented for only instances where it constitutes greater than 5%.
The p‐values in bold format are statistically significant. NA = not applicable.
Studies assessing drugs (147) or vaccines (118) exclusively do not add up to the total number of studies (268) because three studies that investigated both drugs and vaccines are not presented in the table.
Refers to journals that publish wide variety of medical topics (irrespective of specialty).
Journals that do not fit into any of the specified categories i.e. PLOS ONE.
For the age distributions, the proportions do not add up to 100% because some studies included multiple age categories.
World Health Organization‐Anatomic Therapeutic Chemical.
The proportions are based on only those studies that investigated specific compounds either exclusively or in combination with drug/vaccine class.
The proportions are based on only those studies that investigated drug/vaccine class either exclusively or in combination with specific compounds.
The median impact factor of the journals in which the studies were published was 3.8 (3.1–5.3). As seen in Table 1, vaccine‐only studies (4.6 [3.6–5.6]) were published in higher impact journals than drug‐only studies (3.7 [2.6–5.5]) (Mann–Whitney U p‐value 0.01). Few studies were published in pediatric (88 [32.8%]) or pharmacoepidemiological specialty (30 [11.2%]) journals. Only 14 studies (17.5%) were privately funded, vaccine studies were more frequently publicly funded but for a large proportion the type of funding was unknown. Regardless of the type of exposure that was investigated, privately funded studies (journal impact factor = 3.5; 3.1–5.3) were of lower impact than studies receiving public funding (journal impact factor = 5; 3.5–7.8) (Mann–Whitney U p‐value < 0.01).
Methodology of the studies
From the 268 studies, 202 (75.4%) reported the study design(s) and for the remaining study design was classified according to the reviewers' judgment. Cohort studies were the most common (174 [64.9%]), and 23 studies (8.6%) applied more than one design. Case‐only designs were seldom used: the self‐controlled case series (SCCS) design was utilized in only 30 studies (11.2%), to evaluate vaccine‐related outcomes exclusively. Similarly, case‐crossover studies were few (4 [1.5%]).
In most studies (196 [73.1%]), data collection was retrospective. Prospective studies (88 [32.8%]) were usually cohort studies that used mainly primary data (56 [63.6%]) and were smaller than studies with retrospective data. Secondary data was utilized for both drugs and vaccines and concerned 183 studies (68.3%). Studies using secondary data had larger sample sizes than studies using primary data collection. Exposure and outcome data were collected from mainly medical charts ((85 [31.7%]) and (122 [45.5%]) respectively) followed by claims data and primary care medical or dispensing data (Table 2).
Table 2.
Methodology of 268 pharmacoepidemiological studies that evaluated the safety of drugs and vaccines in children (≤18 years)
| Total (268) | Type of exposure investigated | |||
|---|---|---|---|---|
| Only drugs (147) | Only vaccines (118) | |||
| Number(%) or median(Q1–Q3) | Number(%) or median(Q1–Q3) | Number(%) or median(Q1–Q3) | p‐Value | |
| Design * | ||||
| Cohort | 174 (64.9) | 114 (77.6) | 60 (50.8) | <0.01 |
| Case control | 73 (27.2) | 31 (21.1) | 39 (33.1) | 0.03 |
