Abstract
Background: Endometriosis is a gynecologic disease reported to be associated with infertility and, possibly, adverse pregnancy outcomes. While considerable research focuses on pregnancy outcomes following diagnosis and/or treatment, few data actually describe women's reproductive history before diagnosis for a more complete understanding of endometriosis and reproduction.
Materials and Methods: The study sample comprised 473 women (aged 18–44 years) undergoing laparoscopies or laparotomies, irrespective of surgical indication at 14 clinical sites, during the period 2007–2009. Upon enrollment and before surgery, women were queried about pregnancy intentions and the time required to become pregnant for planned pregnancies. Endometriosis was defined as surgically visualized disease. Using discrete time survival analysis, we estimated fecundability odds ratios (FORs) and 95% confidence intervals (CIs) to assess time to pregnancy (TTP) after adjusting for potential confounders (age, body composition, cigarette smoking, site). Generalized estimating equations accounted for multiple pregnancy attempts per woman. FORs <1.0 denote a longer TTP or diminished fecundity.
Results: Approximately 66% and 69% of women with and without endometriosis, respectively, reported having a planned pregnancy before surgery, respectively. After adjustment, an endometriosis diagnosis was associated with ≈29% reduction in fecundity or a longer TTP across all pregnancy-trying attempts (adjusted FOR = 0.71; 95% CI 0.46–1.10). While FORs were consistently <1.0, irrespective of endometriosis staging, CIs included 1.
Conclusions: Women with endometriosis had a longer TTP than unaffected women, irrespective of disease severity, although the findings did not achieve significance. Prior reproductive history may be informative for predicting fecundity and pregnancy outcomes following diagnosis/treatment.
Keywords: : endometriosis, epidemiology, fertility
Introduction
Women's prior reproductive history is a strong predictor of a range of subsequent pregnancy outcomes, including ectopic pregnancies, preterm and low birth weight deliveries, and birth defects.1–4 These findings have led to the inclusion of prior pregnancy history in prenatal risk assessments used by healthcare practitioners in the clinical management of pregnant women to help identify high-risk women. Recent findings suggest female fecundity as measured by time to pregnancy (TTP), or the number of months couples require to become pregnant, also repeats within women.5,6 For example, women with a shorter TTP for their initial pregnancy attempt tend to have shorter TTPs in subsequent attempts, and similarly for women with longer TTPs. Collectively, these data underscore the importance of past reproductive performance in predicting future performance inclusive of adverse pregnancy outcomes. Moreover, increasing evidence suggests that gynecologic diseases may be in the pathway to both gravid and later onset adult diseases, as evident by reported associations between endometriosis and pregnancy complications and also ovarian cancer in some, but not all, studies.7–10 These data underscore the importance of assessing female fecundity in the context of women's health across the life span.
Endometriosis, an estrogen-dependent gynecologic disease characterized by the presence of endometrial glands and stroma outside the uterine cavity,11 affects a sizeable percentage of women, although prevalence varies depending upon the sampling framework, that is, 4% to 43% among asymptomatic women undergoing tubal sterilization12–14 and 30% to 50% among women undergoing various surgical procedures who were fortuitously diagnosed.15,16 Recent population-based incidence estimates suggest that 11% of reproductive age women not seeking clinical care may have moderate to severe endometriosis.17 Despite a plethora of hypothesized etiologies, its pathophysiology remains elusive, in part, complicated by the absence of a biomarker suitable for population-based research.18
Infertility is often described as a classic risk factor for endometriosis, although the exact temporal ordering relative to disease onset and fecundity remains unknown in light of no prospective cohort studies that have followed girls from birth through menopause. This interval is needed to delineate female fecundity, defined as the biologic capacity for reproduction irrespective of pregnancy intentions,19 and endometriosis. In response to this data gap, investigators have utilized medical records to assess cross-sectional associations between fecundity, related impairments such as infertility or pregnancy loss, and endometriosis. Other researchers have utilized novel designs to study women who do and do not seek clinical care for signs and symptoms that might lead to an endometriosis diagnosis. While the relationship between endometriosis and fecundity is of interest to many, much of the available research only focuses on pregnancy outcomes after diagnosis and/or treatment.20–23 Of the many so-called risk factors for endometriosis reported to date, only a prior history of infertility was recently reported to be consistently associated with incident endometriosis among women seeking and not seeking clinical care.24
To address data gaps on fecundity before an endometriosis diagnosis, we assessed TTP and incident endometriosis in the context of women's pregnancy intentions and duration of trying attempts to provide a more complete perspective about reproductive history.
