Abstract
Background
Diabetic forefoot joint deformities are a known risk factor for skin breakdown and amputation, but the causes of deformity are not well understood. The purposes of this study were to determine the effects of intrinsic foot muscle deterioration and limited ankle joint mobility on the severity of metatarsophalangeal joint (MTPJ) deformity, and determine the relationships between these potential contributing factors and indicators of diabetic complications (peripheral neuropathy and advanced glycation end products).
Methods
A total of 34 participants with diabetic neuropathy (average age, 59 years; range 41-73) were studied. MTPJ angle and intrinsic foot muscle deterioration were measured with computed tomography and magnetic resonance imaging, respectively. Maximum ankle dorsiflexion was measured using kinematics. Skin intrinsic fluorescence served as a proxy measure for advanced glycation end product accumulation.
Results
Total forefoot lean muscle volume (r = −0.52, P < .01) and maximum ankle dorsiflexion (r = −0.42, P < .05) were correlated with severity of MTPJ deformity. Together they explained 35% of the variance of MTPJ angle. Neuropathy was correlated with forefoot muscle deterioration (ρ = 0.53, P < .01). Skin intrinsic fluorescence was correlated to severity of neuropathy (r = 0.50, P < .01) but not maximum ankle dorsiflexion, or forefoot deterioration when controlling for neuropathy.
Conclusion
These results suggest that the interplay of intrinsic foot muscle deterioration and limited ankle mobility may be the primary contributor to the development of MTPJ deformity. Identifying these muscle and ankle motion impairments as risk factors for MTPJ deformity supports the need for targeted interventions early in the disease process to slow, or possibly stop the progression of deformity over time and reduce the risk of amputation.
Level of Evidence
Level IV, case series.
Keywords: hammertoe, muscle, intermuscular adipose tissue, neuropathy, advanced glycation end products
Introduction
Up to 80% of the 73 000 diabetes-related amputations performed annually in the United States are preceded by a neuropathic ulcer.8,12 The Task Force of the Foot Care Interest Group of the American Diabetes Association indicated the “most common triad of causes that interact and ultimately result in ulceration has been identified as neuropathy, deformity, and trauma.”7 The most common deformity is at the metatarsophalangeal joint (MTPJ), which has been shown to have a prevalence as high as 85% in persons with a history of ulcers and amputation.31 Characteristics of this forefoot deformity, commonly called “hammer or claw-toe,” are hyperextension of the proximal phalanx on the metatarsal and prominent metatarsal heads on the plantar surface of the foot.32 MTPJ angle has been found to be the most important variable predicting pressure in those with diabetes mellitus (DM).35 Given these statistics, MTPJ deformity is a useful early warning indicator for possible skin breakdown and amputation. Therefore, understanding the etiology of MTPJ deformity should lead to improved techniques for managing and treating diabetic, neuropathic forefoot deformity, reducing the risk of lower extremity ulceration and amputation.
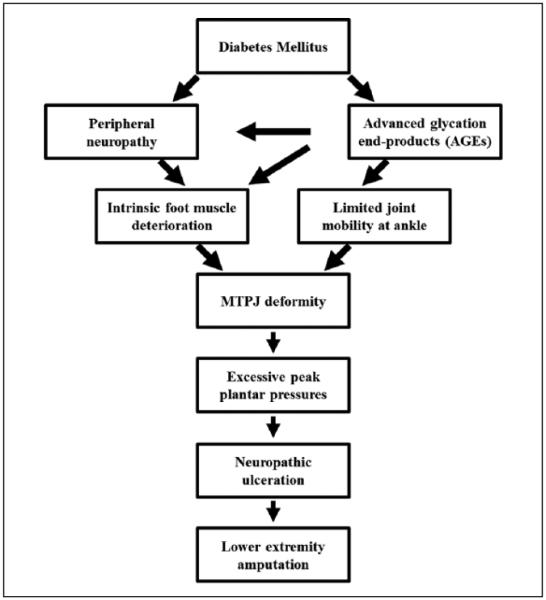
MTPJ deformity has primarily been attributed to intrinsic foot muscle deterioration that precedes extrinsic extensor digitorum longus deterioration as a result of distal to proximal peripheral neuropathy (PN).40 The combined effects of DM and PN are reductions in muscle strength, power, and function, thought to occur from muscle mass loss and the infiltration of intermuscular adipose tissue (IMAT).10,11,30,36 The muscle imbalance of weak intrinsic foot muscles in the presence of relatively stronger extrinsic toe extensors (extensor digitorum longus), results in a force couple that hyperextends the MTPJ.7 Few studies have investigated the relationship between intrinsic foot muscle deterioration and MTPJ deformity, and with conflicting results.9,10,13,32 We hypothesized that there were other factors that combine with muscle deterioration to cause MTPJ deformity and these are depicted in Figure 1. In particular, we hypothesized that limited ankle mobility combines with intrinsic foot muscle deterioration to contribute to MTPJ hyperextension deformity. Limited ankle dorsiflexion is well documented in people with diabetes and likely caused by an accumulation of advanced glycation end products (AGE) and Achilles tendon thickening.24,34,37,38 Muscle deterioration coupled with limited ankle range of motion, exacerbated by DM severity, results in excessive MTPJ extension repeated thousands of times a day during ankle dorsiflexion movement tasks, and causes MTPJ deformity (Figure 1).