| Self‐controlled case series | 30 (11.2) | 0 | 30 (25.4) | NA |
| Case crossover | 4 (1.5) | 3 (2.0) | 1 (0.8) | 0.42 |
| Others† | 14 (5.2) | 6 (4.1) | 8 (6.8) | 0.33 |
| Mode of data collection ‡ | ||||
| Retrospective | 177 (66.0) | 96 (65.3) | 80 (67.8) | 0.66 |
| Prospective | 69 (25.7) | 41 (27.9) | 26 (22.0) | 0.27 |
| Both | 19 (7.1) | 10 (6.8) | 9 (7.6) | 0.80 |
| Unclear§ | 3 (1.1) | 0 | 3 (2.5) | NA |
| Type of data | 0.11 | |||
| Primary | 58 (21.6) | 38 (25.9) | 18 (15.3) | |
| Secondary | 183 (68.3) | 95 (64.6) | 87 (73.7) | |
| Mixed | 27 (10.1) | 14 (9.5) | 13 (11.0) | |
| Source of (collection method for) exposure data ¶ | ||||
| Primary care (prescription) data | 27 (10.1) | 14 (9.5) | 12 (10.2) | 0.85 |
| Outpatient (pharmacy) dispensing data | 19 (7.1) | 7 (4.8) | 12 (10.2) | 0.09 |
| Inpatient dispensing data | 21 (7.8) | 18 (12.2) | 2 (1.7) | <0.01 |
| Paper medical chart | 85 (31.7) | 54 (36.7) | 29 (24.6) | 0.03 |
| Claims data | 55 (20.5) | 25 (17.0) | 30 (25.4) | 0.09 |
| Registry | 35 (13.1) | 16 (10.9) | 19 (16.1) | 0.21 |
| Self‐report questionnaire | 12 (4.5) | 7 (4.8) | 5 (4.2) | 0.82 |
| Telephone call | 13 (4.9) | 5 (3.4) | 8 (6.8) | 0.20 |
| Face to face interview | 17 (6.3) | 6 (4.1) | 10 (8.5) | 0.14 |
| Others∥ | 40 (14.9) | 15 (10.2) | 25 (21.2) | 0.01 |
| Unclear** | 13 (4.9) | 7 (4.8) | 6 (5.1) | 0.91 |
| Source of (collection method for) outcome data †† | ||||
| Primary care data | 29 (10.8) | 14 (9.5) | 15 (12.7) | 0.41 |
| Paper medical charts | 122 (45.5) | 66 (44.9) | 53 (44.9) | 1.00 |
| Institution, administrative or electronic heath records | 60 (22.4) | 23 (15.6) | 37 (31.4) | <0.01 |
| Claims data | 71 (26.5) | 32 (21.8) | 38 (32.2) | 0.06 |
| Registry | 38 (14.2) | 21 (14.3) | 17 (14.4) | 0.98 |
| Self‐report questionnaire | 25 (9.3) | 13 (8.8) | 12 (10.2) | 0.70 |
| Telephone call | 13 (4.9) | 8 (5.4) | 5 (4.2) | 0.65 |
| Face to face interview | 12 (4.5) | 6 (4.1) | 6 (5.1) | 0.70 |
| Others‡‡ | 32 (11.9) | 18 (12.2) | 14 (11.9) | 0.94 |
| Unclear§§ | 4 (1.5%) | 3 (2.0) | 1 (0.8) | 0.42 |
| Size of the study population per design | ||||
| Fixed cohort | ||||
| Exposed, number of participants | 2050 (103–34 544) | 283 (51–12 432) | 44 001 (4009–278 624) | <0.01 |
| Unexposed, number of participants | 1073 (74–27 417) | 372 (58–8533) | 24 175 (1215–227 288) | 0.67 |
| Missing data | 18 (32.2) | 6 (6.2) | 12 (30.8) | |
| Dynamic cohort [person‐years(PY)] | ||||
| Exposed PY | 92 835 (11 931–731 043) | 62 383 (3600–416 018) | 123 287 (14 708–1 220 006) | 0.56 |
| Unexposed PY | 362 142 (9235–1 315 038) | 162 622 (5485–1 728 969) | 535 375 (17 496–1 298 601) | 0.90 |
| Case–control | ||||
| Cases | 189 (68–467) | 79 (30–532) | 252 (133–452) | 0.03 |
| Number of controls per case | 2.2 (1.1–4.2) | 2.1 (1.0–4.4) | 2.8 (2.0–4.1) | 0.24 |
| SCCS and Case Crossover, number of participants | 402 (168–1380) | NA | 369 (173–1334) | NA |
| Missing data | 5 (14.7) | 0 | 5 (16.1) | |
| Control of confounding ¶¶ | ||||
| Matching | 98 (36.6) | 43 (29.3) | 53 (44.9) | <0.01 |
| Stratification | 103 (38.4) | 36 (24.5) | 66 (55.9) | <0.01 |
| Multivariate modeling adjustment | 138 (51.5) | 75 (51.0) | 60 (50.8) | 0.97 |
Note: Missing data is presented for only instances where it constitutes greater than 5%.
The p‐values in bold format are statistically significant. NA = not applicable.