Materials and Methods
Study design and sampling
The study sample comprised 473 women who underwent either laparoscopy or laparotomy at 1 of 14 participating clinical sites located in Salt Lake City, Utah, or San Francisco, California, geographic areas, 2007–2009. Eligibility criteria were scheduled for surgery, aged 18–44 years, and residents of the geographic catchment areas for participating clinical sites. Exclusion criteria were prior surgically diagnosed endometriosis (prevalent cases), previous cancer diagnosis (except nonmelanoma skin cancer), use of injectable contraceptives within the past 2 years, and currently breastfeeding for ≥6 months. The latter two criteria were to remove exposures that may temporarily reduce fecundity. By design, the sample was meant to be inclusive to remove assumptions about fecundity and endometriosis. The sample size was powered for the detection of environmental/lifestyle risk factors. Complete details on study methodology have been previously published.17
Data collection
Upon recruitment, women were interviewed at the clinical sites ∼2 months before undergoing surgery so that they were not aware of their diagnoses at the time of interview. This interval reflected the availability of the woman, surgeon, and surgical suite. Using computer-assisted interviews, women provided information about prior pregnancies, their intentions for each pregnancy (planned vs. unplanned pregnancy), TTP or the number of months required to become pregnant, and use of infertility treatment among other reproductive health characteristics. Height and weight were measured for all women using a standardized protocol25 for the estimation of body–mass index (BMI; weight in kg/height in m2). As a measure of adolescent body composition that has been linked to endometriosis,26,27 women selected the body composition picture that most closely resembled them at 15–19 years of age using the Stunkard Scale.28 Women also provided a serum sample for the quantification of cotinine, a biomarker of nicotine, given its reported inverse association with endometriosis.29,30 Active smoking was defined as ≥10.0 ng/mL cotinine.31 Full institutional review board approval was obtained from all participating institutions, with written informed consent obtained from all women before data collection.
Endometriosis diagnosis
We used the established clinical gold standard definition for endometriosis or surgically visualized disease32,33 with severity staged using the Revised American Society for Reproductive Medicine (r-ASRM) criteria.34 The r-ASRM staging pertains largely to potential surgical challenges and is not correlated with disease severity or infertility per se.35,36 Participating surgeons were not asked to change their operative technique, but were asked to record postoperative diagnosis(es) and other operative findings on standardized forms immediately following surgery.
Statistical analysis
A cross-sectional design was used for analysis. Women were first compared by endometriosis status for the full study sample (n = 473) and then restricted to women who reported ever having planned a pregnancy (n = 214; 45%) to assess the consistency of characteristics by endometriosis status. Significance (p < 0.05) was assessed using the chi-square and nonparametric Wilcoxon tests for categorical and continuous variables, respectively. To assess female fecundity as measured by TTP, we further restricted our analysis to 198 (93%) women with planned pregnancies for whom TTP was reported for each pregnancy. Unadjusted and adjusted fecundability odds ratios (FORs) and 95% confidence intervals (CIs) were estimated using Cox models for discrete survival time, allowing for a cycle-varying intercept.37 TTP ≥13 months was censored for analysis. Correlations between multiple pregnancies for the same woman were modeled using the generalized estimation equation approach.38 FORs estimated the monthly probability of pregnancy for women with endometriosis relative to those without the disease, conditional on not becoming pregnant in the previous month. FORs <1 denote diminished fecundity or a longer TTP, while FORs >1 denote enhanced fecundity or a shorter TTP.
We used the literature to a priori identify potential confounders, chronologic age (in years),39 BMI (categorized <25.0 under/normal weight; 25.0–29.9 over weight; ≥30.0 obese),26,27 cigarette smoking (categorized cotinine ≥10 ng/mL),31 and research site (Utah/California), to account for any varying geographic clinical practices. Trends were assessed using the log-rank test. We undertook sensitivity analyses, in which we replaced chronologic age with gynecologic age (number of years between menarche and age at first pregnancy, categorical) to assess duration at risk, in that endometriosis is a disease of menstruating women, substituting adult BMI with categorized Stunkard body shape at 15–19 years as a proxy of adolescent body composition.
Results
The incidence of endometriosis was 40% (190/473 women) in the full cohort and 34% (72/214 women) in the study sample when restricting to women with planned pregnancies. Among the women participating in the overall study, 45% (214/473) reported having tried for pregnancy before diagnosis. In the overall study sample (n = 473), disease severity was skewed toward milder disease: 50% of women had stage 1, 21% had stage 2, 18% had stage 3, and 11% had stage 4 (Table 1). When restricting to women with a prior pregnancy attempt (n = 214), a similar distribution for disease severity was observed (i.e., 57%, 19%, 15%, 8%, respectively). Pelvic pain was the leading surgical indication for both the full and study samples (44% and 39%, respectively), followed by pelvic mass for the overall cohort (16%) and menstrual irregularities (19%) for the study sample as the second leading indication. Specifically, when comparing women by whether or not they had endometriosis, only pelvic pain achieved significance. Specifically, a higher percentage of women diagnosed with than without endometriosis had pelvic pain as a preoperative diagnosis, that is, 63% and 31% in the full and 57% and 29% in the study samples.
Table 1.
