Abstract
Aims
To assess the performance and safety of an integrated bihormonal artificial pancreas system consisting of one wearable device and two wireless glucose sensor transmitters during short‐term daily use at home.
Methods
Adult patients with type 1 diabetes using an insulin pump were invited to enrol in this randomized crossover study. Treatment with the artificial pancreas started with a day and night in the clinical research centre, followed by 3 days at home. The control period consisted of 4 days of insulin pump therapy at home with blinded continuous glucose monitoring for data collection. Days 2–4 were predefined as the analysis period, with median glucose as the primary outcome.
Results
A total of 10 patients completed the study. The median [interquartile range (IQR)] glucose level was similar for the two treatments [7.3 (7.0–7.6) mmol/l for the artificial pancreas vs. 7.7 (7.0–9.0) mmol/l for the control; p = 0.123]. The median (IQR) percentage of time spent in euglycaemia (3.9–10 mmol/l) was longer during use of the artificial pancreas [84.7 (82.2–87.8)% for the artificial pancreas vs. 68.5 (57.9–83.6)% for the control; p = 0.007]. Time in hypoglycaemia was 1.3 (0.2–3.2)% for the artificial pancreas and 2.4 (0.4–10.3)% for the control treatment (p = 0.139). Separate analysis of daytime and night‐time showed that the improvements were mainly achieved during the night.
Conclusions
The results of this pilot study suggest that our integrated artificial pancreas provides better glucose control than insulin pump therapy in patients with type 1 diabetes at home and that the treatment is safe.
Keywords: clinical trial, glucagon, glycaemic control, insulin therapy, type 1 diabetes
Introduction
The currently available insulin therapies for patients with type 1 diabetes are patient‐controlled. Consequently, achieving near‐normal blood glucose control is challenging for patients and requires substantial effort. An artificial pancreas or closed‐loop system aims to automate blood glucose control, thereby improving glucose control and reducing the disease burden for patients.
Hybrid closed‐loop insulin delivery systems have successfully been used in long‐term home studies overnight 1, during the evening and night 2, and during the day and night 3. These studies reported improved glycaemic control compared with sensor‐augmented pump therapy. The closed‐loop systems are designated as hybrid because they require carbohydrate‐counting and meal announcement by the patient.
An extension to closed‐loop insulin delivery is the bihormonal artificial pancreas which also uses glucagon, being a counter‐regulatory hormone to insulin, to treat or prevent hypoglycaemia. Bihormonal artificial pancreas systems have not yet been used in long‐term studies, but short‐term inpatient 4, 5, supervised outpatient 6, 7 and home 8 studies have shown the potential for further improvement in glycaemic control with a low risk of hypoglycaemia. Although the addition of glucagon increases the complexity of the closed‐loop system, bihormonal closed‐loop glucose control has shown the potential to relieve the burden of carbohydrate‐counting 6, 9 or completely omit meal announcements 8.
Typically, an artificial pancreas consists of a continuous glucose monitor (CGM) to measure blood glucose concentration, a control unit with the control algorithm, and an insulin infusion pump. These components have to communicate with each other to enable automated glucose control. Using separate devices for these components, with for example a smartphone as the control unit, frequently limits the usability of artificial pancreas systems because of connectivity problems 1, 2, 5, 6, 10. At this moment, one study investigating an integrated artificial pancreas device has been published 11. This hybrid closed‐loop system by Medtronic consists of an insulin pump which also contains the control algorithm and receiver of the wireless glucose sensor. For a bihormonal artificial pancreas the integration of devices becomes even more critical since such a system will need a second infusion pump for the glucagon delivery.
To make our bihormonal artificial pancreas 8 suitable for daily use at home, we integrated all components into one wearable device. The aim of this pilot study was to test the performance and safety of our integrated bihormonal artificial pancreas in adults with type 1 diabetes during short‐term daily use at home. We addressed the following research questions: (i) Does this artificial pancreas provide better glucose control than standard insulin pump therapy? and (ii) Is treatment with this artificial pancreas at least as safe as standard therapy?
Materials and Methods
Study Design
This pilot study was a single‐centre, randomized crossover trial to compare home treatment with the bihormonal artificial pancreas with standard insulin pump therapy in patients with type 1 diabetes. Patients were randomized to start with either 4 days of artificial pancreas treatment, followed by 4 days of insulin pump therapy at home, with a wash‐out period of at least 1 week in between, or the reverse order. Both 4‐day periods started on a Saturday. The study protocol was approved by the ethics committee of the Academic Medical Centre at the University of Amsterdam (Amsterdam, the Netherlands) and was performed in concordance with the Declaration of Helsinki. All patients provided written informed consent. The study was registered under number NCT02160275 at ClinicalTrials.gov.
