Abstract
Ribosomal proteins have long been known to serve critical roles in facilitating the biogenesis of the ribosome and its ability to synthesize proteins. However, evidence is emerging that suggests ribosomal proteins are also capable of performing tissue-restricted, regulatory functions that impact normal development and pathological conditions, including cancer. The challenge in studying such regulatory functions is that elimination of many ribosomal proteins also disrupts ribosome biogenesis and/or function, preventing one from unambiguously determining whether developmental abnormalities resulting from ablation of a ribosomal protein result from loss of core ribosome functions or from loss of the regulatory function of the ribosomal protein. Rpl22, a ribosomal protein component of the large 60S subunit, provides insight into this conundrum, as Rpl22 is dispensable for both ribosome biogenesis and protein synthesis, yet its ablation causes tissue-restricted disruptions in development. Here we review evidence supporting the regulatory functions of Rpl22 and other ribosomal proteins.
INTRODUCTION
Mutations in ribosomal proteins (RP) have long been known to cause a collection of diseases termed ribosomopathies, which are characterized by disruptions in hematopoiesis, abnormalities in craniofacial and skeletal structure, and increased cancer risk. The prevailing view has been that these abnormalities result from general perturbations in the assembly or function of the ribosome; however, recent analysis of the pathophysiology of ribosomopathies, and the specific functions of particular RP, has challenged this convention and suggested that, in addition to being key components of the ribosome, RP are also capable of serving regulatory functions1–6. Indeed, evidence is emerging that suggests that the regulatory functions of certain RP may contribute to the constellation of abnormalities observed in ribosomopathies, and may do so from within specialized ribosomes, or alternatively, in an extraribosomal capacity. By exploring these novel regulatory mechanisms and pathways, we can begin to address key questions regarding the tissue-specific defects linked to human diseases. Here we review the evidence that certain RP are capable of regulatory functions, using the RP, Rpl22, as an important example of those capabilities.
BODY
Ribosomes & Ribosomopathies
Ribosomes are intracellular machines that are responsible for protein synthesis and comprise large ribonuceloprotein (RNP) particles containing numerous RNA and protein species. Eukaryotic ribosomes contain 4 ribosomal RNAs (rRNA) and ~78 RP7 and their assembly is facilitated by a large array of additional RNA and protein factors8. Despite the vital role of ribosomes in translation in all living cells, defects in ribosome synthesis are not always lethal. Ribosomopathies are a collection of human genetic disorders resulting from mutations in RP or ribosome biogenesis cofactors, which are thought to cause disruptions in development through inducing ribosome dysfunction9,10. These diseases have recently attracted great interest, since elucidation of the mechanisms underlying this set of diseases could have broad implications for both basic biology and therapeutic intervention1. Ribosomopathies include Diamond-Blackfan anemia (DBA), 5q-syndrome, Shwachman-Diamond syndrome (SDS), cartilage-hair hypoplasia (CHH), X-linked dyskeratosis congenita (DC), Treacher-Collins syndrome (TCS), North American Indian childhood cirrhosis (NAIC), Bowen-Conradi syndrome, and isolated congenital asplenia (ICA)2,11,12.
As a founding member of the collection of diseases termed ribosomopathies, DBA was initially linked to genetic mutation of the RPS19 gene, which encodes a ribosomal protein in the small subunit of ribosome13. Since then, 13 additional RP genes (RPS7, RPS10, RPS17, RPS24, RPS26, RPS28, RPS29, RPL5, RPL11, RPL15, RPL26, RPL31 and RPL35A) have been implicated in DBA14–18. Indeed, more than 50% of patients who are diagnosed with DBA harbor heterozyous mutations in one of the identified RP genes1,3. While it has never been described in DBA, monoallelic loss of RPS14 has been shown to play a critical role in the development of 5q-syndrome19,20, a condition of altered hematopoiesis with increased cancer risk that is an independent subtype of myelodysplastic syndromes (MDS). Remarkably, in contrast to the common anemic manifestations present in DBA and 5q-syndrome, halpoinsufficiency of RPSA (a small subunit RP) was recently linked to isolated congenital asplenia by unbiased exome analysis21. The absence of hematopoietic defects in patients with RPSA haploinsufficiency illustrates how mutations in particular RP can have distinct and tissue-restricted effects.
Despite the diverse clinical manifestations associated with ribosomopathies, the prevailing view is that these diseases result from activation of p53 via cellular stress induced upon ribosome dysfunction3,22,23. Numerous reports have demonstrated that disruption in ribosome biosynthesis, now referred to as “ribosomal/nucleolar stress”, causes cell cycle arrest and apoptosis through p53 activation2,24–27. Indeed, CD34+ bone marrow cells isolated from RPS19-mutant DBA patients generate a higher number of apoptotic cells during in vitro cell culture28. Moreover, shRNA-mediated knockdown of Rps14 or Rps19 in healthy primary human CD34+ cells causes erythroid lineage-restricted induction of p5329. Finally, inactivation of p53 rescues the phenotypes observed in several animal models of ribosomopathies, suggesting that aberrant p53 upregulation may be responsible for their pathogenesis1,22,30–37. These studies raise a key question as to how disrupted ribosome biosynthesis activates p53. One mechanism involves blocking p53 ubiquitination and degradation. Numerous ribosomal proteins, including Rpl5, Rpl11, Rpl23, Rpl26, Rpl37, Rps3, Rps7, Rps14, Rps15, Rps20, Rps24 and Rps25 have been reported to physically interact with HDM2 (or Mdm2 in mouse) and suppress its E3 ubiquitin ligase activity38–53. HDM2 is a pivotal negative regulator of p53 that polyubiquitinates p53 and causes its proteasomal degradation. Hence, the loss of HDM2 E3 ligase activity upon binding of RP results in elevated levels of p53. While numerous RP can interact with HDM2, several studies suggest that only Rpl5 and Rpl11, potentially together with 5S rRNA, are essential for the induction of p5327,54–56. Indeed, only Rpl5 and Rpl11 accumulate in the ribosome-free fraction upon induction of nucleolar stress, while other RP are rapidly degraded by the proteasome57. Regardless, the prevailing view is that defective ribosome biogenesis activates p53 by increasing the pool of free RP, which impair HDM2 function and increase p53 stability1,58. p53 activation, in turn, is postulated to induce anemia because the rapid proliferation of erythroid progenitors renders them hypersensitive to p53 induction23.
While this model may explain the anemia and bone marrow failure seen in may ribosomopathies, it fails to explain the distinct clinical manifestations observed in particular ribosomopathies. Presently, the pathophysiology of ribosomopathies remains largely unknown and is an area of active research9,10. Among the outstanding questions is how mutations in ubiquitously expressed RP are able to cause tissue-restricted defects2. Emerging evidence suggests that RP are not just static passengers on ribosomes that contribute to its structural integrity, thereby generally facilitating translation, but may also actively participate in regulating many biological processes, from within and even separate from the ribosome2,4.
A New Paradigm: Unique Functions of Ribosomal Proteins
In principle, RP are capable of performing regulatory functions either from within specialized ribosomes of differing content or separated from the ribosome in an “extraribosomal” capacity. The “Ribosome Filter Hypothesis” was proposed in 2002 by Mauro and Edeleman and suggested that ribosomes of differing composition exert regulatory influence on the cellular proteome by controlling the extent to which particular mRNA species are translated into protein59. While this is an attractive hypothesis that underpins more recent proposals of specialized ribosomes, it has been difficult to conclusively test; however, recent experimentation, including efforts evaluating the role of Rpl38 in controlling the translation of Hox mRNAs, is beginning to provide evidence in favor of some RP performing particular functions from within the ribosome60,61. The alternative possibility, for which there is already substantial support, is that RP can be separate from the ribosome and regulate cellular processes in an “extraribosomal” capacity. There are three biological contexts where RP would be separated from the ribosome: 1) under conditions of disrupted ribosome biogenesis, as described above; 2) when super-stoichiometric increases in expression of one RP exceed the levels of other RP; and 3) stimulus-induced release from the ribosome. All of these circumstances enable RP, many of which are RNA-binding proteins, to engage cellular RNAs and proteins and regulate their activity.
Interactions with Proteins
Along with interactions between RP and HDM2 (discussed above), RP also interact with other members of this family of p53 regulators. Several RP, including Rps15, Rps20 and Rpl37, can bind to and negatively regulate MDMX, a homologue and partner of MDM24,40. Although there is no evidence of direct binding with MDMX, Rpl11 promotes MDMX degradation by binding MDM262. Interestingly, Rpl11 also binds PICT1, which sequesters Rpl11 in the nucleolus and prevents its association with HDM263.
Rps3 has been shown to interact with E2F1 to induce apoptosis in neuronal cells, and this interaction is inhibited via AKT-binding and phosphorylation of Rps364. Rps3 also interacts with TRADD and is recruited to the death-inducing signaling complex (DISC) to induce apoptosis65. In addition to regulating apoptosis, Rps3 has also been reported to influence the transcriptional activity of NF-κB. Rps3 associates with p65 (RelA) of the NF-κB complex and is required for enhanced DNA binding and regulation of the expression of a subset of NF-κB target genes66. Further studies demonstrated that Rps3 translocation to the nucleus is dependent on IκB kinase β phosphorylation at a site distinct from the site phosphorylated by AKT67. Phosphorylation of Rps3 also mediates activation of an NF-κB-mediated pro-survival pathway in non-small cell lung cancer (NSCLC) cells through disruption of the Rps3-TRAF2 complex, thereby rendering those cells radioresistant68. In addition to phosphorylation, other post-translational modifications, such as methylation and sumoylation, also regulate the extraribosomal functions of Rps365,69,70.
Rps19, the first ribosomal gene linked to DBA13, has been shown to dimerize with the receptor for complement component C5a and induce a respiratory burst reaction in monocytes. Conversely, this same interaction inhibits the C5a-induced respiratory burst in neutrophils71. How this function of Rps19 influences the pathophysiology of DBA remains unknown.
A number of RP have also been implicated in regulating transformation through relatively poorly understood mechanisms. Rpl11 and Rps14 are both able to bind the c-Myc oncogene and inhibit its transcriptional activity by preventing the recruitment of its cofactor TRRAP72,73. Rps7 suppresses ovarian tumorigenesis though regulation of the PI3K/AKT and MAPK pathways74, although the mechanism of how Rps7 regulates those signaling networks has not been elucidated. Finally, Rpl41 regulates the phosphorylation and degradation of ATF4, which is critical for tumor cell survival in response to stress75.