Comparison of the Full and Study Cohort by Reproductive Health Characteristics
| Full cohort (n = 473) | Study sample (n = 214)a | |||
|---|---|---|---|---|
| Reproductive characteristic | Endometriosis (n = 190), n (%) | No endometriosis (n = 283), n (%) | Endometriosis (n = 72), n (%) | No endometriosis (n = 142), n (%) |
| Chronologic age (years) | ||||
| <30 | 75 (39.5) | 88 (31.2)b | 22 (30.6) | 34 (23.9) |
| 30–39 | 83 (43.7) | 120 (42.6) | 29 (40.3) | 65 (45.8) |
| >40 | 32 (16.8) | 74 (26.2) | 21 (29.2) | 43 (30.3) |
| Mean (SD) | 32 (6.8) | 34 (7.1)b | 34 (6.4) | 35 (6.3) |
| Gynecologic age (years) | ||||
| <10 | 68 (62.4) | 118 (57.0) | 41 (56.9) | 74 (52.1) |
| 10–15 | 31 (28.4) | 63 (30.4) | 24 (33.3) | 48 (33.8) |
| >16 | 10 (9.2) | 26 (12.6) | 7 (9.7) | 20 (14.1) |
| Mean gynecologic age (SD) | 8.9 (4.4) | 9.1 (5.3) | 9.4 (4.5) | 9.8 (5.4) |
| BMI (weight in kg/height in m2) | ||||
| Undernormal weight (<25.0) | 105 (55.6) | 98 (35.1)c | 34 (47.2) | 49 (35.0) |
| Overweight (25.0–29.9) | 39 (20.6) | 70 (25.1) | 18 (25.0) | 35 (25.0) |
| Obese (>30.0) | 45 (23.8) | 111 (39.8) | 20 (27.8) | 56 (40.0) |
| Mean (SD) | 26 (7.2) | 29 (8.4)c | 27 (7.8) | 29 (8.1) |
| Body figure rating at 15–19 years26 | ||||
| 1 | 33 (17.4) | 35 (12.4)b | 15 (20.8) | 19 (13.4) |
| 2 | 34 (17.9) | 60 (21.3) | 12 (16.7) | 30 (21.1) |
| 3 | 70 (36.8) | 74 (26.2) | 25 (34.7) | 40 (28.2) |
| 4 | 36 (18.9) | 61 (21.6) | 13 (18.1) | 29 (20.4) |
| >5 | 17 (8.9) | 52 (18.4) | 7 (9.7) | 24 (16.9) |
| Mean (SD) | 2.9 (1.2) | 3.2 (1.5)b | 2.8 (1.3) | 3.2 (1.6) |
| Age at menarche (years) | ||||
| <9 | 1 (0.5) | 0 (0.0) | 1 (1.4) | 0 (0.0) |
| 9–11 | 39 (20.5) | 50 (17.7) | 13 (18.1) | 22 (15.5) |
| 12 | 45 (23.7) | 75 (26.6) | 19 (26.4) | 39 (27.5) |
| 13–15 | 90 (47.4) | 138 (48.9) | 34 (47.2) | 71 (50.0) |
| ≥16 | 15 (7.9) | 19 (6.7) | 5 (6.9) | 10 (7.0) |
| Mean (SD) | 13 (1.8) | 13 (1.6) | 13 (1.9) | 13 (1.5) |
| Ever sexually active | ||||
| No | 27 (14.2) | 37 (13.2) | 7 (9.7) | 18 (12.7) |
| Yes | 163 (85.8) | 244 (86.8) | 65 (90.3) | 124 (87.3) |
| Ever taken emergency contraception | ||||
| No | 157 (82.6) | 237 (85.3) | 60 (83.3) | 125 (88.0) |
| Yes | 33 (17.4) | 41 (14.7) | 12 (16.7) | 17 (12.0) |
| Ever pregnant | ||||
| No | 81 (42.6) | 74 (26.3)c | — | — |
| Yes | 109 (57.4) | 207 (73.7) | 72 (100) | 142 (100) |
| Ever tried for planned pregnancy | ||||
| No | 37 (33.9) | 65 (31.4) | — | — |
| Yes | 72 (66.1) | 142 (68.6) | 72 (100) | 142 (100) |
| Ever tried >6 months for pregnancy | ||||
| No | 96 (50.5) | 209 (74.9)c | 37 (51.4) | 98 (70.0)b |
| Yes | 94 (49.5) | 70 (25.1) | 35 (48.6) | 42 (30.0) |
| Mean longest attempt (SD) | 34 (41) | 28 (29) | 27 (41) | 27 (30) |
| Ever have live birth | ||||
| No | 105 (55.3) | 102 (36.0)c | 9 (12.5) | 9 (6.3) |
| Yes | 85 (44.7) | 181 (64.0) | 63 (87.5) | 133 (93.7) |
| Ever use infertility treatment | ||||
| No | 126 (66.3) | 235 (83.0)c | 47 (65.3) | 118 (83.1)b |
| Yes | 64 (33.7) | 48 (17.0) | 25 (34.7) | 24 (16.9) |
| Active smoking (serum cotinine ng/mL)31 | ||||
| No (<10) | 169 (88.9) | 233 (82.3)b | 67 (93.1) | 124 (87.3) |
| Yes (≥10.0) | 21 (11.1) | 50 (17.7) | 5 (6.9) | 18 (12.7) |
