Abstract
Although executive functioning (EF) difficulties are well documented among children and adolescents with autism spectrum disorder (ASD), little is known about real-world measures of EF among adults with ASD. Therefore, this study examined parent-reported real-world EF problems among 35 adults with ASD without intellectual disability and their correlations with adaptive functioning and co-morbid anxiety and depression symptomatology. A variable EF profile was found with prominent deficits occurring in flexibility and metacognition. Flexibility problems were associated with anxiety-related symptoms while metacognition difficulties were associated with depression symptoms and impaired adaptive functioning (though the metacognition-adaptive functioning relationship was moderated by ADHD symptoms). These persistent EF problems are predictors of broader functioning and therefore remain an important treatment target among adults with ASD.
Keywords: Autism, Adult, Executive function, Adaptive functioning, Anxiety, Depression
Introduction
Autism spectrum disorder (ASD) is a lifelong disorder characterized by impairments in social and communicative functioning and the presence of restricted interests/repetitive behaviors. In spite of recent estimates indicating that nearly 70 % of individuals with ASD do not have an intellectual disability (i.e., IQ > 70; Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators and Centers for Disease Control and Prevention (CDC) 2014), adult outcome in ASD remains poor (Henninger and Taylor 2013; Howlin and Moss 2012; Roux et al. 2013). Over 25 % of young adults with ASD without intellectual disability have no daytime activities of any kind (Taylor and Seltzer 2011).
Executive function (EF) is an omnibus term describing supramodal, higher-order cognitive abilities including working memory, planning, flexibility, and organization, in the service of problem-solving and behavioral regulation. EF difficulties have long been associated with ASD since the publication of seminal theoretical (Damasio and Maurer 1978) and empirical (Rumsey 1985) work. While the earliest empirical studies used EF lab tasks (e.g., the Wisconsin Card Sorting Task) in small samples of adults, the vast majority of studies since then have focused on EF difficulties in ASD during childhood and adolescence. Flexibility and planning deficits are considered most characteristic of the EF profile in ASD as evaluated by lab-based performance tasks; however, findings are somewhat mixed (see Hill 2004; Kenworthy et al. 2008 for review).
In contrast, recent work demonstrates that ecologically valid measures of EF capture robust deficits in children and adolescents with ASD that are characterized by a specific profile of EF subdomain deficits and that relate to important outcomes, such as adaptive behavior and co-morbid psychiatric problems (Blijd-Hoogewys et al. 2014; Gilotty et al. 2002; Granader et al. 2014; Hill and Bird 2006; Pugliese et al. 2015). Ecologically valid measures of EF can include tasks that attempt to replicate real-world scenarios and experiences and/or questionnaires that assess consistent patterns of EF difficulties experienced by various individuals and that detect difficulties experienced in more dynamic settings than assessed in the laboratory/clinic. These ecologically-valid EF measures are therefore viewed as complementary tools that provide separate and additional information not captured by lab-based EF tasks. The literature thus far converges in finding a prototypical EF profile characterized by a peak deficit in behavioral flexibility/shifting on informant reports of real-world EF problems using tools like the Behavior Rating Inventory of Executive Function (BRIEF). For example, in the largest study to date, Granader et al. (2014) found that among 411 children with ASD compared to 467 typically developing children, real-world EF deficits were prominent across all subdomains, but a peak difficulty was noted in behavioral flexibility. Furthermore, several studies of individuals with ASD without intellectual disability have demonstrated links between real-world EF, as assessed by measures like the BRIEF, and adaptive functioning (e.g., social, communication, and daily living skills), over and above the effects of IQ (Gilotty et al. 2002; Pugliese et al. 2015). Recent work also finds that real-world measures of behavioral flexibility in particular are associated with symptoms of anxiety and depression in samples of children with ASD or ADHD (Lawson et al. 2015) and that lab-based measures of flexibility, unlike lab measures of social cognition, are associated with anxiety symptomatology (Hollocks et al. 2014).
The literature on EF among adults with ASD is more limited and what exists is dominated by utilization of lab-based EF tasks (see Table 1 for a review of EF studies among adults with ASD). The seminal empirical work on EF in ASD was conducted with adults (Rumsey 1985; Rumsey and Hamburger 1988, 1990) and demonstrated consistent impairments in cognitive flexibility (as measured by the Wisconsin Card Sorting and Trail Making Tests), but the groups studied were matched for education level, not IQ scores, which were consistently lower for the ASD than the TD groups. When ASD and TD participants are matched for IQ scores, EF deficits are found in some studies, particularly related to flexibility, generativity, and spatial working memory (Ambery et al. 2006; Geurts and Vissers 2012; Sachse et al. 2013; Williams et al. 2005), but are far from universal (Sachse et al. 2013; Geurts and Vissers 2012; Hill and Bird 2006, Nakahachi et al. 2006; Towgood et al. 2009). Notably absent from the extant literature on adults with ASD are studies of real-world EF utilizing ratings scale such as the adult version of the BRIEF (Roth et al. 2005), although Rosenthal et al. (2013) do report age-related declines in some domains of parent-reported EF in later adolescence when compared to childhood and early adolescence.
Table 1.