Figure 1.
Proposed pathway to acquired MTPJ deformity.
The primary purpose of this study was to determine the influence of the combined effects of intrinsic foot muscle deterioration and limited ankle joint mobility on the severity of MTPJ deformity using a hierarchical regression analysis. A secondary purpose was to determine the relationships between these contributing factors (muscle deterioration and limited joint mobility) and indicators of diabetic complications (peripheral neuropathy and AGEs). Identifying risk factors associated with MTPJ deformity would support the need for the development of targeted interventions early in the disease process.
Methods
Thirty-four participants (15 male, 19 female; average age, 59 years; range, 41-73) provided informed consent and participated in this cross-sectional study (Table 1). An a priori sample size prediction was performed using G*power 3.1 (UCLA, Los Angeles, CA) and estimated effect sizes from the literature: 1.0 in maximum ankle dorsiflexion range of motion, 0.88 in skin intrinsic fluorescence, and a correlation of −0.51 between intrinsic foot muscle deterioration and MTPJ angle.14,18,32 A sample of 34 was estimated for a minimal statistical power of 0.80 and α = .05. The inclusion criteria for all participants were the presence of type 2 DM and diabetic PN. PN was assessed using the Michigan Neuropathy Screening Instrument (MNSI) lower extremity exam, where PN was defined as a score >2.22,29 The exam assessed foot appearance, ankle reflexes, and vibration (128Hz tuning fork) and pressure (5.07g Semmes-Weinstein monofilament) sensation. Further sensory testing for descriptive purposes was performed using monofilaments and a biothesiometer (PN ≥ 25 V) to test pressure and vibration perception thresholds of the plantar surfaces of both feet.19 Exclusion criteria included age ≤40 and ≥75 years, nondiabetic PN; dialysis; severe arterial disease (defined as an ankle-brachial index ≤0.90 or >1.30 assessed with a hand-held Doppler ultrasound device and inflatable cuff); contraindications to magnetic resonance imaging (MRI); presence of a neuropathic ulcer; or lower extremity amputations.3
Table 1.
Subject Demographics.
| Variable | Mean (SD) |
|---|---|
| Gender (female/male) | 19/15 |
| Age (y) | 59 (9) |
| Height (cm) | 170 (9) |
| Weight (kg) | 108 (19) |
| BMI (kg/m2) | 38 (6) |
| Duration of DM (y) | 18 (10) |
| HbA1c (%) | 7.5 (1.8) |
| MNSI examination score | 5.0 (1.7) |
| Biothesiometry VPT (volts) | |
| Great toe | 37 (15) |
| First metatarsal head | 36 (15) |
| 5.07 Semmes-Weinstein monofilament (no. of persons / N) | |
| Unable to feel ≥1 plantar location | 32/34 |
| Resting MTPJ angle (degrees) | 59 (16) |
Abbreviations: BMI, body mass index; DM, diabetes mellitus; HbA1c, glycosylated hemoglobin; MNSI, Michigan Neuropathy Screening Instrument; MTPJ, metatarsophalangeal; SD, standard deviation; VPT, vibration perception threshold.