| Pelvic pain >6 months | ||||
| No | 106 (55.8) | 184 (65.2)b | 41 (56.9) | 98 (69.0) |
| Yes | 84 (44.2) | 98 (34.8) | 31 (43.1) | 44 (31.0) |
| Dyspareunia | ||||
| No | 54 (28.4) | 144 (50.9)c | 25 (34.7) | 71 (50.0)b |
| Yes | 136 (71.6) | 139 (49.1) | 47 (65.3) | 71 (50.0) |
| Ever avoid sex due to pelvic pain | ||||
| No | 89 (46.8) | 145 (52.0) | 34 (47.2) | 66 (46.5) |
| Yes | 101 (53.2) | 134 (48.0) | 38 (52.8) | 76 (53.5) |
| Preoperative diagnosis | ||||
| Pelvic pain | 120 (63.2) | 86 (30.5)c | 41 (56.9) | 41 (29.1)b |
| Pelvic mass | 26 (13.7) | 48 (17.0) | 6 (8.3) | 17 (12.0) |
| Irregular menses | 20 (10.5) | 40 (14.2) | 11 (15.3) | 29 (20.6) |
| Fibroids | 9 (4.7) | 40 (14.2) | 5 (7.0) | 12 (8.5) |
| Infertility | 7 (3.7) | 28 (9.9) | 2 (2.8) | 13 (9.2) |
| Tubal ligation | 8 (4.2) | 40 (14.2) | 7 (9.7) | 29 (20.6) |
| Endometriosis severity (rASR1V)34 | ||||
| Stage 1, minimal | 95 (50.0) | — | 41 (57.0) | — |
| Stage 2, mild | 39 (21.0) | — | 14 (19.0) | — |
| Stage 3, moderate | 35 (18.0) | — | 11 (15.0) | — |
| Stage 4, severe | 21 (11.0) | — | 4 (8.0) | — |
Chi-square or Wilcoxon continuous tests were used to compare women with and without endometriosis.
Restricted to women reporting ever having planned a pregnancy.
p < 0.05.
p < 0.001.
SD, standard deviation; —, not applicable for estimation, in that all women were defined as having tried for pregnancy with a known time to pregnancy in the study sample.
In women in both the overall and study samples, women with endometriosis had relatively similar reproductive histories of ever being sexually active, having tried for pregnancy, having taken emergency contraception, or having avoided sex due to pelvic pain in comparison with unaffected women (Table 1). However, several important differences emerged by endometriosis status, including that women with endometriosis were significantly younger, leaner as adults, and at ages 15–19 years, less likely to be smoking, or to ever having been pregnant or to have had a live birth in comparison with women without endometriosis. Conversely, women with endometriosis were more likely to report having tried >6 months to become pregnant, ever experiencing infertility, and to have more pelvic pain and dyspareunia than women without endometriosis. In the restricted sample comprising only women with a prior pregnancy-trying attempt (n = 214), many of these differences disappeared with three exceptions. Women with endometriosis were significantly more likely to report ever having tried >6 months for pregnancy (49% and 30%, respectively), using infertility treatment (35% and 17%), and having experienced dyspareunia (65% and 50%) in comparison with unaffected women, respectively.
Figure 1 illustrates the proportion of women not achieving pregnancy over a year of trying by endometriosis diagnosis (Fig. 1a) and severity of disease (Fig. 1b). A higher proportion of women without endometriosis became pregnant in the first 5 months of trying than affected women, and similarly so when further considering disease severity, although without a clear pattern. However, the findings were not significant when using the log-rank test.
FIG. 1.