Interactions with RNA
One of the best-described extraribosomal functions of RP is that of Rpl13a, which is released from the ribosome by γ-interferon stimulation and regulates the translation of particular mRNA targets76. In response to γ-interferon, Rpl13a is phosphorylated at Ser77, which releases it from the ribosome. Upon release, Rpl13a is able to bind to mRNAs containing “GAIT” elements (γ-interferon activated inhibitor of translation) in their 3′UTR and specifically repress their translation76–79. Rpl13a represses translation as part of the heterotetrameric GAIT complex comprising Rpl13a, GAPDH, EPRS and NSAP1. GAIT-containing targets are highly enriched for mRNAs that encode for pro-inflammatory proteins, adding an additional layer of regulation and contributing to the resolution of inflammation78,80,81. Recently, a virus-activated translation repression complex containing Rpl13a has also been reported82.
In addition to Rpl13 and the GAIT complex, several other RP have been reported to mediate transcript-specific translational control. In particular, emerging evidence suggests that RP may recognize specific IRES (internal ribosome entry site) elements5. For example, Rps6 and Rps25 are critical for IRES-mediated translation of viral RNAs83–86. Rps19 and Rpl11, two DBA-associated RP, selectively regulate IRES-dependent translation of BAG1 and CSDE1, both of which are crucial for erythroid differentiation4,5,87,88. A recent report demonstrated that Rpl38 is required for the IRES-dependent translation of HoxA during skeleton development, possibly explaining the tissue-specific developmental defects caused by Rpl38-haploinsufficiency60,89. While the control of Hox transcript translation has been proposed to result from the function of Rpl38 from within specialized ribosomes, it is remains unclear how this would occur given that Rpl11 and Rpl38 are components of the large 60S subunit and only the small 40S subunit is associated with the mRNA during scanning for start codons.
While impaired ribosome biogenesis has been shown to induce p53 by altering p53 stability, RP have also been implicated in regulating the translation of p53 mRNA. Indeed, Rpl26 enhances p53 translation following DNA damage through interactions with the 5′ and 3′ UTR of p53 mRNA, and this is antagonized by nucleolin90–92. Rpl22 has also been suggested to bind to p53 and repress its translation93. In addition to the regulation of tumor suppressors such as p53, RP also regulate the expression of oncogenes. Indeed, Rpl11 and Rpl5 bind to the 3′-UTR of c-Myc mRNA and promotes its degradation94,95, while Rps14 promotes c-Myc mRNA turnover through miRNA-mediated degradation73. Moreover, Rpl11 has been shown to upregulate the translation of mRNAs carrying a 5′ oligopyrimidine tract (5′-TOP mRNAs), which have been implicated in growth control27,96.
Tissue-specific and divergent functions of RP paralogs
Unlike yeast, where 75% of RP have highly-homologous functional paralogs, most RP in plants, flies, and vertebrates are encoded by a single functional gene5. Nevertheless, a handful of RP paralogs have been preserved in mammals, and such paralogs exhibit tissue specificity in their expression. The paralog of Rpl3, Rpl3l, is selectively expressed in muscle tissue, while that of Rpl10, Rpl10l, is restricted to the testis12,97. The physiologic relevance of the tissue-restricted expression of RP paralogs remains largely unknown, but has been suggested to enable greater control of gene expression in higher eukaryotes97.
Rpl22: not a typical ribosomal protein
Recent reports have demonstrated highly specialized roles for Rpl22 and its paralog Rpl22-like1 (Rpl22l1) in normal development and tumorigenesis98–101. We will now discuss findings on how these two proteins regulate hematopoiesis and transformation. In particular, we will focus on why Rpl22 appears to be critical for normal development and function of only certain tissues, despite its ubiquitous expression. We will also address the functional relationship between Rpl22 and Rpl22l1 and the evidence that they are functioning through extraribosomal interactions with cellular target RNAs and proteins.
Historical perspective: from housekeeping to RNA-binding
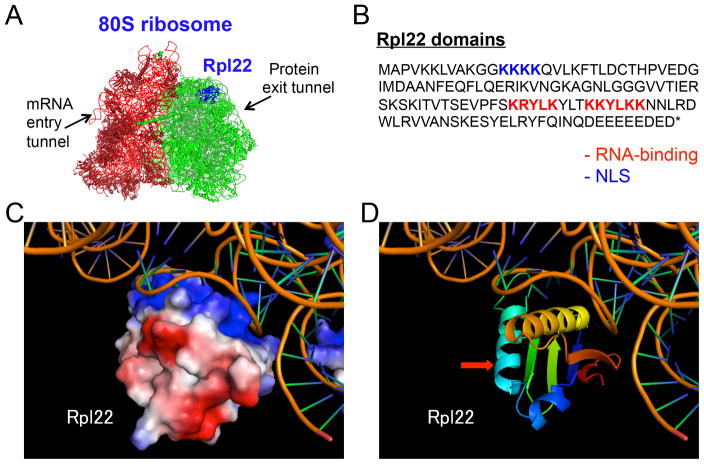
Rpl22 was originally identified as a ubiquitously-expressed protein that was associated with the ribosome, which led to its use as a loading control for normalizing gene expression102,103. Subsequent studies, however, demonstrated that Rpl22 is incorporated into the 60S large subunit of the ribosome at a late stage of ribosome maturation104. Rpl22 is an external, salt-extractable component on the surface of 60S, positioned away from the subunit interface (Figure 1A, blue area). Although it co-localizes with rRNA in the nucleolus and the cytoplasm105–107, Rpl22 is not required for general ribosome assembly or global, cap-dependent translation108. This is consistent with its positioning in the crystal structure of the 80S ribosome, which reveals that Rpl22 is located distal to both the mRNA entry tunnel and the nasecent protein exit tunnel of the 60S subunit (Figure 1A, arrows).
Figure 1. Rpl22 and its location in the ribosome.
A) The position of Rpl22 in the published 80S ribosome model. Red: small 40S subunit, Green: large 60S subunit, Blue: Rpl22. B) Amino acid sequence of murine Rpl22. The RNA-binding helices (Red) and nuclear localization signal (NLS; blue) are indicated. C) Cryo-EM depiction of Rpl22 interacting with 28S rRNA. D) Ribbon diagram of Rpl22 secondary structure as it interacts with 28S rRNA. The red arrow indicates the RNA-binding helices.
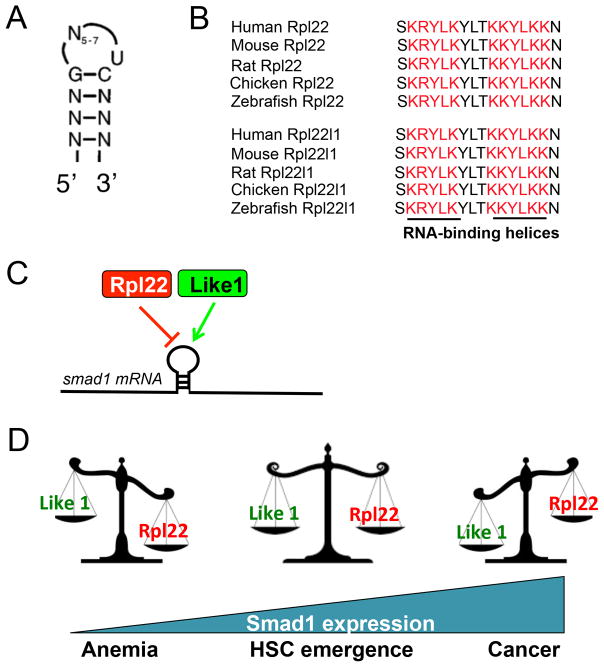
Rpl22 is an RNA-binding protein that not only associates with the 28S ribosomal RNA, but also with the Epstein-Barr Virus EBER-1 RNA in human B cells109 and the 3′ UTR of hepatitis C virus (HCV) RNA110,111. Rpl22 also interacts with human telomerase RNA hTR within the nucleus112,113 and modulates the translation of its paralog, Rpl22l1, by binding to an internal hairpin structure in the Rpl22l1 mRNA114. Rpl22 contains classical RNA-binding helices (Figure 1B–D, red arrow), which are required for RNA-binding, as their mutation abolishes interactions with RNA115. SELEX experiments revealed that Rpl22 binds a consensus RNA secondary structure with three conserved nucleotides (G-CU) in the neck of a stem-loop110 (Figure 2A). This consensus binding motif is present in all Rpl22 RNA targets identified to date, including 28S rRNA, hTR, and the Epstein Barr Virus EBER-1 latency RNA stem-loops III and IV, which direct translocation of Rpl22 from the cytoplasm to the nucleus in EBV infected host cells109, 112, 113.
Figure 2. Functional interplay between Rpl22 and its highly homologous paralog, Rpl22l1.
A) RNA consensus hairpin/motif bound by Rpl22 (adapted from Dobbelstein and Shenk, J Virology, 1995). B) Evolutionary conservation of the RNA-binding domains of Rpl22 and Rpl22l1. C) Rpl22 and Rpl22l1 play distinct, and opposing roles in HSC emergence. Rpl22 and Rpl22l1 both bind to Smad1 mRNA and control its translation, with Rpl22 playing a repressive role and Rpl22l1 acting to oppose that repression. D) HSC emergence is controlled by the opposing actions of Rpl22 and Rpl22l1 on Smad1 expression. When Rpl22 and Rpl22l1 are in balance, HSC emergence occurs normally; however, if Rpl22 dominates, it represses Smad1, blocks HSC emergence and causes anemia, whereas if Rpl22l1 dominates, HSC emergence is uncontrolled, leading to an increased predisposition to transformation.
In addition to its RNA binding ability, emerging evidence suggests that Rpl22 physically interacts with various protein partners. For example, Drosophila Rpl22 specifically interacts with and co-localizes with histone H1, and this complex is tightly associated with condensed chromatin, regulates histone modifications, and epigenetically modulates gene transcription106. In addition, Drosophila Rpl22 can interact with Poly(ADP-ribose) polymerase (PARP), a nuclear enzyme involved in DNA repair and programmed cell death116. Casein kinase 2α was also reported to co-purify with Rpl22 in human lung cancer cells and in Drosophila117,118. Interestingly, Rpl22 was reported to associate with Ago2, a core protein component for the miRNA-mediated translational repression machinery119. Together, these studies highlight the variety of proteins and RNA targets with which Rpl22 is already known to interact. While these interactions can easily be envisioned to contribute to the regulatory functions of Rpl22, their relevance to Rpl22’s role in normal development or in pathological contexts, such as in development, anti-viral immune responses or transformation, remain unknown.