Study Patients
The patients were recruited from the outpatient clinic of Rijnstate Hospital (Arnhem, the Netherlands). Inclusion criteria were: age 18–75 years; diagnosis of type 1 diabetes; and treatment with an insulin pump for at least 6 months. Exclusion criteria were: impaired awareness of hypoglycaemia, according to the questionnaire used by either Gold et al. 12 or Clarke et al. 13; body mass index (BMI) > 35 kg/m2; glycated haemoglobin (HbA1c) > 11% (97 mmol/mol); pregnancy or breastfeeding; and use of heparin, coumarin derivatives or oral corticosteroids.
Study Procedures
On the first day of the artificial pancreas treatment period the patients were admitted to the clinical research centre and received training on the use of the artificial pancreas (Inreda Diabetic BV, Goor, the Netherlands). After this training, the artificial pancreas treatment was started. After an overnight stay at the clinical research centre, the patients went home to use the system for 3 days.
On the first day of the control period the patients came to the clinical research centre. Continuous glucose monitoring with the artificial pancreas' CGM configuration with two glucose sensors was started. Subsequently the patients were sent home for 4 days. During this control period the patients used their own insulin pump (different manufacturers) and the CGM measurements were not visible for the patients. By using the artificial pancreas' CGM configuration during the control period, it was possible to assess and compare the two treatments by means of the same CGM configuration. No insulin optimization was carried out prior to the control period and the patients were not instructed on the timing of meal boluses and whether to use carbohydrate counting for their insulin treatment or not.
During both study periods the patients were allowed to carry out their normal activities and there were no restrictions on meals. For safety reasons, patients were not allowed to operate a motorized vehicle during the artificial pancreas period. The patients were asked to keep a diary to record self‐monitored blood glucose (SMBG) measurements, insulin use (only during the control period), meals and activities. The carbohydrate content of the meals was estimated by the patients or by the research team based on the notes in the diary. SMBG was to be performed before each meal, 2 h after each meal, before bedtime and at 03:00 hours (the latter only during the artificial pancreas period). Additional SMBG measurements were performed if requested by the CGM of the artificial pancreas or according to patient judgement. The Accu‐Chek Mobile system (Roche Diagnostics, Mannheim, Germany) was used for all SMBG measurements. Physical activity was measured using a heart rate belt (HRM1G, Garmin, Olathe, KS, USA; during daytime only) which was wirelessly connected to the artificial pancreas and with a tri‐axial accelerometer incorporated in the artificial pancreas.
Artificial Pancreas
The integrated artificial pancreas was developed by Inreda Diabetic BV. The system consists of a wearable device, which contains the CGM, control algorithm, insulin pump and glucagon pump, and two wireless transmitters to obtain the continuous glucose measurements (Figure 1). This bihormonal closed‐loop system provides automated glucose control without meal and exercise announcements. The artificial pancreas device and transmitters contain custom‐made printed circuit boards with a proprietary operating system. The artificial pancreas device has a main processor and a safety processor which checks all insulin and glucagon doses determined by the control algorithm. The dimensions of the device are 119 × 74 × 35 mm3 and it weighs 350 g with full insulin and glucagon cartridges and batteries.
Figure 1.
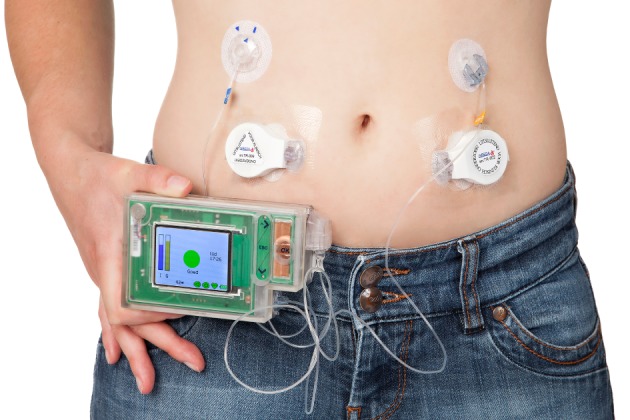
The Inreda integrated bihormonal artificial pancreas. Patients wear the device on a belt. One infusion set is connected to the insulin pump and the other to the glucagon pump. The two transmitters wirelessly sent the glucose measurements to the wearable device.