Studies assessing drugs (147) or vaccines (118) exclusively, do not add up to the total number of studies (268) because three studies that investigated both drugs and vaccines are not presented in the table.
The proportions do not add up to 100% because some studies applied multiple designs.
Includes study designs that are not listed, i.e. case‐time‐control.
The proportions do not add up to 100% because some studies applied multiple data collection modes.
Implies that there was inadequate information to determine if data collection was done prospectively or retrospectively.
The proportions do not add up to 100% because some studies applied multiple sources (or collection modes for) exposure data.
Includes data sources (or collection methods) that are not specified e.g. maternal and child health handbook.
Implies that there was inadequate information to determine the source of (or collection mode for) the exposure data.
The proportions do not add up to 100% because some studies applied multiple sources (or collection modes for) outcome data.
Includes data sources (or collection methods) that are not specified, e.g. maternal and child health handbook.
Implies that there was inadequate information to determine the source of (or collection mode for) the outcome data.
The proportions do not add up to 100% because some studies applied methods to control confounding.
Out of 147 studies that evaluated drugs exclusively, 87 (59.2%) assessed only exposures to specific compounds (i.e. amoxicillin), 51 (34.7%) evaluated exposures to a specific drug class (i.e. ‘antibacterials for systemic use’), only 9 (6.1%) assessed both specific compound and drug class. Regarding the 96 studies that assessed specific compounds, 54 (56.3%) investigated only one compound, 20 (20.8%) assessed two compounds and 22 (22.9%) assessed three or more. Given the 60 studies that assessed drug class, 45 (75.0%) investigated only one class, 7 (11.7%) assessed two classes and 8 (13.3%) investigated three or more. Fourteen studies (23.3%) evaluated ‘antibacterials for systemic use’, 10 (16.7%) assessed psychoanaleptics and 7 (11.7%) assessed psycholeptics. Considering 14 drug safety studies that included preterm newborns, ‘antibacterials for systemic use’ were evaluated in 3 (21.4%) and ‘corticosteroids for systemic use’ in 2 (14.3%). For details of studied drug by age, see electronic Supporting Information.
Across the 150 studies that assessed drugs whether exclusively or with vaccines, a total of 291 unique exposures representing 39 unique classes (WHO‐ATC second level) were investigated. Psychoanaleptics (53 [18.2%]) were the commonest, followed by ‘antibacterials for systemic use’ (40 [13.7%]) and psycholeptics (38 [13.1%]). For further details, see Table 3.
Table 3.
Twenty most frequently investigated drug classes across the 150 studies that investigated drugs (whether exclusively or with vaccines)
| Drug class (WHO‐ATC second level)* | Code | N (%)† |
|---|---|---|
| Psychoanaleptics | N06 | 53 (18.2) |
| Antibacterials for systemic use | J01 | 40 (13.7) |
| Psycholeptics | N05 | 38 (13.1) |
| Antineoplastic agents | L01 | 30 (10.3) |
| Anti‐inflammatory and anti‐rheumatic products | M01 | 18 (6.2) |
| Anti‐epileptics | N03 | 12 (4.1) |
| Corticosteroids for systemic use | H02 | 11 (3.8) |
| Analgesics | N02 | 10 (3.4) |
| Contrast media | V08 | 8 (2.7) |
| Immunosuppresants | L04 | 7 (2.4) |
| Anesthetics | N01 | 6 (2.1) |
| Antihistamines for systemic use | R06 | 6 (2.1) |
| Antihemorrhagics | B02 | 5 (1.7) |
| Pituitary and hypothalamic hormones and analogues | H01 | 5 (1.7) |
| Antivirals for systemic use | J05 | 5 (1.7) |
| Drugs for obstructive airway diseases | R03 | 4 (1.4) |
| Cardiac therapy | C01 | 3 (1.0) |
| Cough and cold preparations | R05 | 3 (1.0) |
| Drugs for functional gastrointestinal disorders | A03 | 2 (0.7) |
| Agents acting on the renin‐angiotensin system | C09 | 2 (0.7) |
World Health Organization‐Anatomic Therapeutic Chemical classification.
Proportion is based on the total number (291) of unique drug exposures that were investigated.
