Abstract
An adequate vitamin D status is essential to optimize muscle strength. However, whether vitamin D directly reduces muscle fiber atrophy or stimulates muscle fiber hypertrophy remains subject of debate. A mechanism that may affect the role of vitamin D in the regulation of muscle fiber size is the local conversion of 25(OH)D to 1,25(OH)2D by 1α‐hydroxylase. Therefore, we investigated in a murine C2C12 myoblast culture whether both 1,25(OH)2D3 and 25(OH)D3 affect myoblast proliferation, differentiation, and myotube size and whether these cells are able to metabolize 25(OH)D3 and 1,25(OH)2D3. We show that myoblasts not only responded to 1,25(OH)2D3, but also to the precursor 25(OH)D3 by increasing their VDR mRNA expression and reducing their proliferation. In differentiating myoblasts and myotubes 1,25(OH)2D3 as well as 25(OH)D3 stimulated VDR mRNA expression and in myotubes 1,25(OH)2D3 also stimulated MHC mRNA expression. However, this occurred without notable effects on myotube size. Moreover, no effects on the Akt/mTOR signaling pathway as well as MyoD and myogenin mRNA levels were observed. Interestingly, both myoblasts and myotubes expressed CYP27B1 and CYP24 mRNA which are required for vitamin D3 metabolism. Although 1α‐hydroxylase activity could not be shown in myotubes, after treatment with 1,25(OH)2D3 or 25(OH)D3 myotubes showed strongly elevated CYP24 mRNA levels compared to untreated cells. Moreover, myotubes were able to convert 25(OH)D3 to 24R,25(OH)2D3 which may play a role in myoblast proliferation and differentiation. These data suggest that skeletal muscle is not only a direct target for vitamin D3 metabolites, but is also able to metabolize 25(OH)D3 and 1,25(OH)2D3. J. Cell. Physiol. 231: 2517–2528, 2016. © 2016 The Authors. Journal of Cellular Physiology Published by Wiley Periodicals, Inc.
Aging is associated with a loss of muscle mass, bone mass, and strength, which may result in reduced mobility and an increased risk for falls and fractures (Cederholm et al., 2013; Rizzoli et al., 2014). An adequate vitamin D status is essential to reduce the risk for falls and fractures and to optimize bone mineral density and muscle strength (Morgan, 2008; Lips and van Schoor, 2011; Bischoff‐Ferrari, 2012). Vitamin D stimulates calcium absorption from the intestine and maintains serum calcium levels which is required for normal bone mineralization and muscle function (Lips, 2006). Regarding bone metabolism, vitamin D reduces osteoblast proliferation, stimulates osteoblast differentiation, and induces RANKL expression in osteoblasts which is involved in stimulation of osteoclast formation and bone resorption (Lips, 2006; Anderson and Atkins, 2008; van der Meijden et al., 2014). However, whether vitamin D directly reduces muscle fiber atrophy or stimulates muscle fiber hypertrophy remains subject of debate.
Several in vivo studies suggest a role for vitamin D in the regulation of muscle mass and function. Observational studies demonstrate that vitamin D deficiency in elderly people is associated with reduced muscle mass (Tieland et al., 2013) and strength (Bischoff et al., 1999; Zamboni et al., 2002), lower physical performance (Wicherts et al., 2007; Tieland et al., 2013), and an increased risk of falling (Snijder et al., 2006). Furthermore, a meta‐analysis of 17 randomized controlled trials showed that vitamin D supplementation in subjects with a baseline serum 25‐hydroxyvitamin D (25(OH)D) lower than 25 nmol/L did have a positive effect on hip muscle strength (Stockton et al., 2011). In animal models, reduced muscle function was reported in vitamin D deficient rats (Rodman and Baker, 1978; Pleasure et al., 1979) and chickens (Bischoff‐Ferrari, 2012) compared to control animals. The studies described above suggest that vitamin D can affect muscle mass and function, however it is not clear whether vitamin D plays a direct or indirect role.
In vitro studies on myoblasts and myotubes show that the active metabolite 1,25‐dihydroxyvitamin D (1,25(OH)2D) is able to directly affect myogenesis (Garcia et al., 2011; Buitrago et al., 2012; Girgis et al., 2014a). Myogenesis, a process that is essential for muscle regeneration, growth and hypertrophy, includes satellite cell activation, myoblast proliferation, differentiation, and myotube formation (Zanou and Gailly, 2013). Regarding myoblast proliferation in vitro, most studies show inhibitory effects of 1,25(OH)2D (Simpson et al., 1985; Garcia et al., 2011; Okuno et al., 2012; Srikuea et al., 2012; Girgis et al., 2014a) likely due to a cell cycle arrest at the G1 to S transition (Girgis et al., 2014a). However, 1,25(OH)2D effects on proliferation have also been reported to be absent (Stio et al., 2002) or stimulatory (Drittanti et al., 1989a; Capiati et al., 1999; Buitrago et al., 2012). Furthermore, whether 1,25(OH)2D affects myoblast differentiation and hypertrophy of differentiated myotubes is not well known. Recently, it has been shown that when myoblasts were cultured in growth medium and subsequently in differentiation medium which were supplemented with 1,25(OH)2D from the start of the culture resulted in less myotubes (Girgis et al., 2014a), but myotubes were larger in diameter than those that were differentiated in medium without supplemented 1,25(OH)2D (Garcia et al., 2011; Girgis et al., 2014a). Since in these experiments the number of myoblasts was not standardized due to the anti‐proliferating effects of 1,25(OH)2D, the larger myotube size could not be ascribed to a direct effect of 1,25(OH)2D per se. As, yet it is still unknown what the effects are of 1,25(OH)2D on myotube formation and size in cultures starting with the same number of cells.
A mechanism that may affect the role of vitamin D in the regulation of muscle fiber size and contractile function in vivo is the local conversion of 25(OH)D to 1,25(OH)2D. The metabolite 1,25(OH)2D is primarily synthesized in the kidney from the precursor 25‐hydroxyvitamin D (25(OH)D) (Lips, 2006). In addition, 1,25(OH)2D synthesis has been demonstrated in several other cell types, such as in osteoblasts (Howard et al., 1981; van Driel et al., 2006a; Atkins et al., 2007; van der Meijden et al., 2014), prostate cells (Schwartz et al., 1998), and monocytes (Bacchetta et al., 2013). In osteoblasts, the function of locally synthesized 1,25(OH)2D is supposed to be regulation of cell proliferation and differentiation (van Driel et al., 2006a; Atkins et al., 2007; Bikle, 2009). Recent studies have shown that rat muscle as well as C2C12 myoblasts and myotubes also express CYP27B1, which encodes the enzyme 1α‐hydroxylase (Testerink et al., 2011b; Srikuea et al., 2012; Girgis et al., 2014a). Moreover, CYP27B1 activity has indirectly been demonstrated in muscle cells by performing luciferase reporter studies (Girgis et al., 2014a) and CYP27B1 silencing experiments (Srikuea et al., 2012). However, to the best of our knowledge, whether C2C12 cells do convert 25(OH)D to 1,25(OH)2D has not been investigated yet. In addition to its possible conversion into 1,25(OH)2D, 25(OH)D may also be converted to 24R,25‐dihydroxyvitamin D (24R,25(OH)2D). This conversion is catalyzed by the 24‐hydroxylase enzyme, encoded by the CYP24 gene (St‐Arnaud, 2010). In osteoblasts in vitro, 24R,25(OH)2D synthesis has been shown (Turner et al., 1980; Howard et al., 1981; van der Meijden et al., 2014) and this metabolite may stimulate cell differentiation through binding to the VDR (van Driel et al., 2006a; Curtis et al., 2014; van der Meijden et al., 2014). In myoblasts and myotubes, CYP24 expression has also been shown (Girgis et al., 2014a) and CYP24 activity may, therefore, affect skeletal muscle tissue as well. However, whether skeletal muscle cells are capable of synthesizing 24R,25(OH)2D is still unknown.
The aim of this study was to investigate in a murine C2C12 myoblast culture model whether both 1,25(OH)2D3 and 25(OH)D3 affect myoblast proliferation, differentiation, and myotube size, and which regulatory mechanisms, including myogenic regulatory factors and signaling pathways, are involved. We hypothesized that 1,25(OH)2D3 affects the expression of genes in the regulation of vitamin D3 signaling in myoblasts and myotubes, inhibits myoblast proliferation and stimulates myoblast differentiation and myotube hypertrophy. Moreover, we hypothesized that actions of 25(OH)D3 occur via its conversion to 1,25(OH)2D3. Therefore, we investigated in C2C12 myoblast and myotube cultures whether supplementation of 1,25(OH)2D3 or 25(OH)D3 to culture medium alters mRNA levels of genes involved in vitamin D metabolism and/or signaling pathways for protein synthesis. We further tested whether myotubes were able to synthesize 1,25(OH)2D3 and 24R,25(OH)2D3 from supplemented 25(OH)D3.
Materials and Methods
Cell culture
Mouse C2C12 myoblast cell line was obtained from ATCC (American Type Culture Collection; Manassas, VA). Myoblasts were cultured in growth medium consisting of Dulbecco's Modified Eagle Medium (DMEM‐31885, low glucose, phenol red; Gibco, Life Technologies, Grand Island, NY) supplemented with 10% Fetal Bovine Serum (FBS; Gibco), 10 µg/ml penicillin (Sigma–Aldrich, St. Louis, MO), 10 µg/ml streptomycin (Sigma–Aldrich), 50 µg/ml fungizone (Gibco) and incubated at 37°C in humidified air with 5% CO2. Passages between 4 and 10 were used for experiments and all culture media, including those of treated and control groups, contained 0.1% ethanol (vehicle).
