Abstract
The increasing regulatory burden and cost of doing clinical trials in cardiac emergencies has greatly impacted the development of novel therapies resulting in increased morbidity and mortality of patients. A new regulatory framework is required.
Keywords: cardiac arrest, ischemia-reperfusion injury, IRB, informed consent
“Good judgment seeks balance and progress; lack of it eventually finds imbalance and frustration”.
- President Dwight D. Eisenhower, January 1961 speech on the growing military-industrial complex.
Over the last 2 decades, great progress has been made in the treatment of ST-elevation myocardial infarction (STEMI). The development of regional systems of care for delivery of primary PCI (1), advances in pharmacotherapy and public education have significantly reduced the time to reperfusion. Although the rapid restoration of coronary blood flow is the most effective means of reducing infarct size and preserving left-ventricular (LV) function, it is not without consequence as reperfusion may also be associated with further injury to the myocardium and vasculature. Reperfusion injury may increase infarct size to a degree that is similar to the initial ischemic insult (2) by increasing myocyte cell death, activating apoptosis and promoting endothelial dysfunction. Improvements in reducing ischemia – reperfusion (I/R) injury are urgently needed as this represent the largest remaining target available to reduce infarct size. This achievement could significantly improve patient’s quality of life and reduce the great expenditures associated with the development of congestive heart failure.
Unfortunately, efforts at reducing I/R injury by methods successful in animal models have largely been unsuccessful in clinical trials (3). To date, clinical trials to limit reperfusion injury have targeted many areas including reactive oxygen species, reductions in calcium overload, inflammation, mitochondrial permeability transition pore to name just a few. The reasons for disappointing results in the majority of trials may include the frequently longer duration of ischemia and co-morbidities in humans with STEMI compared to animals, the frequent inability to accurately quantify an area-at-risk (AAR) to measure myocardial salvage, and the failure to deliver the therapy at the immediate onset of reperfusion. This point is essential since even a delay of several minutes following reperfusion may render these therapies ineffective.
This unique temporal requirement in an emergency setting currently poses a significant regulatory burden to conducting research in I/R injury, especially in the United States. Although current regulations appropriately require patients participating in research trials be fully informed of the risks and benefits of the proposed research, how does a patient with ongoing chest pain and potential hemodynamic instability or arrhythmias read, and let alone, comprehend a 15-page consent form and HIPPA documentation? Research suggests they do not! Our efforts at targeting I/R injury in a clinical trial of postconditioning are illustrative of the issues now facing researchers in the United States studying I/R injury and methods to reduce infarct size.
In 2003 Zhao and Vinten-Johansen (4) introduced a modified reperfusion technique called postconditioning that reduced infarct size in dogs by 44% following a 60-minute occlusion of the left-anterior descending artery. Postconditioning was demonstrated to be as powerful as ischemic preconditioning, the most potent endogenous form of cardioprotection yet discovered. Postconditioning involves repeated brief occlusions of the artery (30–60 s) followed by reperfusion (30–60 s) over several cycles. To be effective, the protocol must be implemented immediately upon reperfusion. It has great clinical potential since it can be performed in the cardiac catheterization laboratory using a PTCA balloon during the setting of primary PCI for STEMI where reperfusion can be controlled. Whether it can be effective in humans is not clear, as several clinical trials from Europe have delivered inconsistent findings regarding its benefit.
We hypothesized that one reason for its inconsistent demonstration of benefit arose from patient selection as trials frequently included patients with ongoing limited reperfusion (TIMI 1), prolonged ischemic times and the failure to exclude patients pre-infarction angina (5), a strong mitigator of infarct size that may occur in up to 30% of patients with STEMI. We developed a single center trial funded by the NHLBI (6) with strict enrollment criteria that included only patients with 100% occlusion of a major epicardial artery (TIMI 0) and no collateral filling of the infarct zone with ischemic times between 1 and 6 hours and no evidence of pre-infarction angina. We began enrollment in 2011 after our Health System’s Institutional Review Board agreed that the low risk imposed by standardizing 4, 30-sec PTCA inflations/deflations could qualify for a verbal consent process in the catheterization lab after initial angiography confirmed the presence of an occluded artery. Full, informed consent was obtained within 24-hrs when the patient was stable and could comprehend a 15-page document.