The input for the control algorithm is provided by the CGM of the artificial pancreas that receives the measurements from two subcutaneous glucose sensors (Enlite®, Medtronic Inc., Northridge, CA, USA). The glucose sensors were calibrated by entering three SMBG values with at least 6‐min intervals into the system. One sensor was used as the main sensor and the other sensor as back‐up and to check the main sensor. During both study periods, the SMBG values before breakfast and before bedtime were entered into the system to verify the sensor glucose measurements. In addition, the system requested a SMBG value in case the difference between the two sensors was >15% for 15 min. The patients were instructed to consult the research team before entering an SMBG into the system to ensure stable glucose before verification or calibration of the CGM. If an SMBG that was entered into the system deviated from one of the two sensors, the system corrected the calibration factor of that sensor and selected the other sensor as the main sensor. A full calibration procedure, using three SMBG values with at least 6 min in between, was requested by the system if both sensors deviated from an entered SMBG. The automated glucose control remained active only if measurements from at least one sensor were available.
The control algorithm was slightly revised from the previous report 8. In particular, glucagon doses were reduced using the results of Blauw et al. 14. The algorithm determined the insulin and glucagon delivery using the difference between current and target glucose level and the rate of change of the glucose level (proportional integral derivative controller). Insulin administration was reduced in case the measured heart rate exceeded a threshold indicating physical activity. In addition, glucose thresholds triggered the delivery of corrective insulin or glucagon boluses. The initial setting for an individual's insulin sensitivity factor was based on the weight of the patient. Every day the insulin sensitivity factor setting was evaluated and, if necessary, manually adapted. Glucagon dosing was equal for all patients and was not adapted. If the glucose level fell below 3.2 mmol/l, an auditory alarm advised the patient to take oral carbohydrates.
For the insulin administration a 3‐ml prefilled insulin cartridge (U‐100, Humalog, Eli Lilly, Indianapolis, IN, USA) was placed in the device. The glucagon cartridge was replaced every 24 h with 2 ml freshly reconstituted glucagon (1 mg/ml; GlucaGen, Novo Nordisk, Bagsvaerd, Denmark) as there is no stable glucagon commercially available. The occlusion detection mechanism gave an alarm in case the insulin or glucagon infusion set occluded.
Every 10 min, logged data were sent via WiFi through a dedicated access point (a mobile access point with 3G or connected to the home internet network) to a database for remote monitoring. Until the first night at home the glucose levels and performance of the system were continuously monitored. Subsequently, the monitoring was less intensive and based on the judgement of the research team. The research team was available for 24 h per day to assist the patients in using the artificial pancreas.
Data Analysis and Statistics
The primary endpoint was the median glucose concentration. Secondary performance endpoints are the mean glucose concentration, percentage of time spent in euglycaemia (3.9–10 mmol/l) and glycaemic variability [expressed as interquartile range (IQR) and Blood Glucose Risk Index (BGRI) 15]. Secondary safety endpoints include the percentage of time spent in hypoglycaemia (<3.9 and <3.3 mmol/l), percentage of time spent in hyperglycaemia (>10 and >13.9 mmol/l), and the number of carbohydrate‐treated hypoglycaemic events. Daytime was defined as the period from 07:00 till 24:00 hours, night‐time from 24:00 till 07:00 hours, and the postprandial periods as 3 h from the start time of a main meal, as noted in the diary.
The glucose measurement accuracy of the artificial pancreas' CGM was expressed as the mean absolute relative error (MARD). The first sensor glucose measurement after an SMBG measurement was paired with that SMBG value. If there was no sensor glucose measurement available <10 min after a SMBG measurement, that SMBG value was excluded from the MARD calculation.
Other study endpoints were the daily insulin and glucagon use, daily carbohydrate intake, median heart rate during daytime, and the percentage of time that the control algorithm was active (artificial pancreas period only). In addition, adaptations in the insulin sensitivity setting of the control algorithm, adverse events and technical issues were recorded.
Sample size calculation was based on the results of the previous study 8 and a two group t‐test (crossover anova) with a 0.05 two‐sided significance level. A sample size of six patients in each group would provide 80% power to detect a difference between the treatments of 1.3 mmol/l in median glucose. As predefined, the first day of both study periods was excluded from analysis because this was considered as a start‐up day; therefore, 72 h of artificial pancreas use was compared with 72 h of insulin pump treatment. We used a modified intention‐to‐treat analysis including all patients with at least 24 h of evaluable data for both study periods. Glucose endpoints were calculated from the artificial pancreas' CGM measurements. Unless stated otherwise, all endpoints were calculated per patient and summarized as median (IQR). We assessed carry‐over and period effects for all endpoints using Mann–Whitney U‐tests to compare, respectively, the sum and difference of the treatments between the two randomization groups. The Wilcoxon signed rank test for paired data was used for comparison between the two treatments. A p value ≤0.05 indicated statistical significance. matlab (version R2014a and R2015b;The MathWorks, Natick, MA, USA) was used to calculate the endpoints and statistical analysis was performed with IBM spss Statistics (version 20; IBM Corp., Armonk, NY, USA).