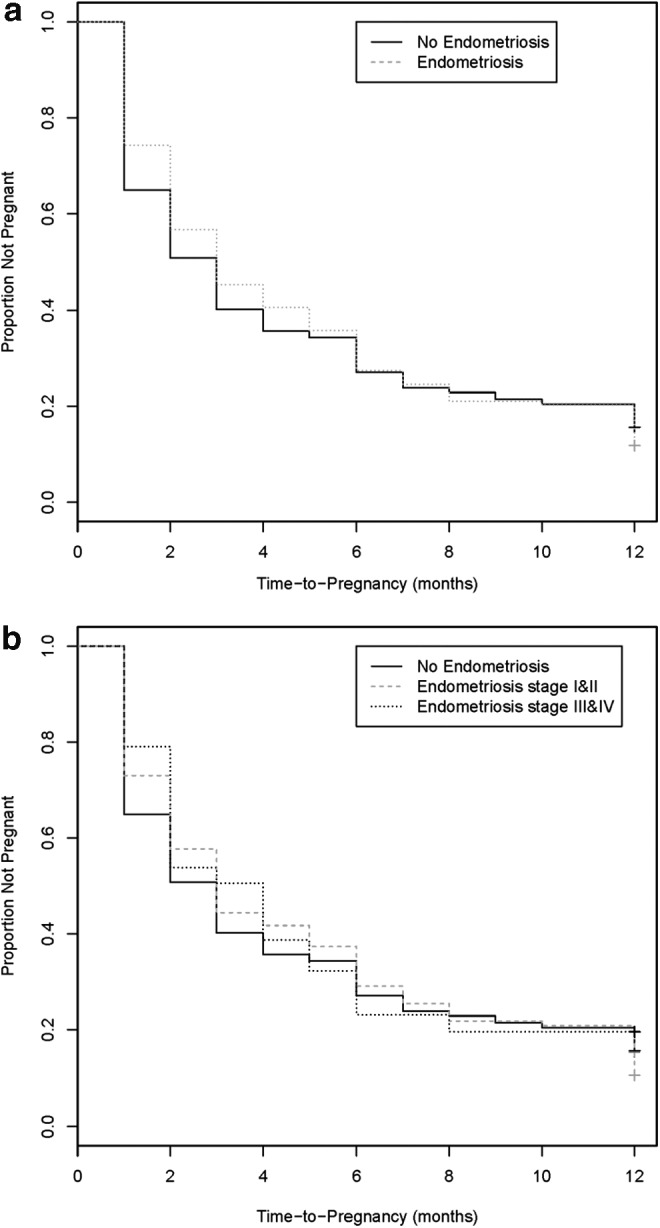
Survival curves for TTP by endometriosis status (a) and severity (b). Survival curves for TTP distributions for women with (dark dash line) and without (solid line) endometriosis and by r-ASRM disease severity categorized as stages 1 and 2 (dark dash line) and stages 3 and 4 (light dash line). Differences in TTP by endometriosis status or severity did not achieve significance. TTP, time to pregnancy; r-ASRM, Revised American Society for Reproductive Medicine.
Surgically visualized endometriosis was associated with a 26% reduction in female fecundity (FOR 0.74; 95% CI 0.48–1.14) reflecting a longer TTP in unadjusted models, with little change after adjustment (FOR 0.71; 95% CI 0.46–1.10) (Table 2). FORs were further reduced with advancing disease severity, but test for trends was not significant. Moreover, a generalized pattern of FORs <1.0 with 95% CIs, including 1.0, remained in sensitivity analyses where gynecologic age replaced chronologic age and body shape at 15–19 years replaced current BMI (Table 3).
Table 2.
Fecundability Odds Ratios for Endometriosis and by Severity of Disease—Primary Analysis
| Endometriosis | Unadjusted FOR (95% CI) | Adjusted FOR (95% CI)a | Adjusted FOR (95% CI)b |
|---|---|---|---|
| Surgically visualized disease (yes/no) | 0.74 (0.48–1.14) | 0.71 (0.46–1.10) | 0.70 (0.45–1.09) |
| Minimal disease (stage 1) | 0.78 (0.47–1.30) | 0.74 (0.44–1.23) | 0.74 (0.44–1.24) |
| Mild disease (stage 2) | 0.79 (0.38–1.62) | 0.78 (0.39–1.57) | 0.74 (0.36–1.50) |
| Moderate disease (stage 3) | 0.59 (0.28–1.22) | 0.58 (0.27–1.26) | 0.56 (0.26–1.23) |
| Severe disease (stage 4) | 0.54 (0.16–1.85) | 0.57 (0.16–2.05) | 0.55 (0.15–1.99) |
| Trend test | p = 0.10 | p = 0.10 | p = 0.08 |
| Minimal and mild disease (stages 1–2) | 0.78 (0.49–1.25) | 0.75 (0.47–1.20) | 0.74 (0.46–1.19) |
| Moderate and severe disease (stages 3–4) | 0.57 (0.28–1.14) | 0.58 (0.29–1.17) | 0.56 (0.27–1.14) |
| Trend test | p = 0.10 | p = 0.09 | p = 0.07 |
Women without surgically visualized endometriosis are the referent group for all disease and severity comparisons.
Adjusted for chronologic age (continuous, in years) and BMI (categorical: <25.0, 25.0–29.9, >30.0), smoking (serum cotinine >10 ng/mL, yes/no), and research site.
Adjusted for chronologic age (continuous in years) and BMI (categorical: <25.0, 25.0–29.9, >30.0).
BMI, body–mass index; CI, confidence interval; FOR, fecundability odds ratio.
Table 3.