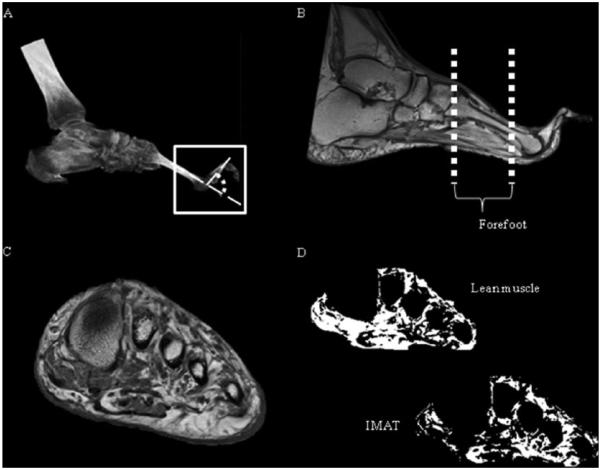
Spiral computed tomography (CT) images were used to measure second MTPJ alignment at rest on the target lower extremity, determined visually as the foot with the most severe deformity. The second MTPJ was chosen because it is the most common site of deformity.32 Participants were positioned supine on the CT scanner table using previously published reliable and precise methods.32,41 A Siemens Sensation 64 CT scanner (Siemens Medical Systems, Inc, Iselin, NJ) was used to acquire the images with the following parameters: 0.5 second rotation time, 64 × 0.6 mm collimation, 220 mAs, 120 kVp, a pitch of 1, and a 512 × 512 matrix. The calf and foot were placed on a positioning device to standardize ankle position to 30 degrees plantarflexion to approximate the midposition of physiological range of motion. MTPJ angle was defined as the complement of the angle between the longitudinal axes of the second metatarsal and second proximal phalanx (Figure 2A). A larger angle corresponded to more severe hyperextension deformity. The MTPJ angle was measured using Analyze software (Biomedical Imaging Resource, Rochester, MN).15
Figure 2.
(A) Metatarsophalangeal angle from computed tomography, defined as the angle between the second metatarsal and proximal phalanx; (B) intrinsic foot muscle regions of interest; (C) cross-sectional magnetic resonance image through forefoot; (D) lean muscle–only and intermuscular adipose tissue (IMAT)–only volumes calculated after magnetic resonance imaging segmentation.
Coronal plane MR images through the foot were collected using a Siemens Magnetom Trio 3T scanner (Siemens Medical Systems, Malvern, PA). The scans were T1 weighted for best adipose and muscle tissue discrimination, with the following parameters: spin echo pulse sequence, repetition time/echo time (TR/TE) = 700/11 milliseconds, field of view = 120 mm, bandwidth = 244 Hz/pixel, signal averages = 1, flip angle = 128 degrees, matrix = 320 × 320, echo train length = 4, thickness = 3.5 mm, and pixel size 0.375 × 0.375 mm.13 The participants were supine with the target foot (foot with the most severe MTPJ deformity) perpendicular to the table in a head coil for an acquisition time of approximately 9.5 minutes. The primary region of interest was from the second tarsometatarsal joint through the sesamoids (forefoot) (Figure 2B).
A custom, semi-automatic program described previously was developed using MatLab (Mathworks, Natick, MA) to measure lean muscle and IMAT volumes from MRI.13 Cross-sectional images (Figure 2C) over the forefoot were inputted individually, and histograms of voxel intensities were produced. Utilizing a multiple Gaussian function fitting algorithm, an intensity threshold between tissue types unique to each image was objectively calculated. The intrinsic foot muscles were segmented using an edge detection algorithm and separated into lean muscle-only and IMAT-only volumes (Figure 2D). The lean muscle volume, IMAT volume, and average deterioration ratio were calculated over the regions of interest. The deterioration ratio was a measure of intrinsic foot muscle quality, defined as the ratio of IMAT volume to lean muscle volume of each MR image slice, averaged over all the slices spanning the region of interest. Higher values indicated higher fat infiltration and greater muscle deterioration.
Ankle motion was calculated from kinematic data captured with an 8 camera, 200Hz Vicon 3D motion analysis system (Vicon MX, Los Angeles, CA). Marker placement followed methods previously described.27 Ankle dorsiflexion was calculated from the hindfoot on shank from the target lower extremity. Participants were positioned long sitting with knees extended and feet off the end of the plinth. They completed 3 trials of 3 repetitions through the full ankle range of motion. The repetition within each trial with the greatest magnitude of dorsiflexion was selected for analysis. The kinematic trials were processed in Visual3D (C-Motion Inc, Germantown, MD) and Microsoft Excel (Microsoft, Redmond, WA).
Skin intrinsic fluorescence (SIF) was measured as an indicator of advanced glycation end products using the SCOUT DS skin fluorescence spectrometer (VeraLight, Albuquerque, NM).20 The spectrometer was built into an arm cradle that the volar forearm rested on, allowing the participant to sit comfortably during the testing. Two measures of SIF were taken from the left volar forearm, an area that is usually protected from excessive sunlight (that can affect SIF values) and easily accessible for testing. SIF was excited with a light emitting diode (LED) centered at 375 nm and detected over an emission range of 375-600 nm. Skin reflectance was measured over both the excitation and emission regions to compensate for absorbance caused by melanin and hemoglobin.18 An intrinsic fluorescence correction was used to compensate for distortion of raw fluorescence by skin absorption, adjusted in these analyses with the dimensionless exponents of kx = 0.6 and km = 0.2.17 The resulting fluorescence was integrated and reported in arbitrary units (AU).