Results
Baseline Characteristics
We included 16 patients in the study, of whom 10 completed the study. After inclusion, one patient was not available in the period of conduct of the study. Four patients completed the control period only and were not able to participate in the second part of the study for personal reasons not related to the study. For one patient the artificial pancreas treatment was terminated during the first night at home and for days 3 and 4 of the control period the artificial pancreas' CGM measurements were not evaluable, both caused by highly irregular sensor readings and sensor failures in this particular patient. Thus, the study results of the remaining 10 patients were analysed. The median (IQR) amount of evaluable data was 71.4 (70.9–71.6) h out of the theoretical maximum of 72 h during the artificial pancreas period and 71.0 (69.6–71.5) h during the control period. Five of the 10 patients started with the artificial pancreas treatment and the other five with the control treatment. No carry‐over and period effects were found.
Half of the patients were male. At baseline, the median (IQR) age was 41.0 (26.5–52.3) years and the BMI was 24.5 (22.6–26.6) kg/m2. The median (IQR) HbA1c level was 7.7 (7.4–8.0)% or 60.5 (57.3–63.8) mmol/mol, with a self‐reported daily insulin dose of 39.0 (34.8–43.3) U. The median (IQR) type 1 diabetes duration was 18.0 (14.8–29.5) years and the patients had used an insulin pump for 9.5 (6.5–14.0) years.
Glucose Control Endpoints
The primary and secondary glucose control endpoints are shown in Table 1. Figure 2 shows the glucose profile over 24 h. The median glucose level was not different between the two treatments. A statistically significant increase in the percentage of time in euglycaemia was found for the artificial pancreas period. The time in euglycaemia for each patient is given in Figure 3. For daytime only, the glucose control endpoints were not significantly different between the two treatments, while the glucose control was significantly improved with the artificial pancreas during the night‐time. Glycaemic variability during the postprandial periods was not different for the two treatments (data not shown).
Table 1.
Primary and secondary endpoints.
| Endpoint | Artificial pancreas period | Control period | p value* |
|---|---|---|---|
| Day and night | |||
| Median glucose, mmol/l | 7.3 (7.0–7.6) | 7.7 (7.0–9.0) | 0.123 |
| Mean glucose, mmol/l | 7.4 (7.3–8.1) | 8.1 (7.4–9.3) | 0.059 |
| Time spent in euglycaemia, % | 84.7 (82.2–87.8) | 68.5 (57.9–83.6) | 0.007 |
| Time spent in hypoglycaemia (blood glucose <3.9 mmol/l), % | 1.3 (0.2–3.2) | 2.4 (0.4–10.3) | 0.139 |
| Time spent in hypoglycaemia (blood glucose <3.3 mmol/l), % | 0.0 (0.0–0.8) | 0.5 (0.0–4.8) | 0.069 |
| Time spent in hyperglycaemia (blood glucose >10 mmol/l), % | 11.9 (10.6–17.6) | 24.3 (15.5–39.3) | 0.022 |