Considering drug evaluations exclusively, only three studies (2.0%) assessed the effect of rarely used drugs (i.e. ciprofloxacin) and only 30 (20.0%) assessed dose‐effects.
Altogether, 588 outcomes were evaluated with a median of 1(1, 2) outcome per study; 36 studies (13.4%) did not state the outcome definition. Most events (68 [39.5%]) were acute, and defined as symptoms, signs or ill‐defined conditions (i.e. diarrhea) (Figure 3). Rare outcomes (i.e. Stevens–Johnson syndrome) were evaluated in only 17 studies (6.3%). Expert validation of the outcomes was frequent (172 [64.2%]) but only in 46 of those (26.7%) the experts were blinded to exposure.
Figure 3.

Distribution of papers according to the main divisions of the International Classification of diseases (ninth edition), and type of exposure. Note: ‘Certain conditions originating in the perinatal period’ includes ‘Other conditions originating in the perinatal period’ (764–779) which does not include maternal causes, i.e. Necrotizing enterocolitis (777.5); ‘Injury and poisoning’ includes ‘unspecified adverse effect of drug medicinal and biological substance not elsewhere classified’ (995.2)
Out of 174 cohort studies, the follow‐up time was inadequate to observe the investigated outcomes in 76 (43.7%).
Only 27 studies (10.1%) reported sample size or power calculations. Cohort studies were the largest unlike SCCS and case crossover studies which included few participants. Most studies (229 [85.4%]) adjusted for confounding either by stratification (mainly vaccine safety studies), matching or by multivariate modeling (mainly drug safety studies).
Only 133 studies (49.6%) specified a primary objective and 129 studies (48.1%) reported at least one statistically significant result. This proportion increased to 59.0% when only 183 child‐specific studies were considered. Most studies with significant statistical results (97 [75.2%]) were published after 2002. Among the 75 child‐specific studies that did not present any statistically significant result, 41 (54.7%) did not discuss lack of power.
Discussion
We have conducted a systematic review to assess the characteristics of pediatric pharmacoepidemiological safety studies that were published over 34 years, while aiming to identify areas for improvement of these much needed studies. The review also highlights differences in drug versus vaccine pediatric studies. Some previous reviews have summarized evidence regarding specific drug or vaccine safety issues that affect children12, 13, 14, 15 while others have focused on specific methodological aspects of pediatric pharmacoepidemiology16, 17 but to the best of our knowledge no review has attempted to provide a general overview of these studies.
Our main findings are: the absolute number of pediatric pharmacoepidemiological safety studies is low; in 2012 only 33 studies concerned pediatrics compared to a total of 3197 published studies (data not presented but utilized in constructing Figure 2). Such studies are almost exclusively conducted in developed countries and receive very little private funding. Evaluated exposures concern few pharmaceuticals while investigating mainly intermediate clinical outcomes (signs/symptoms). As areas of improvement we recommend better global spread, interaction between pharmacoepidemiologists evaluating drugs and vaccines to apply designs more broadly, more focus and funding of such studies, and collaboration between investigators so that larger size studies can be conducted that may have enough power to study the rarer and potentially more serious safety issues.
Although paper medical charts may be regarded as the gold standard source of patient information electronic health records and claims data comprise vast amounts of routine care information that can be readily utilized for pharmacoepidemiological safety studies, as demonstrated by several authors.18, 19, 20, 21 More extensive use of such data may be needed to overcome the problem of inadequate follow‐up for many cohort studies as demonstrated in this review, especially if this is related to the high costs that is associated with long follow‐up time in some prospective studies utilizing primary data collection. Generally, the potential of secondary data has been recognized by FDA in Sentinel,22 by Health Canada in their CNODES project23 and in Europe by the GRIP consortium and other projects.24 We should now focus the potential of these powerful resources on pediatric studies specifically in order to quickly fill the existing gap in knowledge of drug use.