Review of studies examining executive functioning in adults with autism spectrum disorder
| Study | N | Age (years) | Sex ratio: M/F | Mean IQ | Matching criteria | EF measures | EF domains | ASD impaired compared to TD/Othera |
|---|---|---|---|---|---|---|---|---|
| Rumsey (1985) | ASD: 9 TD: 10 |
ASD: 27 TD: 28 |
ASD: 9/0 TD: 10/0 |
ASD FSIQ: 104 TD FSIQ: 113 |
Highest level of education | WCST | Flexibility | Y |
| Rumsey and Hamburger (1988) | ASD: 10 TD: 10 |
ASD: 26 TD: 28 |
ASD: 10/0 TD: 10/0 |
ASD VIQ: 103 ASD PIQ: 104 TD VIQ: 113 TD PIQ: 111 |
Age Sex IQ Level of education Headedness |
Trail making test Part B WCST |
Flexibility Flexibility |
Y Y |
| Rumsey and Hamburger (1990) | ASD: 10 Dyslexic: 15 TD: 25 |
ASD: 26 Dyslexic: 22 TD: 24 |
ASD: 10/0 Dyslexic: 15/0 TD: 25/0 |
ASD FSIQ: 96 Dyslexic FSIQ: 104 TD FSIQ: 107 |
Age Sex Level of education Headedness |
WCST Word fluency |
Flexibility Generativity |
Y/Yb Y/N |
| Goldstein et al. (2002) | ASD: 31 Schizophrenia: 80 |
ASD: 21 Schizophrenia High functioning: 40 Moderately impaired: 42 Severely impaired: 48 Severe psychomotor: 43 |
ASD: not reported Schizophrenia: 80/0 |
ASD: VIQ: 102 PIQ: 97 FSIQ: 100 Schizophrenia High functioning: VIQ: 102 PIQ: 98 FSIQ: 99 Moderately impaired: VIQ: 92 PIQ: 87 FSIQ: 89 Severely impaired: VIQ: 85 PIQ: 81 FSIQ: 82 Severe psychomotor: VIQ: 102 PIQ: 77 FSIQ: 80 |
Verbal and Full Scale IQ scores of 70 or higher | Trail Making Test Part B WCST |
Flexibility Flexibility |
N/N/N/Nc N/N/N/N |
| Lopez et al. (2005) | ASD: 17 TD: 17 |
ASD: 29.0 TD: 29.0 |
ASD: 14/3 TD: 11/6 |
ASD FSIQ: 77 TD FSIQ: 89 |
Age Sex PIQ |
Design Fluency LNS Tower-DKEFS The California Stroop test Verbal fluency WCST |
Generativity Working Memory Planning Inhibition Generativity Flexibility |
Y N Y N N Y |
| Williams et al. (2005) | ASD: 31 TD: 25 |
ASD: 26.6 TD: 26.8 |
ASD: 29/2 TD: 21/4 |
ASD FSIQ: 107 TD FSIQ: 110 |
Age Sex IQ |
Letter-number sequencing N-Back Spatial span |
Working Memory Working Memory Working memory |
N N Y |
| Ambery et al. (2006) | ASD: 27 TD: 20 |
ASD: 37.6 TD: 33.5 |
ASD: 22/5 TD: 16/4 |
ASD VIQ: 106 PIQ: 104 TD VIQ: 107 PIQ: 109 |
Age Sex VIQ PIQ Years of education |
COWAT Stroop test WCST |
Generativity Inhibition Flexibility |
Y N Y |
| Hill and Bird (2006) | ASD: 22 TD: 22 |
ASD: 31.1 TD: 33.5 |
ASD: 16/6 TD: 14/8 |
ASD FSIQ: 110 TD FSIQ: 108 |
Age Sex IQ |
MCST Stroop Test Trail making test Part B Verbal fluency |
Flexibility Inhibition Flexibility Generativity |
N N N N |
| Nakahachi et al. (2006) | ASD: 16 TD: 28 |
ASD: 28.0 TD: 28.3 |
ASD: 12/4 TD: 21/7 |
ASD FSIQ: 101 TD FSIQ: 103 |
Age Sex IQ |
ATMT Digit span |
Flexibility Working memory |
N N |
| Barnard et al. (2008) | ASD + ID: 20 ID: 23 |
ASD + ID: 18–45 years ID: 18–45 years |
ASD + ID: 20/0 ID: 23/0 |
ASD + ID: VIQ: 68 PIQ: 69 FSIQ: 67 ID: VIQ: 69 PIQ: 71 FSIQ: 69 |
Chronological age VIQ PIQ FSIQ |
COWAT Knock and tap task Mazes task MCST Non-verbal fluency (from NEPSY) Spatial Span Task Tower of London Test Verbal Conflict Task |
Generativity Inhibition Planning Flexibility Generativity Working memory Planning Inhibition |
N N Y N N Y Y N |
| Towgood et al. (2009) | ASD: 21 TD: 22 |
ASD: 31.76 TD: 30.64 |
ASD: 17/4 TD: 18/4 |
ASD FSIQ: 116 TD FSIQ: 118 |
Age Sex |
CET COWAT Hayling Test MCST Proverbs subtest (from DKEFS) Six Elements subtests (from BADS) Trail Making Test Part B Zoo Map (from BADS) |
Multiple Generativity Inhibition Flexibility Flexibility Planning Flexibility Planning |
N N N N N N N N |
| Nydén et al. (2010) | ASD: 55 ADHD: 73 ASD/ADHD: 33 |
ASD: 32.02 ADHD: 33.18 ASD/ADHD: 32.37 |
ASD: 38/17 ADHD: 39/34 ASD/ADHD: 20/13 |
ASD FSIQ: 98 ADHD FSIQ: 93 ASD/ADHD FSIQ: 92 |
Age Sex |
TOVA Tower of London Test |
Inhibition Planning |
N/Nd N/N |
| Johnston et al. (2011) | ASD: 24 ADHD: 24 TD: 14 |
ASD: 27.8 ADHD: 27.3 TD: 28.7 |
ASD: 19/5 ADHD: 19/5 TD: 10/4 |
ASD VIQ: 102 ADHD VIQ: 98 TD VIQ: 108 |
Age Sex VIQ |
Hayling Test MFF Stroop Test |
Inhibition Inhibition Inhibition |
Y/Ye N/N N/N |
| Geurts and Vissers (2012) | ASD: 23 TD: 23 |
ASD: 63.6 TD: 63.7 |
ASD: 18/5 TD: 18/5 |
ASD: DART-IQ: 110 TD DART-IQ: 110 |
Age Sex Estimated IQ and educational level |
COWAT Modified WCST SART Spatial Span Subtest Trail Making Test Part B The Tower of London of the Drexel University |
Generativity Flexibility Attention Working memory Flexibility Planning |
Y N Y Y N N |
| Lai et al. (2012) | ASD: 17 TD: 17 |
ASD: 27.2 TD: 28.3 |
ASD: 14/3 TD: 11/6 |
ASD VIQ: 73 ASD FSIQ: 77 TD VIQ: 92 TD FSIQ: 89 |
Age PIQ |
F-A-S Go/No-Go |
Generativity Inhibition |
N Y |
| Sachse et al. (2013) | ASD: 30 TD: 28 |
ASD: 19.2 TD: 19.9 |
ASD IQ: 105 TD ID: 109 |
Age Sex Non-verbal IQ |
ID/ED Stroop Test Stockings of Cambridge SWM |
Flexibility Inhibition Planning Working memory |
Y N N N |
|
| Wilson et al. (2014) | ASD: 89 TD: 89 |
ASD: 26 TD: 28 |
ASD: 89/0 TD: 89/0 |
ASD FSIQ: 110 VIQ: 110 PIQ: 108 TD FSIQ: 114 VIQ: 109 PIQ: 116 |
Sex IQ |
F-A-S TOVA |
Generativity Attention |
N N |
N sample size, EF executive function, ASD autism spectrum disorder, TD typically developing, ID intellectual disability, VIQ verbal intelligence quotient, PIQ performance intelligence quotient, FSIQ full scale intelligence quotient, WCST Wisconsin Card Sorting Test, COWAT Controlled Oral Word Association Test, SART Sustained Attention to Response Test, CANTAB Cambridge Neuropsychological Test Automated Battery, IED Intradimensional/extradimensional set shifting task, SWM spatial working memory, DART Dutch Adult Reading Test IQ, MCST Modified Card Sorting Task, MFF Matching familiar figures test, BADS Behavioral Assessment of Dysexecutive Syndrome, ADHD attention-deficit/hyperactivity disorder, DKEFS Delis Kaplan Executive function scale, CET Cognitive elimination test, F-A-S word generativity task, LNS letter number sequencing, ATMT advanced trail making task, TOVA test of variables of attention, HFA high functioning autism, RTC mean reaction time of correct answers, RTE mean reaction time incorrect “no” responses, RTE + 1 Mean reaction time post-error responses