C2C12 myoblast proliferation
C2C12 cells were plated out in 96‐well plates or 6‐well plates at a density of 500 cells per cm2. After 24 h cells were cultured in medium with 1000 nmol/L 25(OH)D3 (Sigma–Aldrich), 100 nmol/L 1,25(OH)2D3 (Sigma–Aldrich) or without supplements (i.e., control). Medium was replaced every day by growth medium containing 25(OH)D3, 1,25(OH)2D3, or control. At day 1 and 4, the proliferation of C2C12 myoblasts in the 96‐well plate was measured using XTT Cell Proliferation Kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's protocol. Briefly, cells were incubated with XTT solution at 37°C, whereby the viable cells formed an orange formazan dye by cleaving the yellow tetrazolium salt XTT. After 2 h the orange formazan solution was quantified by a photospectrometer (Berthold Technologies, Bad Wildbad, Germany) at 450 nm. Cells from the 6‐well plate were lysed and stored at −80°C until total RNA isolation.
C2C12 myoblast differentiation
C2C12 cells were plated out in 6‐well plates and were grown until 90% confluence. To induce myotube formation, growth medium was changed to differentiation medium consisting of DMEM supplemented with 2% horse serum (Gibco), 10 µg/ml penicillin (Sigma–Aldrich), 10 µg/ml streptomycin (Sigma–Aldrich) and 50 µg/ml fungizone (Gibco) At day 1 and 3 of differentiation, cells were lysed and stored at −80°C until total RNA isolation or western blotting. Myotube thickness was measured at day 3 of the differentiation by obtaining images using a Leica inverted microscope type DM‐IL. Subsequently, myotube thickness was determined from four images per well using ImageJ (v.1.41o, National Institute of Health, USA; http://rsbweb.nih.gov/ij/). Myotubes (>90 per experiment) were measured at three locations along their lengths (25%, 50%, and 75% of the length).
RNA isolation and RT‐qPCR
Total RNA of C2C12 cells was isolated using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. For removing residual DNA amounts an additional on‐column DNase treatment was accomplished. Total RNA concentration was measured by the Nanodrop spectrophotometer (Nanodrop Technologies, Wilmington, DE).
RNA was reverse transcribed from 500 ng total RNA in a 20 µl reaction mixture using the High Capacity RNA‐to‐DNA Master Mix (Applied Biosystems, Foster City, CA). Reverse transcription was performed using the following thermal cycler conditions: 5 min at 25°C, 30 min at 42°C, and 5 min at 85°C. The PCR reaction of total 20 µl contained 5 µl cDNA, 200 nmol/L reverse and forward primer (Table 1) and SYBR Green Master Mix (Applied Biosystems). cDNA was diluted 1:10. qPCR was performed in duplicate on a StepOne real‐time PCR System (Applied Biosystems): 20 sec at 95°C, 40 cycles consisting of 3 sec at 95°C, and 30 sec at 60°C. Several housekeeping genes were tested (18S, hypoxanthine phosphoribosyltransferase and glyceraldehyde 3‐phosphate dehydrogenase) of which assessment of 18S rRNA expression was shown to be the more reproducible. Therefore, 18S rRNA was used as reference and the relative gene expression was calculated by the 2−ΔCt method.
Table 1.
Primer Sequence
| Target Gene | Primer Sequence (5′‐ 3′) |
|---|---|
| CYP27B1 | Forward: CATCATGGGCAGAGCACCGT |
| Reverse: TCACCATCCGCCGTTAGCAA | |
| Vitamin D receptor (VDR) | Forward: TCCTGCTCGATGCCCACCACA |
| Reverse: TGCACGAATTGGAGGCCGGAA | |
| CYP24 | Forward: AACAGCACGACACACTGGCAGA |
| Reverse: CTCGGCGAGCCCAGATGCAG | |
| MyoD | Forward: CATCCAGCCCGCGCTCCAAC |
| Reverse: GGGCCGCTGTAATCCATCATGCC | |
| Myogenin | Forward: CCAGCCCATGGTGCCCAGTGA |
| Reverse: CCAGTGCATTGCCCCACTCCG | |
| PGC1α | Forward: ACACAACCGCAGTCGCAACA |
| Reverse: GGGAACCCTTGGGGTCATTTGG | |
| Ki67 | Forward: GGTGGGCACCTAAGACCTGAA |
| Reverse: TCCTAGGACTAGGAGCTGGAG | |
| MHC‐I (MYH7) | Forward: AGATCCGAAAGCAACTGGAG |
| Reverse: CTGCCTTGATCTGGTTGAAC | |
| MHC‐IIA (MYH2) | Forward: GCAGAGACCGAGAAGGAG |
| Reverse: CTTTCAAGAGGGACACCATC | |
| MHC‐IIX (MYH1) | Forward: GCGACAGACACCTCCTTCAAG |
| Reverse: TCCAGCCAGCCAGCGATG | |
| MHC‐IIB (MYH4) | Forward: CAACTGAGTGAAGTGAAGACC |
| Reverse: AGCTGAGAAACCATAGCGTC | |
| MHC embryonic (MYH8) | Forward: ACTGAGGAAGACCGCAAGAA |
| Reverse: CAGGTTGGCATTGGATTGTTC | |
| 18S rRNA | Forward: GTAACCCGTTGAACCCCATT |
| Reverse: CCATCCAATCGGTAGTAGCG |
Western blot
C2C12 cells for western blot were scraped in cold radioimmunoprecipitation assay (RIPA) buffer (Thermo Fisher Scientific, Rockford, IL) containing protease and phosphatase inhibitors (Thermo Fisher Scientific). Protein concentrations were measured by the bicinchoninic acid (BCA) protein assay (Thermo Fisher Scientific). Samples (one per experiment) were denaturated in SDS–PAGE sample buffer for 5 min at 90°C, loaded onto a SDS PAGE gel and transferred to a nitrocellulose membrane (GE Healthcare, Little Chalfont, UK). After transfer, the membrane was blocked overnight at 4°C with 2% ECL Advance Blocking Agent (GE Healthcare) in TBS with 0.01% Tween 20 (Sigma–Aldrich). Subsequently, the membrane was washed and incubated for 1 h at room temperature with primary antibody against p‐Akt (Ser473; 1:4000), total Akt (1:4000), p‐S6 (Ser235/236; 1:1000), total S6 (1:1000), and β‐tubulin (1:2000) (all Cell Signaling Technology). After washing, the membrane was incubated for 1 h at room temperature with horseradish peroxidase‐conjugated polyclonal goat anti‐rabbit secondary antibody (1:4000; DakoCytomation, Glostrup, Denmark) and the membrane was analyzed with the enhanced chemiluminescence method (ECL Advance; GE Healthcare). Western blots were quantified using ImageJ. Total Akt, p‐Akt, total S6, and p‐S6 were normalized to β‐tubulin.
Measurement of medium concentrations of 1,25(OH)2D3, 25(OH)D3, and 24R,25(OH)2D3
C2C12 myoblasts were seeded into 6‐well plates and were grown until 90% confluence. Cells were induced to form myotubes by changing growth medium to differentiation medium. After 3 days of differentiation, cells were cultured in medium supplemented with 0, 400, 1000, or 2000 nmol/L 25(OH)D3 for 24 h. Medium was collected and stored at −20°C until measurement of vitamin D3 metabolites. As positive control, primary human osteoblasts were cultured in medium supplemented with 0, 400, and 1000 nmol/L 25(OH)D3 in 6‐well plates with a cell density of 500.000 cells per well for 24 h, as described previously (van der Meijden et al., 2014). Medium was collected and stored at −20°C until measurement of vitamin D3 metabolites.
The metabolite 1,25(OH)2D3 was measured in non‐conditioned and conditioned medium using a radioimmunoassay (Immunodiagnostic Systems, Boldon, UK). Cross reactivity with 25(OH)D3 and 24R,25(OH)2D3 was 0.01% and <0.01% respectively. The intra‐assay variation was 8% at a level of 25 pmol/L and 9% at a level of 70 pmol/L. The inter‐assay variation was 11% at a concentration of 25 and 70 pmol/L.
The metabolites 25(OH)D3 and 24R,25(OH)2D3 were analyzed in non‐conditioned and conditioned medium using liquid chromatography‐tandem mass spectrometry (LC‐MS/MS). Briefly, samples were incubated with deuterated internal vitamin D standards (d6‐25(OH)D3 and d6‐24R,25(OH)2D3) and protein‐precipitated using acetonitrile. Supernatant was, after PTAD derivatization, purified using a Symbiosis online solid phase extraction (SPE) system (Spark Holland, Emmen, The Netherlands), followed by detection with a Quattro Premier XE tandem mass spectrometer (Waters Corp., Milford, MA). Intra‐assay variation of 25(OH)D3 was 9.6%, 6.0%, and 8.5% at a level of 58, 191, and 516 nmol/L, respectively. Intra‐assay variation of 24R,25(OH)2D3 was 5.4% and 9.1% at a level of 46 and 150 nmol/L, respectively.
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). Data were analyzed using SPSS version 20.0 software (SPSS Inc., Chicago, IL). Differences between groups were assessed using a one‐way ANOVA followed by Bonferroni's post hoc test to examine the effects of treatment on myotube diameter and myotube number. A two‐way ANOVA followed by Bonferroni's post hoc test was used to examine the effects of time and vitamin D3 treatment on absorbance values, mRNA expression levels and protein expression levels. A three‐way ANOVA was used to examine whether time and vitamin D3 treatment affect expression levels of myosin heavy chain isoforms. A P‐value < 0.05 was considered to be significant (*P < 0.05, **P < 0.01, ***P < 0.001).
Results
Both 25(OH)D3 and 1,25(OH)2D3 attenuated C2C12 myoblast proliferation
Figure 1 shows effects of 1 and 4 days of supplementation of 25(OH)D3 and 1,25(OH)2D3 on estimates of viable cell numbers determined by the absorbance of the colored formazan product which is directly proportional to the number of viable cells. Two‐way ANOVA revealed a significant effect of time as well as an interaction between time and vitamin D3 treatment (P < 0.001). As expected, for all treatment groups the number of viable cells was significantly increased at day 4 compared to day 1 (P < 0.001; Fig. 1D). At day 4, both 1000 nmol/L 25(OH)D3 and 100 nmol/L 1,25(OH)2D3 significantly reduced the number of viable cells compared to control culture (P < 0.001). In the presence of 1000 nmol/L 25(OH)D3 and 100 nmol/L 1,25(OH)2D3 the proportion of viable cells was shown to be 30.1% and 27.6% lower than in the control condition, respectively.