| Time spent in hyperglycaemia (blood glucose >13.9 mmol/l), % | 1.8 (0.9–3.7) | 5.2 (0.6–9.9) | 0.059 |
| Glycaemic variability (IQR), mmol/l | 2.8 (2.6–2.9) | 3.7 (3.1–5.3) | 0.011 |
| Glycaemic variability (BGRI), score | 6.6 (5.9–7.2) | 10.8 (7.4–15.9) | 0.005 |
| Daytime (07:00–24:00 hours) | |||
| Median glucose, mmol/l | 7.5 (7.2–8.2) | 7.8 (6.8–8.9) | 0.799 |
| Mean glucose, mmol/l | 7.8 (7.5–8.3) | 8.1 (7.4–9.6) | 0.508 |
| Time spent in euglycaemia, % | 79.9 (76.4–82.8) | 69.7 (53.5–83.1) | 0.074 |
| Time spent in hypoglycaemia (blood glucose <3.9 mmol/l), % | 1.8 (0.0–4.5) | 1.3 (0.4–12.9) | 0.285 |
| Time spent in hyperglycaemia (blood glucose >10 mmol/l), % | 16.9 (13.6–22.0) | 24.3 (16.2–40.8) | 0.169 |
| Glycaemic variability (IQR), mmol/l | 3.3 (3.1–3.4) | 3.7 (2.9–5.0) | 0.074 |
| Glycaemic variability (BGRI), score | 8.1 (6.7–8.5) | 10.2 (7.2–15.5) | 0.093 |
| Night‐time (24:00–07:00 hours) | |||
| Median glucose, mmol/l | 7.0 (6.4–7.4) | 7.7 (6.8–8.0) | 0.028 |
| Mean glucose, mmol/l | 6.8 (6.6–7.5) | 7.8 (7.4–8.7) | 0.007 |
| Time spent in euglycaemia, % | 97.7 (94.7–100.0) | 71.2 (60.3–85.9) | 0.005 |
| Time spent in hypoglycaemia (blood glucose <3.9 mmol/l), % | 0.0 (0.0–0.6) | 1.7 (0.0–10.3) | 0.069 |
| Time spent in hyperglycaemia (blood glucose >10 mmol/l), % | 0.0 (0.0–4.9) | 18.7 (11.9–36.0) | 0.007 |
| Glycaemic variability (IQR), mmol/l | 1.6 (1.4–1.8) | 3.3 (2.6–5.7) | 0.005 |
| Glycaemic variability (BGRI), score | 2.4 (2.0–4.5) | 12.3 (7.6–15.7) | 0.005 |
| Postprandial median glucose, mmol/l | |||
| Breakfast | 8.9 (7.8–10.1) | 7.7 (7.2–10.2) | 0.139 |
| Lunch | 7.3 (6.8–8.6) | 8.5 (7.5–11.0) | 0.153 |
| Dinner | 7.9 (7.3–8.3) | 7.4 (6.8–9.4) | 0.594 |
| Postprandial mean glucose, mmol/l | |||
| Breakfast | 8.9 (8.2–10.5) | 7.8 (7.2–10.8) | 0.333 |
| Lunch | 7.5 (6.9–8.7) | 8.5 (8.0–11.2) | 0.028 |
| Dinner | 8.0 (7.0–8.4) | 7.4 (7.0–9.0) | 0.646 |
| Postprandial time spent in hyperglycaemia (blood glucose >10 mmol/l), % | |||
| Breakfast | 30.6 (15.0–50.8) | 21.8 (3.8–53.2) | 0.241 |
| Lunch | 9.0 (7.4–30.2) | 30.8 (19.8–59.7) | 0.037 |
| Dinner | 17.5 (2.8–26.0) | 18.5 (4.0–40.1) | 0.374 |
Data are median (interquartile range).
Euglycaemia: 3.9–10 mmol/l. Postprandial control during the 3 h from start of the meal.
Wilcoxon signed rank tests.
Figure 2.
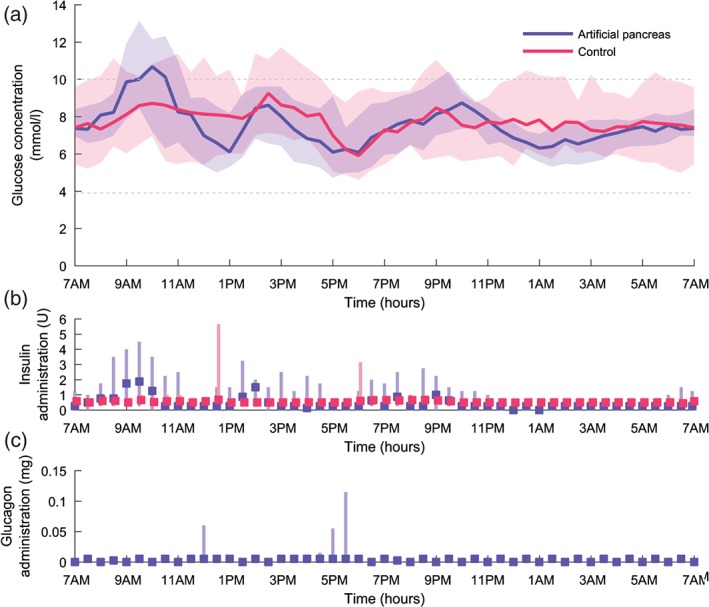
Glucose profile, insulin and glucagon administration over 24 h for the artificial pancreas and control period. For each treatment period this figure summarizes the data of 30 days (3 days for 10 patients). (a) Median glucose profile, the shaded bands indicate interquartile range (IQR). The grey dashed lines indicate the euglycaemic range. (b and c) Median administration (sum per 30 min). The lines indicate IQR.
Figure 3.