This column refers to whether there is an impairment reported for the ASD group relative to the comparison group(s). The possibilities are: Y = yes differences are reported for particular domain(s)/EF test(s); N = no differences are reported for particular domain(s)/EF test(s)
First comparison is between ASD and TD and the second comparison is between ASD and the dyslexic group (e.g., Y/N means ASD group exhibited deficits compared to TD and Y/Y means ASD exhibited deficits relative to both groups
First comparison is between ASD and moderate schizophrenia, second comparison is between ASD and high functioning schizophrenia, third comparison is between ASD and severely impaired schizophrenia, fourth comparison is between ASD and severe psychomotor schizophrenia (e.g., N/N/N/N means ASD group did not exhibit deficits compared to all four groups)
First comparison is between ASD and ADHD, second comparison is between ASD ASD/ADHD. Comparisons between both groups do not indicate deficits
First comparison is between ASD and TD and second comparison is between ASD and ADHD (e.g., Y/Y means ASD group exhibited unique deficits relative to both groups, while Y/N means ASD group exhibited deficits compared to TD but was not different from ADHD group)
Assessment of adaptive behavior, including daily living, social, and communication skills, is one key way to quantify the failure of many individuals with ASD to integrate effectively into society. Another impediment to optimal functioning in adulthood in ASD is co-morbid psychopathology, particularly anxiety and depression (Mazurek 2014). Because EF has been shown to be a predictor of key outcomes, including adaptive behavior and co-morbid anxiety/depression, in ASD during childhood and adolescence, it could also be a key contributor to these in adulthood, and therefore, an important treatment target. Studies have not yet been published exploring these relationships among adults with ASD, however.
The present study is the first to examine real-world EF profiles utilizing the informant version of the Behavior Rating Inventory of Executive Function—Adult among 35 adults with ASD without intellectual disability. We also investigated the links between real-world EF difficulties and both adaptive functioning and internalizing psychopathology (i.e., anxiety and depression symptoms) in this group. Consistent with prior studies of children and adolescents with ASD, we expected to find a peak real-world EF deficit in flexibility problems and that these flexibility impairments would be associated with co-morbid anxiety symptomatology in adults with ASD. Further, we expected that EF problems would be associated with adaptive behavior impairments.
Methods
Participants
Thirty-five adults with ASD (31 males) ranging in age from 18–40 years (M = 21.55, SD = 4.12) participated in the study. All participants had average or better intellectual functioning (IQ > 85; Full Scale IQ range = 88–133; M = 112.47, SD = 11.21) and met Diagnostic and Statistical Manual of Mental Disorders-5 diagnostic criteria for Autism Spectrum Disorder as assessed by an experienced clinician. Thirty-one participants received the Autism Diagnostic Interview or Autism Diagnostic Interview-Revised (Le Couteur et al. 1989; Lord et al. 1994) and all 35 participants received the Autism Diagnostic Observation Schedule (Lord et al. 2000), Module 4. Both of these instruments were administered by a trained, research-reliable clinician. All ASD participants’ scores met cut-off for the category designated as ‘Broad ASD’ according to criteria established by the NICHD/NIDCD Collaborative Programs for Excellence in Autism (CPEA; see Lainhart et al. 2006). ‘Broad ASD’ is defined as meeting the Autism Diagnostic Interview or Autism Diagnostic Interview-Revised cut-off for ‘autism’ in the social domain and at least one other domain or meeting the Autism Diagnostic Observation Schedule cut-off for the combined social and communication score. See Table 2. Exclusion criteria included an IQ < 85 or any known comorbid medical conditions, such as fragile X syndrome or other genetic disorders, and brain trauma/injury.
Table 2.
Subject characteristics including mean age and IQ as well as executive function index scores and mean adaptive functioning and internalizing behavior scores
| Measure | M | SD | n |
|---|---|---|---|
| Age | 21.55 | 4.12 | 35 |
| Full Scale IQ | 112.47 | 11.21 | 34 |
| Verbal IQ | 110.59 | 12.80 | 34 |
| Performance IQ | 111.62 | 11.32 | 34 |
| ADOS social + communication total | 11.91 | 3.31 | 35 |
| ADOS restricted and repetitive behaviors | 1.17 | 1.45 | 35 |
| ADI reciprocal social interaction | 17.87 | 6.42 | 31 |
| ADI verbal communication | 15.42 | 4.50 | 31 |
| ADI restricted, repetitive, stereotyped behaviors | 4.71 | 2.34 | 31 |
| BRIEF-A Global Executive Composite | 62.20 | 11.44 | 35 |
| BRIEF-A Behavior Regulation Index | 57.06 | 11.44 | 35 |
| BRIEF-A Metacognition Index | 64.86 | 12.04 | 35 |
| ABAS-II Global Adaptive Composite | 72.84 | 14.71 | 31 |
| ABAS-II conceptual skills | 80.29 | 13.04 | 34 |
| ABAS-II social skills | 72.38 | 11.36 | 32 |
| ABAS-II practical skills | 76.21 | 16.27 | 33 |
| ABCL attention deficit/hyperactivity problems | 64.53 | 10.05 | 30 |
| ABCL depression | 63.67 | 8.67 | 30 |
| ABCL anxiety | 58.17 | 8.15 | 30 |
Both IQ and ABAS scores are reported as standard scores (M = 100, SD = 15), BRIEF and ABCL scores are reported as T scores (M = 50, SD = 10), and ADOS and ADI scores are raw scores
ADOS Autism Diagnostic Observation Schedule, ADI Autism Diagnostic Interview, BRIEF-A Behavior Rating Inventory of Executive Function—Adult version; ABAS-II Adaptive Behavior Assessment System-Second edition; ABCL Adult Behavior CheckList
Procedures
This project was part of a larger study examining brain and behavioral functioning in ASD. This study was conducted in compliance with standards established by the institution’s IRB including procedures for informed consent.