The number of pediatric safety studies started increasing steadily after 2002, following the introduction of the ‘Best Pharmaceuticals for children Act’ (BPCA).3 Under the BPCA, the US National Institutes of Health sponsored several pharmacoepidemiological studies in children.25 The pediatric regulation was introduced in the European Union in 2007, perhaps explaining the even steeper increase in the number of pediatric studies that is observed after 2007 (Figure 2). The predominance of US and EU studies may be explained partially by these legislations but also by the number of epidemiologists and data resources. Whatever the explanation may be, this review points to a large public health need for more human capacity building and studies in many children that live in other parts of the world, particularly lower and middle income countries. From a publication and academic perspective it should be noted that the studies were published mostly in more general journals and the impact factors were well above the median in the pharmacology field.
Where should funding for such studies come from? Generally, studies relying on secondary data are affordable. In this review we observed that few studies were privately funded. We recommend that the politicians who passed the BPCA and other legislations to stimulate generation of efficacy data in children see the potential of pharmacoepidemiology rather than clinical trials to generate safety data and oblige long‐term postmarketing studies in children for newly marketed drugs specifically. Studies on off‐patent drugs that are frequently used in children should be investigated through public funding in both developing and developed countries.
Almost half of the evaluated drugs belong to only three classes (Table 3): anti‐infectives, psychoanaleptics and psycholeptics. Although anti‐infectives are often prescribed in pediatrics across all age groups, psychoanaleptics and psycholeptics represent a minority of drug exposure in children.26 However, these drugs have been surrounded by specific safety issues.27, 28 Specifically, psychoanaleptics (i.e. atomoxetine and methylphenidate) were commonly mentioned in pediatric case reports submitted to FAERS between 2004 and 2011,29 probably reflecting its increased use and high risk of toxicity, notably cardiovascular toxicity, as demonstrated by several authors in our review.27, 28, 30, 31, 32
Very few studies evaluated rare drug exposures possibly because such studies would not have been adequately powered to detect an association. Specifically, in preterm newborns, the investigated drugs (i.e. sildenafil and morphine) are possibly associated with serious safety issues;33, 34, 35 however, these studies could not confirm safety associations possibly because of their limited sample size. Inadequate sample size may account for the lack of at least one statistically significant result in 41% of the child‐specific studies, even if majority of these studies did not discuss lack of power. Size issues may be addressed by implementing case‐only (i.e. SCCS) designs.36 In our review, few studies applied case‐only analysis essentially to evaluate vaccine safety. However, case‐only designs present strengths that are suited for the drug utilization patterns and characteristics of outcomes in children.37, 38 Further, multi‐site data pooling may be necessary to acquire adequate power to study rare events in children.39, 40 International collaboration on a global scale may be required; this is the main aim of the Global Research in Pediatrics (GRIP) project.
The strength of this review is the systematic assessment of pharmacoepidemiological safety studies in children, with broad inclusion criteria. Regarding limitations, we applied minimum follow‐up periods (to cohort studies) according to the type of investigated outcome; this may not have been accurate for some specific outcomes. Yet, standardization was necessary because the outcomes were numerous and highly heterogeneous. Also, the findings we have presented are based on published data reported by authors of the studies; therefore, if such reporting was incomplete and/or inaccurate, this may have impacted our findings. For example, several irrelevant drug exposures were assessed in neonates (electronic Supporting Information), like anti‐obesity preparations or antineoplastic drugs merely because authors stated that included pediatric population started at 0 years of age. Such imprecisions inevitably lead to erroneous conclusions. Also, we used country of corresponding author as a proxy for the study setting; by doing this, study setting for multi‐country database studies may not have been accurately captured. Further, we used the journal impact factor as a proxy for the quality of the studies that we reviewed; the limitations of this measure have been described in the literature.
Based on the reviewed literature, we conclude that there is a need to build global collaborative capacity and funding opportunities for pediatric pharmacoepidemiology because this is one of the most powerful ways to provide evidence of drug safety in children.
Conflict of Interest
MS is heading a research group that occasionally conducts post‐authorization safety studies for pharmaceutical companies; none is related to this topic. FK has received funding from the ‘Priority Medicines Kinderen project ZONMW: EVIPED: Novel methods to assess and compare drug effects in pediatrics’ (Grant agreement number 113201007), the funders had no role in designing and conducting the study, collecting and managing data, and preparation, review or approval of the manuscript. AP is an employee of Dutch Medicines Evaluation Board. The views expressed in this article are the personal views of the author(s) and may not be understood or quoted as being made on behalf of or reflecting the position of the Dutch Medicines Agency.