Figure 1.
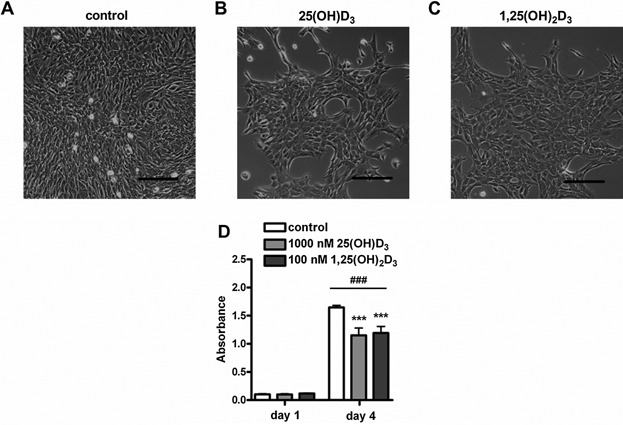
Both 25(OH)D3 and 1,25(OH)2D3 attenuated C2C12 myoblast proliferation. Micrographs of C2C12 myoblasts cultured for 4 days in growth medium (A), in growth medium supplemented with 1000 nmol/L 25(OH)D3 (B), or 100 nmol/L 1,25(OH)2D3 (C). Myoblast proliferation was quantified at day 1 and 4 (D). Scale bar indicates 100 µm. Data were analyzed using a two‐way ANOVA followed by Bonferroni's post hoc comparisons test. Values are mean ± SEM (n = 20). ***P < 0.001; ### P < 0.001 (# between time period, * between vitamin D3 concentrations).
Both 25(OH)D3 and 1,25(OH)2D3 increased CYP24 and VDR mRNA levels and reduced myogenin mRNA levels in proliferating myoblasts
Since myoblast proliferation was reduced by vitamin D3 metabolites, we next investigated whether myoblasts expressed mRNA of proteins involved in vitamin D3 metabolism and whether expression levels were modulated by vitamin D3 metabolites. Myoblasts did express CYP27B1, CYP24, and VDR. At day 4, CYP27B1 mRNA levels were lower compared to those at day 1 (P < 0.05; Fig. 2A), but significant effects of 25(OH)D3 or 1,25(OH)2D3 were not observed. CYP24 mRNA levels were higher at day 4 compared to day 1 (P < 0.01; Fig. 2B). At day 1 and day 4, CYP24 and VDR mRNA levels were both significantly increased by 25(OH)D3 (P < 0.01) as well as by 1,25(OH)2D3 (P < 0.01 and P < 0.05, respectively; Fig. 2B and C). To investigate mechanisms underlying the anti‐proliferative effects of both vitamin D3 metabolites, we determined mRNA levels of ki67 and MyoD. Ki67 mRNA was significantly reduced at day 4 compared to day 1 (P < 0.001; Fig. 2D), but 25(OH)D3 and 1,25(OH)2D3 supplementation did not change these mRNA levels. MyoD mRNA levels were also not affected by 25(OH)D3 or 1,25(OH)2D3 supplementation (Fig. 2E). For myogenin, two‐way ANOVA showed a significant interaction between the effects of time and vitamin D3 treatment (P < 0.05; Fig. 2F). Post hoc analysis revealed that 25(OH)D3 and 1,25(OH)2D3 reduced myogenin mRNA levels at day 4 of proliferation (P < 0.01). In addition, myogenin mRNA levels in control cultures were significantly increased at day 4 compared to day 1 (P < 0.001), but mRNA levels at day 4 were still 44 times lower than myogenin mRNA levels in myotubes (Fig. 4E).
Figure 2.
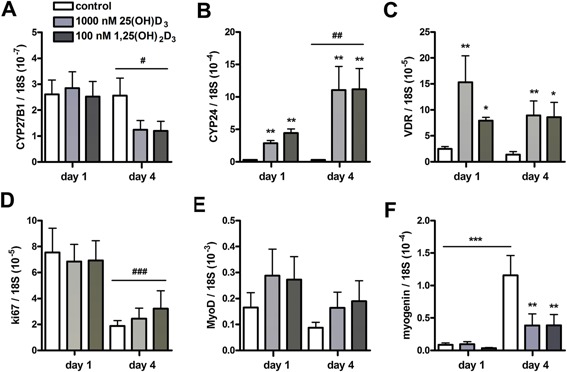
Both 25(OH)D3 and 1,25(OH)2D3 increased CYP24 and VDR mRNA levels and reduced myogenin mRNA levels in myoblasts. Myoblasts were cultured for 4 days in growth medium supplemented with 1000 nmol/L 25(OH)D3, 100 nmol/L 1,25(OH)2D3, or without any supplements. After 1 and 4 days, mRNA levels of CYP27B1 (A), CYP24 (B), VDR (C), ki67 (D), MyoD (E), and myogenin (F) were determined. Data were analyzed using a two‐way ANOVA followed by Bonferroni's post hoc comparisons test. Values are mean ± SEM (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001; # P < 0.05, ### P < 0.01, ### P < 0.001 (# between time period, * between vitamin D3 concentrations).
Figure 4.
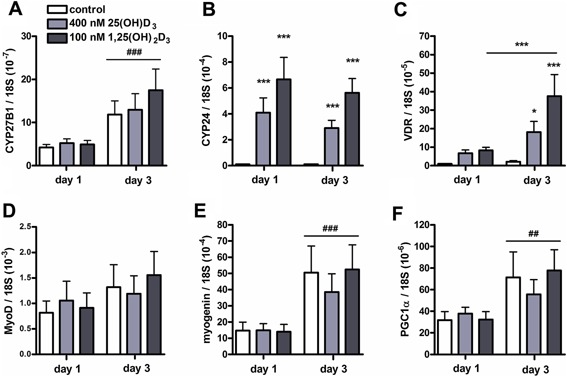
Both 25(OH)D3 and 1,25(OH)2D3 increased CYP24 and VDR mRNA levels in differentiating C2C12 cells. C2C12 cells were cultured for 3 days in differentiation medium supplemented with 400 nmol/L 25(OH)D3, 100 nmol/L 1,25(OH)2D3, or without any supplements. After 1 and 3 days of culture, mRNA levels of CYP27B1 (A), CYP24 (B), VDR (C), MyoD (D), myogenin (E), and PGC1α (F) were determined. Data were analyzed using a two‐way ANOVA followed by Bonferroni's post hoc comparisons test. Values are mean ± SEM (n = 6). *P < 0.05, ***P < 0.001; ## P < 0.01, ### P < 0.001 (# between time period, * between vitamin D3 concentrations).
Effects of 25(OH)D3 and 1,25(OH)2D3 on myotube diameter
Confluent (90%) cultures of myoblasts differentiated into myotubes during 3 days of culture in differentiation medium. To examine whether 25(OH)D3 and 1,25(OH)2D3 stimulated myotube hypertrophy, we measured myotube diameter at day 3 of the differentiation. Figure 3 shows micrographs of control myotubes, and 25(OH)D3 or 1,25(OH)2D3 treated cells taken at day 3 of treatment (Fig. 3A–C). The diameter of myotubes exposed to 25(OH)D3 was slightly increased compared to control myotubes (19%; P < 0.05; Fig. 3D), but an effect of 1,25(OH)2D3 on myotube diameter was not observed. The number of myotubes per mm2 was not altered by both vitamin D3 metabolites (Fig. 3E).
Figure 3.
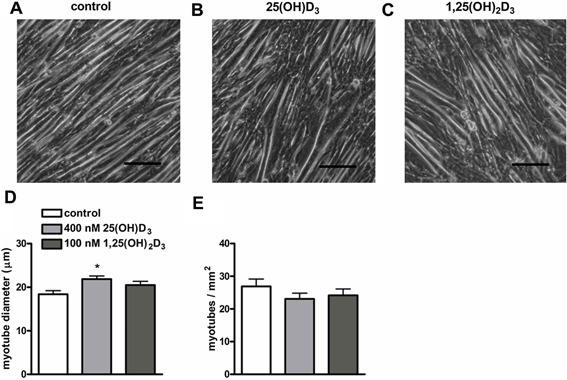
Effects of 25(OH)D3 and 1,25(OH)2D3 on myotube diameter. Micrographs of C2C12 myoblasts cultured for 3 days in differentiation medium (A), in differentiation medium supplemented with 400 nmol/L 25(OH)D3 (B), or 100 nmol/L 1,25(OH)2D3 (C). After 3 days of culture, myotube diameter (µm) (D) and myotubes/mm2 (E) were determined. Scale bar indicates 100 µm. Data were analyzed using a one‐way ANOVA followed by Bonferroni's post hoc test. Values are mean ± SEM (n = 8). *P < 0.05.
To investigate whether the lack of hypertrophy was associated with the high dose of the vitamin D3 metabolites, we tested whether low concentrations of vitamin D3 metabolites would induce myotube hypertrophy. For this purpose myoblasts were differentiated into myotubes in the presence of 100 nmol/L 25(OH)D3 and 1 nmol/L 1,25(OH)2D3. However, these low concentrations of 25(OH)D3 and 1,25(OH)2D3 did not affect myotube diameter or the number of myotubes per mm2 (Fig. S1A and B, Supplementary Data).