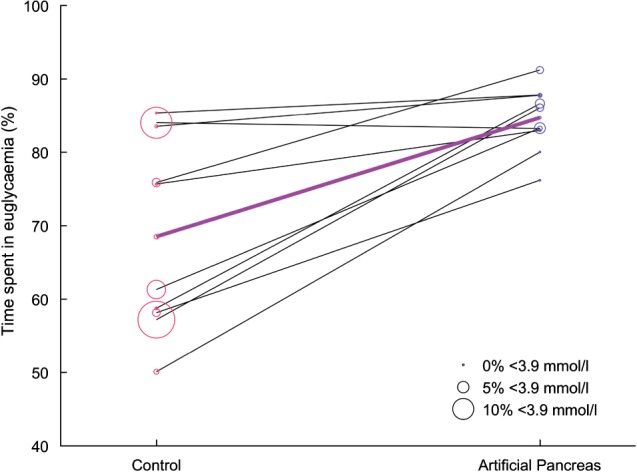
Percentage of time spent in euglycaemia per patient for both treatment periods. The bold purple line indicates the median time spent in euglycaemia. The size of each circle is proportional to the time spent in hypoglycaemia.
Safety Endpoints
The safety endpoints are shown in Table 1. We found no statistically difference in the time in hypoglycaemia. The time in hyperglycaemia (blood glucose >10 mmol/l) was shorter for the artificial pancreas in comparison with the control treatment. For daytime only, however, there was no significant difference in the time in hyperglycaemia. In total, there were 12 carbohydrate‐treated events during the artificial pancreas treatment (median 1, range 0–4) and 21 during the control period (median 1, range: 0–10; p = 0.551).
Glucose Measurement Performance
The MARD for the artificial pancreas' CGM during the artificial pancreas period was 11.6 (10.4–17.2)%, while it was 15.5 (13.0–21.9)% during the control period (p = 0.241). During the artificial pancreas period a median (IQR) of 12.0 (9.6–15.1) SMBG measurements per day were performed and 10.0 (9.1–10.3) during the control period (p = 0.028).
Other Endpoints
Median (IQR) daily insulin use was higher during the artificial pancreas period [51.9 (41.7–65.3) U] than during the control period [42.2 (31.6–48.6) U; p = 0.022]. There was no difference in the insulin administration overnight between the two periods. The median amount of glucagon that was administered by the artificial pancreas per 24 h was 0.74 (0.53–1.03) mg. The artificial pancreas administered 0.005 mg glucagon as a ‘maintenance’ bolus if no glucagon was given the last hour to prevent clotting of the infusion set. The median (IQR) daily maintenance bolus was 0.09 (0.08–0.09) mg; therefore, the daily glucagon dose requested by the control algorithm was 0.65 (0.45–0.94) mg. The median (IQR) daily carbohydrate intake was 159.0 (133.4–198.6) g during the artificial pancreas period and 186.5 (153.5–202.0) g during the control period (p = 0.646). In addition, the carbohydrate intake for the three main meals separately was not significantly different between the two treatments (data not shown). The median (IQR) heart rate during the daytime was similar for the two treatment periods [79.5 (69.5–82.5)] during the artificial pancreas period and 80 (72.3–83.0) during the control period; p = 0.608]. The control algorithm of the artificial pancreas was active for a median (IQR) of 95.4 (90.2–97.9)% of the time. The insulin sensitivity setting of the control algorithm was adapted in all but one patient before the patients went home during the artificial pancreas period. At home, the insulin sensitivity was further adapted to a median (IQR) of 1.5 (1.0–3.3) times.
Adverse Events and Technical Issues
Severe hypoglycaemia or ketoacidosis did not occur during the study. One female patient reported nausea on two occasions during 1 day of the artificial pancreas treatment. The patient received 0.88 mg glucagon that day. No insulin infusion set occlusions were detected or noticed during treatment with the artificial pancreas. A glucagon infusion set occlusion was detected six times by the artificial pancreas. In all six cases the tubing of the infusion set turned out to be occluded. Furthermore, one patient noted leakage around the cannula of the glucagon infusion set, after which the infusion set was replaced. One artificial pancreas had to be replaced on the last day because the glucagon pump became stuck, which was caused by a loose wire in the device. Three glucose sensors of the artificial pancreas' CGM had to be replaced during the control period and two during the artificial pancreas treatment. During the control period one insulin pump failed, and was replaced within a few hours.
Discussion
The results of this short‐term home study firstly suggest that our integrated bihormonal artificial pancreas provides better glucose control than insulin pump therapy in patients with type 1 diabetes. The percentage of time spent in euglycaemia was higher during use of the artificial pancreas. The median glucose level was lower for the artificial pancreas during the night only. Together with the very high time spent in euglycaemia during the night, this indicates that the improvement in glucose control with the artificial pancreas was mainly achieved during the night period.