Loss of Rpl22 in model organisms
In humans, haploinsufficiency of RP is the pathophysiological basis for DBA, leading to clinical phenotypes such as organ malformations, defective hematopoiesis and increased cancer risk11. To gain insight into the normal physiologic role of Rpl22 in development, Rpl22 has been ablated in a number of model systems. Due to the ubiquitous expression of Rpl22 in all organisms, it would be predicted that the loss of Rpl22 would be the lethal. Indeed, deletion of Rpl22 in Drosophila and knockdown of Rpl22 in C. elegans are both embryonic lethal11,120. However, ablation of Rpl22a or Rpl22b in yeast does not affect their viability, and such yeast exhibit only mild defects in bud site selection and slowed growth121,122. The discrepant results in these model systems prompted an assessment in mammals. The Rpl22 knockout mouse, which was generated by gene-trap mutagenesis, was viable and fertile with no obvious morphologic defects98, which is quite unusual as RP-deficiencies are generally embryonic lethal or at least display gross morphologic abnormalities123,124. Interestingly, the most obvious initial phenotype in Rpl22-deficient mice was a highly selective arrest in development of a subset of T lymphocytes98,125. This raises two critical questions: 1) Why is Rpl22-deficiency not lethal; and 2) Why do only selected tissues exhibit Rpl22-dependency, when Rpl22 is ubiquitiously expressed?
The most likely explanations are either that: 1) Rpl22 is dispensable for ribosome biogenesis and function and the tissue-restricted developmental abnormalities result from the loss of its tissue-restricted regulatory functions; or 2) Rpl22l1, the highly homologous paralog of Rpl22, might compensate for Rpl22 loss in some tissues. In Drosophila, Rpl22l1 is specifically enriched in testes126 but not in the ovary or other tissues127. Rpl22l1 can incorporate into the ribosome and associate with active translational machinery127. The RNA-binding helices in Rpl22l1 are highly conserved and identical to those in Rpl22, suggesting the potential for Rpl2 and Rpl22l1 to bind a similar set of RNA targets (Figure 2B). Moreover, in Rpl22-deficient mice, Rpl22l1 expression is increased and it is more extensively incorporated into the ribosome, suggesting that the potential for compensation exists114. Conversely, while knockdown of Rpl22l1 in MEF impairs growth, the knockdown of Rpl22 promotes MEF growth and proliferation, raising the possibility that these paralogs perform distinct functions114. Accordingly, the potential for compensation for Rpl22 loss by Rpl22l1 exists, but at least in some tissues, the functions of these paralogs may be distinct. The surprising possibility that Rpl22 and Rpl22l1 may have distinct and even antagonistic functions is addressed below.
Antagonistic Regulatory Functions of Rpl22 and Rpl22l1 in Hematopoiesis
The question of whether Rpl22 and Rpl22l1 are functionally redundant was evaluated using the zebrafish model, which has a number of attributes that make it particularly useful for such comparisons99. Zebrafish are extremely amenable to assessing the regulatory roles of RP in development because: 1) blood cell development in zebrafish is extremely well conserved with that in mammals; 2) development of all blood cell lineages can be easily monitored in the transparent embyros using transgenic fluorescent reporters that mark each blood cell lineage128–130; and 3) gene expression can be easily manipulated by knocking genes down using morpholinos or by overexpressing them through injection of mRNA or heat inducible expression vectors.
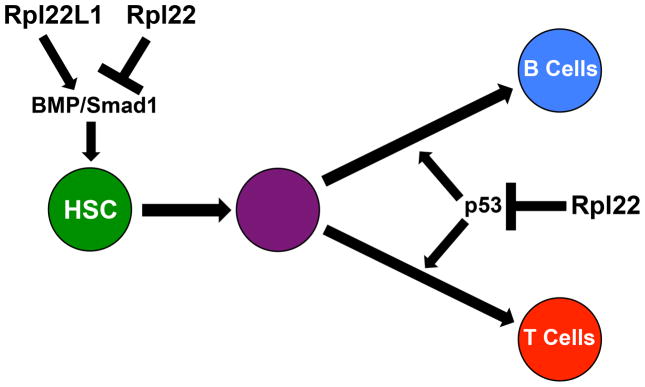
Both Rpl22 and Rpl22l1 are ubiquitously expressed in all tissues in zebrafish. Their functional relationships were assessed by knockdown using morpholino oligonucleotides, which revealed that both are essential for development of T cell progenitors in the thymus99; however, Rpl22 knockdown arrested T cell development in a p53-dependent manner, whereas the arrest of T cell development caused by Rpl22l1 knockdown was p53-independent. Rpl22 knockdown arrested the development of T cell progenitors after their arrival in the thymus, but knockdown of Rpl22l1 arrested T cell development indirectly, by arresting the emergence of hematopoietic stem cells (HSC) in the aorta-gonad-mesonephros (AGM). Because of the arrest of HSC emergence caused by Rpl22l1 knockdown, it was not possible to determine in zebrafish if Rpl22l1 function was also required in downstream progeny, including T cell progenitors in the thymus. Nevertheless, it was definitively demonstrated that Rpl22 and Rpl22l1 are not functionally interchangeable in the hematopoiesis, since enforced expression of Rpl2211 was unable to alleviate the arrest of T cell progenitors caused by knockdown or Rpl22, nor was enforced expression of Rpl22 able to restore HSC emergence in zebrafish embryos in which Rpl22l1 had been knocked down. Together, these data make clear that Rpl22 and Rpl22l1 are performing distinct functions in hematopoiesis.
The arrest of HSC emergence caused by Rpl22l1 knockdown is independent of effects on p53 and instead results from impaired BMP4/Smad1 signaling, which is required for induction of the essential transcriptional regulator for definitive hematopoiesis, Runx199. Rpl22l1 knockdown interferes with BMP4/Smad1 signaling by post-transcriptionally reducing the expression of Smad1. This reduction in Smad1 expression is associated with a shift of smad1 mRNA out of the polysome fraction99. Moreover, smad1 mRNA contains consensus Rpl22/Rpl22l1 binding sites (Figure 2A) and can be bound by Rpl22l1, as well as Rpl22. These data suggest that Rpl22l1 controls the translation of smad1 mRNA by direct binding. Moreover, the repression of Smad1 protein expression caused by knockdown of Rpl22l1 is reversed by simultaneous knockdown of Rpl22, indicating that Smad1 expression is reduced upon Rpl22l1 knockdown because the Rpl22 that remains represses the translation of smad1 mRNA. Thus, Smad1 expression is antagonistically controlled by Rpl22 and Rpl22l1, with Rpl22l1 acting to facilitate Smad1 expression and Rpl22 acting to interfere with Rpl22l1 and block Smad1 expression (Figure 2C). By extension, HSC emergence is controlled by the antagonistic balance of Rpl22 and Rpl22l1. When Rpl22 and Rpl22l1 expression is balanced, HSC emergence occurs normally in a tightly regulated manner (Figure 2D); however, when Rpl22 and Rpl22l1 are not balanced, pathology results, with excess Rpl22 blocking HSC emergence and causing anemia, while Rpl22l1 dominance leads to excessive generation of HSC that are predisposed to transformation (Harris et al., In preparation). More generally, these findings suggest that Rpl22 and Rpl22l1 are likely to bind an extensively overlapping set of RNA targets, but they have distinct and in many instances antagonistic effects on those targets (Figure 2C).
Rpl22 in Lymphocyte Development and Transformation
While the networks of cell surface receptors and transcription factors that regulate the development and function of B and T cells have been extensively studied131–133, relatively little is known regarding the influence of post-transcriptional regulation on lymphocyte development and function by RNA-binding proteins such as Rpl22. Despite the ubiquitous expression of Rpl22, its germline ablation does not result in embryonic lethality or gross abnormalities in mice, but does result in profound reductions in selected peripheral lymphocyte populations98. Indeed, Rpl22-deficient mice have striking decreases in splenic CD4 and CD8 T cells, and somewhat more modest reductions in splenic B2 cells and B1 B cells present in the peritoneal cavity98,134. Surprisingly, Rpl22-deficient peripheral B cells appear fully functional, as they are able to proliferate in response to mitogenic stimulation, upregulate activation markers, and undergo class switch recombination. Initial studies reported that Rpl22-deficient T cells were unable to proliferate in response to TCR stimulation98; however, this is likely to be due to the extensive homeostatic proliferation that occurs in Rpl22-deficient mice, which are lymphopenic because of limited thymic output134. While these studies do not rule out a role for Rpl22 in peripheral immune responses, they suggest that the predominant role of Rpl22 is likely to be in supporting normal lymphoid development.
B cell development
The development of B cells occurs in the bone marrow of adult mammals132. The first committed B cell progenitor in the bone marrow is the pro-B cell, in which rearrangements at the immunoglobulin heavy chain (IGH) locus occur. Successful rearrangement of the IGH locus results in production of μ heavy chain protein, which pairs with the surrogate light chains, λ5 and VpreB, to form the pre-BCR. Expression of the pre-BCR initiates the signals required for traversal of the pre-BCR checkpoint, which ensures that only progenitors with productive IGH rearrangements are selected to differentiate to the large pre-B cell stage. Following several rounds of cellular division to expand the pool of B cell progenitors with successful IGH rearrangements, the large pre-B cells differentiate to the small pre-B cell stage where immunoglobulin light chain (IGL) rearrangements occur, first at the κ locus and then at the λ locus. Successful rearrangement of the IGL locus results in expression of IGL chain protein, which pairs with μ IGH protein to form the BCR, which initiates differentiation to the immature B cell stage. Immature B cells are vetted through a tolerance checkpoint to ensure that only non-autoreactive B cells are selected into the peripheral B cell pool.
While initial studies reported potential defects in B cell development in Rpl22-deficient mice98,100, it was only recently that the stages of B cell development impacted by the loss of Rpl22 were reported134. Rpl22-deficiency causes a mild reduction in pro-B cells that becomes more pronounced at the pre-B and immature B cell stages, demonstrating that Rpl22 is required for early B cell development. Pro-B cells are highly dependent on the cytokine interleukin 7 (IL-7) for their survival and proliferation135. Despite expressing normal cell surface levels of the IL-7 receptor α chain, Rpl22-deficient pro-B cells were unable to respond to IL-7, exhibiting both increased apoptosis and reduced proliferation. Responsiveness of Rpl22-deficient pro-B cells to IL-7 signaling in vitro is restored by the knockdown of p53, and ablation of p53 completely restores the development of B cells in Rpl22-deficient mice. The mechanistic basis by which Rpl22 loss induces p53 in developing B cells remains unknown.
T cell development
T cells develop in the thymus through characteristic stages defined by the expression of cell surface molecules133. The earliest T cell progenitors lack surface expression for the co-receptors CD4 and CD8 and are termed double negative (DN) progenitors. The DN compartment is further segregated by CD44 and CD25 expression into DN1 (CD44+ CD25−), DN2 (CD44+ CD25+), DN3 (CD44− CD25+) and DN4 (CD44− CD25−) stages. The early thymus-seeding progenitors are contained with the DN1 compartment. Upon entry into the thymus, interactions of these progenitors with Notch ligands initiate commitment to the T cell lineage. At this stage, rearrangement of the Tcrb, Tcrg, and Tcrd loci begins. Successful rearrangement of the Tcrg and Tcrd loci results in surface expression of the γδ T cell receptor (TCR) and commitment to the γδ T cell lineage. Successful rearrangement of the Tcrb locus results in production of TCRβ protein, which pairs with the pre-T cell receptor α (pTα) to form the pre-TCR. Expression of the pre-TCR initiates the signaling events required for traversal of the β-selection checkpoint at the DN3 stage, which ensures that only those progenitors with productive Tcrb rearrangements are selected to differentiate. β-selected T cell progenitors undergo robust proliferation and downregulate CD25 as they differentiate to the DN4 stage, following which they upregulate CD4 and CD8 as they differentiate to the CD4+ CD8+ double positive (DP) stage. In DP thymocytes, the Tcra locus is rearranged. Successful rearrangement of the Tcra locus initiates two selection checkpoints, positive and negative selection, which ensures generation of T cells that are not autoreactive, yet recognize peptides in the context of self MHC molecules.