Both 25(OH)D3 and 1,25(OH)2D3 increased CYP24 and VDR mRNA levels in differentiating myotubes
Figure 4 shows mRNA levels of CYP27B1, CYP24, VDR, MyoD, myogenin, and PGC1α during myotube formation. CYP27B1 mRNA levels after 3 days of culture in differentiation medium were increased compared to those after 1 day (P < 0.001; Fig. 4A). CYP24 mRNA levels were substantially increased by both 25(OH)D3 and 1,25(OH)2D3 (P < 0.001; Fig. 4B). For mRNA levels of VDR, two‐way ANOVA showed significant interaction effects between time and vitamin D3 treatment (P < 0.05; Fig. 4C). Post hoc analyses showed that after 3 days of differentiation both 25(OH)D3 and 1,25(OH)2D3 increased VDR mRNA levels (P < 0.05 and P < 0.001, respectively). In addition, within 1,25(OH)2D3 treated myotubes, VDR mRNA levels at day 3 were increased compared to those measured at day 1 (P < 0.001). MyoD mRNA levels were not affected by time or vitamin D3 treatment (Fig. 4D). After 3 days of culture in differentiation medium, myogenin and PGC1α mRNA levels were significantly higher than those after 1 day of culture in differentiation medium (P < 0.001 and P < 0.01, respectively; Fig. 4E and F), but effects of vitamin D3 treatment could not be shown.
We also verified whether low concentrations of 25(OH)D3 (100 nmol/L) and 1,25(OH)2D3 (1 nmol/L) were able to affect mRNA levels of CYP27B1, CYP24, VDR, MyoD, and myogenin during myotube formation (Fig. S2A–E, Supplementary Data). Low concentrations of 25(OH)D3 and 1,25(OH)2D3 did not affect CYP27B1 mRNA (Fig. S2A). CYP24 and VDR mRNA levels were markedly increased by 1 nmol/L 1,25(OH)2D3 (P < 0.001; Fig. S2B and C), but 100 nmol/L 25(OH)D3 did not induce CYP24 or VDR mRNA. MyoD and myogenin mRNA levels were not affected by low concentrations of 25(OH)D3 and 1,25(OH)2D3 (Fig. S2D and E).
Effects of 25(OH)D3 and 1,25(OH)2D3 on mRNA levels of myosin heavy chain (MHC)
Using a three‐way ANOVA, we tested whether vitamin D3 metabolites altered myotube phenotype by changing MHC isoform expression. After 3 days of culture in differentiation medium, mRNA levels of MHC‐I, MHC‐IIA, MHC‐IIX, MHC‐IIB, and MHC embryonic were increased compared to those at day 1 (P < 0.001; Fig. 5A–E). Since MHC expression levels are a hallmark of differentiation, effects of vitamin D3 metabolites may have been obscured due to variations in MHC expression levels between experiments as the degree of differentiation may differ from experiment to experiment. Therefore, data were also normalized (treatment/control ratio). Three‐way ANOVA on normalized data showed a significant interaction between time and vitamin D3 treatment (P < 0.001). A main effect of vitamin D3 treatment on MHC mRNA levels was significant at day 3, but not at day 1. Post hoc analysis revealed that for all conditions MHC mRNA levels were higher at day 3 than those at day 1 (P < 0.001) and revealed also that at day 3, 1,25(OH)2D3 significantly increased mRNA levels of MHC compared to control (P < 0.01).
Figure 5.
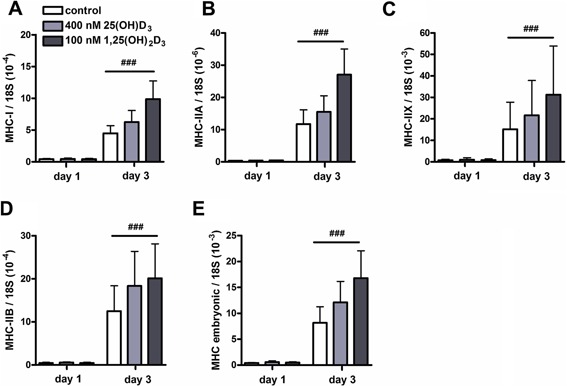
Effects of 25(OH)D3 and 1,25(OH)2D3 on mRNA levels of myosin heavy chain. C2C12 cells were cultured for 3 days in differentiation medium supplemented with 400 nmol/L 25(OH)D3, 100 nmol/L 1,25(OH)2D3, or without any supplements. After 1 and 3 days of culture, mRNA levels of MHC‐I (A), MHC‐IIA (B), MHC‐IIX (C), MHC‐IIB (D), and MHC embryonic (E) were determined. Data were analyzed using a three‐way ANOVA. Values are mean ± SEM (n = 6). ### P < 0.001 (# between time period).
We also verified whether low concentrations of 25(OH)D3 (100 nmol/L) and 1,25(OH)2D3 (1 nmol/L) were able to affect mRNA levels of different types of MHC during myotube formation (Fig. S3A–E, Supplementary Data). We found that MHC mRNA levels were higher at day 3 than those at day 1 (P < 0.05), but low concentrations of 25(OH)D3 and 1,25(OH)2D3 did not affect mRNA levels of any of the MHC isoforms.
Both 25(OH)D3 and 1,25(OH)2D3 did not affect levels of p‐Akt, total Akt, p‐S6, and total S6 during myotube formation
To explain the positive effect of 25(OH)D3 on myotube size, we examined whether 25(OH)D3 and 1,25(OH)2D3 activate components of the Akt/mTOR signaling pathway, including Akt and S6. Levels of p‐Akt and p‐S6 were not affected by time and treatment with vitamin D3 metabolites (Fig. 6A and B). Total Akt protein levels after 3 days of culture were increased compared to those at day 1 (P < 0.01; Fig. 6C), but the two vitamin D3 metabolites did not change this. Total S6 levels were not affected by time and vitamin D3 treatment (Fig. 6D). Ratio of p‐Akt/total Akt was lower at day 3 compared to day 1 (P < 0.01; Fig. 6E), whereas a higher ratio of p‐S6/total S6 was found at day 3 compared to day 1 (P < 0.05; Fig. 6F). These results indicate that both vitamin D3 metabolites did not enhance Akt/mTOR signaling.
Figure 6.
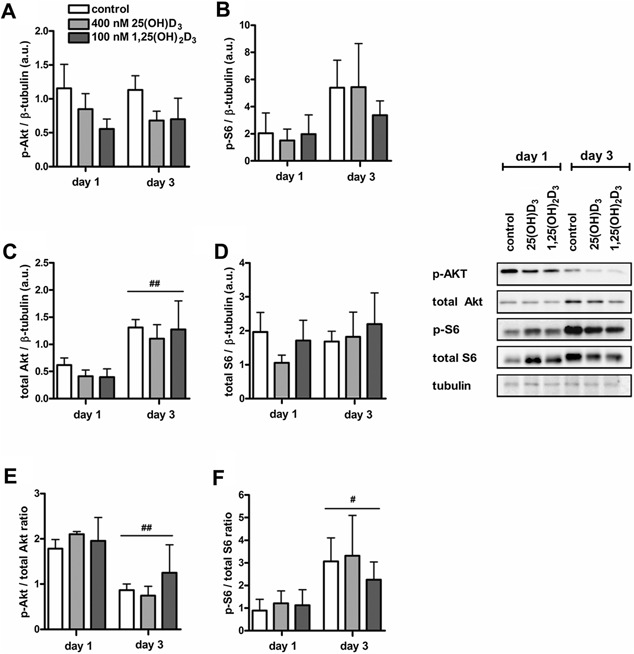
Both 25(OH)D3 and 1,25(OH)2D3 did not affect levels of p‐Akt, total Akt, p‐S6, and total S6 in differentiating C2C12 cells. C2C12 cells were cultured for 3 days in differentiation medium supplemented with 400 nmol/L 25(OH)D3, 100 nmol/L 1,25(OH)2D3, or without any supplements. After 1 and 3 days of culture, levels of p‐Akt (A), p‐S6 (B), total Akt (C), total S6 (D) the ratio of p‐Akt/total Akt (E), and p‐S6/total S6 (F) were determined. Data were analyzed using a two‐way ANOVA followed by Bonferroni's post hoc comparisons test. Values are mean ± SEM (n = 4). # P < 0.05, ## P < 0.01 (# between time period, * between vitamin D3 concentrations).
Myotubes did not synthesize detectable 1,25(OH)2D3 levels, but synthesized 24R,25(OH)2D3 after exposure to 25(OH)D3
To test whether the effects of 25(OH)D3 occur via conversion to 1,25(OH)2D3, we investigated whether C2C12 cells were able to synthesize 1,25(OH)2D3 from 25(OH)D3. Because CYP27B1 mRNA levels in cells at day 3 of the differentiation (myotubes) were higher than those at day 1 (myoblasts), we chose to examine the conversion in myotubes. Myotubes were exposed to 0, 400, 1000, or 2000 nmol/L 25(OH)D3 and after 24 h 25(OH)D3 concentrations were strongly reduced to, respectively, 42%, 34%, and 36% of non‐conditioned values (data not shown). However, after 24 h of culture the metabolite 1,25(OH)2D3 could not be detected in medium (Table 2A). As positive control, osteoblasts were cultured in medium supplemented with 0, 400, or 1000 nmol/L 25(OH)D3. After 24 h, mean concentrations of, respectively, <10.0, 110.3, and 183.0 pmol/L 1,25(OH)2D3 were measured in medium, whereas mean 1,25(OH)2D3 concentrations in non‐conditioned medium of, respectively, <10.0, 43.3, and 64.7 pmol/L were measured. These results indicate that primary human osteoblasts do convert 25(OH)D3 to 1,25(OH)2D3, whereas C2C12 myotubes do not.
Table 2.