Measures
Executive Functioning
Behavior Rating Inventory of Executive Functioning—Adult version (BRIEF-A; Roth et al. 2005): The informant rated version of the BRIEF-A was utilized to assess real-world EF. The BRIEF-A, a standardized rating scale composed of 75 items, assesses the frequency (‘often,’ ‘sometimes,’ or ‘never’) of problems related to EF that have occurred in the last 4 weeks. The BRIEF-A provides both global indices and specific subdomains of real-world EF based on factor analytic studies. There is a Global Executive Composite that is broken down into two index scores, the Behavioral Regulation Index (BRI), which in turn consists of four subscales (i.e., Inhibit, Shift, Emotional Control, and Self-Monitor) and the Metacognition Index (MCI), which in turn consists of five subscales (i.e., Initiate, Working Memory, Plan/Organize, Task Monitor, and Organization of Materials). All of these ratings are expressed as T scores (M = 50; SD = 10) derived from comparisons with normative age expectations. Higher scores are indicative of more EF difficulties and T scores of 65 or higher are categorized as clinically significant.
Adaptive Functioning
Adaptive Behavior Assessment System—Second Edition (ABAS-II; Harrison and Oakland 2003): The ABAS-II is a measure of adaptive behavior with national standardization samples representative of the English speaking US population. The informant report adult form of the ABAS-II (Harrison and Oakland 2003) used in the present study was standardized on an age stratified sample and provided information in the areas of Conceptual (including Communication, Functional Academics, Self-Direction), Social (including not only Social but also Leisure), and Practical (including Community Use, Home Living, Health and Safety, Self-Care) Skills, all of which are presented as norm-referenced standard scores (M = 100; SD = 15) and were used as correlates of interest in the present study.
Co-morbid Internalizing Psychopathology
Adult Behavior CheckList (ABCL; Achenbach and Rescorla 2003): The ABCL is an 118 item scale composed of statements of behavior rated as ‘not true’, ‘somewhat or sometimes true’ and ‘very true’. The current study utilized the informant-rating version of the ABCL (appropriate for ages 18–59 years), which was filled out by parents/guardians. Two DSM-oriented subscales from the ABCL measuring anxiety problems and depression problems were utilized as correlates of interest. A third metric from the ABCL, Attention Deficit/Hyperactivity Deficit (ADHD) problems, was used as a nuisance variable. These ratings are expressed as T scores (M = 50; SD = 10) derived from comparisons with normative age expectations. Higher scores are indicative of more problem behaviors and T scores of 65 or higher are categorized as clinically significant.
Data Analysis
Analyses were conducted using IBM SPSS Statistics, version 22. One-sample t-tests were run to examine the degree of impairment on the BRIEF-A scores relative to the population mean of 50. Furthermore, a repeated measures ANOVA was run to examine the profile of EF scores within the ASD group. Hierarchical multiple regressions were then completed with ABAS-II domain scores (see Table 2) and ABCL anxiety and depression problems scores serving as the dependent variables (see Table 3). Demographic predictors were entered in the first block, followed by the BRIEF MCI and BRI scores in the second block. To examine the contribution of specific EF domains above and beyond the influences of age and IQ, a second set of regressions was run in which the independent variables of interest were the peak scales from within the MCI and BRI (defined by the scale within each index that exhibits greater deficits than the other scales based on post-hoc analyses obtained from the initial repeated measures ANOVA). As in the first set of regressions, the BRIEF scales were entered in the second block (after age and FSIQ).
Table 3.
Adaptive functioning scores regressed onto age, IQ, and executive function scores
| Predictor | Conceptual skills
|
Social skills
|
Practical skills
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| B | SE B | t | B | SE B | t | B | SE B | t | |
| BRIEF index analyses | |||||||||
| Step 1 | |||||||||
| Age | 0.10 | 0.84 | 0.12 | 0.25 | 0.76 | 0.33 | 0.70 | 1.07 | 0.66 |
| FSIQ | 0.26 | 0.20 | 1.30 | 0.09 | 0.20 | 0.47 | −0.02 | 0.26 | −0.07 |
| Step 2 | |||||||||
| Age | 0.04 | 0.73 | 0.06 | 0.18 | 0.72 | 0.25 | 0.58 | 0.92 | 0.62 |
| FSIQ | 0.20 | 0.18 | 1.10 | 0.01 | 0.19 | 0.05 | −0.11 | 0.23 | −0.47 |
| BRI | −0.05 | 0.27 | −0.17 | −.013 | 0.28 | −0.45 | −0.16 | 0.36 | −0.44 |
| MCI | −0.56 | 0.25 | −2.23* | −0.31 | 0.25 | −1.27 | −0.65 | 0.32 | −2.04* |
| BRIEF subscale analyses | |||||||||
| Step 1 | |||||||||
| Age | 0.10 | 0.84 | 0.12 | 0.25 | 0.76 | 0.33 | 0.70 | 1.07 | 0.66 |
| FSIQ | 0.26 | 0.20 | 1.30 | 0.09 | 0.20 | 0.47 | −0.02 | 0.26 | −0.07 |
| Step 2 | |||||||||
| Age | −0.04 | 0.72 | −0.06 | 0.16 | 0.70 | 0.23 | 0.54 | 0.96 | 0.56 |
| FSIQ | 0.16 | 0.18 | 0.89 | −0.01 | 0.19 | −0.07 | −0.13 | 0.24 | −0.56 |
| Shift | 0.24 | 0.25 | 0.96 | 0.05 | 0.25 | 0.22 | 0.25 | 0.34 | 0.76 |
| Plan/Org | −0.77 | 0.27 | −2.81** | −0.46 | 0.26 | −1.74 | −0.85 | 0.37 | −2.33* |
Bold values are statistically significant
FSIQ Full Scale IQ, BRI Behavior Regulation Index, MCI Metacognition Index, Plan/Org Planning/Organization
p ≤ .05;
p < .01
Results
Although one-sample t-tests suggested that adults with ASD were impaired on all nine domains of EF (based on the BRIEF-A) relative to the population mean of 50 (ps < .05), a repeated measures ANOVA demonstrated a variable EF profile (F = 10.20, p < .001) with the most prominent deficits occurring in flexibility (based on the Shift score from the Behavioral Regulation Index) and metacognition more broadly (in particular, Initiation, Working Memory, Plan/Org and Task Monitoring; see Fig. 1). Indeed, paired samples t-tests revealed that the Shift score was significantly higher than the scores on the other three BRI scales (ts > 3, ps < .005), but none of the MCI scores. The Plan/Organize score was significantly higher than scores found on three of the four BRI scales (not Shift) and three of the other four MCI scales (ts > 2.09, ps < .05; not Task Monitor). At the index score level, 26 and 60 % of the sample exhibited a clinically significant impairment (i.e., a T score ≥ 65) on the BRI and MCI, respectively, while at the subscale level 46 and 57 % exhibited a clinically significant impairment on the Shift and Plan/Organize scales, respectively. Since Shift was the peak score within the BRI and Plan/Organize was the peak score in the MCI (i.e., the only one that was significantly different from a majority of other subscales within its respective index), they were selected to examine the contribution of specific BRIEF subscale scores to the outcome variables and entered into the second block (after age and FSIQ) in the regression analyses that follow. Another series of regressions was run that were identical to those listed above except that an additional nuisance variable was added in the second block (ADHD problems from the ABCL) to assess the robustness of these findings to the influence of co-morbid ADHD symptomatology.