Unfortunately, in 2014, our Health System began utilizing outside IRB agencies to approve the renewals of clinical trials. These IRBs now stipulated that our initial verbal consent process followed by full, informed consent the next day was no longer permissible and that the only way we could continue the trial was by obtaining an “emergency waiver of informed consent”. As a result, our trial was placed on clinical hold despite not having a single complication or adverse event. At that time we had enrolled 90 patients and had no safety issues identified by the DSMB.
The emergency waiver of consent was implemented by HHS in 1996 (45 CFR Part 46) to allow research to be conducted in patients in need of emergency medical treatment who cannot give informed consent because of their life-threatening condition and no legal authorized representative of the patient is available. The therapy should include the prospect of direct patient benefit. These waivers are required for cardiac arrest and resuscitation studies. Obtaining this waiver is an arduous and expensive process. It requires the completion of expensive community phone surveys, talks and public notices of the research to be performed and the opportunities for people in the community to prospectively opt-out from participating. We spent over one year in this endeavor monitored by a 3rd outside IRB at the cost of over $15,000. We lost one year of NIH funding of our trial because of this delay. Of interest, we found that very few IRBs would agree to review our application for this waiver.
In Europe, this would never have been an issue and reflects the ongoing difficulties in doing emergency clinical research in the United States compared to other countries. Review of several recently completed or ongoing I/R clinical trials for STEMI in Europe administering beta-blockers (7), sodium nitrite (8) or melatonin (9) have all been permitted to utilize verbal consent in the catheterization laboratory at the time of primary PCI followed by full informed consent before discharge. Certainly, the standardization of PTCA inflations in postconditioning would hardly seem riskier than the agents delivered in these European trials?
A recent out of hospital cardiac arrest (OHCA) trial from France (10) was able to administer cyclosporine during resuscitation in an attempt to mitigate multiple organ failure from I/R injury. They received a consent waiver from their ethics committee. Unfortunately, this would never be permitted in the United States. Each year approximately 347,000 adults experience EMS-assessed OHCA (11) in the United States. The survival to hospital discharge is dismal, between 8 and 12% depending on the registry analyzed. Thus, a miniscule 1% improvement in survival would save 3000 lives. Unfortunately, research in cardiac arrest that could improve survival has been decreasing due to the high regulatory burden. This cost in human life is substantial. Preventing or delaying research results in vastly more suffering and death than occurs from researchers’ ethical lapses (12).
I’m sure most of us would allow reasonable emergency research to be conducted on ourselves without informed consent when faced with a 90% mortality if it offered the prospects of potentially improving our survival. Indeed, this appears to be the attitude of most patients who were interviewed in an outpatient setting where 84% agreed that they were willing to be recruited to an emergency research trial without their consent should this situation arise (13).
Obtaining consent in an emergency situation is often impractical and results in unnecessary delays in administering therapy, making it less effective. The CRASH-multicenter trial administered corticosteroids in severe head injury and observed that those hospitals that required informed consent experienced a treatment delay of 1.2 hours compared to those centers where consent was waived. The CRASH-2 trial demonstrated a significant reduction in mortality with administration of tranexamic acid to bleeding trauma patients. Roberts et al (14) showed that a 1-hr delay in obtaining consent would have increased mortality in that trial from a relative risk of 0.85 to 0.96. The authors concluded that consent rituals result in avoidable mortality and probably morbidity and that “ far from protecting the interests of patients participating in research, requirements for written informed consent and the resultant delay in starting treatment could be lethal and obscure a real treatment benefit”.