OO, JD, CF, CD, WB, GJ and DM have no conflicts of interest that are directly related to the content of this study.
Key points.
The number of pharmacoepidemiological safety studies is steadily increasing in pediatrics
We identified various challenges including funding, design, type and source of data, mode of data collection, age and geographic spread of the investigated population, studied drugs and outcomes, sample size, control of confounding and reporting of results.
Pharmacoepidemiological safety studies in children can be improved in several ways including global collaboration.
Prior Presentation
Preliminary results were presented at the 9th Asian Conference on Pharmacoepidemiology, Bangkok, Thailand.
Funding
The Global Research in Pediatrics‐Network of Excellence is funded under the European Union's Seventh Framework Program (FP7/2007–2013) for research, technological development and demonstration under grant agreement number 261060. Funding for this study was also received from the ‘Priority Medicines Kinderen project ZONMW: EVIPED: Novel methods to assess and compare drug effects in pediatrics’ (Grant agreement number 113201007). The funders had no role whatsoever in designing and conducting the study, the collection and management of data, and preparation, review or approval of the manuscript.
Supporting information
Table 1: Number of pediatric pharmacoepidemiological safety studies according to investigated drug (including vaccines) class (World Health Organization‐Anatomic Therapeutic Chemical classification second level) and age category
Appendix 1: Full search strategy
Appendix 2: Data collection form
Supporting info item
Osokogu, O. U. , Dukanovic, J. , Ferrajolo, C. , Dodd, C. , Pacurariu, A. C. , Bramer, W. M. , 'tJong, G. , Weibel, D. , Sturkenboom, M. C. J. M. , and Kaguelidou, F. (2016) Pharmacoepidemiological safety studies in children: a systematic review. Pharmacoepidemiol Drug Saf, 25: 861–870. doi: 10.1002/pds.4041.
References
- 1. United States of America . Pediatric Research Equity Act of 2003. 2003; http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM077853.pdf. Accessed 22nd December, 2015.
- 2. European Union . REGULATION (EC) No 1901/2006 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 12 December 2006 on medicinal products for paediatric use and amending Regulation (EEC) No 1768/92, Directive 2001/20/EC, Directive 2001/83/EC and Regulation (EC) No 726/2004. 2006; http://ec.europa.eu/health/files/eudralex/vol‐1/reg_2006_1901/reg_2006_1901_en.pdf. Accessed 26th June, 2015.
- 3. FDA . Best Pharmaceuticals for Children Act. 2002; http://www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/ucm148011.htm. Accessed 26th June, 2015.
- 4. Turner MA, Catapano M, Hirschfeld S, Giaquinto C. Paediatric drug development: the impact of evolving regulations. Adv Drug Deliv Rev 2014; 73 ((Turner M.A., mark.turner@liv.ac.uk) University of Liverpool, Department of Women's and Children's Health, Institute of Translational Medicine, Liverpool Women's NHS Foundation Trust, Liverpool L8 7SS, United Kingdom): 2–13. [DOI] [PubMed] [Google Scholar]
- 5. Booth CM, Tannock IF. Randomised controlled trials and population‐based observational research: partners in the evolution of medical evidence. Br J Cancer 2014; 110(3): 551–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. D'Agostino RB, Jr , D'Agostino RB, Sr . Estimating treatment effects using observational data. JAMA 2007; 297(3): 314–6. [DOI] [PubMed] [Google Scholar]
- 7. Vandenbroucke JP. When are observational studies as credible as randomised trials? Lancet 2004; 363(9422): 1728–31. [DOI] [PubMed] [Google Scholar]
- 8. Juurlink D. Studying drug safety in the real world. Clin Toxicol 2009; 47(5): 489. [Google Scholar]
- 9. Kooblal Y, Kruger M. Unlicensed and off label drug use in children an a large central hospital in South Africa. Basic Clin Pharmacol Toxicol 2014; 115(1):337. [Google Scholar]
- 10. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med 2009; 151(4): 264–269. [DOI] [PubMed] [Google Scholar]
- 11. INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE. CLINICAL INVESTIGATION OF MEDICINAL PRODUCTS IN THE PEDIATRIC POPULATION E11. Current Step 4 version 2000; http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E11/Step4/E11_Guideline.pdf. Accessed 28th February, 2016.