SPSS (IBM, Armonk, NY) was used to determine correlations between the variables of interest. The Shapiro-Wilk test was used to test for normality. Pearson r and Spearman rho correlation coefficients were calculated where appropriate. A significance level of P <.05 was used for all analyses. A hierarchical multiple regression analysis was conducted to explain the variance of MTPJ deformity. The independent variables entered were total lean muscle volume in the forefoot and maximum ankle dorsiflexion. The independent variable was left in the overall equation if (1) the overall P value (for the F value) was less than 0.05; (2) the individual P value (based on the t value) was less than 0.10; and (3) the variable added at least 5% unique variance beyond the preceding variable.
Results
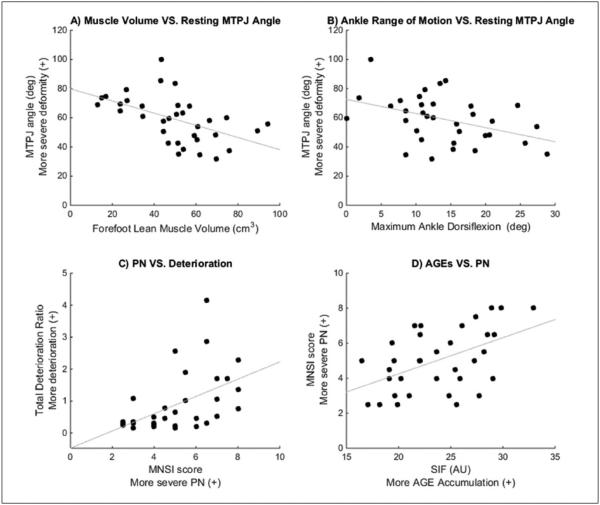
Forefoot lean muscle volume was inversely associated with MTPJ hyperextension; that is, less forefoot lean muscle tissue was associated with greater MTPJ deformity (r = −0.52, P < .01, Figure 3A). Maximum ankle dorsiflexion also was inversely correlated with severity of MTPJ deformity (r = −0.42, P < .05, Figure 3B). From the regression analysis, forefoot lean muscle volume (R2 = .27; P < .01) and maximum ankle dorsiflexion (R2 change = .08; P < .10) explained 35% of the variance of severity of MTPJ deformity.
Figure 3.
Scatterplots of (A) forefoot lean muscle volume versus MTPJ angle (r = −0.52); (B) ankle dorsiflexion versus MTPJ angle (r = −0.42); (C) PN versus forefoot deterioration ratio (ρ = 0.53); (D) SIF versus severity of PN (r = 0.50). All correlations are significant (P < .05). MTPJ, metatarsophalangeal; PN, peripheral neuropathy; SIF, skin intrinsic fluorescence.
PN, assessed using the MNSI score, was correlated with forefoot deterioration ratio (ρ = 0.53, P < .01, Figure 3C). The SIF was correlated to MNSI score (r = 0.50, P < .01) but not correlated to maximum ankle dorsiflexion (r = 0.13, P = .48, Figure 3D). SIF was not correlated with any measures of intrinsic foot muscle deterioration when controlling for MNSI score.
Discussion
The results of this study showed that reduced lean muscle volume in the forefoot and decreased ankle dorsiflexion were associated with increased hyperextension at the MTPJ, consistent with our hypotheses (Figure 1). Increased AGEs, assessed with the proxy measure of SIF, was associated with increased severity of PN, which in turn was associated with an increased ratio of IMAT to lean muscle volume (deterioration ratio) in the forefoot.
Previous literature has shown conflicting results on the relationship between intrinsic foot muscle deterioration and MTPJ deformity. Studies by Ledoux et al and Robertson et al support this relationship showing decreased plantar muscle density (indicative of muscle deterioration) related to MTPJ hyperextension in the neuropathic foot.33,39 Bus et al found no relationship between muscle volume and MTPJ deformity, perhaps because they did not quantify volume of lean tissue and IMAT beyond a 5-point visual atrophy scale to assess deterioration on a single image slice from the forefoot.10 Our previous work showed a correlation between MTPJ angle and midfoot deterioration ratio similar to the correlation found in this current study (r = −0.51, P < .05) despite using different methods to measure MTPJ deformity and collected on a different cohort of participants primarily with a midfoot deformity.14 Our current study helps to clarify the discrepancies in previous literature because we have measured multislice volumes of both lean muscle and IMAT, over the entire forefoot, in a larger sample of participants with DMPN and a wide spectrum of MTPJ deformity severity, in a reliable and valid way.13 In addition, we have considered the relationship of other potential contributing factors to deformity as outlined in Figure 1.