Myotubes did not synthesize detectable 1,25(OH)2D3 levels after exposure to 25(OH)D3
| A | Myotubes | Osteoblasts positive control | ||
|---|---|---|---|---|
| 1,25(OH)2D3 (pM) PRE | 1,25(OH)2D3 (pM) POST | 1,25(OH)2D3 (pM) PRE | 1,25(OH)2D3 (pM) POST | |
| 0 nM 25(OH)D3 | 13.0 | 12.0 ± 1.0 | <10.0 | <10.0 |
| 400 nM 25(OH)D3 | 43.0 | 30.5 ± 0.5 | 43.3 ± 2.2 | 110.3 ± 13.5 |
| 1000 nM 25(OH)D3 | 76.0 | 52.5 ± 0.5 | 64.7 ± 4.8 | 183.0 ± 26.6 |
| 2000 nM 25(OH)D3 | 125.0 | 105.0 ± 3.0 | – | – |
| B | Myotubes | Osteoblasts positive control | ||
|---|---|---|---|---|
| 24R,25(OH)2D3 (nM) PRE | 24R,25(OH)2D3 (nM) POST | 24R,25(OH)2D3 (nM) PRE | 24R,25(OH)2D3 (nM) POST | |
| 0 nM 25(OH)D3 | <3.0 | <3.0 | <3.0 | <3.0 |
| 400 nM 25(OH)D3 | <3.0 | 6.8 ± 0.1 | <3.0 | 70.2 ± 4.4 |
| 1000 nM 25(OH)D3 | <3.0 | 7.4 ± 1.3 | <3.0 | 105.4 ± 8.2 |
| 2000 nM 25(OH)D3 | <3.0 | 14.5 ± 2.1 | – | – |
Myotubes and osteoblasts (positive control) were cultured in medium supplemented with increasing concentrations of 25(OH)D3. After 24 h, 1,25(OH)2D3 (A) and 24R,25(OH)2D3 (B) concentrations in non‐conditioned (PRE) and conditioned (POST) culture medium were measured. Data are presented as mean ± SEM. Regarding the osteoblast culture, concentrations of 1,25(OH)2D3 and 24R,25(OH)2D3 in non‐conditioned and conditioned medium have been published previously (van der Meijden et al., 2014).
Because CYP24 mRNA levels were strongly induced after 25(OH)D3 treatment, we examined whether myotubes were able to synthesize 24R,25(OH)2D3 from 25(OH)D3. Myotubes were exposed to 0, 400, 1000, or 2000 nmol/L 25(OH)D3 and after 24 h of culture we, respectively, measured mean concentrations of <3, 6.8, 7.4, and 14.5 nmol/L 24R,25(OH)2D3 in medium (Table 2B). In non‐conditioned medium, 24R,25(OH)2D3 concentrations were below their detection limit (<3 nmol/L). As positive control, osteoblasts were cultured in medium supplemented with 0, 400, or 1000 nmol/L 25(OH)D3. After 24 h, mean concentrations of, respectively, <3, 70.2, and 105.4 nmol/L 24R,25(OH)2D3 were measured in medium, whereas 24R,25(OH)2D3 concentrations in non‐conditioned medium were below their detection limit (<3 nmol/L). These results indicate that myotubes are able to convert 25(OH)D3 to 24R,25(OH)2D3.
Discussion
The aim of this study was twofold: (i) to investigate the effects of 1,25(OH)2D3 and 25(OH)D3 on proliferation and differentiation of myoblasts and myotube size; and (ii) to investigate 25(OH)D3 metabolism within C2C12 muscle cells. With respect to our first aim, we demonstrated in myoblasts that both 25(OH)D3 and 1,25(OH)2D3 increased VDR mRNA levels, reduced proliferation and decreased myogenin mRNA levels. During differentiation, both 25(OH)D3 and 1,25(OH)2D3 increased VDR mRNA levels, but did not activate the Akt/mTOR pathway. Only 25(OH)D3 slightly increased myotube size. Regarding our second aim, we hypothesized that effects of 25(OH)D3 occur after its conversion to 1,25(OH)2D3, but despite the presence of CYP27B1 mRNA in myoblasts and myotubes we could not demonstrate 1,25(OH)2D3 synthesis in medium of myotubes after exposure to 25(OH)D3. Interestingly, in myoblasts and myotubes CYP24 mRNA levels were increased in response to 25(OH)D3 and accompanied by elevated 24R,25(OH)2D3 levels in medium. These results suggest that skeletal muscle cells not only respond to vitamin D3 metabolites, but are also able to reduce vitamin D signaling by the activity of CYP24.
Proliferation
During myoblast proliferation, VDR mRNA expression was higher in myoblasts treated with 1,25(OH)2D3 than those without treatment. This observation is consistent with previous studies (Garcia et al., 2011; Srikuea et al., 2012; Girgis et al., 2014b) and suggests not only the presence of genomic transcriptional effects via the VDR, but also an increased responsiveness to 1,25(OH)2D3. Genomic effects of 1,25(OH)2D3 via the VDR were confirmed by strongly increased mRNA levels of CYP24, which is a target gene of the VDR. Treatment with 1,25(OH)2D3 also resulted in a reduction of myoblast number which is in line with several other studies (Simpson et al., 1985; Garcia et al., 2011; Okuno et al., 2012; Srikuea et al., 2012; Girgis et al., 2014a). This reduction of cell number in our study may in part be regulated by the genomic pathway of 1,25(OH)2D3, since it has been shown that expression of cell cycle genes is altered by 1,25(OH)2D3 (Drittanti et al., 1989b; Girgis et al., 2014a). In addition to genomic actions, non‐genomic actions of 1,25(OH)2D3 such as stimulation of ERK1/2 (Ronda et al., 2007) and p38 MAPK (Buitrago et al., 2006) have been reported to modulate proliferation of myoblasts. In addition to the inhibitory effects of 1,25(OH)2D3, a few studies reported a stimulatory effect of 1,25(OH)2D3 (Bellido et al., 1987; Buitrago et al., 2012). These stimulatory effects of 1,25(OH)2D3 on myoblast proliferation were only demonstrated at early time‐points (4–24 h), whereas inhibitory effects of 1,25(OH)2D3 were mainly found at later time‐points suggesting that the effect of 1,25(OH)2D3 on proliferation is time‐dependent. Serum concentration in medium is also important for the effects of 1,25(OH)2D3 on proliferation, since it has been reported that 1,25(OH)2D3 induces inhibitory effects in cultures with lower serum concentrations (5–10%), while higher serum concentrations (15–20%) result in stimulatory effects (Drittanti et al., 1989a). Both factors, serum and time, do probably affect the differentiation state of the cell which may determine the response of the cell to 1,25(OH)2D3. Furthermore, we observed that myoblast number was not only lower after treatment with 1,25(OH)2D3, but also after treatment with its precursor 25(OH)D3. This result confirms recent studies that found an anti‐proliferative effect of 25(OH)D3 on myoblasts as well (Srikuea et al., 2012; Girgis et al., 2014a). It shows that muscle cells have the capacity to take up 25(OH)D3 (Abboud et al., 2013) and that 25(OH)D3 is directly or indirectly able to trigger mechanisms to reduce cell number or inhibit proliferation. Effects of 25(OH)D3 may occur via conversion to 1,25(OH)2D3 since C2C12 myoblasts express CYP27B1, however as our results on myotubes show that myotubes do not convert 25(OH)D3 to 1,25(OH)2D3 a direct effect may also be possible. Although 25(OH)D3 has a low affinity for the VDR (Lips, 2007), supra‐physiological concentrations of 25(OH)D3 may activate the VDR leading to altered gene expression levels.
MyoD and ki67 were not significantly affected by both 25(OH)D3 or 1,25(OH)2D3, but myogenin mRNA levels were lower after treatment with both metabolites compared to non‐treated myoblasts which suggests that 25(OH)D3 and 1,25(OH)2D3 inhibit the differentiation in growth medium. However, it is also possible that the higher mRNA levels of myogenin in control cultures were due to the almost confluent cell culture at the end of the proliferation experiment. An increased cell density will lead to more cell–cell contact which results in an earlier initiation of the differentiation (Mudera et al., 2010).
Differentiation and hypertrophy
As in myoblasts, differentiated myotubes also showed increased VDR mRNA levels by treatment with 25(OH)D3 or 1,25(OH)D3, suggesting the presence of genomic transcriptional effects via the VDR. However, vitamin D3 signaling did not result in hypertrophic effects; we observed only a minor increase in myotube diameter (19% in 3 days) by 25(OH)D3. Other studies demonstrated increases in myotube diameter by 80–100% after 1,25(OH)2D3 or 25(OH)D3 treatment (Garcia et al., 2011; Girgis et al., 2014a). Differences in experimental set up may clarify the conflicting results. Studies which observed an effect of 1,25(OH)2D3 or 25(OH)D3 on myotube diameter, used a prolonged cell culture model in which proliferation was immediately followed by myotube formation. Due to anti‐proliferative effects of 1,25(OH)2D3 or 25(OH)D3, a lower number of cells was present at the start of the differentiation in myotubes in the cultures that had been treated with 1,25(OH)2D3 or 25(OH)D3 compared to cell cultures without treatment. The lower number of myoblasts may have resulted in a lower number of myotubes, and thicker myotubes due to extra space in the culture well. This hypothesis is supported by data showing an optimal seeding density of human myoblasts in a 3D engineered collagen construct to obtain maximal force production of myotubes (Mudera et al., 2010). A high myoblast density may have a negative impact on myoblast force generating capacity and is associated with slow myosin expression (Mudera et al., 2010). In our study, myotube formation was investigated after starting with the same cell number and we did not observe any effect of 1,25(OH)2D3 and only a minor effect of 25(OH)D3 on myotube number and size suggesting that both metabolites are not potent hypertrophic agents like for instance insulin‐like growth factor‐1 (IGF‐1) (Stitt et al., 2004). To verify whether the lack of substantial hypertrophy was due to a lack of hypertrophic signaling, we investigated the activity of the Akt/mTOR signaling pathway, a key pathway involved in skeletal myotube hypertrophy (van Wessel et al., 2010) in response to 1,25(OH)2D3 and 25(OH)D3. Akt mediates a wide range of cellular functions including cell proliferation, differentiation, gene transcription, and the rate of mRNA translation (Bodine et al., 2001; Glass, 2003; Gardner et al., 2012). Akt activation has been demonstrated by 1,25(OH)2D3 during proliferation and differentiation of C2C12 myoblasts (Buitrago et al., 2013, 2012). However, in our study during differentiation no effect of 1,25(OH)2D3 or 25(OH)D3 on the phosphorylation of Akt was observed. Moreover, downstream p‐S6 was also not affected by both metabolites. Therefore, these results suggest that both 1,25(OH)2D3 and 25(OH)D3 did not activate the Akt/mTOR signaling pathway in our model. These observations are in line with those of an in vivo rat study in which supra‐physiological 1,25(OH)2D3 levels did not result in muscle hypertrophy, but rather in muscle atrophy (Testerink et al., 2011a). This in vivo negative effect on muscle mass could be indirect, but in our cell culture model we investigated direct hypertrophic effects of 1,25(OH)2D3 and 25(OH)D3 which were not present.