The striking decreases in peripheral CD4 and CD8 αβ T cells, but not γδ T cells, in Rpl22-deficient mice suggested that Rpl22 might play a crucial role specifically during the development of αβ T cells98. Consistent with this notion, γδ T cell development is minimally affected by Rpl22-deficiency; however, Rpl22-deficiency profoundly impairs the development of αβ lineage T cells. Indeed, αβ T cell development in Rpl22-deficient mice is arrested at the β-selection checkpoint, leading to severe reductions in DP, as well as CD4 and CD8 single positive, thymocytes. The arrest at the β-selection checkpoint in Rpl22-deficient mice does not result from impaired rearrangement of the Tcrb locus or pre-TCR signaling, but rather resulted from p53-induced apoptosis that began to manifest in DN3 thymocytes. Thus, Rpl22-deficiency impaired the development of both B and αβ T lymphocytes in a p53-dependent manner, as p53-deficiency completely reverses the developmental arrest of both cell lineages.
Enhanced p53 protein expression in the absence of Rpl22
The induction of p53 is frequently associated with mutations that inactivate RP53,98,134,136; however, a major distinction is that the induction of p53 in response to loss of other RP is widespread, explaining the embryonic lethality of most RP mutations, whereas p53-induction in Rpl22-deficient mice is highly selective, even among closely related cell lineages. While p53 induction was observed in B and αβ T lineage progenitors, it was not induced in closely related γδ T progenitors. p53 causes the developmental arrest of B and αβ T lineage progenitors through the induction of proapoptotic targets, including Puma, Noxa, and Bax, as well as the cell cycle regulator p21134,136. p53 also induces the target microRNA miR-34a, which is able to replicate the developmental arrest of p53 induction upon ectopic expression in Rpl22-expressing αβ T lineage progenitors. Of the potential downstream targets of p53, Puma was the only molecular effector whose elimination by itself was able to partially rescue the developmental defects observed in Rpl22-deficient mice136. The combined loss of Puma and Bim (not a p53 target) resulted in a near complete restoration of T cell development in the absence of Rpl22, further demonstrating that p53 induction in Rpl22-deficient progenitors controls early T cell development primarily through effects on cell survival. Collectively, these studies demonstrated that Rpl22 regulates survival during early B and T cell development by repression of p53 (Figure 3).
Figure 3. Rpl22 and Rpl22l1 have multiple roles during hematopoiesis.
Rpl22 and Rpl22l1 have opposing effects on the translation of Smad1 mRNA, and the balance of their antagonistic activities control HSC emergence by controlling the expression of Smad1. Rpl22 also controls key developmental checkpoints during B and T cell development by controlling p53 expression in a lineage-restricted manner.
A critical remaining question is why the inactivation of other RP causes widespread induction of p53 and embryonic lethality, while Rpl22-deficiency selectively induces p53 expression in only selected lineages. One important factor is that Rpl22 is not essential for ribosome biogenesis or global protein synthesis, suggesting that the developmental defects caused by Rpl22-deficiency resulted from the loss of some lineage-restricted regulatory function of Rpl2298,100. Rpl22 loss does not increase p53 expression by enhancing its stability, as occurs upon loss of other RP. Instead, Rpl22 regulates p53 expression by altering its translation98. Indeed, a recent study reported that Rpl22 can directly bind to p53 mRNA and control its translation93, suggesting direct repression. However, this finding is insufficient to explain why p53 translation is increased upon Rpl22 loss in only certain cell types, such as in αβ, but not γδ, T cell progenitors. p53 is activated in response to a variety of distinct stimuli, including DNA damage and the unfolded protein response137,138. The DNA damage response is activated and leads to activation of p53 during early lymphocyte progenitors in response to V(D)J recombination of the antigen receptor loci139. Moreover, DNA-damage or nucleotoxic stress unrelated to V(D)J recombination might also be expected to occur during the robust proliferation undergone during development of susceptible αβ and B cell lineages, but not by γδ T cell progenitors140. The unfolded protein response is also activated during early B and T cell development, possibly as a consequence of the robust proliferation required following successful antigen receptor rearrangements141. Thus, the lineages that are susceptible to developmental arrest by Rpl22-deficiency may be those whose development entails abrupt and stress-inducing transitions, such as those that occur at the pre-BCR and β-selection checkpoints. The mechanistic basis for lineage restriction of p53 induction remains a critical, unanswered question.
Transformation
RP mutations are often associated with an increased risk for hematologic malignancies11; however, the underlying mechanistic basis by which RP mutations facilitate transformation remain elusive. Interestingly, haploinsufficiency of Rpl24 and Rpl38 delay the development of B and T cell lymphomas in mouse models142,143. In contrast, Rpl22 haploinsufficiency results in the rapid development of thymic lymphoma in a mouse model of T acute lymphoblastic leukemia (T-ALL) driven by enforced expression of oncogenic Akt2 in T cell progenitors101. Rpl22 appears to be functioning as a haploinsufficient tumor suppressor, as the remaining Rpl22 allele in Rpl22+/− lymphomas is both intact and expressed. The link between Rpl22 inactivation and enhanced transformation and/or accelerated tumor progression is also observed in human cancer. Indeed, RPL22 is inactivated in approximately 10% of primary T-ALL and specific inactivating point mutations in RPL22 have been observed in relapsed T-ALL samples and are associated with reduced survival101. In addition, Rpl22 mRNA levels are reduced in a number of T cell malignancies, including adult T-cell lymphoma/leukemia144. These studies suggest that, unlike Rpl24 and Rpl38, Rpl22 acts as a tumor suppressor.
The mechanism by which Rpl22 haploinsufficiency accelerates the development and progression of T cell malignancies is distinct from the mechanism by which it controls traversal of the β-selection checkpoint as it is unrelated to effects on p53. Indeed, p53 was not inactivated in Rpl22+/− thymic lymphomas, suggesting that Rpl22 suppresses transformation by an alternative mechanism101. Instead, Rpl22 inactivation enhances transformation potential by inducing NF-κB, which then transactivates the stemness factor Lin28B145. Lin28B, a negative regulator of processing of let-7 miRNA, is overexpressed in 15% of human primary tumors and is often associated with the advanced stages of disease146. Importantly, among the downstream targets of let-7 are the critical oncogenes, Myc and Ras, which are upregulated upon Rpl22 inactivation. Consequently, Rpl22 haploinsufficiency appears to promote transformation by activating the NF-κB/Lin28B axis, which induces Myc and Ras in a let-7 dependent manner101. The mechanistic basis by which Rpl22 inactivation induces NF-κB activity to initiate this oncogenic cascade remains unclear.
CONCLUSIONS
The ability of Rpl22 and its paralog Rpl22l1 to play critical roles in regulating hematopoiesis, while not adversely affecting ribosome biogenesis or global, CAP-dependent translation, illustrates the regulatory, moonlighting functions that can be attributed to at least certain RP. These observations underscore an emerging paradigm shift in the way that RP are viewed. Indeed, because most RP are RNA-binding proteins, one can view the ribosome as a large collection of RNA-binding proteins with the potential to exist separate from the ribosome and in that state to bind cellular proteins and RNAs and regulate their activities, thereby critically influencing a variety of biological processes. Studying these regulatory functions remains challenging, as simple loss-of-function approaches for most RP will compromise both the core function of the RP in facilitating ribosome biogenesis and function, as well as its regulatory function, thereby preventing clear attribution of the cause of any resultant developmental abnormality. Fortunately, because Rpl22 and Rpl22l1 are dispensable for ribosome biogenesis and function, their analysis is not impeded by this complication, and the developmental defects caused by their loss can reasonably be attributed to their moonlighting functions. Nevertheless, a number of critical questions regarding their roles in development remain to be addressed. How are such highly homologous proteins as Rpl22 and Rpl22l1 (73% identical) able to perform distinct and even antagonistic functions? What are the cellular target RNAs through which they mediate their effects? How are these widely expressed proteins capable of exerting such lineage-restricted effects on p53 induction? How is Rpl22 and Rpl22l1 incorporation into the ribosome controlled and does Rpl22 and Rpl22l1 found outside the ribosome the result of stimulus-induced release or blocked incorporation? Addressing these questions represents a small part of an emerging effort to understand the ribosome and its protein components as a critical regulatory platform.
Acknowledgments
The work on which this review was based was supported by NIH grants R01AI110985 and P30CA006927, Leukemia and Lymphoma Society grant 6057-14, and an appropriation from the Commonwealth of Pennsylvania, under the Pennsylvania Department of Health. The Department specifically disclaims responsibility for any analyses, interpretations or conclusions.