The results of this study support that the lean muscle tissue remaining in the foot is important to MTPJ alignment. The intrinsic foot muscles are primarily responsible for flexion at the MTPJ. This flexion force is important for balance, propulsion, and providing structural stability to the foot and medial longitudinal arch. The forefoot muscle volume region of interest included the muscle bellies of the lumbricals, dorsal and plantar interossei, flexor digiti minimi, and other individual muscles deeper to the plantar surface, most related to controlling the second MTPJ. Optimal MTPJ alignment at rest is present when there is a muscular balance between extrinsic extensors and intrinsic flexors.6 When intrinsic foot muscle deterioration increases, there is an increased amount of noncontractile tissue, which is negatively related to the number of functioning motor units.2 The remaining lean muscle tissue provides an inadequate amount of force, resulting in an imbalance and MTPJ hyperextension.
The correlation between the muscle deterioration ratio and the MNSI (Figure 3C) underscores the relationship between PN and lower extremity muscular changes. Chronic hyperglycemia damages the sensory, motor, and autonomic nerves.11 Previous work suggested that in the intrinsic foot muscle compartment of persons with DMPN, the volume previously occupied by functional lean muscle tissue is replaced by IMAT due to PN.14 Although functional muscle volume is critical to structure and joint motions, IMAT may be detrimental to general lower extremity performance during functional tasks, though this is not independent of DM and/or PN. IMAT may serve as a more sensitive indicator of the progression and severity of DMPN; previous work has shown that IMAT is associated with insulin resistance and an increased risk of metabolic impairments.5,16,23,25 Additionally, IMAT has been associated with several negative effects in muscle functional capacity; fascicle arrangement, pennation angle, and excursion during contraction may all be negatively impacted by IMAT infiltration, and exacerbated by the presence of PN.4 General losses in strength, power, and balance due to IMAT could explain changes in walking speed, fall risk, proprioception, and joint mobility.11 A higher volume of IMAT in the calf muscles of persons with DMPN was shown to be associated with lower Physical Performance Test scores, an assessment of the severity of physical frailty.30 Future work is needed to determine if interventions could slow, stop, or even reverse IMAT infiltration in the intrinsic foot muscles.
Our regression results support that the interplay of intrinsic foot muscle deterioration and limited ankle joint mobility are associated with the development of MTPJ deformity. Adequate dorsiflexion range of motion is required to clear the foot during the swing phase of gait. Individuals may develop an MTPJ hyperextension movement strategy in which the extrinsic toe extensor muscle (extensor digitorum longus) is recruited to assist with ankle dorsiflexion for proper clearance. However, increased reliance on the extensor digitorum longus, combined with insufficient stabilization of the MTPJ by the intrinsic foot muscles, may cause the extensor digitorum longus to shorten and produce hyperextension at the MTPJ. Although beyond the scope of this paper, we have characterized the full MTPJ hyperextension movement pattern using 3-dimensional kinematic analysis in the same cohort as in the present study (unpublished manuscript). The results showed that MTPJ hyperextension deformity was associated with a pattern of motion characterized by an increase in MTPJ extension during active ankle dorsiflexion. The amount of MTPJ extension excursion during daily tasks (ie, walking, sit-to-stand) was related to the severity of resting MTPJ extension alignment. A progressive exercise and movement retraining program may help to improve specific muscle strength, joint range of motion, and reduce MTPJ extension with active dorsiflexion to minimize the risk of development of deformity, but additional research is required to test that intervention. Furthermore, other potential risk factors may exist that account for the 65% unexplained variance in MTPJ angle. These include traumatic injury, arthritis, changes in bone shape and capsule laxity that impact joint position and mobility, ill-fitting footwear (eg, high-heeled shoes, tight toe box), and a genetic predisposition for abnormal foot structure (ie, high or low arch) and/or biomechanics.26,32
The relationship between SIF and MNSI score shows that AGE accumulation is associated with severity of PN. This supports previous work that demonstrated a strong association between SIF and PN in a large sample of persons with type 1 DM.18 AGEs are protein-sugar complexes that accumulate rapidly in DM, and are thought to contribute to the high incidence of musculoskeletal complications.1 The buildup of AGEs on peripheral nerve tissue is accelerated in the presence of high glucose and leads to damage and worsening PN.21 There was a nonsignificant correlation between SIF and the intrinsic foot muscle deterioration measures, when controlling for the MNSI score. The direct effect of AGEs may be on skeletal muscle function rather than volume changes in lean muscle and IMAT, which are indirectly influenced through the PN pathway. AGEs lead to excessive collagen crosslinking in multiple tissue types, including muscle.9 Increased muscle connective tissue protein stiffness may contribute to altered force transmission and function, outcomes that may show better association with SIF.28
We did not find a significant correlation between SIF and maximum ankle dorsiflexion. Limited ankle dorsiflexion has primarily been attributed to increased stiffness from AGE accumulation and thickening of the Achilles tendon.38 The lack of association in our results could be due to a couple of reasons. Limitations in dorsiflexion could be due to muscular rather than tendon changes from a loss of contractile protein due to PN and the protein catabolic state of insulin deficiency.37 Muscle atrophy coupled with increased connective tissue volume may contribute to increased passive stiffness, where the effect of AGEs may depend on the collagen composition of the connective tissue. Also, SIF from the dermal collagen of the volar forearm may not be the best proxy measure of AGEs in the Achilles tendon. Although AGEs accumulate in multiple tissue types over the entire body, the SIF measure may be more reflective of systemic changes rather than local changes at the tendon level. Fluorescence analysis of Achilles tendon biopsies may show relationships between AGEs, tendon thickness, and tendon stiffness.