Fecundability Odds Ratios for Endometriosis and by Severity of Disease—Sensitivity Analysis
| Endometriosis | Unadjusted FOR (95% CI) | Adjusted FOR (95% CI)a | Adjusted FOR (95% CI)b |
|---|---|---|---|
| Surgically visualized disease (yes/no) | 0.74 (0.48–1.14) | 0.76 (0.50–1.17) | 0.75 (0.48–1.17) |
| Minimal disease (stage 1) | 0.78 (0.47–1.30) | 0.78 (0.47–1.29) | 0.78 (0.46–1.32) |
| Mild disease (stage 2) | 0.79 (0.38–1.62) | 0.87 (0.44–1.73) | 0.82 (0.41–1.66) |
| Moderate disease (stage 3) | 0.59 (0.28–1.22) | 0.64 (0.31–1.32) | 0.62 (0.30–1.30) |
| Severe disease (stage 4) | 0.54 (0.16–1.85) | 0.58 (0.16–2.08) | 0.56 (0.15–2.02) |
| Trend test | p = 0.10 | p = 0.18 | p = 0.14 |
| Minimal and mild disease (stages 1–2) | 0.78 (0.49–1.25) | 0.80 (0.51–1.27) | 0.79 (0.49–1.27) |
| Moderate and severe disease (stages 3–4) | 0.57 (0.28–1.14) | 0.62 (0.31–1.24) | 0.60 (0.30–1.21) |
| Trend test | p = 0.10 | p = 0.15 | p = 0.13 |
Sensitivity analyses replace chronologic age with gynecologic age and measured BMI with body shape during adolescence.
Adjusted for gynecologic age (categorical: <10, 10–15, >16 years), body shape at ages 15–19 years (categorical: 1, 2, 3, 4, >5), smoking (serum cotinine >10 ng/mL, yes/no), and research site.
Adjusted for gynecologic age (categorical: <10, 10–15, >16 years) and body shape at ages 15–19 years (categorical: 1, 2, 3, 4, >5).
Discussion
To our knowledge, this is the first study to assess reproductive history (intentions and duration of pregnancy attempts) before the diagnosis of incident endometriosis in women recruited from various clinical settings. Among women with prior pregnancy attempts before diagnosis, we found that most women reported being sexually active and having similar pregnancy intentions, irrespective of endometriosis status. Consistent with a higher percentage of women with incident endometriosis reporting having tried for >6 months to become pregnant compared with women without endometriosis, fecundity also was reduced as measured by longer TTPs for women with endometriosis. The consistency of FORs <1 across all models is suggestive of an association between diminished fecundity and an eventual endometriosis diagnosis. The lack of significance for FORs may suggest either no association or limited statistical power, possibly a function of fewer prior pregnancy attempts reported by women with endometriosis or that disease severity was skewed toward minimal/mild versus moderate/severe disease. In terms of TTP, a lower proportion of women with endometriosis achieved pregnancy during the first 5 months of trying for pregnancy in comparison with unaffected women. Our study findings suggest that diminished fecundity may be more sensitive to endometriosis than age or body composition, two known risk factors for endometriosis, as adjustment for these two risk factors did not change estimates relative to the modeling of endometriosis alone. Moreover, the findings for diminished fecundity remained across all sensitivity analyses.
We were unable to find previous studies that assessed women's reproductive histories and pregnancy intentions before diagnosis or treatment, precluding a more complete interpretation of our findings. There is rather equivocal literature focusing on pregnancy outcomes following endometriosis diagnosis and by type of treatment. For example, some studies report poorer pregnancy outcomes for women with endometriosis in comparison with unaffected women,40 while others report comparable per cycle pregnancy rates following diagnosis for women with and without minimal endometriosis undergoing donor insemination ∼2 months following laparoscopy.41A recent meta-analysis concluded that women with endometriosis have similar chances of achieving a clinical pregnancy and live birth in comparison with women with other causes of infertility.40 Two recent systematic reviews concluded that endometriosis may be associated with adverse pregnancy outcomes such as miscarriage, placenta previa, preterm, and small-for-gestational age births.42,43 A recent population-based linkage study concluded that pregnancy outcomes such as miscarriage differed between women with and without endometriosis, as did pregnancy complications.44 None of this earlier work considered women's reproductive history before diagnosis, a consideration we believe that may also aid the design and analysis of future research in light of the observed clustering of fecundity within women.5,6 Our results support the need for a more in-depth reproductive history during clinical examinations, inclusive of querying women about pregnancy intentions and TTP. Such information is likely to become increasingly available to women and their healthcare providers given the number of web-based apps and other methods for tracking menstruation and TTP. The extent to which infertility remains a marker for undiagnosed endometriosis awaits corroboration from other cohorts.