Literature Cited
- 1.Raiser DM, Narla A, Ebert BL. The emerging importance of ribosomal dysfunction in the pathogenesis of hematologic disorders. Leukemia & lymphoma. 2014;55:491–500. doi: 10.3109/10428194.2013.812786. [DOI] [PubMed] [Google Scholar]
- 2.Nakhoul H, Ke J, Zhou X, Liao W, Zeng SX, Lu H. Ribosomopathies: mechanisms of disease. Clinical medicine insights. Blood disorders. 2014;7:7–16. doi: 10.4137/CMBD.S16952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellis SR. Nucleolar stress in Diamond Blackfan anemia pathophysiology. Biochim Biophys Acta. 2014;1842:765–768. doi: 10.1016/j.bbadis.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Zhou X, Liao WJ, Liao JM, Liao P, Lu H. Ribosomal proteins: functions beyond the ribosome. Journal of molecular cell biology. 2015;7:92–104. doi: 10.1093/jmcb/mjv014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xue S, Barna M. Specialized ribosomes: a new frontier in gene regulation and organismal biology. Nature reviews. Molecular cell biology. 2012;13:355–369. doi: 10.1038/nrm3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruggero D, Shimamura A. Marrow failure: a window into ribosome biology. Blood. 2014;124:2784–2792. doi: 10.1182/blood-2014-04-526301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor DJ, Devkota B, Huang AD, Topf M, Narayanan E, Sali A, Harvey SC, Frank J. Comprehensive molecular structure of the eukaryotic ribosome. Structure. 2009;17:1591–1604. doi: 10.1016/j.str.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woolford JL, Jr, Baserga SJ. Ribosome biogenesis in the yeast Saccharomyces cerevisiae. Genetics. 2013;195:643–681. doi: 10.1534/genetics.113.153197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCann KL, Baserga SJ. Genetics. Mysterious ribosomopathies. Science. 2013;341:849–850. doi: 10.1126/science.1244156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Keersmaecker K, Sulima SO, Dinman JD. Ribosomopathies and the paradox of cellular hypo- to hyperproliferation. Blood. 2015;125:1377–1382. doi: 10.1182/blood-2014-10-569616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narla A, Ebert BL. Ribosomopathies: human disorders of ribosome dysfunction. Blood. 2010;115:3196–3205. doi: 10.1182/blood-2009-10-178129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warner JR. Twenty years of ribosome assembly and ribosomopathies. Rna. 2015;21:758–759. doi: 10.1261/rna.050435.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Draptchinskaia N, Gustavsson P, Andersson B, Pettersson M, Willig TN, Dianzani I, Ball S, Tchernia G, Klar J, Matsson H, Tentler D, Mohandas N, Carlsson B, Dahl N. The gene encoding ribosomal protein S19 is mutated in Diamond-Blackfan anaemia. Nature genetics. 1999;21:169–175. doi: 10.1038/5951. [DOI] [PubMed] [Google Scholar]
- 14.Ellis SR, Gleizes PE. Diamond Blackfan anemia: ribosomal proteins going rogue. Seminars in hematology. 2011;48:89–96. doi: 10.1053/j.seminhematol.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Gazda HT, Preti M, Sheen MR, O’Donohue MF, Vlachos A, Davies SM, Kattamis A, Doherty L, Landowski M, Buros C, Ghazvinian R, Sieff CA, Newburger PE, Niewiadomska E, Matysiak M, Glader B, Atsidaftos E, Lipton JM, Gleizes PE, Beggs AH. Frameshift mutation in p53 regulator RPL26 is associated with multiple physical abnormalities and a specific pre-ribosomal RNA processing defect in diamond-blackfan anemia. Human mutation. 2012;33:1037–1044. doi: 10.1002/humu.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landowski M, O’Donohue MF, Buros C, Ghazvinian R, Montel-Lehry N, Vlachos A, Sieff CA, Newburger PE, Niewiadomska E, Matysiak M, Glader B, Atsidaftos E, Lipton JM, Beggs AH, Gleizes PE, Gazda HT. Novel deletion of RPL15 identified by array-comparative genomic hybridization in Diamond-Blackfan anemia. Human genetics. 2013;132:1265–1274. doi: 10.1007/s00439-013-1326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farrar JE, Quarello P, Fisher R, O’Brien KA, Aspesi A, Parrella S, Henson AL, Seidel NE, Atsidaftos E, Prakash S, Bari S, Garelli E, Arceci RJ, Dianzani I, Ramenghi U, Vlachos A, Lipton JM, Bodine DM, Ellis SR. Exploiting pre-rRNA processing in Diamond Blackfan anemia gene discovery and diagnosis. American journal of hematology. 2014;89:985–991. doi: 10.1002/ajh.23807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gripp KW, Curry C, Olney AH, Sandoval C, Fisher J, Chong JX, Pilchman L, Sahraoui R, Stabley DL, Sol-Church K. Diamond-Blackfan anemia with mandibulofacial dystostosis is heterogeneous, including the novel DBA genes TSR2 and RPS28. American journal of medical genetics. Part A. 2014;164A:2240–2249. doi: 10.1002/ajmg.a.36633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebert BL, Pretz J, Bosco J, Chang CY, Tamayo P, Galili N, Raza A, Root DE, Attar E, Ellis SR, Golub TR. Identification of RPS14 as a 5q- syndrome gene by RNA interference screen. Nature. 2008;451:335–339. doi: 10.1038/nature06494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pellagatti A, Boultwood J. The molecular pathogenesis of the myelodysplastic syndromes. European journal of haematology. 2015;95:3–15. doi: 10.1111/ejh.12515. [DOI] [PubMed] [Google Scholar]
- 21.Bolze A, Mahlaoui N, Byun M, Turner B, Trede N, Ellis SR, Abhyankar A, Itan Y, Patin E, Brebner S, Sackstein P, Puel A, Picard C, Abel L, Quintana-Murci L, Faust SN, Williams AP, Baretto R, Duddridge M, Kini U, Pollard AJ, Gaud C, Frange P, Orbach D, Emile JF, Stephan JL, Sorensen R, Plebani A, Hammarstrom L, Conley ME, Selleri L, Casanova JL. Ribosomal protein SA haploinsufficiency in humans with isolated congenital asplenia. Science. 2013;340:976–978. doi: 10.1126/science.1234864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fumagalli S, Thomas G. The role of p53 in ribosomopathies. Seminars in hematology. 2011;48:97–105. doi: 10.1053/j.seminhematol.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Narla A, Hurst SN, Ebert BL. Ribosome defects in disorders of erythropoiesis. International journal of hematology. 2011;93:144–149. doi: 10.1007/s12185-011-0776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pestov DG, Strezoska Z, Lau LF. Evidence of p53-dependent cross-talk between ribosome biogenesis and the cell cycle: effects of nucleolar protein Bop1 on G(1)/S transition. Mol Cell Biol. 2001;21:4246–4255. doi: 10.1128/MCB.21.13.4246-4255.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sulic S, Panic L, Barkic M, Mercep M, Uzelac M, Volarevic S. Inactivation of S6 ribosomal protein gene in T lymphocytes activates a p53-dependent checkpoint response. Genes & development. 2005;19:3070–3082. doi: 10.1101/gad.359305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barkic M, Crnomarkovic S, Grabusic K, Bogetic I, Panic L, Tamarut S, Cokaric M, Jeric I, Vidak S, Volarevic S. The p53 tumor suppressor causes congenital malformations in Rpl24-deficient mice and promotes their survival. Mol Cell Biol. 2009;29:2489–2504. doi: 10.1128/MCB.01588-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fumagalli S, Di Cara A, Neb-Gulati A, Natt F, Schwemberger S, Hall J, Babcock GF, Bernardi R, Pandolfi PP, Thomas G. Absence of nucleolar disruption after impairment of 40S ribosome biogenesis reveals an rpL11-translation-dependent mechanism of p53 induction. Nature cell biology. 2009;11:501–508. doi: 10.1038/ncb1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyake K, Utsugisawa T, Flygare J, Kiefer T, Hamaguchi I, Richter J, Karlsson S. Ribosomal protein S19 deficiency leads to reduced proliferation and increased apoptosis but does not affect terminal erythroid differentiation in a cell line model of Diamond-Blackfan anemia. Stem Cells. 2008;26:323–329. doi: 10.1634/stemcells.2007-0569. [DOI] [PubMed] [Google Scholar]
- 29.Dutt S, Narla A, Lin K, Mullally A, Abayasekara N, Megerdichian C, Wilson FH, Currie T, Khanna-Gupta A, Berliner N, Kutok JL, Ebert BL. Haploinsufficiency for ribosomal protein genes causes selective activation of p53 in human erythroid progenitor cells. Blood. 2011;117:2567–2576. doi: 10.1182/blood-2010-07-295238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruggero D, Grisendi S, Piazza F, Rego E, Mari F, Rao PH, Cordon-Cardo C, Pandolfi PP. Dyskeratosis congenita and cancer in mice deficient in ribosomal RNA modification. Science. 2003;299:259–262. doi: 10.1126/science.1079447. [DOI] [PubMed] [Google Scholar]
- 31.Danilova N, Sakamoto KM, Lin S. Ribosomal protein S19 deficiency in zebrafish leads to developmental abnormalities and defective erythropoiesis through activation of p53 protein family. Blood. 2008;112:5228–5237. doi: 10.1182/blood-2008-01-132290. [DOI] [PubMed] [Google Scholar]
- 32.McGowan KA, Li JZ, Park CY, Beaudry V, Tabor HK, Sabnis AJ, Zhang W, Fuchs H, de Angelis MH, Myers RM, Attardi LD, Barsh GS. Ribosomal mutations cause p53-mediated dark skin and pleiotropic effects. Nature genetics. 2008;40:963–970. doi: 10.1038/ng.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones NC, Lynn ML, Gaudenz K, Sakai D, Aoto K, Rey JP, Glynn EF, Ellington L, Du C, Dixon J, Dixon MJ, Trainor PA. Prevention of the neurocristopathy Treacher Collins syndrome through inhibition of p53 function. Nature medicine. 2008;14:125–133. doi: 10.1038/nm1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakai D, Trainor PA. Treacher Collins syndrome: unmasking the role of Tcof1/treacle. The international journal of biochemistry & cell biology. 2009;41:1229–1232. doi: 10.1016/j.biocel.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barlow JL, Drynan LF, Hewett DR, Holmes LR, Lorenzo-Abalde S, Lane AL, Jolin HE, Pannell R, Middleton AJ, Wong SH, Warren AJ, Wainscoat JS, Boultwood J, McKenzie AN. A p53-dependent mechanism underlies macrocytic anemia in a mouse model of human 5q- syndrome. Nature medicine. 2010;16:59–66. doi: 10.1038/nm.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaako P, Flygare J, Olsson K, Quere R, Ehinger M, Henson A, Ellis S, Schambach A, Baum C, Richter J, Larsson J, Bryder D, Karlsson S. Mice with ribosomal protein S19 deficiency develop bone marrow failure and symptoms like patients with Diamond-Blackfan anemia. Blood. 2011;118:6087–6096. doi: 10.1182/blood-2011-08-371963. [DOI] [PubMed] [Google Scholar]