Expression of transcription factors that are essential for differentiation, including MyoD and myogenin, were also not affected by 25(OH)D3 or 1,25(OH)2D3. However, at day 3 of differentiation MHC mRNA levels were increased by high concentrations of 1,25(OH)2D3. Effects of 1,25(OH)2D3 on MHC expression levels have been reported before, but those were not consistent (Okuno et al., 2012; Tanaka et al., 2014). In differentiating C2C12 myoblasts 1,25(OH)2D3 decreased embryonic MHC, while in C2C12 differentiated myotubes 1,25(OH)2D3 increased MHC‐IIA mRNA expression (Okuno et al., 2012). In vivo injection of 1,25(OH)2D in steers also showed an increased MHC‐IIA expression (Korn et al., 2013). In contrast, in differentiating C2C12 myoblasts 1,25(OH)2D3 supplementation has also been reported to increase mRNA levels of MHC‐I, MHC‐IIB, and MHC embryonic, without an effect on type IIA mRNA (Tanaka et al., 2014). Such differences may be due to differences in differentiation phases, medium composition or species differences (Okuno et al., 2012; Tanaka et al., 2014). In our study, 1,25(OH)2D3 increased MHC mRNA levels in general, however an effect on myotube diameter was not observed. This suggests that the mRNA availability was sufficient and that the rate of mRNA translation was likely not affected yet. The mechanism by which 1,25(OH)2D affects mRNA levels of MHC is not fully elucidated. Direct regulation of MHC mRNA levels by 1,25(OH)2D is possible through binding to its receptor (Tanaka et al., 2014), but non‐genomic actions of 1,25(OH)2D such as the increase in intracellular calcium concentrations (de Boland and Boland, 1987) may also play indirectly a role in the regulation of MHC mRNA expression. Thus, based on our results and those reported in above mentioned studies, we conclude that 1,25(OH)2D is able to increase mRNA levels of MHC isoforms, however effects seem to be determined by multiple factors. Effects of 25(OH)D3 on MHC expression were not observed.
Vitamin D3 metabolism
CYP27B1 mRNA and protein expression have recently been shown in C2C12 myoblasts and C2C12 myotubes (Girgis et al., 2014a,2014b) as well as in primary murine myotubes (Girgis et al., 2014b) and regenerating murine muscle fibers in vivo (Srikuea et al., 2012). We confirmed the presence of CYP27B1 mRNA levels in both myoblasts and myotubes. Moreover, we also showed that myotubes have even higher levels of CYP27B1 mRNA compared to myoblasts, which suggests that myotubes were able to synthesize higher quantities of 1,25(OH)2D3 than myoblasts. In addition, myotubes also have a higher uptake of 25(OH)D3 than myoblasts (Abboud et al., 2013). However, myotubes exposed to 25(OH)D3 did not synthesize detectable levels of 1,25(OH)2D3. This is an unexpected finding as the presence of functional CYP27B1 has been reported in C2C12 myoblasts and primary mouse myotubes by performing luciferase reporter studies (Girgis et al., 2014a,2014b). Furthermore, it has been shown that CYP27B1 knockdown in C2C12 myoblasts abolishes the anti‐proliferative effects of 25(OH)D3 (Garcia et al., 2011), which suggests that CYP27B1 is required for the actions of 25(OH)D3. The questions arises why in muscle cells in our study the presence of 1α‐hydroxylase activity did not result in the synthesis of detectable 1,25(OH)2D3 after 25(OH)D3 treatment. A possible explanation is that 1,25(OH)2D3 was soon converted to 1,24R,25(OH)3D3 by 24‐hydroxylase, which is supported by the finding that CYP24 mRNA levels were strongly increased by 1,25(OH)2D3. This explanation is supported by the observation that after 24 h 25(OH)D3 levels were strongly reduced to 34–42% of the non‐conditioned concentrations, suggesting the presence of a very high vitamin D3 metabolism in muscle cells. In osteoblasts, 25(OH)D3 levels are also reduced to 16–33% of non‐conditioned concentrations (van der Meijden et al., 2014), but medium of these osteoblasts did show detectable levels of 1,25(OH)2D3. Therefore, it is also possible that in C2C12 muscle cells 1α‐hydroxylase activity was inhibited causing extremely low or absent 1,25(OH)2D3 levels. In primary human osteoblast cultures, medium was supplemented with bovine serum albumin (BSA), but medium used in C2C12 cell cultures was supplemented with horse serum which may contain inhibiting factors such as transforming growth factor‐β (TGF‐β) (Turner et al., 2007) or growth factor independent‐1 (GFI‐1) (Dwivedi et al., 2005). Another explanation for the absent 1,25(OH)2D3 levels in C2C12 cells may be a loss of 1α‐hydroxylase activity due to post‐transcriptional abnormalities or deficient cofactors such as ferredoxin reductase or ferredoxin (Henry et al., 1992).
In addition to CYP27B1 expression, myoblasts and myotubes also expressed CYP24 mRNA. We show that 25(OH)D3 strongly increased CYP24 mRNA in myotubes and that myotubes were able to metabolize 25(OH)D3 to 24R,25(OH)2D3. This result shows that muscle cells have a functional enzyme, that is, 24‐hydroxylase, to regulate local 25(OH)D3 and and 1,25(OH)2D3 concentrations. The 24‐hydroxylase has been proposed to be responsible for the first step in degradation of 25(OH)D3 and 1,25(OH)2D3, but several studies demonstrate that 24R,25(OH)2D3 and 1,24R,25(OH)3D3 may also play a role in bone tissue (Galus et al., 1980; Yamate et al., 1994; Erben et al., 1997; Seo et al., 1997; Yamamoto et al., 1998; van Driel et al., 2006b). The metabolites 24R,25(OH)2D3 and 1,24R,25(OH)3D3 stimulate osteoblast differentiation in vitro (van Driel et al., 2006b; van der Meijden et al., 2014). This raises the question whether the synthesized 24R,25(OH)2D3 from 25(OH)D3 in our model is able to affect myoblast proliferation and differentiation. To the best of our knowledge, there is no literature available about 24R,25(OH)2D3 actions on skeletal muscle cell proliferation and differentiation. Only in cardiac and vascular smooth muscle cells actions of 24R,25(OH)2D3 have been reported, but these actions are all associated with calcium uptake by the cells and not with myogenesis. In vascular smooth muscle cells, 24R,25(OH)2D3 is able to stimulate Ca2+‐ATPase and to reduce membrane L‐type calcium channel activity as well as the intracellular calcium concentration (Shan et al., 1996). In cardiac myocytes, 24R,25(OH)2D3 stimulates the calcium uptake by these cells, but less efficiently than 1,25(OH)2D3 (Selles et al., 1994). Thus, it is possible that 24R,25(OH)2D3 affects calcium uptake by skeletal muscle cells. Regarding the actions of 24R,25(OH)2D3 in bone cells (van Driel et al., 2006b; van der Meijden et al., 2014), it is also possible that 24R,25(OH)2D3 plays a role in skeletal muscle cell development or regeneration. Therefore, additional research is needed to investigate whether 24R,25(OH)2D3 affects calcium uptake by skeletal muscle cells as well as skeletal muscle cell proliferation, differentiation and hypertrophy.
Limitations
Doses of 25(OH)D3 and 1,25(OH)2D3 used in this study were relatively high compared to normal serum concentrations and care should be taken in the translation of these results to in vivo. However, differentiation experiments were also performed with low concentrations of 1,25(OH)2D3 (1 nM) and 25(OH)D3 (100 nM) (see Figs. S1–S3). Low concentrations of 1,25(OH)2D3 increased VDR and CYP24 mRNA levels in differentiating myoblasts similarly as the higher concentrations. Incubation of differentiating myoblasts with low concentrations of 25(OH)D3 and 1,25(OH)2D3 did not lead to altered MyoD or myogenin mRNA levels nor to myotube hypertrophy. Thus, low concentrations of 1,25(OH)2D3 and 25(OH)D3 also did not have marked effects in our cell culture model with respect to differentiation and myotube size. Regarding the rate of proliferation, the anti‐proliferative effects of 25(OH)D3 and 1,25(OH)2D3 at lower concentrations have been reported previously in literature (1–100 nmol/L 25(OH)D3; 1–100 nmol/L 1,25(OH)2D3) (Okuno et al., 2012; Girgis et al., 2014a). Note, however, that tissue concentrations of 1,25(OH)2D3 can be higher than serum concentrations because of the local conversion of 25(OH)D3 to 1,25(OH)2D3. The use of relatively high doses of 25(OH)D3 and 1,25(OH)2D3 did allow us to compare our results with those from other studies using C2C12 myoblasts and myotubes (Garcia et al., 2011; Srikuea et al., 2012; Girgis et al., 2014a).
The metabolite 1,25(OH)2D3 was not detected in medium after treatment of C2C12 myotubes with 25(OH)D3 which was probably due to very fast vitamin D3 metabolism in these cells. Synthesized 1,25(OH)2D3 may be rapidly converted to 1,24R,25(OH)3D3 and therefore further research is needed to examine whether there is detectable 1,25(OH)2D3 synthesis on earlier time points.