Our findings are strengthened by the inclusive nature of our sample irrespective of surgical indication(s) from diverse clinical sites, ascertainment of reproductive history before diagnosis and before women were aware of their diagnoses, reliance on the clinical gold standard for endometriosis diagnosis and staging, reliable clinical diagnosis and staging of endometriosis for our study sample,45 high enrollment and completion rates for the overall study,17 and measured rather than self-reported BMI. Of particular note is our inclusive design relative to surgical indication, in that we made no assumptions about the association between fecundity and endometriosis and, thereby, did not restrict eligibility to gravid or parous women. Because women were unaware of their eventual surgical diagnosis at the time of interview, systematic reporting differences by endometriosis status are believed to be minimal. Still, we recognize the potential for diagnostic or staging errors as with any observational study. We did not have information on any previously diagnostic subtypes of infertility. By restricting our analysis to focus on women reporting a TTP for a previous pregnancy, we were able to assess pregnancy intentions and time required for achieving pregnancy, which are important considerations when assessing reproductive history, more globally.
Still, there are important limitations necessitating the need for cautious interpretation of our findings, including reliance on retrospectively reported TTP, which is subject to reporting errors.46 We relied on surgically visualized disease, but did not have histologic confirmation for all women as we did not require participating surgeons to change practice—at the time of this study, the diagnostic standard in the United States was surgical visualization without histological confirmation.34 In the absence of a biomarker for endometriosis18 and imaging modalities incapable of reliable diagnosis as per the conclusions of a recent Cochrane Report,47 we believe surgical visualization is suitable for study purposes and was used in a consistent manner, irrespective of endometriosis diagnosis. In previous work, we empirically assessed the validity of stage 3 and 4 endometriosis given that a random sample of women also underwent pelvic magnetic resonance imaging (MRI) for the detection of endometriosis.48 All women with MRI-diagnosed endometriosis also had surgically visualized disease, despite the blinding of clinicians. We do recognize that MRI does not capture less severe disease.49 Still, cautious interpretation of r-ASRM endometriosis staging is needed in light of its poor predictability of future fertility.35,36Another important limitation is that disease was skewed toward milder disease, most likely a function of our inclusive sample comprising incident disease. Given this study's observational design, we also cannot eliminate possible residual confounding that might further impact results, nor can we readily generalize findings to other populations.
Our inability to observe significant FORs may suggest the need for even larger cohorts to detect subtle differences in fecundity, as measured by TTP. Cohort study designs with prospective measurement of TTP would eliminate reporting errors and potential biases. The extent to which reproductive history varies by endometriosis staging in the population remains to be established given that we may have been underpowered to assess disease severity. In addition, our study did not follow women past diagnosis, so we are unable to interpret our findings more globally to women's fecundity status following diagnosis and treatment. The extent to which our findings are generalizable to other study populations remains to be established as other investigators consider this question.
In moving the field forward for continued discovery, novel study designs such as matched exposure cohort designs44 and use of causal analysis techniques are needed to delineate and quantify the relationship between female fecundity, its impairments such as conception delay, pregnancy loss, or infertility, and endometriosis. Given that female reproductive age spans from ∼15 to 44 years of age, it is important for researchers to consider a longitudinal approach for better understanding fecundity and fertility in the context of gynecologic disorders such as endometriosis. We concur with an earlier call for a life course approach for studying endometriosis50 to better delineate the implications of endometriosis on fecundity and fertility and health status more generally, as increasing evidence suggests it has implications for health across the life span,51 as conceptualized in the ovarian dysgenesis syndrome.52
Conclusions
While our findings suggest that women diagnosed with endometriosis may have diminished fecundity as measured by longer TTP in comparison with unaffected women, our findings did not achieve significance and await corroboration. Considering reproductive history before diagnosis may improve the prediction of pregnancy probabilities and outcomes following diagnosis and treatment.