- 37.Griffin JN, Sondalle SB, Del Viso F, Baserga SJ, Khokha MK. The ribosome biogenesis factor Nol11 is required for optimal rDNA transcription and craniofacial development in Xenopus. PLoS genetics. 2015;11:e1005018. doi: 10.1371/journal.pgen.1005018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ofir-Rosenfeld Y, Boggs K, Michael D, Kastan MB, Oren M. Mdm2 regulates p53 mRNA translation through inhibitory interactions with ribosomal protein L26. Mol Cell. 2008;32:180–189. doi: 10.1016/j.molcel.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Wang J, Yuan Y, Zhang W, Guan W, Wu Z, Jin C, Chen H, Zhang L, Yang X, He F. Negative regulation of HDM2 to attenuate p53 degradation by ribosomal protein L26. Nucleic Acids Res. 2010;38:6544–6554. doi: 10.1093/nar/gkq536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daftuar L, Zhu Y, Jacq X, Prives C. Ribosomal proteins RPL37, RPS15 and RPS20 regulate the Mdm2-p53-MdmX network. PLoS One. 2013;8:e68667. doi: 10.1371/journal.pone.0068667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu Y, Poyurovsky MV, Li Y, Biderman L, Stahl J, Jacq X, Prives C. Ribosomal protein S7 is both a regulator and a substrate of MDM2. Mol Cell. 2009;35:316–326. doi: 10.1016/j.molcel.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou X, Hao Q, Liao J, Zhang Q, Lu H. Ribosomal protein S14 unties the MDM2-p53 loop upon ribosomal stress. Oncogene. 2013;32:388–396. doi: 10.1038/onc.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X, Wang W, Wang H, Wang MH, Xu W, Zhang R. Identification of ribosomal protein S25 (RPS25)-MDM2-p53 regulatory feedback loop. Oncogene. 2013;32:2782–2791. doi: 10.1038/onc.2012.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhat KP, Itahana K, Jin A, Zhang Y. Essential role of ribosomal protein L11 in mediating growth inhibition-induced p53 activation. The EMBO journal. 2004;23:2402–2412. doi: 10.1038/sj.emboj.7600247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dai MS, Lu H. Inhibition of MDM2-mediated p53 ubiquitination and degradation by ribosomal protein L5. The Journal of biological chemistry. 2004;279:44475–44482. doi: 10.1074/jbc.M403722200. [DOI] [PubMed] [Google Scholar]
- 46.Dai MS, Zeng SX, Jin Y, Sun XX, David L, Lu H. Ribosomal protein L23 activates p53 by inhibiting MDM2 function in response to ribosomal perturbation but not to translation inhibition. Mol Cell Biol. 2004;24:7654–7668. doi: 10.1128/MCB.24.17.7654-7668.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen D, Zhang Z, Li M, Wang W, Li Y, Rayburn ER, Hill DL, Wang H, Zhang R. Ribosomal protein S7 as a novel modulator of p53-MDM2 interaction: binding to MDM2, stabilization of p53 protein, and activation of p53 function. Oncogene. 2007;26:5029–5037. doi: 10.1038/sj.onc.1210327. [DOI] [PubMed] [Google Scholar]
- 48.Jin A, Itahana K, O’Keefe K, Zhang Y. Inhibition of HDM2 and activation of p53 by ribosomal protein L23. Mol Cell Biol. 2004;24:7669–7680. doi: 10.1128/MCB.24.17.7669-7680.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lohrum MA, Ludwig RL, Kubbutat MH, Hanlon M, Vousden KH. Regulation of HDM2 activity by the ribosomal protein L11. Cancer cell. 2003;3:577–587. doi: 10.1016/s1535-6108(03)00134-x. [DOI] [PubMed] [Google Scholar]
- 50.Marechal V, Elenbaas B, Piette J, Nicolas JC, Levine AJ. The ribosomal L5 protein is associated with mdm-2 and mdm-2-p53 complexes. Mol Cell Biol. 1994;14:7414–7420. doi: 10.1128/mcb.14.11.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yadavilli S, Mayo LD, Higgins M, Lain S, Hegde V, Deutsch WA. Ribosomal protein S3: A multi-functional protein that interacts with both p53 and MDM2 through its KH domain. DNA repair. 2009;8:1215–1224. doi: 10.1016/j.dnarep.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Wolf GW, Bhat K, Jin A, Allio T, Burkhart WA, Xiong Y. Ribosomal protein L11 negatively regulates oncoprotein MDM2 and mediates a p53-dependent ribosomal-stress checkpoint pathway. Mol Cell Biol. 2003;23:8902–8912. doi: 10.1128/MCB.23.23.8902-8912.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, Lu H. Signaling to p53: ribosomal proteins find their way. Cancer cell. 2009;16:369–377. doi: 10.1016/j.ccr.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Teng T, Mercer CA, Hexley P, Thomas G, Fumagalli S. Loss of tumor suppressor RPL5/RPL11 does not induce cell cycle arrest but impedes proliferation due to reduced ribosome content and translation capacity. Mol Cell Biol. 2013;33:4660–4671. doi: 10.1128/MCB.01174-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Donati G, Peddigari S, Mercer CA, Thomas G. 5S ribosomal RNA is an essential component of a nascent ribosomal precursor complex that regulates the Hdm2-p53 checkpoint. Cell reports. 2013;4:87–98. doi: 10.1016/j.celrep.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sloan KE, Bohnsack MT, Watkins NJ. The 5S RNP couples p53 homeostasis to ribosome biogenesis and nucleolar stress. Cell reports. 2013;5:237–247. doi: 10.1016/j.celrep.2013.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bursac S, Brdovcak MC, Pfannkuchen M, Orsolic I, Golomb L, Zhu Y, Katz C, Daftuar L, Grabusic K, Vukelic I, Filic V, Oren M, Prives C, Volarevic S. Mutual protection of ribosomal proteins L5 and L11 from degradation is essential for p53 activation upon ribosomal biogenesis stress. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:20467–20472. doi: 10.1073/pnas.1218535109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teng T, Thomas G, Mercer CA. Growth control and ribosomopathies. Current opinion in genetics & development. 2013;23:63–71. doi: 10.1016/j.gde.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 59.Mauro VP, Edelman GM. The ribosome filter hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:12031–12036. doi: 10.1073/pnas.192442499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kondrashov N, Pusic A, Stumpf CR, Shimizu K, Hsieh AC, Xue S, Ishijima J, Shiroishi T, Barna M. Ribosome-mediated specificity in Hox mRNA translation and vertebrate tissue patterning. Cell. 2011;145:383–397. doi: 10.1016/j.cell.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shi Z, Barna M. Translating the Genome in Time and Space: Specialized Ribosomes, RNA Regulons, and RNA-Binding Proteins. Annual review of cell and developmental biology. 2015;31:31–54. doi: 10.1146/annurev-cellbio-100814-125346. [DOI] [PubMed] [Google Scholar]
- 62.Gilkes DM, Chen L, Chen J. MDMX regulation of p53 response to ribosomal stress. The EMBO journal. 2006;25:5614–5625. doi: 10.1038/sj.emboj.7601424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sasaki M, Kawahara K, Nishio M, Mimori K, Kogo R, Hamada K, Itoh B, Wang J, Komatsu Y, Yang YR, Hikasa H, Horie Y, Yamashita T, Kamijo T, Zhang Y, Zhu Y, Prives C, Nakano T, Mak TW, Sasaki T, Maehama T, Mori M, Suzuki A. Regulation of the MDM2-P53 pathway and tumor growth by PICT1 via nucleolar RPL11. Nature medicine. 2011;17:944–951. doi: 10.1038/nm.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee SB, Kwon IS, Park J, Lee KH, Ahn Y, Lee C, Kim J, Choi SY, Cho SW, Ahn JY. Ribosomal protein S3, a new substrate of Akt, serves as a signal mediator between neuronal apoptosis and DNA repair. The Journal of biological chemistry. 2010;285:29457–29468. doi: 10.1074/jbc.M110.131367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jang CY, Kim HD, Kim J. Ribosomal protein S3 interacts with TRADD to induce apoptosis through caspase dependent JNK activation. Biochem Biophys Res Commun. 2012;421:474–478. doi: 10.1016/j.bbrc.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 66.Wan F, Anderson DE, Barnitz RA, Snow A, Bidere N, Zheng L, Hegde V, Lam LT, Staudt LM, Levens D, Deutsch WA, Lenardo MJ. Ribosomal protein S3: a KH domain subunit in NF-kappaB complexes that mediates selective gene regulation. Cell. 2007;131:927–939. doi: 10.1016/j.cell.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 67.Wan F, Weaver A, Gao X, Bern M, Hardwidge PR, Lenardo MJ. IKKbeta phosphorylation regulates RPS3 nuclear translocation and NF-kappaB function during infection with Escherichia coli strain O157:H7. Nature immunology. 2011;12:335–343. doi: 10.1038/ni.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang HJ, Youn H, Seong KM, Jin YW, Kim J, Youn B. Phosphorylation of ribosomal protein S3 and antiapoptotic TRAF2 protein mediates radioresistance in non-small cell lung cancer cells. The Journal of biological chemistry. 2013;288:2965–2975. doi: 10.1074/jbc.M112.385989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shin HS, Jang CY, Kim HD, Kim TS, Kim S, Kim J. Arginine methylation of ribosomal protein S3 affects ribosome assembly. Biochem Biophys Res Commun. 2009;385:273–278. doi: 10.1016/j.bbrc.2009.05.055. [DOI] [PubMed] [Google Scholar]
- 70.Jang CY, Shin HS, Kim HD, Kim JW, Choi SY, Kim J. Ribosomal protein S3 is stabilized by sumoylation. Biochem Biophys Res Commun. 2011;414:523–527. doi: 10.1016/j.bbrc.2011.09.099. [DOI] [PubMed] [Google Scholar]
- 71.Revollo I, Nishiura H, Shibuya Y, Oda Y, Nishino N, Yamamoto T. Agonist and antagonist dual effect of the cross-linked S19 ribosomal protein dimer in the C5a receptor-mediated respiratory burst reaction of phagocytic leukocytes. Inflammation research: official journal of the European Histamine Research Society … [et al.] 2005;54:82–90. doi: 10.1007/s00011-004-1327-4. [DOI] [PubMed] [Google Scholar]
- 72.Dai MS, Arnold H, Sun XX, Sears R, Lu H. Inhibition of c-Myc activity by ribosomal protein L11. The EMBO journal. 2007;26:3332–3345. doi: 10.1038/sj.emboj.7601776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou X, Hao Q, Liao JM, Liao P, Lu H. Ribosomal protein S14 negatively regulates c-Myc activity. The Journal of biological chemistry. 2013;288:21793–21801. doi: 10.1074/jbc.M112.445122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Z, Hou J, Lu L, Qi Z, Sun J, Gao W, Meng J, Wang Y, Sun H, Gu H, Xin Y, Guo X, Yang G. Small ribosomal protein subunit S7 suppresses ovarian tumorigenesis through regulation of the PI3K/AKT and MAPK pathways. PLoS One. 2013;8:e79117. doi: 10.1371/journal.pone.0079117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang A, Xu S, Zhang X, He J, Yan D, Yang Z, Xiao S. Ribosomal protein RPL41 induces rapid degradation of ATF4, a transcription factor critical for tumour cell survival in stress. The Journal of pathology. 2011;225:285–292. doi: 10.1002/path.2918. [DOI] [PubMed] [Google Scholar]