Conclusion
This in vitro study shows that C2C12 myoblasts not only respond to 1,25(OH)2D3, but also to the precursor 25(OH)D3 by reducing their proliferation and increasing their VDR expression. In differentiating myoblasts and myotubes, 1,25(OH)2D3 as well as 25(OH)D3 stimulate VDR mRNA and in myotubes 1,25(OH)2D3 also stimulates MHC mRNA expression. However, this occurs without notable effects on expression of myogenic regulatory factors and myotube size. Interestingly, C2C12 myoblasts and myotubes express CYP27B1 and CYP24 mRNA which are required for vitamin D3 metabolism. Although CYP27B1 activity could not be shown in myotubes, after treatment with 1,25(OH)2D3 or 25(OH)D3 C2C12 muscle cells showed strongly increased CYP24 mRNA levels and were able to synthesize 24R,25(OH)2D3 from 25(OH)D3. Since 24R,25(OH)2D3 stimulates osteoblast differentiation in vitro, this metabolite may play a role in myoblast differentiation as well. These data suggest that skeletal muscle is not only a direct target for vitamin D3 metabolites, but is also able to metabolize 25(OH)D3 and 1,25(OH)2D3.
Supporting information
Additional supporting information may be found in the online version of this article at the publisher's web‐site.
Figure S1. Effects of low concentrations of 25(OH)D3 and 1,25(OH)2D3 on myotube diameter.
Figure S2. Low concentrations of 1,25(OH)2D3 increased CYP24 and VDR mRNA levels in differentiating C2C12 cells.
Figure S3. Effects of low concentrations of 25(OH)D3 and 1,25(OH)2D3 on mRNA levels of myosin heavy chain.
Acknowledgments
We thank Huib van Essen for his excellent technical advice. We also thank the technicians of the Endocrine Laboratory for performing 1,25(OH)2D3 measurements.
Conflicts of interest: All authors disclose no conflict of interest.
Literature Cited
- Abboud M, Puglisi DA, Davies BN, Rybchyn M, Whitehead NP, Brock KE, Cole L, Gordon‐Thomson C, Fraser DR, Mason RS. 2013. Evidence for a specific uptake and retention mechanism for 25‐Hydroxyvitamin d (25OHD) in SkelMuscle cells. Endocrinology 154:3022–3030. [DOI] [PubMed] [Google Scholar]
- Anderson PH, Atkins GJ. 2008. The skeleton as an intracrine organ for vitamin D metabolism. Mol Aspects Med 29:397–406. [DOI] [PubMed] [Google Scholar]
- Atkins GJ, Anderson PH, Findlay DM, Welldon KJ, Vincent C, Zannettino AC, O'Loughlin PD, Morris HA. 2007. Metabolism of vitamin D3 in human osteoblasts: Evidence for autocrine and paracrine activities of 1α, 25‐dihydroxyvitamin D3 . Bone 40:1517–1528. [DOI] [PubMed] [Google Scholar]
- Bacchetta J, Sea JL, Chun RF, Lisse TS, Wesseling‐Perry K, Gales B, Adams JS, Salusky IB, Hewison M. 2013. Fibroblast growth factor 23 inhibits extrarenal synthesis of 1,25‐dihydroxyvitamin D in human monocytes. J Bone Miner Res 28:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellido T, Drittanti L, Boland R, de Boland AR. 1987. The phospholipid and fatty acid composition of skeletal muscle cells during culture in the presence of vitamin D‐3 metabolites. Biochim Biophys Acta 922:162–169. [DOI] [PubMed] [Google Scholar]
- Bikle D. 2009. Nonclassic actions of vitamin D. J Clin Endocrinol Metab 94:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff HA, Stahelin HB, Urscheler N, Ehrsam R, Vonthein R, Perrig‐Chiello P, Tyndall A, Theiler R. 1999. Muscle strength in the elderly: Its relation to vitamin D metabolites. Arch Phys Med Rehabil 80:54–58. [DOI] [PubMed] [Google Scholar]
- Bischoff‐Ferrari HA. 2012. Relevance of vitamin D in muscle health. Rev Endocr Metab Disord 13:71–77. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. 2001. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3:1014–1019. [DOI] [PubMed] [Google Scholar]
- Buitrago C, Pardo VG, Boland R. 2013. Role of VDR in 1α,25‐dihydroxyvitamin D3‐dependent non‐genomic activation of MAPKs, Src and Akt in skeletal muscle cells. J Steroid Biochem Mol Biol 136:125–130. [DOI] [PubMed] [Google Scholar]
- Buitrago CG, Arango NS, Boland RL. 2012. 1α,25(OH)2D3‐dependent modulation of Akt in proliferating and differentiating C2C12 skeletal muscle cells. J Cell Biochem 113:1170–1181. [DOI] [PubMed] [Google Scholar]
- Buitrago CG, Ronda AC, de Boland AR, Boland R. 2006. MAP kinases p38 and JNK are activated by the steroid hormone 1α,25(OH)2‐vitamin D3 in the C2C12 muscle cell line. J Cell Biochem 97:698–708. [DOI] [PubMed] [Google Scholar]
- Capiati DA, Tellez‐Inon MT, Boland RL. 1999. Participation of protein kinase C alpha in 1,25‐dihydroxy‐vitamin D3 regulation of chick myoblast proliferation and differentiation. Mol Cell Endocrinol 153:39–45. [DOI] [PubMed] [Google Scholar]
- Cederholm T, Cruz‐Jentoft AJ, Maggi S. 2013. Sarcopenia and fragility fractures. Eur J Phys Rehabil Med 49:111–117. [PubMed] [Google Scholar]
- Curtis KM, Aenlle KK, Roos BA, Howard GA. 2014. 24R,25‐dihydroxyvitamin D3 promotes the osteoblastic differentiation of human mesenchymal stem cells. Mol Endocrinol 28:644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boland AR, Boland RL. 1987. Rapid changes in skeletal muscle calcium uptake induced in vitro by 1,25‐dihydroxyvitamin D3 are suppressed by calcium channel blockers. Endocrinology 120:1858–1864. [DOI] [PubMed] [Google Scholar]
- Drittanti L, de Boland AR, Boland R. 1989a. Modulation of DNA synthesis in cultured muscle cells by 1, 25‐dihydroxyvitamin D‐3. Biochim Biophys Acta 1014:112–119. [DOI] [PubMed] [Google Scholar]
- Drittanti LN, Boland RL, de Boland AR. 1989b. Induction of specific proteins in cultured skeletal muscle cells by 1, 25‐dihydroxyvitamin D‐3. Biochim Biophys Acta 1012:16–23. [DOI] [PubMed] [Google Scholar]
- Dwivedi PP, Anderson PH, Omdahl JL, Grimes HL, Morris HA, May BK. 2005. Identification of growth factor independent‐1 (GFI1) as a repressor of 25‐hydroxyvitamin D 1‐α hydroxylase (CYP27B1) gene expression in human prostate cancer cells. Endocr Relat Cancer 12:351–365. [DOI] [PubMed] [Google Scholar]
- Erben RG, Bante U, Birner H, Stangassinger M. 1997. Prophylactic effects of 1,24,25‐trihydroxyvitamin D3 on ovariectomy‐induced cancellous bone loss in the rat. Calcif Tissue Int 60:434–440. [DOI] [PubMed] [Google Scholar]
- Galus K, Szymendera J, Zaleski A, Schreyer K. 1980. Effects of 1α‐hydroxyvitamin D3 and 24R,25‐dihydroxyvitamin D3 on bone remodeling. Calcif Tissue Int 31:209–213. [DOI] [PubMed] [Google Scholar]
- Garcia LA, King KK, Ferrini MG, Norris KC, Artaza JN. 2011. 1,25(OH)2vitamin D3 stimulates myogenic differentiation by inhibiting cell proliferation and modulating the expression of promyogenic growth factors and myostatin in C2C12 skeletal muscle cells. Endocrinology 152:2976–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner S, Anguiano M, Rotwein P. 2012. Defining Akt actions in muscle differentiation. Am J Physiol Cell Physiol 303:C1292–C1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis CM, Clifton‐Bligh RJ, Mokbel N, Cheng K, Gunton JE. 2014a. Vitamin D signaling regulates proliferation, differentiation, and myotube size in C2C12 skeletal muscle cells. Endocrinology 155:347–357. [DOI] [PubMed] [Google Scholar]
- Girgis CM, Mokbel N, Cha KM, Houweling PJ, Abboud M, Fraser DR, Mason RS, Clifton‐Bligh RJ, Gunton JE. 2014b. The vitamin D receptor (VDR) is expressed in skeletal muscle of male mice and modulates 25‐hydroxyvitamin D (25OHD) uptake in myofibers. Endocrinology 155:3227–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass DJ. 2003. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol 5:87–90. [DOI] [PubMed] [Google Scholar]
- Henry HL, Dutta C, Cunningham N, Blanchard R, Penny R, Tang C, Marchetto G, Chou SY. 1992. The cellular and molecular regulation of 1,25(OH)2D3 production. J Steroid Biochem Mol Biol 41:401–407. [DOI] [PubMed] [Google Scholar]
- Howard GA, Turner RT, Sherrard DJ, Baylink DJ. 1981. Human bone cells in culture metabolize 25‐hydroxyvitamin D3 to 1, 25‐dihydroxyvitamin D3 and 24, 25‐dihydroxyvitamin D3 . J Biol Chem 256:7738–7740. [PubMed] [Google Scholar]
- Korn KT, Lemenager RP, Claeys MC, Waddell JN, Engstrom M, Schoonmaker JP. 2013. Supplemental vitamin D3 and zilpaterol hydrochloride. II. Effect on calcium concentration, muscle fiber type, and calpain gene expression of feedlot steers. J Anim Sci 91:3332–3340. [DOI] [PubMed] [Google Scholar]
- Lips P. 2006. Vitamin D physiology. Prog Biophys Mol Biol 92:4–8. [DOI] [PubMed] [Google Scholar]
- Lips P. 2007. Relative value of 25(OH)D and 1, 25(OH)2D measurements. J Bone Miner Res 22:1668–1671. [DOI] [PubMed] [Google Scholar]