- 12. Etminan M, Sadatsafavi M, Jafari S, Doyle‐Waters M, Aminzadeh K, FitzGerald JM. Acetaminophen use and the risk of asthma in children and adults: a systematic review and metaanalysis. Chest 2009; 136(5): 1316–23. [DOI] [PubMed] [Google Scholar]
- 13. Li L, Shen J, Bala MM, et al. Incretin therapy and risk of pancreatitis in type 2 diabetes mellitus: systematic review of randomized and non‐randomized studies. Pharmacoepidemiol Drug Saf 2014; 23 ((Li L.; Sun X.) Chinese Evidence‐based Medicine Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China): 334–5. [Google Scholar]
- 14. Mannion ML, Beukelman T. Risk of malignancy associated with biologic agents in pediatric rheumatic disease. Curr Opin Rheumatol 2014; 26(5): 538–42. [DOI] [PubMed] [Google Scholar]
- 15. Weibel D, Dodd C, Romio S, Bonhoeffer J, Black S, Sturkenboom MCJM. Guillain‐barre syndrome and pandemic influenza A(H1N1) 2009 vaccines: meta‐analysis of observational risk estimate studies from 2010 and 2011. Pharmacoepidemiol Drug Saf 2012; 21 ((Weibel D.; Romio S.; Sturkenboom M.C.J.M.) Erasmus University Medical Center, Rotterdam, Netherlands): 363. [Google Scholar]
- 16. Murray ML, Insuk S, Banaschewski T, et al. An inventory of European data sources for the long‐term safety evaluation of methylphenidate. Euro Child Adolescent Psychiatry 2013; 22(10): 605–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Verhamme K, Sturkenboom M. Study designs in paediatric pharmacoepidemiology. Eur J Clin Pharmacol 2011; 67(Suppl. 1): S67–74. [DOI] [PubMed] [Google Scholar]
- 18. Neubert A, Sturkenboom MCJM, Murray ML, et al. Databases for pediatric medicine research in Europe—assessment and critical appraisal. Pharmacoepidemiol Drug Saf 2008; 17(12): 1155–67. [DOI] [PubMed] [Google Scholar]
- 19. Leal I, Masclee GMC, Scotti L, et al. Identification of type 2 diabetes mellitus in different electronic databases within the safeguard project. Pharmacoepidemiol Drug Saf 2014; 23 ((Leal I.; Masclee G.M.C.; Trifiro G.; Rijnbeek P.; Sturkenboom M.C.J.M.; Romio S.) Medical Informatics, Erasmus University Medical Center, Rotterdam, Netherlands): 193–194. [Google Scholar]
- 20. Ferrajolo C, Trifiro G, Coloma PM, et al. Pediatric acute liver injury: signal detection using multiple healthcare databases from EU‐ADR network. Pharmacoepidemiol Drug Saf 2012; 21 ((Ferrajolo C.; Trifiro G.; Coloma P.M.; Verhamme K.M.C.; Schuemie M.J.; Van Der Lei J.; Sturkenboom M.C.J.M.) Medical Informatics, Erasmus University Medical Center, Rotterdam, Netherlands): 182–3. [Google Scholar]
- 21. Coloma PM, Valkhoff VE, Mazzaglia G, et al. Accuracy of coding‐based algorithms in identification of acute myocardial infarction from multi‐country electronic healthcare records (EHR) databases. Pharmacoepidemiol Drug Saf 2012; 21 ((Coloma P.M.; Mosseveld B.; Schuemie M.J.; Sturkenboom M.C.J.M.) Medical Informatics, Erasmus Medical Center, Rotterdam, Netherlands): 395–6. [Google Scholar]
- 22. US Food and Drug Administration . FDA's Sentinel Initiative. http://www.fda.gov/Safety/FDAsSentinelInitiative/ucm2007250.htm. Accessed 12th January, 2016.
- 23. CNODES . Canadian Network for Observational Drug Effect Studies. 2013; https://www.cnodes.ca/. Accessed 12th January, 2016.