- 76.Mazumder B, Sampath P, Seshadri V, Maitra RK, DiCorleto PE, Fox PL. Regulated release of L13a from the 60S ribosomal subunit as a mechanism of transcript-specific translational control. Cell. 2003;115:187–198. doi: 10.1016/s0092-8674(03)00773-6. [DOI] [PubMed] [Google Scholar]
- 77.Sampath P, Mazumder B, Seshadri V, Fox PL. Transcript-selective translational silencing by gamma interferon is directed by a novel structural element in the ceruloplasmin mRNA 3′ untranslated region. Mol Cell Biol. 2003;23:1509–1519. doi: 10.1128/MCB.23.5.1509-1519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mukhopadhyay R, Jia J, Arif A, Ray PS, Fox PL. The GAIT system: a gatekeeper of inflammatory gene expression. Trends in biochemical sciences. 2009;34:324–331. doi: 10.1016/j.tibs.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fox PL. Discovery and investigation of the GAIT translational control system. Rna. 2015;21:615–618. doi: 10.1261/rna.050187.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ray PS, Fox PL. A post-transcriptional pathway represses monocyte VEGF-A expression and angiogenic activity. The EMBO journal. 2007;26:3360–3372. doi: 10.1038/sj.emboj.7601774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mukhopadhyay R, Ray PS, Arif A, Brady AK, Kinter M, Fox PL. DAPK-ZIPK-L13a axis constitutes a negative-feedback module regulating inflammatory gene expression. Mol Cell. 2008;32:371–382. doi: 10.1016/j.molcel.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mazumder B, Poddar D, Basu A, Kour R, Verbovetskaya V, Barik S. Extraribosomal l13a is a specific innate immune factor for antiviral defense. J Virol. 2014;88:9100–9110. doi: 10.1128/JVI.01129-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang JY, Su WC, Jeng KS, Chang TH, Lai MM. Attenuation of 40S ribosomal subunit abundance differentially affects host and HCV translation and suppresses HCV replication. PLoS pathogens. 2012;8:e1002766. doi: 10.1371/journal.ppat.1002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nishiyama T, Yamamoto H, Uchiumi T, Nakashima N. Eukaryotic ribosomal protein RPS25 interacts with the conserved loop region in a dicistroviral intergenic internal ribosome entry site. Nucleic Acids Res. 2007;35:1514–1521. doi: 10.1093/nar/gkl1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Landry DM, Hertz MI, Thompson SR. RPS25 is essential for translation initiation by the Dicistroviridae and hepatitis C viral IRESs. Genes & development. 2009;23:2753–2764. doi: 10.1101/gad.1832209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Muhs M, Yamamoto H, Ismer J, Takaku H, Nashimoto M, Uchiumi T, Nakashima N, Mielke T, Hildebrand PW, Nierhaus KH, Spahn CM. Structural basis for the binding of IRES RNAs to the head of the ribosomal 40S subunit. Nucleic Acids Res. 2011;39:5264–5275. doi: 10.1093/nar/gkr114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Horos R, Ijspeert H, Pospisilova D, Sendtner R, Andrieu-Soler C, Taskesen E, Nieradka A, Cmejla R, Sendtner M, Touw IP, von Lindern M. Ribosomal deficiencies in Diamond-Blackfan anemia impair translation of transcripts essential for differentiation of murine and human erythroblasts. Blood. 2012;119:262–272. doi: 10.1182/blood-2011-06-358200. [DOI] [PubMed] [Google Scholar]
- 88.Garcon L, Ge J, Manjunath SH, Mills JA, Apicella M, Parikh S, Sullivan LM, Podsakoff GM, Gadue P, French DL, Mason PJ, Bessler M, Weiss MJ. Ribosomal and hematopoietic defects in induced pluripotent stem cells derived from Diamond Blackfan anemia patients. Blood. 2013;122:912–921. doi: 10.1182/blood-2013-01-478321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xue S, Tian S, Fujii K, Kladwang W, Das R, Barna M. RNA regulons in Hox 5′ UTRs confer ribosome specificity to gene regulation. Nature. 2015;517:33–38. doi: 10.1038/nature14010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Takagi M, Absalon MJ, McLure KG, Kastan MB. Regulation of p53 translation and induction after DNA damage by ribosomal protein L26 and nucleolin. Cell. 2005;123:49–63. doi: 10.1016/j.cell.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 91.Chen J, Kastan MB. 5′-3′-UTR interactions regulate p53 mRNA translation and provide a target for modulating p53 induction after DNA damage. Genes & development. 2010;24:2146–2156. doi: 10.1101/gad.1968910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen J, Guo K, Kastan MB. Interactions of nucleolin and ribosomal protein L26 (RPL26) in translational control of human p53 mRNA. The Journal of biological chemistry. 2012;287:16467–16476. doi: 10.1074/jbc.M112.349274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rashkovan M, Vadnais C, Ross J, Gigoux M, Suh WK, Gu W, Kosan C, Moroy T. Miz-1 regulates translation of Trp53 via ribosomal protein L22 in cells undergoing V(D)J recombination. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E5411–5419. doi: 10.1073/pnas.1412107111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Challagundla KB, Sun XX, Zhang X, DeVine T, Zhang Q, Sears RC, Dai MS. Ribosomal protein L11 recruits miR-24/miRISC to repress c-Myc expression in response to ribosomal stress. Mol Cell Biol. 2011;31:4007–4021. doi: 10.1128/MCB.05810-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liao JM, Zhou X, Gatignol A, Lu H. Ribosomal proteins L5 and L11 co-operatively inactivate c-Myc via RNA-induced silencing complex. Oncogene. 2014;33:4916–4923. doi: 10.1038/onc.2013.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lempiainen H, Shore D. Growth control and ribosome biogenesis. Current opinion in cell biology. 2009;21:855–863. doi: 10.1016/j.ceb.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 97.Wong QW, Li J, Ng SR, Lim SG, Yang H, Vardy LA. RPL39L is an example of a recently evolved ribosomal protein paralog that shows highly specific tissue expression patterns and is upregulated in ESCs and HCC tumors. RNA biology. 2014;11:33–41. doi: 10.4161/rna.27427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Anderson SJ, Lauritsen JP, Hartman MG, Foushee AM, Lefebvre JM, Shinton SA, Gerhardt B, Hardy RR, Oravecz T, Wiest DL. Ablation of ribosomal protein L22 selectively impairs alphabeta T cell development by activation of a p53-dependent checkpoint. Immunity. 2007;26:759–772. doi: 10.1016/j.immuni.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 99.Zhang Y, Duc AC, Rao S, Sun XL, Bilbee AN, Rhodes M, Li Q, Kappes DJ, Rhodes J, Wiest DL. Control of hematopoietic stem cell emergence by antagonistic functions of ribosomal protein paralogs. Dev Cell. 2013;24:411–425. doi: 10.1016/j.devcel.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.O’Leary MN, Schreiber KH, Zhang Y, Duc AC, Rao S, Hale JS, Academia EC, Shah SR, Morton JF, Holstein CA, Martin DB, Kaeberlein M, Ladiges WC, Fink PJ, Mackay VL, Wiest DL, Kennedy BK. The ribosomal protein Rpl22 controls ribosome composition by directly repressing expression of its own paralog, Rpl22l1. PLoS genetics. 2013;9:e1003708. doi: 10.1371/journal.pgen.1003708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rao S, Lee SY, Gutierrez A, Perrigoue J, Thapa RJ, Tu Z, Jeffers JR, Rhodes M, Anderson S, Oravecz T, Hunger SP, Timakhov RA, Zhang R, Balachandran S, Zambetti GP, Testa JR, Look AT, Wiest DL. Inactivation of ribosomal protein L22 promotes transformation by induction of the stemness factor, Lin28B. Blood. 2012;120:3764–3773. doi: 10.1182/blood-2012-03-415349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.de Jonge HJ, Fehrmann RS, de Bont ES, Hofstra RM, Gerbens F, Kamps WA, de Vries EG, van der Zee AG, te Meerman GJ, ter Elst A. Evidence based selection of housekeeping genes. PLoS One. 2007;2:e898. doi: 10.1371/journal.pone.0000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hruz T, Wyss M, Docquier M, Pfaffl MW, Masanetz S, Borghi L, Verbrugghe P, Kalaydjieva L, Bleuler S, Laule O, Descombes P, Gruissem W, Zimmermann P. RefGenes: identification of reliable and condition specific reference genes for RT-qPCR data normalization. BMC Genomics. 2011;12:156. doi: 10.1186/1471-2164-12-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Auger-Buendia MA, Longuet M, Tavitian A. Kinetic studies on ribosomal proteins assembly in preribosomal particles and ribosomal subunits of mammalian cells. Biochim Biophys Acta. 1979;563:113–128. doi: 10.1016/0005-2787(79)90012-1. [DOI] [PubMed] [Google Scholar]
- 105.Sanz E, Yang L, Su T, Morris DR, McKnight GS, Amieux PS. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13939–13944. doi: 10.1073/pnas.0907143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ni JQ, Liu LP, Hess D, Rietdorf J, Sun FL. Drosophila ribosomal proteins are associated with linker histone H1 and suppress gene transcription. Genes & development. 2006;20:1959–1973. doi: 10.1101/gad.390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Toczyski DP, Matera AG, Ward DC, Steitz JA. The Epstein-Barr virus (EBV) small RNA EBER1 binds and relocalizes ribosomal protein L22 in EBV-infected human B lymphocytes. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:3463–3467. doi: 10.1073/pnas.91.8.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lavergne JP, Marzouki A, Reboud JP, Reboud AM. Reconstitution of the active rat liver 60 S ribosomal subunit from different preparations of core particles and split proteins. FEBS Lett. 1988;236:345–351. doi: 10.1016/0014-5793(88)80053-x. [DOI] [PubMed] [Google Scholar]