Fig. 1.
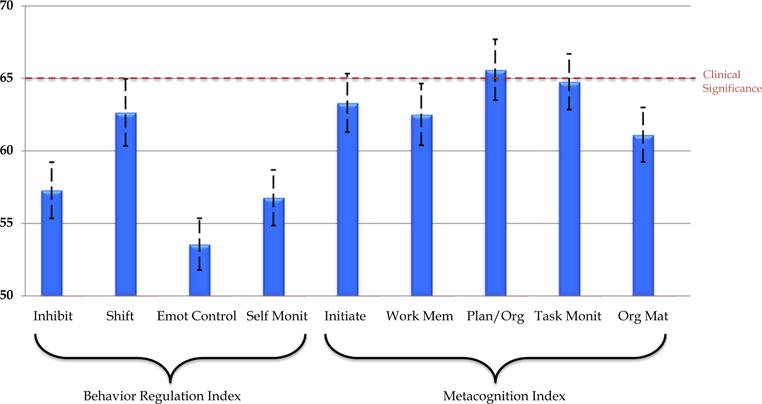
Profile of informant ratings of real-world executive functioning among 35 adults with autism spectrum disorder by subscale of the Behavior Rating Inventory of Executive Function—Adult (T-scores; M = 50, SD = 10)
Neither age nor IQ was a significant predictor of adaptive functioning across the ABAS-II domains of Conceptual, Social, and Practical Skills. When BRIEF index scores were added to the Conceptual Skills and Practical Skills models, they accounted for an additional 29 % (F = 6.42, p = .005) and 32 % (F = 6.66, p = .004) of the variance; the MCI was a significant predictor but the BRI was not (see Table 3). Similarly, Plan/Org (a subscale of the MCI) was the only significant predictor of Conceptual Skills scores, accounting for an additional 29 % of the variance beyond age and IQ (F = 6.49, p = .005) in the BRIEF subscales model (see Table 3). Fewer EF problems on the MCI and Plan/Org subscale of the BRIEF were significant predictors of better Conceptual Skills, and fewer EF problems on the MCI only was a significant predictor of better Practical Skills on the ABAS-II. Adding ADHD problems to the regression models as a nuisance variable resulted in EF problems no longer significantly predicting adaptive functioning impairments (ts < 1.66, ps > .11), however.
In contrast, results for the regression models examining the association between EF problems and internalizing behavior remained unchanged whether or not ADHD problems were included as a nuisance variable. Therefore, only results of the regression models including ADHD as a nuisance variable are reported below. Neither age nor IQ was a significant predictor of anxiety or depression symptomatology from the ABCL, but ADHD problems significantly predicted depression (not anxiety) symptoms when these three factors were modeled together (F = 5.47, p = .005). Adding the BRIEF index scores resulted in similarly predictive and significant regression model (F = 4.62, p = .005) with MCI becoming a significant predictor (see Table 4) explaining an additional 11 % of the variance of depression symptomatology scores, though this model was not significantly changed from the one including age, IQ, and ADHD problems alone (F = 2.41, p = .11). There were no significant predictors of anxiety symptomatology in the BRIEF index scores model. When the Shift and Planning Organization subscales were substituted for BRIEF indices, Plan/Org was the only other significant predictor (beyond ADHD problems) of depression symptomatology (F = 4.62, p = .02, ΔR2 = .17) and Shift was the only significant predictor (F = 8.56, p = .002; ΔR2 = .38) of anxiety symptomatology (see Table 4). More EF problems were associated with greater depression and anxiety symptomatology.
Table 4.
Internalizing behavior problem scores regressed onto age, IQ, ADHD problems, and executive function scores
| Predictor | Depression symptomatology
|
Anxiety symptomatology
|
||||
|---|---|---|---|---|---|---|
| B | SE B | t | B | SE B | t | |
| BRIEF index analyses | ||||||
| Step 1 | ||||||
| Age | 0.15 | 0.53 | 0.27 | 0.19 | 0.56 | 0.32 |
| FSIQ | 0.03 | 0.12 | 0.23 | 0.05 | 0.13 | 0.37 |
| ADHD problems | 0.54 | 0.14 | 4.02*** | 0.25 | 0.15 | 1.66 |
| Step 2 | ||||||
| Age | 0.25 | 0.52 | 0.47 | 0.48 | 0.58 | 0.82 |
| FSIQ | 0.04 | 0.12 | 0.38 | 0.08 | 0.13 | 0.63 |
| ADHD problems | 0.22 | 0.23 | 0.96 | −0.16 | 0.26 | −0.62 |
| BRI | −0.07 | 0.19 | −0.35 | 0.32 | 0.21 | 1.49 |
| MCI | 0.46 | 0.22 | 2.16* | 0.18 | 0.24 | 0.74 |
| BRIEF subscale analyses | ||||||
| Step 1 | ||||||
| Age | 0.15 | 0.53 | 0.27 | 0.19 | 0.59 | 0.32 |
| FSIQ | 0.03 | 0.12 | 0.23 | 0.05 | 0.13 | 0.37 |
| ADHD problems | 0.54 | 0.14 | 4.02*** | 0.25 | 0.15 | 1.66 |
| Step 2 | ||||||
| Age | 0.41 | 0.48 | 0.87 | 0.41 | 0.47 | 0.88 |
| FSIQ | 0.08 | 0.11 | 0.77 | 0.09 | 0.11 | 0.84 |
| ADHD problems | 0.20 | 0.16 | 1.25 | −0.10 | 0.16 | −0.61 |
| Shift | −0.05 | 0.14 | −0.35 | 0.39 | 0.14 | 2.74** |
| Plan/Org | 0.50 | 0.20 | 2.52* | 0.11 | 0.19 | 0.57 |
Bold values are statistically significant
FSIQ Full Scale IQ, BRI Behavior Regulation Index, MCI Metacognition Index, Plan/Org Planning/Organization
p < .05;
p ≤ .01;
p ≤ .001
Discussion
In accord with the growing literature documenting real-world EF difficulties in children and adolescents with ASD, the present study is one of the first to demonstrate that these problems persist into adulthood. The profile of real-world EF difficulties captured on the informant report of the BRIEF-A closely mirrors that found among children and adolescents, with peak weaknesses in flexibility and plan/org. Furthermore, these problems are robustly associated with both adaptive functioning deficits (above and beyond the influence of age and IQ) and co-morbid symptoms of depression and anxiety (above and beyond the influence of age, IQ, and co-morbid ADHD symptoms) in ASD. This suggests that EF problems should be a focus of evaluation and intervention amongst adults with ASD, just as they are in children and adolescents with ASD.