Secondly, this study indicates that treatment with this artificial pancreas is at least as safe as insulin pump therapy. The percentage of time spent in hypoglycaemia was low during use of the artificial pancreas (1.3%), although this percentage and the lower number of carbohydrate‐treated events during the artificial pancreas period did not significantly differ from the control treatment. The relatively high frequency of glucagon infusion set occlusion probably affected the ability of the artificial pancreas to prevent hypoglycaemic events.
Although we acknowledge all the caveats of between‐study comparisons, the median glucose concentration found in the present study was similar to the glucose level in adults reported by Russel et al. 6, who investigated a bihormonal artificial pancreas for 5 days in a supervised outpatient setting, while the percentage time spent in euglycaemia was 79.5% during the day‐ and night‐time and 86.5% during the night‐time in that study. With hybrid closed‐loop insulin delivery systems, a time in euglycaemia of 67.0–72.9% was achieved in long‐term home studies 1, 2, 3. The improved glucose control found in the studies by Kropff et al. 2 and Nimri et al. 1 was accompanied by a reduction in insulin dose. In the present study, however, insulin dose was increased during use of the artificial pancreas. To reach even higher percentages of time in euglycaemia, it might be necessary to administer more insulin so that the time in hyperglycaemia can be further reduced. This can be safely achieved with a bihormonal artificial pancreas because of the addition of glucagon. Glucose control during the control period was remarkably good in the present study and was better than expected from the baseline HbA1c levels.
The overall glucose measurement performance of the artificial pancreas seems to be acceptable, with an MARD of 11.6% during the artificial pancreas period and 15.5% during the control period; however, the MARD was rather variable between patients. During the artificial pancreas period, more SMBG measurements were performed, partly because an additional SMBG measurement during the night was prescribed in the protocol for safety reasons. In both study treatment periods the regularly taken SMBG measurements could have affected patients' behaviour and thus glucose control. With the automated glucose control provided by the artificial pancreas, it was, however, not possible to manually command insulin or glucagon infusion. In addition, no correlation was found between percentage of time spent in euglycaemia and the number of SMBGs (data not shown).
A key difference from other artificial pancreas systems is that the investigated device is a fully closed‐loop system and therefore releases the patient from carbohydrate‐counting and announcing meals. Because of the time lag between plasma glucose and interstitial glucose concentration and the delayed action of subcutaneously administered insulin, postprandial glucose peaks are unavoidable. Despite these difficulties, postprandial glucose control was found to be acceptable in the present study while there were no restrictions in diet, which anecdotally led to patients to challenge the artificial pancreas with, for example, exceptionally large meals. Glucose control after breakfast seemed to be most challenging, which may be partly explained by lower insulin sensitivity in the morning and already increasing blood glucose (dawn phenomenon) before breakfast 16. The integration of the different components in one device avoids connectivity problems between the CGM, control algorithm and infusion pumps.
The disadvantage of the current system design is the four subcutaneous insertion points for the infusion sets and glucose sensors. If more accurate and reliable glucose sensors or combined glucose sensors and infusion sets become available, this aspect may be improved. The use of two sensors does, however, enable continuation of the closed‐loop glucose control in case one sensor needs to be replaced and facilitates detection of sensor inaccuracies. In addition, the glucagon had to be reconstituted every day and occluded the tubing of the infusion set several times. Fortunately, pharmaceutical companies are working on the development of stable glucagon 17. An important limitation of the present trial is that the insulin setting was evaluated daily and, if needed, manually adapted. Furthermore, the use of the artificial pancreas was irregularly telemonitored by the research team and, if needed, they assisted the patients in using the device. For future trials, the consultation with the research team before entering an SMBG will not be necessary because of CGM software improvements and the manual adaptations of the insulin sensitivity factor will be automated to cope with day‐to‐day variations in insulin sensitivity.
In the present study, the artificial pancreas system was compared with insulin pump therapy and not with sensor‐augmented pump therapy. We did not choose sensor‐augmented pump therapy because it is used by a limited number of patients only, because of reimbursement 18, while insulin pump therapy is a standard diabetes therapy. In future studies, we plan to also include patients with impaired awareness of hypoglycaemia. The bihormonal artificial pancreas may especially benefit patients at risk of hypoglycaemia.
In conclusion, we have developed a wearable bihormonal artificial pancreas with the CGM, control algorithm and infusion pumps integrated into one device for daily use at home. The present pilot study indicates that our artificial pancreas provides good glucose control and is safe.
Conflict of Interest
H. B. and R. K. are employees of Inreda Diabetic. R. K. holds patents related to the bihormonal artificial pancreas. J. H. D. V. has served on advisory panel for Johnson & Johnson and received research support from Abbott Diabetes Care, Dexcom, and Medtronic. A. C. V. B. has no relevant conflicts of interest.