- 24. Trifirò G, Coloma PM, Rijnbeek PR, et al. Combining multiple healthcare databases for postmarketing drug and vaccine safety surveillance: why and how? J Intern Med 2014; 275(6): 551–61. [DOI] [PubMed] [Google Scholar]
- 25. NIH . Eunice Kennedy Shriver National Institute of Child Health and Human Development. Best Pharmaceuticals for Children Act. Literature and Data Resources. 2011; http://bpca.nichd.nih.gov/resources/Pages/index.aspx. Accessed 15th July, 2015.
- 26. Sturkenboom MCJM, Verhamme KMC, Nicolosi A, et al. Drug use in children: cohort study in three European countries. BMJ 2008; 337(7682): 1338–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gould MS, Walsh BT, Munfakh JL, et al. Sudden death and use of stimulant medications in youths. AmJ Psychiatry 2009; 166(9): 992–1001. [DOI] [PubMed] [Google Scholar]
- 28. Schelleman H, Bilker WB, Strom BL, et al. Cardiovascular events and death in children exposed and unexposed to ADHD agents. Pediatrics 2011; 127(6): 1102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Bie S, Ferrajolo C, Straus SMJM, et al. Pediatric drug safety surveillance in FDA‐AERS: a description of adverse events from GRiP project. PLoS One 2015; 10(6): e0130399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Olfson M, Huang C, Gerhard T, et al. Stimulants and cardiovascular events in youth with attention‐deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2012; 51(2): 147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Winterstein AG, Gerhard T, Shuster J, Johnson M, Zito JM, Saidi A. Cardiac safety of central nervous system stimulants in children and adolescents with attention‐deficit/hyperactivity disorder. Pediatrics 2007; 120(6): e1494–501. [DOI] [PubMed] [Google Scholar]
- 32. Winterstein AG, Gerhard T, Shuster J, Saidi A. Cardiac safety of methylphenidate versus amphetamine salts in the treatment of ADHD. Pediatrics 2009; 124(1): e75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Enders J, Gebauer C, Pulzer F, Robel‐Tillig E, Knupfer M. Morphine‐related apnoea in CPAP‐treated preterm neonates. Acta Paediatr Int J Paediatr 2006; 95(9): 1087–92. [DOI] [PubMed] [Google Scholar]
- 34. Fang AYW, Guy KJ, Konig K. The effect of sildenafil on retinopathy of prematurity in very preterm infants. J Perinatol 2013; 33(3): 218–21. [DOI] [PubMed] [Google Scholar]
- 35. Drossou‐Agakidou V, Roilides E, Papakyriakidou‐Koliouska P, et al. Use of ciprofloxacin in neonatal sepsis: lack of adverse effects up to one year. Pediatr Infect Dis J 2004; 23(4): 346–9. [DOI] [PubMed] [Google Scholar]
- 36. Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self‐controlled case series method. Stat Med 2006; 25(10): 1768–97. [DOI] [PubMed] [Google Scholar]
- 37. Ueyama H, Hinotsu S, Tanaka S, et al. Application of a self‐controlled case series study to a database study in children. Drug Saf 2014; 37(4): 259–68. [DOI] [PubMed] [Google Scholar]
- 38. Kuhnert R, Hecker H, Poethko‐Müller C, et al. A modified self‐controlled case series method to examine association between multidose vaccinations and death. Stat Med 2011; 30(6): 666–77. [DOI] [PubMed] [Google Scholar]
- 39. Valkhoff VE, Schade R, T Jong GW, et al. Population‐based analysis of non‐steroidal anti‐inflammatory drug use among children in four European countries in the SOS project: what size of data platforms and which study designs do we need to assess safety issues? BMC Pediatr 2013; 13(1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. De Bie S, Coloma PM, Ferrajolo C, et al. The role of electronic healthcare record databases in paediatric drug safety surveillance: a retrospective cohort study. Br J Clin Pharmacol 2015; 80(2): 304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1: Number of pediatric pharmacoepidemiological safety studies according to investigated drug (including vaccines) class (World Health Organization‐Anatomic Therapeutic Chemical classification second level) and age category
Appendix 1: Full search strategy
Appendix 2: Data collection form
Supporting info item