In this study, adults with ASD without intellectual disability exhibited variable EF difficulties with more elevated scores on the Metacognition Index (MCI) than the Behavior Regulation Index (BRI). Closer examination of the profile of EF subdomains revealed particular difficulties in flexibility (Shift subscale) from the BRI and planning and organization (Plan/Organize subscale) from the MCI of the BRIEF-A. This pattern is generally similar to that found amongst children and adolescents with ASD without intellectual disability (e.g., Granader et al. 2014). However, it does appear when comparing the present results to those of Granader et al. (2014), the largest sample assessed to date, that impairments on the BRI subscales (excluding Shift, the subscale assessing behavioral flexibility) are more muted among adults than children with ASD. This might be a function of everyday demands experienced during adulthood given that metacognitive skills such as plan/org ability and task monitoring become more important in the context of higher education and/or occupational pursuits. It is also worth noting that the participants in the current study had high average mean IQ (while Granader et al. did not report IQ, but excluded anyone with scores <70), which might provide compensatory mechanisms for EF difficulties. It is also possible that this pattern of results could be be due to some other factor(s), such as the composition of the standardization sample for the BRIEF-A, different developmental trajectories for some indices/subscales, such as increasing working memory (from the MCI) problems (Rosenthal et al. 2013), or idiosyncrasies of the current sample.
While many of the studies employing laboratory tasks fail to find EF impairments in adults with ASD (see Table 1), the present study finds clear EF deficits in adults with ASD without intellectual disability using informant report of real-world EF problems. This discrepancy between laboratory-based and everyday EF assessment is unsurprising and consistent with the broader literature (for review, see Kenworthy et al. 2008).
The present study also finds that real-world EF deficits amongst adults with ASD are associated with two key factors in adult independence/outcome: adaptive functioning and internalizing behavioral (i.e., depression and anxiety) symptoms, even after accounting for the influences of age and IQ. These findings are also largely consistent with the extant literature. For example, both Gilotty et al. (2002), among 35 youth, and Pugliese et al. (2015), among 357 youth with ASD, found that global and subscale BRIEF ratings correlated with adaptive functioning as measured by the Vineland Adaptive Behavior Scales (Sparrow et al. 1984, 2005). Similarly, in recent work, Lawson et al. (2015) showed that real-world flexibility difficulties were associated with anxiety and depression symptoms among pooled samples of children with ASD or ADHD. However, it should be noted that adding ADHD symptomatology as an additional nuisance variable in the present analyses abolished the significant associations between real-world EF and adaptive functioning. This is perhaps unsurprising given the emerging literature demonstrating, for example, exacerbated adaptive functioning deficits among children with both ASD and ADHD when compared to children with ASD alone (e.g., Yerys et al. 2009) or ADHD alone (e.g., Ashwood et al. 2015).
On the other hand, accounting for ADHD symptoms did not significantly impact the relationship between real-world EF and internalizing behavioral problems in this sample of adults with ASD. Moreover, there appears to be specificity wherein different forms of internalizing psychopathology are associated with distinct components of EF. While inflexibility in ASD is specifically associated with anxiety symptoms, metacognitive impairments (including plan/org problems) are linked with depression symptomatology. There is some precedence for this dissociation. For example, previous studies have linked planning ability, but not cognitive flexibility (using lab-based tasks such as the Tower of London and Wisconsin Card Sorting Test, respectively), with depressive symptoms in a community sample of adolescents (Vergara-Lopez et al. 2013). There is also a growing literature linking anxiety and repetitive behaviors, particularly insistence on sameness, in ASD (e.g., Gotham et al. 2013). Flexibility could be a mediating link between anxiety and insistence on sameness, though future research is needed to address such speculation. However, the mechanisms underlying these associations are largely unclear. Future research employing longitudinal designs to tease apart driving forces in these associations are needed as are studies of the shared and unique genetic, environmental, and neural underpinnings for these behaviors.
The present study had several limitations. Although concern for the role of rater bias in these findings is mitigated by variability in scores across and within informant report measures, and by study findings of specific, as opposed to global, EF predictors of adaptive behavior and psychiatric symptoms, reliance on informant report alone should be alleviated in the future through assessment of self-reports in adults with ASD and use of performance based tasks. Additionally, this study compared EF ratings for individuals with ASD to those from the BRIEF-A standardization sample instead of a control group. Although this provides a robust comparison group, future research should include separately assessed comparison groups to better understand the profile of EF impairments in ASD and control for differences in IQ, sex ratio and age distribution. Finally, the current study included only individuals with ASD without intellectual disability and reports on a sample for which the mean IQ is in the high average range. Whether the same pattern of results would be found amongst individuals with both ASD and intellectual disability or even borderline to low average intelligence, remains to be explored.
Our findings of real-world EF problems and demonstration of links between EF impairments and both adaptive functioning and co-morbid internalizing psychopathology suggests that EF is an important treatment target and that as these difficulties are ameliorated, they could have a cascading effect on other important areas of functioning among adults with ASD. Recent treatment development has extended beyond modular training programs, such as Cogmed (Olesen et al. 2004) for working memory difficulties, to interventions catered to the EF profile of specific disorders. In the case of ASD, one recent example is Unstuck and On Target!, which is a school-based intervention for children and adolescents with ASD (Cannon et al. 2011; Kenworthy et al. 2014a). It utilizes a cognitive-behavioral approach to teach flexibility and metacognitive skills as well as compensatory strategies for EF difficulties to youth with ASD that generalizes beyond the classroom. Initial randomized control trial work is promising (Kenworthy et al. 2014b); however, larger trials are needed. Perhaps most pertinent to the present study, upward developmental extensions of this work to adulthood could prove most promising for individuals with ASD who continue to experience EF difficulties in their everyday lives. This is particularly important during a period of time when external supports (e.g., afforded by the educational environment) are no longer available, and many individuals with ASD find themselves falling “off a cliff” into unstructured and overwhelming adult environments for which they lack the tools for successful integration. Moreover, how these EF-based treatments impact upon co-morbid anxiety and depression symptomatology in ASD remains unknown. If, for example, EF-based interventions are shown to diminish internalizing psychopathology in ASD, this would provide not only invaluable clinical insights but also evidence for mechanistic links between these constructs.