This study has several limitations. The cross-sectional design only allows for correlations and not cause-and-effect relationships to be determined. There could be other potential risk factors for acquired MTPJ deformity, including externally applied forces from ill-fitting footwear and ruptures of the plantar fascia or MTPJ capsule.10 The study sample size was small for regression analysis. Additional prospective studies with larger sample sizes are necessary to confirm the findings of the present study, and clarify the causal relationships between the variables outlined in Figure 1.
In conclusion, the lean muscle tissue volume in the intrinsic foot muscles and limited ankle dorsiflexion were associated with the severity of MTPJ deformity. Intrinsic foot muscle deterioration was associated with the DM complications of PN and AGEs. Identifying impaired intrinsic foot muscles and ankle dorsiflexion as risk factors would support the need for targeted interventions early in the disease process.
Acknowledgments
The authors thank Kathryn Bohnert and Darrah Snozek for their assistance with data collection.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Authors report grants from the National Institute of Diabetes and Digestive and Kidney Diseases (F31 DK101286), the National Institute of Child Health and Human Development (T32 HD007434 and K12 HD055931), and the National Center for Advancing Translational Sciences (KL2 TR000450), US Department of Health and Human Services, National Institutes of Health during the conduct of the study.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Abate M, Schiavone C, Pelottf P, Salini V. Limited joint mobility in diabetes and ageing: recent advances in pathogenesis and therapy. Int J Immunopathol Pharmacol. 2010;23(4):997–1003. doi: 10.1177/039463201002300404. [DOI] [PubMed] [Google Scholar]
- 2.Allen MD, Major B, Kimpinski K, Doherty TJ, Rice CL. Skeletal muscle morphology and contractile function in relation to muscle denervation in diabetic neuropathy. J Appl Physiol. 2014;116(5):545–552. doi: 10.1152/japplphysiol.01139.2013. doi:10.1152/japplphysiol.01139.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26(12):3333–3341. doi: 10.2337/diacare.26.12.3333. doi:10.2337/diacare.26.12.3333. [DOI] [PubMed] [Google Scholar]
- 4.Bittel DC, Bittel AJ, Tuttle LJ, et al. Adipose tissue content, muscle performance and physical function in obese adults with type 2 diabetes mellitus and peripheral neuropathy. J Diabetes Complications. 2015;29(2):250–257. doi: 10.1016/j.jdiacomp.2014.11.003. doi:10.1016/j.jdiacomp.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boettcher M, Machann J, Stefan N, et al. Intermuscular adipose tissue (IMAT): association with other adipose tissue compartments and insulin sensitivity. J Magn Reson Imaging. 2009;29(6):1340–1345. doi: 10.1002/jmri.21754. doi:10.1002/jmri.21754. [DOI] [PubMed] [Google Scholar]
- 6.Boulton AJ, Betts RP, Franks CI, Ward JD, Duckworth T. The natural history of foot pressure abnormalities in neuropathic diabetic subjects. Diabetes Res. 1987;5(2):73–77. [PubMed] [Google Scholar]
- 7.Boulton AJM, Armstrong DG, Albert SF, et al. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31(8):1679–1685. doi: 10.2337/dc08-9021. doi:10.2337/dc08-9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boulton AJM. The pathway to foot ulceration in diabetes. Med Clin North Am. 2013;97(5):775–790. doi: 10.1016/j.mcna.2013.03.007. doi:10.1016/j.mcna.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Brownlee M. Glycation products and the pathogenesis of diabetic complications. Diabetes Care. 1992;15(12):1835–1843. doi: 10.2337/diacare.15.12.1835. doi:10.2337/diacare.15.12.1835. [DOI] [PubMed] [Google Scholar]