- Lips P, van Schoor NM. 2011. The effect of vitamin D on bone and osteoporosis. Best Pract Res Clin Endocrinol Metab 25:585–591. [DOI] [PubMed] [Google Scholar]
- Morgan KT. 2008. Nutritional determinants of bone health. J Nutr Elder 27:3–27. [DOI] [PubMed] [Google Scholar]
- Mudera V, Smith AS, Brady MA, Lewis MP. 2010. The effect of cell density on the maturation and contractile ability of muscle derived cells in a 3D tissue‐engineered skeletal muscle model and determination of the cellular and mechanical stimuli required for the synthesis of a postural phenotype. J Cell Physiol 225:646–653. [DOI] [PubMed] [Google Scholar]
- Okuno H, Kishimoto KN, Hatori M, Itoi E. 2012. 1α,25‐dihydroxyvitamin D3 enhances fast‐myosin heavy chain expression in differentiated C2C12 myoblasts. Cell Biol Int 36:441–447. [DOI] [PubMed] [Google Scholar]
- Pleasure D, Wyszynski B, Sumner A, Schotland D, Feldman B, Nugent N, Hitz K, Goodman DB. 1979. Skeletal muscle calcium metabolism and contractile force in vitamin D‐deficient chicks. J Clin Invest 64:1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli R, Stevenson JC, Bauer JM, van Loon LJ, Walrand S, Kanis JA, Cooper C, Brandi ML, Diez‐Perez A, Reginster JY. 2014. The role of dietary protein and vitamin D in maintaining musculoskeletal health in postmenopausal women: A consensus statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Maturitas 79:122–132. [DOI] [PubMed] [Google Scholar]
- Rodman JS, Baker T. 1978. Changes in the kinetics of muscle contraction in vitamin D‐depleted rats. Kidney Int 13:189–193. [DOI] [PubMed] [Google Scholar]
- Ronda AC, Buitrago C, Colicheo A, de Boland AR, Roldan E, Boland R. 2007. Activation of MAPKs by 1α,25(OH)2‐Vitamin D3 and 17beta‐estradiol in skeletal muscle cells leads to phosphorylation of Elk‐1 and CREB transcription factors. J Steroid Biochem Mol Biol 103:462–466. [DOI] [PubMed] [Google Scholar]
- Schwartz GG, Whitlatch LW, Chen TC, Lokeshwar BL, Holick MF. 1998. Human prostate cells synthesize 1, 25‐dihydroxyvitamin D3 from 25‐hydroxyvitamin D3 . Cancer Epidemiol Biomarkers Prev 7:391–395. [PubMed] [Google Scholar]
- Selles J, Bellido T, Boland R. 1994. Modulation of calcium uptake in cultured cardiac muscle cells by 1, 25‐dihydroxyvitamin D3 . J Mol Cell Cardiol 26:1593–1599. [DOI] [PubMed] [Google Scholar]
- Seo EG, Einhorn TA, Norman AW. 1997. 24R, 25‐dihydroxyvitamin D3: An essential vitamin D3 metabolite for both normal bone integrity and healing of tibial fracture in chicks. Endocrinology 138:3864–3872. [DOI] [PubMed] [Google Scholar]
- Shan JJ, Li B, Taniguchi N, Pang PK. 1996. Inhibition of membrane L‐type calcium channel activity and intracellular calcium concentration by 24R, 25‐dihydroxyvitamin D3 in vascular smooth muscle. Steroids 61:657–663. [DOI] [PubMed] [Google Scholar]
- Simpson RU, Thomas GA, Arnold AJ. 1985. Identification of 1,25‐dihydroxyvitamin D3 receptors and activities in muscle. J Biol Chem 260:8882–8891. [PubMed] [Google Scholar]
- Snijder MB, van Schoor NM, Pluijm SM, van Dam RM, Visser M, Lips P. 2006. Vitamin D status in relation to one‐year risk of recurrent falling in older men and women. J Clin Endocrinol Metab 91:2980–2985. [DOI] [PubMed] [Google Scholar]
- Srikuea R, Zhang X, Park‐Sarge OK, Esser KA. 2012. VDR and CYP27B1 are expressed in C2C12 cells and regenerating skeletal muscle: Potential role in suppression of myoblast proliferation. Am J Physiol Cell Physiol 303:C396–C405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St‐Arnaud R. 2010. CYP24A1‐deficient mice as a tool to uncover a biological activity for vitamin D metabolites hydroxylated at position 24. J Steroid Biochem Mol Biol 121:254–256. [DOI] [PubMed] [Google Scholar]
- Stio M, Celli A, Treves C. 2002. Synergistic effect of vitamin D derivatives and retinoids on C2C12 skeletal muscle cells. IUBMB Life 53:175–181. [DOI] [PubMed] [Google Scholar]
- Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. 2004. The IGF‐1/PI3K/Akt pathway prevents expression of muscle atrophy‐induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14:395–403. [DOI] [PubMed] [Google Scholar]
- Stockton KA, Mengersen K, Paratz JD, Kandiah D, Bennell KL. 2011. Effect of vitamin D supplementation on muscle strength: A systematic review and meta‐analysis. Osteoporos Int 22:859–871. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Kishimoto KN, Okuno H, Saito H, Itoi E. 2014. Vitamin D receptor gene silencing effects on differentiation of myogenic cell lines. Muscle Nerve 49:700–708. [DOI] [PubMed] [Google Scholar]
- Testerink J, Degens H, Rittweger J, Shiraishi A, Jaspers RT, de Haan A. 2011a. Effects of alfacalcidol on the contractile properties of the gastrocnemius medialis muscle in adult and old rats. J Physiol Pharmacol 62:111–118. [PubMed] [Google Scholar]
- Testerink J, Jaspers RT, Rittweger J, de Haan A, Degens H. 2011b. Effects of alfacalcidol on circulating cytokines and growth factors in rat skeletal muscle. J Physiol Sci 61:525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieland M, Brouwer‐Brolsma EM, Nienaber‐Rousseau C, van Loon LJ, de Groot LC. 2013. Low vitamin D status is associated with reduced muscle mass and impaired physical performance in frail elderly people. Eur J Clin Nutr 67:1050–1055. [DOI] [PubMed] [Google Scholar]
- Turner AG, Dwivedi PP, May BK, Morris HA. 2007. Regulation of the CYP27B1 5'‐flanking region by transforming growth factor‐beta in ROS 17/2.8 osteoblast‐like cells. J Steroid Biochem Mol Biol 103:322–325. [DOI] [PubMed] [Google Scholar]
- Turner RT, Puzas JE, Forte MD, Lester GE, Gray TK, Howard GA, Baylink DJ. 1980. In vitro synthesis of 1α,25‐dihydroxycholecalciferol and 24,25‐dihydroxycholecalciferol by isolated calvarial cells. Proc Natl Acad Sci USA 77:5720–5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meijden K, Lips P, van Driel M, Heijboer AC, Schulten EA, den Heijer M, Bravenboer N. 2014. Primary human osteoblasts in response to 25‐Hydroxyvitamin D3, 1,25‐Dihydroxyvitamin D3 and 24R, 25‐Dihydroxyvitamin D3. PLoS ONE 9:e110283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Driel M, Koedam M, Buurman CJ, Hewison M, Chiba H, Uitterlinden AG, Pols HA, van Leeuwen JP. 2006a. Evidence for auto/paracrine actions of vitamin D in bone: 1α‐hydroxylase expression and activity in human bone cells. FASEB J 20:2417–2419. [DOI] [PubMed] [Google Scholar]
- van Driel M, Koedam M, Buurman CJ, Roelse M, Weyts F, Chiba H, Uitterlinden AG, Pols HA, van Leeuwen JP. 2006b. Evidence that both 1α, 25‐dihydroxyvitamin D3 and 24‐hydroxylated D3 enhance human osteoblast differentiation and mineralization. J Cell Biochem 99:922–935. [DOI] [PubMed] [Google Scholar]
- van Wessel T, de Haan A, van der Laarse WJ, Jaspers RT. 2010. The muscle fiber type‐fiber size paradox: Hypertrophy or oxidative metabolism? Eur J Appl Physiol 110:665–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicherts IS, van Schoor NM, Boeke AJ, Visser M, Deeg DJ, Smit J, Knol DL, Lips P. 2007. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab 92:2058–2065. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Ozono K, Shima M, Yamaoka K, Okada S. 1998. 24R,25‐dihydroxyvitamin D3 increases cyclic GMP contents, leading to an enhancement of osteocalcin synthesis by 1,25‐dihydroxyvitamin D3 in cultured human osteoblastic cells. Exp Cell Res 244:71–76. [DOI] [PubMed] [Google Scholar]
- Yamate T, Tanaka H, Nagai Y, Yamato H, Taniguchi N, Nakamura T, Seino Y. 1994. Bone‐forming ability of 24R,25‐dihydroxyvitamin D3 in the hypophosphatemic mouse. J Bone Miner Res 9:1967–1974. [DOI] [PubMed] [Google Scholar]
- Zamboni M, Zoico E, Tosoni P, Zivelonghi A, Bortolani A, Maggi S, Di F V, Bosello O. 2002. Relation between vitamin D, physical performance, and disability in elderly persons. J Gerontol A Biol Sci Med Sci 57:M7–11. [DOI] [PubMed] [Google Scholar]
- Zanou N, Gailly P. 2013. Skeletal muscle hypertrophy and regeneration: Interplay between the myogenic regulatory factors (MRFs) and insulin‐like growth factors (IGFs) pathways. Cell Mol Life Sci 70:4117–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article at the publisher's web‐site.
Figure S1. Effects of low concentrations of 25(OH)D3 and 1,25(OH)2D3 on myotube diameter.
Figure S2. Low concentrations of 1,25(OH)2D3 increased CYP24 and VDR mRNA levels in differentiating C2C12 cells.
Figure S3. Effects of low concentrations of 25(OH)D3 and 1,25(OH)2D3 on mRNA levels of myosin heavy chain.