Acknowledgments
This work was supported by the Intramural Research Program at NIMH, NIH under Grant Number 1-ZIAMH002920. CEP was supported by a T32 Grant HD046388-01A2. Ethics approval for this study was granted by the NIH Combined Neuroscience Institutional Review Board under Protocol Number 10-M-0027. We would like to express our gratitude to the individuals and families who volunteered their time to contribute to this research.
Footnotes
Author contributions Dr. Wallace designed the study, analyzed the data, wrote the initial draft of the paper, and participated in revising the manuscript and addressing the reviewers’ comments. Drs. Kenworthy, Pugliese, and Martin as well as Ms. Brodsky assisted with manuscript development. Drs. Kenworthy, Pugliese, and Martin also participated in revising the manuscript and addressing the reviewers’ comments. Mr. Popal and Ms. White collected data, built the database, and reviewed the manuscript.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA adult forms & profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2003. [Google Scholar]
- Ambery FZ, Russell AJ, Perry K, Morris R, Murphy DG. Neuropsychological functioning in adults with Asperger syndrome. Autism. 2006;10:551–564. doi: 10.1177/1362361306068507. [DOI] [PubMed] [Google Scholar]
- Ashwood KL, Tye C, Azadi B, Cartwright S, Asherson P, Bolton P. Brief report: Adaptive functioning in children with ASD, ADHD and ASD + ADHD. Journal of Autism and Developmental Disorders. 2015;45:2235–2242. doi: 10.1007/s10803-014-2352-y. [DOI] [PubMed] [Google Scholar]
- Barnard L, Muldoon K, Hasan R, O’Brien G, Stewart M. Profiling executive dysfunction in adults with autism and comorbid learning disability. Autism. 2008;12:125–141. doi: 10.1177/1362361307088486. [DOI] [PubMed] [Google Scholar]
- Blijd-Hoogewys EM, Bezemer ML, van Geert PL. Executive functioning in children with ASD: An analysis of the BRIEF. Journal of Autism and Developmental Disorders. 2014;44:3089–3100. doi: 10.1007/s10803-014-2176-9. [DOI] [PubMed] [Google Scholar]
- Cannon L, Kenworthy L, Alexander KC, Werner MA, Anthony LG. Unstuck and on target! An executive function curriculum to improve flexibility for children with autism spectrum disorders. Baltimore, MD: Paul H. Brookes; 2011. [Google Scholar]
- Damasio AR, Maurer RG. A neurological model for childhood autism. Archives of Neurology. 1978;35:777–786. doi: 10.1001/archneur.1978.00500360001001. [DOI] [PubMed] [Google Scholar]
- Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators; Centers for Disease Control and Prevention (CDC) Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveillance Summaries. 2014;63:1–21. [PubMed] [Google Scholar]
- Geurts HM, Vissers ME. Elderly with autism: Executive functions and memory. Journal of Autism and Developmental Disorders. 2012;42:665–675. doi: 10.1007/s10803-011-1291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilotty L, Kenworthy L, Sirian L, Black DO, Wagner AE. Adaptive skills and executive function in autism spectrum disorders. Child Neuropsychology. 2002;8:241–248. doi: 10.1076/chin.8.4.241.13504. [DOI] [PubMed] [Google Scholar]
- Goldstein G, Minshew NJ, Allen DN, Seaton BE. High-functioning autism and schizophrenia a comparison of an early and late onset neurodevelopmental disorder. Archives of Clinical Neuropsychology. 2002;17:461–475. [PubMed] [Google Scholar]
- Gotham K, Bishop SL, Hus V, Huerta M, Lund S, Buja A, et al. Exploring the relationship between anxiety and insistence on sameness in autism spectrum disorders. Autism Research. 2013;6:33–41. doi: 10.1002/aur.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granader Y, Wallace GL, Hardy KK, Yerys BE, Lawson RA, Rosenthal M, et al. Characterizing the factor structure of parent reported executive function in autism spectrum disorders: The role of cognitive inflexibility. Journal of Autism and Developmental Disorders. 2014;44:3056–3062. doi: 10.1007/s10803-014-2169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PL, Oakland T. Adaptive behavior assessment—Second edition manual (ABAS-II) San Antonio, TX: Harcourt Assessment; 2003. [Google Scholar]
- Henninger NA, Taylor JL. Outcomes in adults with autism spectrum disorders: A historical perspective. Autism. 2013;17:103–116. doi: 10.1177/1362361312441266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill EL. Executive dysfunction in autism. Trends in Cognitive Sciences. 2004;8:26–32. doi: 10.1016/j.tics.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Hill EL, Bird CM. Executive processes in Asperger syndrome: Patterns of performance in a multiple case series. Neuropsychologia. 2006;44:2822–2835. doi: 10.1016/j.neuropsychologia.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Hollocks MJ, Jones CR, Pickles A, Baird G, Happé F, Charman T, Simonoff E. The association between social cognition and executive functioning and symptoms of anxiety and depression in adolescents with autism spectrum disorders. Autism Research. 2014;7:216–228. doi: 10.1002/aur.1361. [DOI] [PubMed] [Google Scholar]
- Howlin P, Moss P. Adults with autism spectrum disorders. Canadian Journal of Psychiatry. 2012;57:275–283. doi: 10.1177/070674371205700502. [DOI] [PubMed] [Google Scholar]
- Johnston K, Madden AK, Bramham J, Russell AJ. Response inhibition in adults with autism spectrum disorder compared to attention deficit/hyperactivity disorder. Journal of Autism and Developmental Disorders. 2011;41:903–912. doi: 10.1007/s10803-010-1113-9. [DOI] [PubMed] [Google Scholar]
- Kenworthy L, Anthony LG, Alexander KC, Werner MA, Cannon L, Greenman L. Solving executive functioning challenges: Simple ways to get kids with autism unstuck and on target. Baltimore, MD: Brookes Publishing Company; 2014a. [Google Scholar]