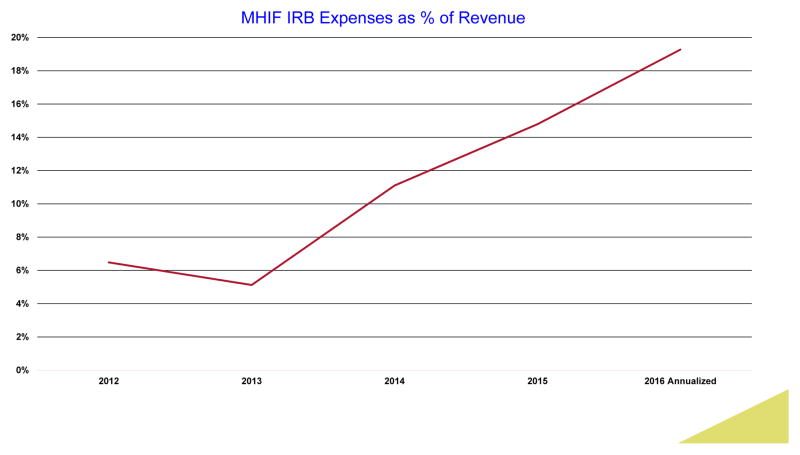
The OHRP crafting of ambiguous rules and regulations has created so much uncertainty amongst researchers and their staffs that a whole new industry of IRB consultants and compliance agencies has been created that devour research dollars that are increasingly diverted from improving the health and lives of our patients. In the last 3 years our Foundation’s HIPPA and IRB-associated costs have increased 3-fold (Figure 1) with no change in the number of clinical research protocols we enroll (approximately 140). We now employ attorneys, consultants and IRB specialists where none existed before. I suspect we are not alone.
Figure 1.
The regulatory costs including salaries for IRB and HIPPA compliance over a 5-year period at the Minneapolis Heart Institute Foundation (MHIF) as percent of total clinical trial revenue.
These issues represent a microcosm of the growing and unreasonable regulatory burden facing investigators performing cardiovascular research in the United States. Investigators and research nurses at our Foundation live in fear of the IRB and our IRBs appear to live in fear of the OHRP for their power to completely shut down research because some form was filled out incorrectly. Rarely is it because of harm to patients. In his book The Censor’s Hand, The Misregulation of Human Subject Research (15), Carl Schneider writes “ In sum, IRBs’, most authoritative law – the federal regulations- is effectively lawless, a failure manifest in the regulation’s opacity, the Guidebook’s refusal to clarify them, regulationists’ complaints about regulatory obscurity, and IRBs’ incompatible treatment of identical protocols”. Our Institutions’s IRB staff and physician members are extremely dedicated and hard-working but remain handcuffed by ill-defined regulations.
Going Forward
It is clear that there is a great element of mistrust between the regulatory agencies and researchers. The spectre of another Tuskeegee continues to haunt this relationship. In the area of emergency research the regulators have thrown the proverbial baby out with the bath water. Emergency research is unique and differs significantly from traditional clinical research because of the urgent time constraints and frequent incapacitation of the patient. A new enlightened approach is needed since so many lives are at stake.
Our initial verbal consent process in the catheterization lab followed by full, informed consent the next day appeared to work well until a new IRB outlawed it. Sahan et al. (16) has proposed the addition of a patient advocate, independent the research team, who could monitor the verbal consent process to ensure its integrity. This seems like an excellent idea although staffing in the catheterization lab could be difficult given the frequency of off-hour presentations of STEMI patients. Clearly, the current pathway of obtaining waivers of informed consent significantly hinders research efforts due to its high cost, regulatory burden and the additional months to years required to attain. Unfortunately, it would appear that any meaningful change must originate at the Federal level via legislation given their one-sided relationship with local IRBs. So how can change be implemented if we are going to improve patient outcomes?
We would propose the following ideas to be considered:
Creation and empowerment of a federally funded National panel of researchers, bioethicists and patient advocates to review, approve and monitor all emergency research. This would relieve the local IRBs from the conflicts with current guidelines and regulations.
In settings such as STEMI where patients are conscious, allow verbal consent process to be implemented in an initial small number of patients to test its feasibility and patient satisfaction. If no issues are apparent allow the verbal consent process to be continued for the remainder of the study. This would be analogous to the FDA’s frequent and appropriate requirement of a lead-in safety study of novel therapies such as stem cells therapy.
As Wootton and colleagues wrote in the journal Pediatrics, (17) – “ thousands of unnecessary deaths may be caused by undue regulatory barriers or consent requirements that reduce enrollment in trials of effective therapies or that obscure their treatment effects due to selection biases or delays in treatment”. Clearly the regulatory pendulum has swung too far away from what is best for our patients. It’s time to restore balance.