- 10.Bus S, Maas M, Michels R, Levi M. Role of intrinsic muscle atrophy in the etiology of claw toe deformity in diabetic neuropathy may not be as straightforward as widely believed. Diabetes Care. 2009;32(6):1063–1067. doi: 10.2337/dc08-2174. doi:10.2337/dc08-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bus SA, Yang QX, Wang JH, Smith MB, Wunderlich R, Cavanagh PR. Intrinsic muscle atrophy and toe deformity in the diabetic neuropathic foot: a magnetic resonance imaging study. Diabetes Care. 2002;25(8):1444–1450. doi: 10.2337/diacare.25.8.1444. doi:10.2337/diacare.25.8.1444. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention . National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta: 2014. [Google Scholar]
- 13.Cheuy VA, Commean PK, Hastings MK, Mueller MJ. Reliability and validity of a MR-based volumetric analysis of the intrinsic foot muscles. J Magn Reson Imaging. 2013;38(5):1083–1093. doi: 10.1002/jmri.24069. doi:10.1002/jmri.24069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheuy VA, Hastings MK, Commean PK, Ward SR, Mueller MJ. Intrinsic foot muscle deterioration is associated with metatarsophalangeal joint angle in people with diabetes and neuropathy. Clin Biomech (Bristol, Avon) 2013;28(9-10):1055–1060. doi: 10.1016/j.clinbiomech.2013.10.006. doi:10.1016/j.clinbiomech.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Commean PK, Mueller MJ, Smith KE, et al. Reliability and validity of combined imaging and pressures assessment methods for diabetic feet. Arch Phys Med Rehabil. 2002;83(4):497–505. doi: 10.1053/apmr.2002.30923. doi:10.1053/apmr.2002.30923. [DOI] [PubMed] [Google Scholar]
- 16.Commean PK, Tuttle LJ, Hastings MK, Strube MJ, Mueller MJ. Magnetic resonance imaging measurement reproducibility for calf muscle and adipose tissue volume. J Magn Reson Imaging. 2011;34:1285–1294. doi: 10.1002/jmri.22791. doi:10.1002/jmri.22791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conway B, Edmundowicz D, Matter N, Maynard J, Orchard T. Skin fluorescence correlates strongly with coronary artery calcification severity in type 1 diabetes. Diabetes Technol Ther. 2010;12(5):339–345. doi: 10.1089/dia.2009.0152. doi:10.1089/dia.2009.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conway BN, Aroda VR, Maynard JD, et al. Skin intrinsic fluorescence correlates with autonomic and distal symmetrical polyneuropathy in individuals with type 1 diabetes. Diabetes Care. 2011;34(4):1000–1005. doi: 10.2337/dc10-1791. doi:10.2337/dc10-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diamond JE, Mueller MJ, Delitto A, Sinacore DR. Reliability of a diabetic foot evaluation. Phys Ther. 1989;69(10):797–802. doi: 10.1093/ptj/69.10.797. http://www.ncbi.nlm.nih.gov/pubmed/2780806. [DOI] [PubMed] [Google Scholar]
- 20.Ediger MN, Olson BP, Maynard JD. Noninvasive optical screening for diabetes. J Diabetes Sci Technol. 2009;3(4):776–780. doi: 10.1177/193229680900300426. doi:10.1177/193229680900300426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Espiritu DJ, Huang ZH, Zhao Y, Mazzone T. Hyperglycemia and advanced glycosylation end products suppress adipocyte apoE expression: implications for adipocyte triglyceride metabolism. Am J Physiol Endocrinol Metab. 2010;299(4):E615–E623. doi: 10.1152/ajpendo.00273.2010. doi:10.1152/ajpendo.00273.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17(11):1281–1289. doi: 10.2337/diacare.17.11.1281. doi:10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- 23.Gallagher D, Kuznia P, Heshka S, et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr. 2005;81(4):903–910. doi: 10.1093/ajcn/81.4.903. doi:81/4/903 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giacomozzi C, D’Ambrogi E, Uccioli L, Macellari V. Does the thickening of Achilles tendon and plantar fascia contribute to the alteration of diabetic foot loading? Clin Biomech (Bristol, Avon) 2005;20(5):532–539. doi: 10.1016/j.clinbiomech.2005.01.011. doi:10.1016/j.clinbiomech.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71(4):885–892. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- 26.Hannan MT, Menz HB, Jordan JM, Cupples LA, Cheng CH, Hsu YH. High heritability of hallux valgus and lesser toe deformities in adult men and women. Arthritis Care Res. 2013;65:1515–1521. doi: 10.1002/acr.22040. doi:10.1002/acr.22040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hastings MK, Woodburn J, Mueller MJ, Strube MJ, Johnson JE, Sinacore DR. Kinematics and kinetics of single-limb heel rise in diabetes related medial column foot deformity. Clin Biomech. 2014;29(9):1016–1022. doi: 10.1016/j.clinbiomech.2014.08.011. doi:10.1016/j.clinbiomech.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haus JM, Carrithers JA, Trappe SW, Trappe TA. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol. 2007;103(6):2068–2076. doi: 10.1152/japplphysiol.00670.2007. doi:10.1152/japplphysiol.00670.2007. [DOI] [PubMed] [Google Scholar]