- Kenworthy L, Anthony LG, Naiman DQ, Cannon L, Wills MC, Werner MA, et al. Randomized controlled effectiveness trial of executive function intervention for children on the autism spectrum. Journal of Child Psychology and Psychiatry. 2014b;55:374–383. doi: 10.1111/jcpp.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy L, Yerys BE, Anthony LG, Wallace GL. Understanding executive control in autism spectrum disorders in the lab and in the real world. Neuropsychology Review. 2008;18:320–338. doi: 10.1007/s11065-008-9077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MC, Lombardo MV, Ruigrok AN, Chakrabarti B, Wheelwright SJ, Auyeung B, et al. Cognition in males and females with autism: Similarities and differences. PLoS One. 2012;7 doi: 10.1371/journal.pone.0047198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainhart JE, Bigler ED, Bocian M, Coon H, Dinh E, Dawson G, et al. Head circumference and height in autism: A study by the Collaborative Program of Excellence in Autism. American Journal of Medical Genetics. 2006;140:2257–2274. doi: 10.1002/ajmg.a.31465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson RA, Papadakis AA, Higginson CI, Barnett JE, Wills MC, Strang JF, et al. Specific executive function impairments predict comorbid psychopathology in autism spectrum and attention-deficit/hyperactivity disorders. Neuropsychology. 2015;29:445–453. doi: 10.1037/neu0000145. [DOI] [PubMed] [Google Scholar]
- Le Couteur A, Rutter M, Lord C, Rios P, Robertson S, Holdgrafer M, McLennan J. Autism diagnostic interview: A standardized investigator-based instrument. Journal of Autism and Developmental Disorders. 1989;19:363–387. doi: 10.1007/BF02212936. [DOI] [PubMed] [Google Scholar]
- Lopez B, Lincoln A, Ozonoff S, Lai Z. Examining the relationship between executive functions and restricted, repetitive symptoms of autistic disorder. Journal of Autism and Developmental Disorders. 2005;35:445–460. doi: 10.1007/s10803-005-5035-x. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, Dilavore PC, et al. The autism diagnostic observation schedule—generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Mazurek MO. Loneliness, friendship, and well-being in adults with autism spectrum disorders. Autism. 2014;18:223–232. doi: 10.1177/1362361312474121. [DOI] [PubMed] [Google Scholar]
- Nakahachi T, Iwase M, Takahashi H, Honaga E, Sekiyama R, Ukai S, et al. Discrepancy of performance among working memory-related tasks in autism spectrum disorders was caused by task characteristics, apart from working memory, which could interfere with task execution. Psychiatry and Clinical Neurosciences. 2006;60:312–318. doi: 10.1111/j.1440-1819.2006.01507.x. [DOI] [PubMed] [Google Scholar]
- Nydén A, Niklasson L, Stahlberg O, Anckarsater H, Wentz E, Rastam M, Gillberg C. Adults with autism spectrum disorders and ADHD neuropsychological aspects. Research in Developmental Disabilities. 2010;31:1659–1668. doi: 10.1016/j.ridd.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nature Neuroscience. 2004;7:75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- Pugliese CE, Anthony L, Strang JF, Dudley K, Wallace GL, Kenworthy L. Increasing adaptive behavior skill deficits from childhood to adolescence in autism spectrum disorder: Role of executive function. Journal of Autism and Developmental Disorders. 2015;45:1579–1587. doi: 10.1007/s10803-014-2309-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal M, Wallace GL, Lawson R, Wills MC, Dixon E, Yerys BE, Kenworthy L. Impairments in realworld executive function increase from childhood to adolescence in autism spectrum disorders. Neuropsychology. 2013;27:13–18. doi: 10.1037/a0031299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth RM, Isquith PK, Gioia GA. Behavior rating inventory of executive function—adult version (BRIEF-A) Lutz, FL: Psychological Assessment Resources; 2005. [Google Scholar]
- Roux AM, Shattuck PT, Cooper BP, Anderson KA, Wagner M, Narendorf SC. Postsecondary employment experiences among young adults with an autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52:931–939. doi: 10.1016/j.jaac.2013.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumsey JM. Conceptual problem-solving in highly verbal, nonretarded autistic men. Journal of Autism and Developmental Disorders. 1985;15:23–36. doi: 10.1007/BF01837896. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Hamburger SD. Neuropsychological findings in high-functioning men with infantile autism, residual state. Journal of Clinical and Experimental Neuropsychology. 1988;10:201–221. doi: 10.1080/01688638808408236. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Hamburger SD. Neuropsychological divergence of high-level autism and severe dyslexia. Journal of Autism and Developmental Disorders. 1990;20:155–168. doi: 10.1007/BF02284715. [DOI] [PubMed] [Google Scholar]
- Sachse M, Schlitt S, Hainz D, Ciaramidaro A, Schirman S, Walter H, et al. Executive and visuo-motor function in adolescents and adults with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2013;43:1222–1235. doi: 10.1007/s10803-012-1668-8. [DOI] [PubMed] [Google Scholar]
- Sparrow S, Balla DA, Cicchetti D. Vineland adaptive behavior scales (expanded form) Circle Pine, MN: American Guidance Service; 1984. [Google Scholar]
- Sparrow SS, Cicchetti D, Balla DA. Vineland adaptive behavior scales— 2nd edition manual. Minneapolis, MN: NCS Pearson Inc; 2005. [Google Scholar]
- Taylor JL, Seltzer MM. Employment and post-secondary educational activities for young adults with autism spectrum disorders during the transition to adulthood. Journal of Autism and Developmental Disorders. 2011;40:1431–1446. doi: 10.1007/s10803-010-1070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towgood KJ, Meuwese JD, Gilbert SJ, Turner MS, Burgess PW. Advantages of the multiple case series approach to the study of cognitive deficits in autism spectrum disorder. Neuropsychologia. 2009;47:2981–2988. doi: 10.1016/j.neuropsychologia.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara-Lopez C, Lopez-Vergara HI, Colder CR. Executive functioning moderates the relationship between motivation and adolescent depressive symptoms. Personality and Individual Differences. 2013;54:18–22. doi: 10.1016/j.paid.2012.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Goldstein G, Carpenter PA, Minshew NJ. Verbal and spatial working memory in autism. Journal of Autism and Developmental Disorders. 2005;35:747–756. doi: 10.1007/s10803-005-0021-x. [DOI] [PubMed] [Google Scholar]
- Wilson CE, Happé F, Wheelwright SJ, Ecker C, Lombardo MV, Johnston P, et al. The neuropsychology of male adults with high-functioning autism or Asperger syndrome. Autism Research. 2014;7:568–581. doi: 10.1002/aur.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerys BE, Wallace GL, Sokoloff J, Shook DA, James JD, Kenworthy L. Attention deficit/hyperactivity disorder symptoms moderate cognition and behavior in children with autism spectrum disorders. Autism Research. 2009;2:322–333. doi: 10.1002/aur.103. [DOI] [PMC free article] [PubMed] [Google Scholar]