Acknowledgments
Sources of Funding: Supported by the NHLBI (5RO1 HL103927)
Footnotes
DISCLOSURES: none
References
- 1.Henry TD, Sharkey SW, Burke MN, Chavez IJ, Graham KJ, Henry CR, Lips DL, Madison JD, Menssen KM, Mooney MR, Newell MC, Pedersen WR, Poulose AK, Traverse JH, Unger BT, Wang YL, Larson DM. A regional system to provide timely access to percutaneous coronary intervention for ST-elevation myocardial infarction. Circulation. 2007;116:721–728. doi: 10.1161/CIRCULATIONAHA.107.694141. [DOI] [PubMed] [Google Scholar]
- 2.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–35. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 3.Rossello X, Yellon DM. Cardioprotection – The disconnect between bench and bedside. Circulation. 2016;134:574–575. doi: 10.1161/CIRCULATIONAHA.116.022829. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Z-Q, Corvera JS, Halkos ME, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 5.Reiter R, Henry TD, Traverse JH. Preinfarction angina reduces infarct size in ST-elevation myocardial infarction treated with percutaneous coronary intervention. Circ Cardiovasc Interv. 2013;6:52–8. doi: 10.1161/CIRCINTERVENTIONS.112.973164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Traverse JH, Swingen C, Henry TD, et al. NHLBI-sponsored trial of postconditioning during PCI for ST-elevation myocardial infarction. To be presented at the 2016 Scientific Sessions of the American Heart Association; November 14, 2016; New Orleans, LA. [Google Scholar]
- 7.Roolvink V, Ibanez B, Ottervanger JP, et al. Early intravenous beta-blockers in patients with ST-segment elevation myocardial infarction before primary percutaneous coronary intervention. J Am Coll Cardiol. 2016;67:2705–15. doi: 10.1016/j.jacc.2016.03.522. [DOI] [PubMed] [Google Scholar]
- 8.Siddiqi N, Neil C, Bruce M, et al. Intravenous sodium nitrite in acute ST-elevation myocardial infarction: a randomized controlled trial (NIAMI) Eur Heart J. 2014;35:1255–1262. doi: 10.1093/eurheartj/ehu096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dominguez-Rodriquez A, Abreu-Gonzalez P, Garcia-Gonzalez MJ, et al. The Melatonin Adjunct in the acute myocaRdial Infarction treated with Angioplasty (MARIA) trial: study design and rationale. Contemp Clin Trials. 2007;28:532–539. doi: 10.1016/j.cct.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Argaud L, Cour M, Dubien P-Y, et al. Effect of cyclosporine in nonshockable out-of-hospital cardiac arrest. The CYRUS randomized clinical trial. JAMA Cardiol. 2016;1:557–565. doi: 10.1001/jamacardio.2016.1701. [DOI] [PubMed] [Google Scholar]
- 11.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2016 update. A report from the American Heart Association. Circulation. 2015;132:000–000. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 12.Heimer CA, Petty J. Bureaucratic ethics:IRBs and the legal regulation of human subjects research. Annu Rev Law Soc Sci. 2010;6:601–626. [Google Scholar]
- 13.Booth MG, Lind A, Read E, Kinsella J. Public perception of emergency research: a questionnaire. Eur J of Anesthesiology. 2005;22:933–937. doi: 10.1017/S0265021505001596. [DOI] [PubMed] [Google Scholar]
- 14.Roberts I, Prieto-Merino D, Shakur H, Chalmers I, Nicholl J. Effect of consent rituals on mortality in emergency care research. Lancet. 2011;377:1071–2. doi: 10.1016/S0140-6736(11)60317-6. [DOI] [PubMed] [Google Scholar]
- 15.Schneider CE. The misregulation of human-subject research. The MIT Press; Cambridge MA: 2015. The Censor’s Hand. [Google Scholar]
- 16.Sahan KM, Channon KM, Choudhury RP, Kharbanda RK, Lee R, Sheehan M. Refining the enrolment process in emergency medicine research. Eur J Cardiovasc Med. 2016;4:506–510. doi: 10.5083/ejcm.20424884.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wootton SH, Evans PW, Tyson JE. Unproven therapies in clinical research and practice: The necessity to change the regulatory paradigm. Pediatrics. 2013;132:599–601. doi: 10.1542/peds.2013-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]