H. B. contributed to the study design, conduct of the study, data analysis and manuscript writing and editing. A. C. V. B. contributed to the study design, conduct of the study, and manuscript review and editing. R. K. contributed to the study design, provided technical support during the study and reviewed the manuscript. J. H. D. V. contributed to the study design and manuscript review and editing. All authors have seen and approved the final version of the manuscript.
Acknowledgements
This study was supported by the European Commission under the Seventh Framework Programme (PCDIAB, grant agreement number 305654). Medtronic provided Enlite glucose sensors for a reduced rate. We thank Roche Diabetes Care for providing research material support.
References
- 1. Nimri R, Muller I, Atlas E et al. MD‐logic overnight control for 6 weeks of home use in patients with type 1 diabetes: randomized crossover trial. Diabetes Care 2014; 37: 3025–3032. [DOI] [PubMed] [Google Scholar]
- 2. Kropff J, Del Favero S, Place J et al. 2 month evening and night closed‐loop glucose control in patients with type 1 diabetes under free‐living conditions: a randomised crossover trial. Lancet Diabetes Endocrinol 2015; 3: 939–947. [DOI] [PubMed] [Google Scholar]
- 3. Thabit H, Tauschmann M, Allen JM et al. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med 2015; 373: 2129–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haidar A, Legault L, Messier V, Mitre TM, Leroux C, Rabasa‐Lhoret R. Comparison of dual‐hormone artificial pancreas, single‐hormone artificial pancreas, and conventional insulin pump therapy for glycaemic control in patients with type 1 diabetes: an open‐label randomised controlled crossover trial. Lancet Diabetes Endocrinol 2015; 3: 17–26. [DOI] [PubMed] [Google Scholar]
- 5. Jacobs PG, El Youssef J, Castle J et al. Automated control of an adaptive bihormonal, dual‐sensor artificial pancreas and evaluation during inpatient studies. IEEE Trans Biomed Eng 2014; 61: 2569–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Russell SJ, El‐Khatib FH, Sinha M et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med 2014; 371: 313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Russell SJ, Hillard MA, Balliro C et al. Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol 2016; 4: 233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Bon AC, Luijf YM, Koebrugge R, Koops R, Hoekstra JB, Devries JH. Feasibility of a portable bihormonal closed‐loop system to control glucose excursions at home under free‐living conditions for 48 hours. Diabetes Technol Ther 2014; 16: 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gingras V, Rabasa‐Lhoret R, Messier V, Ladouceur M, Legault L, Haidar A. Efficacy of dual‐hormone artificial pancreas to alleviate the carbohydrate‐counting burden of type 1 diabetes: a randomized crossover trial. Diabetes Metab 2016; 42: 47–54. [DOI] [PubMed] [Google Scholar]
- 10. Thabit H, Lubina‐Solomon A, Stadler M et al. Home use of closed‐loop insulin delivery for overnight glucose control in adults with type 1 diabetes: a 4‐week, multicentre, randomised crossover study. Lancet Diabetes Endocrinol 2014; 2: 701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ly TT, Roy A, Grosman B et al. Day and night closed‐loop control using the integrated medtronic hybrid closed‐loop system in type 1 diabetes at diabetes camp. Diabetes Care 2015; 38: 1205–1211. [DOI] [PubMed] [Google Scholar]
- 12. Gold AE, MacLeod KM, Frier BM. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care 1994; 17: 697–703. [DOI] [PubMed] [Google Scholar]
- 13. Clarke WL, Cox DJ, Gonder‐Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care 1995; 18: 517–522. [DOI] [PubMed] [Google Scholar]
- 14. Blauw H, Wendl I, DeVries JH, Heise T, Jax T, consortium P. Pharmacokinetics and pharmacodynamics of various glucagon dosages at different blood glucose levels. Diabetes Obes Metab 2016; 18: 34–39. [DOI] [PubMed] [Google Scholar]
- 15. Clarke W, Kovatchev B. Statistical tools to analyze continuous glucose monitor data. Diabetes Technol Ther 2009; 11: S45–S54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carroll MF, Schade DS. The dawn phenomenon revisited: implications for diabetes therapy. Endocr Pract 2005; 11: 55–64. [DOI] [PubMed] [Google Scholar]
- 17. Jackson MA, Caputo N, Castle JR, David LL, Roberts CT Jr, Ward WK. Stable liquid glucagon formulations for rescue treatment and bi‐hormonal closed‐loop pancreas. Curr Diab Rep 2012; 12: 705–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heinemann L, DeVries JH. Reimbursement for continuous glucose monitoring. Diabetes Technol Ther 2016; 18: S248–S252. [DOI] [PMC free article] [PubMed] [Google Scholar]