- 109.Fok V, Mitton-Fry RM, Grech A, Steitz JA. Multiple domains of EBER 1, an Epstein-Barr virus noncoding RNA, recruit human ribosomal protein L22. Rna. 2006;12:872–882. doi: 10.1261/rna.2339606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dobbelstein M, Shenk T. In vitro selection of RNA ligands for the ribosomal L22 protein associated with Epstein-Barr virus-expressed RNA by using randomized and cDNA-derived RNA libraries. J Virol. 1995;69:8027–8034. doi: 10.1128/jvi.69.12.8027-8034.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wood J, Frederickson RM, Fields S, Patel AH. Hepatitis C virus 3′X region interacts with human ribosomal proteins. J Virol. 2001;75:1348–1358. doi: 10.1128/JVI.75.3.1348-1358.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Houmani JL, Davis CI, Ruf IK. Growth-promoting properties of Epstein-Barr virus EBER-1 RNA correlate with ribosomal protein L22 binding. J Virol. 2009;83:9844–9853. doi: 10.1128/JVI.01014-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Le S, Sternglanz R, Greider CW. Identification of two RNA-binding proteins associated with human telomerase RNA. Mol Biol Cell. 2000;11:999–1010. doi: 10.1091/mbc.11.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.O’Leary MN, Schreiber KH, Zhang Y, Duc AC, Rao S, Hale JS, Academia EC, Shah SR, Morton JF, Holstein CA, Martin DB, Kaeberlein M, Ladiges WC, Fink PJ, Mackay VL, Wiest DL, Kennedy BK. The ribosomal protein Rpl22 controls ribosome composition by directly repressing expression of its own paralog, Rpl22l1. PLoS Genet. 2013;9:e1003708. doi: 10.1371/journal.pgen.1003708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Houmani JL, Ruf IK. Clusters of basic amino acids contribute to RNA binding and nucleolar localization of ribosomal protein L22. PLoS One. 2009;4:e5306. doi: 10.1371/journal.pone.0005306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Koyama Y, Katagiri S, Hanai S, Uchida K, Miwa M. Poly(ADP-ribose) polymerase interacts with novel Drosophila ribosomal proteins, L22 and l23a, with unique histone-like amino-terminal extensions. Gene. 1999;226:339–345. doi: 10.1016/s0378-1119(98)00529-0. [DOI] [PubMed] [Google Scholar]
- 117.Yang M, Sun H, He J, Wang H, Yu X, Ma L, Zhu C. Interaction of ribosomal protein L22 with casein kinase 2alpha: a novel mechanism for understanding the biology of non-small cell lung cancer. Oncol Rep. 2014;32:139–144. doi: 10.3892/or.2014.3187. [DOI] [PubMed] [Google Scholar]
- 118.Zhao W, Bidwai AP, Glover CV. Interaction of casein kinase II with ribosomal protein L22 of Drosophila melanogaster. Biochem Biophys Res Commun. 2002;298:60–66. doi: 10.1016/s0006-291x(02)02396-3. [DOI] [PubMed] [Google Scholar]
- 119.Zhou R, Hotta I, Denli AM, Hong P, Perrimon N, Hannon GJ. Comparative analysis of argonaute-dependent small RNA pathways in Drosophila. Mol Cell. 2008;32:592–599. doi: 10.1016/j.molcel.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Boutros M, Kiger AA, Armknecht S, Kerr K, Hild M, Koch B, Haas SA, Paro R, Perrimon N. Genome-wide RNAi analysis of growth and viability in Drosophila cells. Science. 2004;303:832–835. doi: 10.1126/science.1091266. [DOI] [PubMed] [Google Scholar]
- 121.Steffen KK, McCormick MA, Pham KM, MacKay VL, Delaney JR, Murakami CJ, Kaeberlein M, Kennedy BK. Ribosome deficiency protects against ER stress in Saccharomyces cerevisiae. Genetics. 2012;191:107–118. doi: 10.1534/genetics.111.136549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Giaever G, Chu AM, Ni L, Connelly C, Riles L, Veronneau S, Dow S, Lucau-Danila A, Anderson K, Andre B, Arkin AP, Astromoff A, El-Bakkoury M, Bangham R, Benito R, Brachat S, Campanaro S, Curtiss M, Davis K, Deutschbauer A, Entian KD, Flaherty P, Foury F, Garfinkel DJ, Gerstein M, Gotte D, Guldener U, Hegemann JH, Hempel S, Herman Z, Jaramillo DF, Kelly DE, Kelly SL, Kotter P, LaBonte D, Lamb DC, Lan N, Liang H, Liao H, Liu L, Luo C, Lussier M, Mao R, Menard P, Ooi SL, Revuelta JL, Roberts CJ, Rose M, Ross-Macdonald P, Scherens B, Schimmack G, Shafer B, Shoemaker DD, Sookhai-Mahadeo S, Storms RK, Strathern JN, Valle G, Voet M, Volckaert G, Wang CY, Ward TR, Wilhelmy J, Winzeler EA, Yang Y, Yen G, Youngman E, Yu K, Bussey H, Boeke JD, Snyder M, Philippsen P, Davis RW, Johnston M. Functional profiling of the Saccharomyces cerevisiae genome. Nature. 2002;418:387–391. doi: 10.1038/nature00935. [DOI] [PubMed] [Google Scholar]
- 123.Matsson H, Davey EJ, Draptchinskaia N, Hamaguchi I, Ooka A, Leveen P, Forsberg E, Karlsson S, Dahl N. Targeted disruption of the ribosomal protein S19 gene is lethal prior to implantation. Mol Cell Biol. 2004;24:4032–4037. doi: 10.1128/MCB.24.9.4032-4037.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Panic L, Tamarut S, Sticker-Jantscheff M, Barkic M, Solter D, Uzelac M, Grabusic K, Volarevic S. Ribosomal protein S6 gene haploinsufficiency is associated with activation of a p53-dependent checkpoint during gastrulation. Mol Cell Biol. 2006;26:8880–8891. doi: 10.1128/MCB.00751-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stadanlick JE, Zhang Z, Lee SY, Hemann M, Biery M, Carleton MO, Zambetti GP, Anderson SJ, Oravecz T, Wiest DL. Developmental arrest of T cells in Rpl22-deficient mice is dependent upon multiple p53 effectors. J Immunol. 2011;187:664–675. doi: 10.4049/jimmunol.1100029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shigenobu S, Arita K, Kitadate Y, Noda C, Kobayashi S. Isolation of germline cells from Drosophila embryos by flow cytometry. Dev Growth Differ. 2006;48:49–57. doi: 10.1111/j.1440-169X.2006.00845.x. [DOI] [PubMed] [Google Scholar]
- 127.Kearse MG, Chen AS, Ware VC. Expression of ribosomal protein L22e family members in Drosophila melanogaster: rpL22-like is differentially expressed and alternatively spliced. Nucleic Acids Res. 2011;39:2701–2716. doi: 10.1093/nar/gkq1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Trede NS, Langenau DM, Traver D, Look AT, Zon LI. The use of zebrafish to understand immunity. Immunity. 2004;20:367–379. doi: 10.1016/s1074-7613(04)00084-6. [DOI] [PubMed] [Google Scholar]
- 129.Langenau DM, Zon LI. The zebrafish: a new model of T-cell and thymic development. Nat Rev Immunol. 2005;5:307–317. doi: 10.1038/nri1590. [DOI] [PubMed] [Google Scholar]
- 130.Carradice D, Lieschke GJ. Zebrafish in hematology: sushi or science? Blood. 2008;111:3331–3342. doi: 10.1182/blood-2007-10-052761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nutt SL, Kee BL. The transcriptional regulation of B cell lineage commitment. Immunity. 2007;26:715–725. doi: 10.1016/j.immuni.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 132.Hardy RR, Kincade PW, Dorshkind K. The protean nature of cells in the B lymphocyte lineage. Immunity. 2007;26:703–714. doi: 10.1016/j.immuni.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 133.Carpenter AC, Bosselut R. Decision checkpoints in the thymus. Nature immunology. 2010;11:666–673. doi: 10.1038/ni.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fahl SP, Harris B, Coffey F, Wiest DL. Rpl22 Loss Impairs the Development of B Lymphocytes by Activating a p53-Dependent Checkpoint. J Immunol. 2015;194:200–209. doi: 10.4049/jimmunol.1402242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Corfe SA, Paige CJ. The many roles of IL-7 in B cell development; mediator of survival, proliferation and differentiation. Seminars in immunology. 2012;24:198–208. doi: 10.1016/j.smim.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 136.Stadanlick JE, Zhang Z, Lee SY, Hemann M, Biery M, Carleton MO, Zambetti GP, Anderson SJ, Oravecz T, Wiest DL. Developmental arrest of T cells in Rpl22-deficient mice is dependent upon multiple p53 effectors. J Immunol. 2011;187:664–675. doi: 10.4049/jimmunol.1100029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Meek DW. Tumour suppression by p53: a role for the DNA damage response? Nature reviews. Cancer. 2009;9:714–723. doi: 10.1038/nrc2716. [DOI] [PubMed] [Google Scholar]
- 138.Zhang F, Hamanaka RB, Bobrovnikova-Marjon E, Gordan JD, Dai MS, Lu H, Simon MC, Diehl JA. Ribosomal stress couples the unfolded protein response to p53-dependent cell cycle arrest. The Journal of biological chemistry. 2006;281:30036–30045. doi: 10.1074/jbc.M604674200. [DOI] [PubMed] [Google Scholar]
- 139.Guidos CJ, Williams CJ, Grandal I, Knowles G, Huang MT, Danska JS. V(D)J recombination activates a p53-dependent DNA damage checkpoint in scid lymphocyte precursors. Genes & development. 1996;10:2038–2054. doi: 10.1101/gad.10.16.2038. [DOI] [PubMed] [Google Scholar]
- 140.Taghon T, Yui MA, Pant R, Diamond RA, Rothenberg EV. Developmental and molecular characterization of emerging beta- and gammadelta-selected pre-T cells in the adult mouse thymus. Immunity. 2006;24:53–64. doi: 10.1016/j.immuni.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 141.Brunsing R, Omori SA, Weber F, Bicknell A, Friend L, Rickert R, Niwa M. B- and T-cell development both involve activity of the unfolded protein response pathway. The Journal of biological chemistry. 2008;283:17954–17961. doi: 10.1074/jbc.M801395200. [DOI] [PubMed] [Google Scholar]
- 142.Barna M, Pusic A, Zollo O, Costa M, Kondrashov N, Rego E, Rao PH, Ruggero D. Suppression of Myc oncogenic activity by ribosomal protein haploinsufficiency. Nature. 2008;456:971–975. doi: 10.1038/nature07449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hsieh AC, Costa M, Zollo O, Davis C, Feldman ME, Testa JR, Meyuhas O, Shokat KM, Ruggero D. Genetic dissection of the oncogenic mTOR pathway reveals druggable addiction to translational control via 4EBP-eIF4E. Cancer cell. 2010;17:249–261. doi: 10.1016/j.ccr.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Choi YL, Tsukasaki K, O’Neill MC, Yamada Y, Onimaru Y, Matsumoto K, Ohashi J, Yamashita Y, Tsutsumi S, Kaneda R, Takada S, Aburatani H, Kamihira S, Nakamura T, Tomonaga M, Mano H. A genomic analysis of adult T-cell leukemia. Oncogene. 2007;26:1245–1255. doi: 10.1038/sj.onc.1209898. [DOI] [PubMed] [Google Scholar]
- 145.Yuan J, Nguyen CK, Liu X, Kanellopoulou C, Muljo SA. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science. 2012;335:1195–1200. doi: 10.1126/science.1216557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Viswanathan SR, Powers JT, Einhorn W, Hoshida Y, Ng TL, Toffanin S, O’Sullivan M, Lu J, Phillips LA, Lockhart VL, Shah SP, Tanwar PS, Mermel CH, Beroukhim R, Azam M, Teixeira J, Meyerson M, Hughes TP, Llovet JM, Radich J, Mullighan CG, Golub TR, Sorensen PH, Daley GQ. Lin28 promotes transformation and is associated with advanced human malignancies. Nature genetics. 2009;41:843–848. doi: 10.1038/ng.392. [DOI] [PMC free article] [PubMed] [Google Scholar]