- 29.Herman WH, Pop-Busui R, Braffett BH, et al. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med. 2012;29(7):937–944. doi: 10.1111/j.1464-5491.2012.03644.x. doi:10.1111/j.1464-5491.2012.03644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hilton TN, Tuttle LJ, Bohnert KL, Mueller MJ, Sinacore DR. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function. Phys Ther. 2008;88(11):1336–1344. doi: 10.2522/ptj.20080079. doi:10.2522/ptj.20080079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holewski JJ, Moss KM, Stess RM, Graf PM, Grunfeld C. Prevalence of foot pathology and lower extremity complications in a diabetic outpatient clinic. J Rehabil Res Dev. 1989;26(3):35–44. http://www.ncbi.nlm.nih.gov/pubmed/2666642. [PubMed] [Google Scholar]
- 32.Kwon OY, Tuttle LJ, Johnson JE, Mueller MJ. Muscle imbalance and reduced ankle joint motion in people with hammer toe deformity. Clin Biomech (Bristol, Avon) 2009;24(8):670–675. doi: 10.1016/j.clinbiomech.2009.05.010. doi:10.1016/j.clinbiomech.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ledoux WR, Schoen J, Lovell M, Huff E. Clawed toes in the diabetic foot: neuropathy, intrinsic muscle volume, and plantar aponeurosis thickness. J Foot Ankle Res. 2008;1(suppl 1):O2. doi:10.1186/1757-1146-1-S1-O2. [Google Scholar]
- 34.Mueller MJ, Diamond JE, Delitto A, Sinacore DR. Insensitivity, limited joint mobility, and plantar ulcers in patients with diabetes mellitus. Phys Ther. 1989;69:453–459. doi: 10.1093/ptj/69.6.453. discussion 459-462. [DOI] [PubMed] [Google Scholar]
- 35.Mueller MJ, Hastings M, Commean PK, et al. Forefoot structural predictors of plantar pressures during walking in people with diabetes and peripheral neuropathy. J Biomech. 2003;36(7):1009–1017. doi: 10.1016/s0021-9290(03)00078-2. doi:10.1016/S0021-9290(03)00078-2. [DOI] [PubMed] [Google Scholar]
- 36.Park CN, Zuiderbaan HA, Chang A, Khamaisy S, Pearle AD, Ranawat AS. Role of magnetic resonance imaging in the diagnosis of the painful unicompartmental knee arthroplasty. Knee. 2015;22(4):341–346. doi: 10.1016/j.knee.2015.03.007. doi:10.1016/j.knee.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Rao SR, Saltzman CL, Wilken J, Yak HJ. Increased passive ankle stiffness and reduced dorsiflexion range of motion in individuals with diabetes mellitus. Foot Ankle Int. 2006;27(8):617–622. doi: 10.1177/107110070602700809. doi:936105 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reddy GK. Cross-linking in collagen by nonenzymatic glycation increases the matrix stiffness in rabbit achilles tendon. Exp Diabesity Res. 2004;5(2):143–153. doi: 10.1080/15438600490277860. doi:10.1080/15438600490277860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robertson DD, Mueller MJ, Smith KE, Commean PK, Pilgram T, Johnson JE. Structural changes in the forefoot of individuals with diabetes and a prior plantar ulcer. J Bone Jt Surg Am. 2002;84-A(8):1395–1404. doi: 10.2106/00004623-200208000-00016. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12177270. [DOI] [PubMed] [Google Scholar]
- 40.Van Schie CHM, Vermigli C, Carrington AL, Boulton A. Muscle weakness and foot deformities in diabetes: relationship to neuropathy and foot ulceration in Caucasian diabetic men. Diabetes Care. 2004;27(7):1668–1673. doi: 10.2337/diacare.27.7.1668. doi:10.2337/diacare.27.7.1668. [DOI] [PubMed] [Google Scholar]
- 41.Smith KE, Commean PK, Robertson DD, Pilgram T, Mueller MJ. Precision and accuracy of computed tomography foot measurements. Arch Phys Med Rehabil. 2001;82(7):925–929. doi: 10.1053/apmr.2001.23894. doi:10.1053/apmr.2001.23894. [DOI] [PubMed] [Google Scholar]