Acknowledgments
This research was supported by the Intramural Research Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development (contracts NO1-DK-6-3428; NO1-DK-6-3427; 10001406-02). Dr. U.B. was supported as a predoctoral fellow by the National Institute for Nursing Research's Graduate Partnership Program during the course of this research and is now supported, in part, by the National Institutes of Health, National Library of Medicine (NLM) Biomedical and Health Informatics Training Program at the University of Washington (Grant Nr. T15LM007442).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Carr-Hill RA, Hall MH. The repetition of spontaneous preterm labor. Br J Obstet Gynecol 1985;92:921–928 [DOI] [PubMed] [Google Scholar]
- 2.Khoury M, Calle F, Joesoef R. Recurrence of low birthweight in siblings. J Clin Epidemiol 1989;42:1171–1178 [DOI] [PubMed] [Google Scholar]
- 3.Levin AA, Shoenbaum SC, Stubblefield PG, Zimicki S, Monson RR, Ryan KJ. Ectopic pregnancy and prior induced abortion. Am J Public Health 1982;72:253–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lie RT, Wilcox AJ, Skjerven R. A population based study of the risk of recurrence of birth defects. N Engl J Med 1994;331:1–4 [DOI] [PubMed] [Google Scholar]
- 5.Sapra KJ, McLain AC, Maisog JM, Sundaram R, Buck Louis GM. Clustering of retrospectively reported and prospectively observed time-to-pregnancy. Ann Epidemiol 2015;pii:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLain AC, Sundaram R, Cooney MA, Gollenberg AL, Buck Louis GM. Clustering of fecundability within women. Paediatr Perinatal Epidemiol 2011;25: 460–465 [DOI] [PubMed] [Google Scholar]
- 7.Aris A. A 12 year cohort study on adverse pregnancy outcomes in Eastern townships of Canada: Impact of endometriosis. Gynecol Endocrinol 2014;30:34–37 [DOI] [PubMed] [Google Scholar]
- 8.Hadfield RM, Lain SJ, Raynes-Greenow CH, Morris JM, Roberts CL. Is there an association between endometriosis and risk of preeclampsia? A population-based study. Hum Reprod 2009;24:2348–2352 [DOI] [PubMed] [Google Scholar]
- 9.Buis CC, van Leeuwen FE, Mooij TM, Burger CW, Omega Project Group. Increased risk for ovarian cancer and borderline ovarian tumours in subfertile women with endometriosis. Hum Reprod 2013;28:3358–3369 [DOI] [PubMed] [Google Scholar]
- 10.Pearce CL, Templeman C, Rossing MA, et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: A pooled analysis of case–control studies. Lancet Oncol 2012;13:385–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guidice LC. Clinical practice: Endometriosis. New Engl J Med 2010;362:2389–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balasch J, Creus M, Fabregues F, et al. Visible and non-visible endometriosis at laparoscopy in fertile and infertile women and in patients with chronic pelvic pain: A prospective study. Hum Reprod 1996;11:387–391 [DOI] [PubMed] [Google Scholar]
- 13.Sangi-Haghpeykar H, Poindexter AN., 3rd Epidemiology of endometriosis among parous women. Obstet Gynecol 1995;85:983–992 [DOI] [PubMed] [Google Scholar]
- 14.Rawson JM. Prevalence of endometriosis in asymptomatic women. J Reprod Med 1991;36:513–515 [PubMed] [Google Scholar]
- 15.Williams TJ, Pratt JH. Endometriosis in 1,000 consecutive celiotomies: Incidence and management. Am J Obstet Gynecol 1977;29:245–250 [DOI] [PubMed] [Google Scholar]
- 16.Matorras R, Rodriquez F, Pijoan JI, et al. Women who are not exposed to spermatozoa and infertile women have similar rates of stage I endometriosis. Fertil Steril 2001;76:923–928 [DOI] [PubMed] [Google Scholar]
- 17.Buck Louis GM, Hediger ML, Peterson CM, et al. Estimated incidence of endometriosis by diagnostic method and study population: The ENDO Study. Fertil Steril 2011;96:360–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.May KE, Conduit-Hulbert SA, Villar J, Kirtley S, Kennedy SH, Becker CM. Peripheral biomarkers of endometriosis: A systematic review. Hum Reprod Update 2010;16:651–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buck Louis GM. Fecundity and fertility. In: Buck Louis GM, Platt RW, eds. Reproductive and perinatal epidemiology. New York: Oxford University Press, 2011:16–61 [Google Scholar]
- 20.Opøien HK, Fedorcsak P, ÅByholm T, Tanbo T. Complete surgical removal of minimal and mild endometriosis improves outcome of subsequent IVF/ICSI treatment. Reprod Biomed Online 2011;23:389–395 [DOI] [PubMed] [Google Scholar]
- 21.Daraï E, Marpeau O, Thomassin I, Dubernard G, Barranger E, Bazot M. Fertility after laparoscopic colorectal resection for endometriosis: Preliminary results. Fertil Steril 2005;84:945–950 [DOI] [PubMed] [Google Scholar]
- 22.Nardo LG, Moustafa M, Gareth Beynon DW. Reproductive outcome after laparoscopic treatment of minimal and mild endometriosis using Helica Thermal Coagulator. Eur J Obstet Gynecol 2005;126:264–267 [DOI] [PubMed] [Google Scholar]
- 23.Porpora MG, Pultrone DC, Bellavia M, Franco C, Crobu M, Cosmi EV. Reproductive outcome after laparoscopic treatment of endometriosis. Clin Exp Obstet Gynecol 2002;29:271–273 [PubMed] [Google Scholar]
- 24.Peterson CM, Boiman Johnstone E, Hammoud AO, et al. Risk factors associated with endometriosis: Importance of study population for characterizing disease—The ENDO Study. Am J Obstet Gynecol 2013;451:e1–e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lohman TG, Roche AF, Martorell R, eds. Anthropometric standardization reference manual. Champaign, IL: Human Kinetics Books, 1988 [Google Scholar]
- 26.Vitonis AF, Baer HJ, Hankinson SE, Laufer MR, Missmer SA. A prospective study of body size during childhood and early adulthood and the incidence of endometriosis. Hum Reprod 2010;25:1325–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hediger ML, Hartnett HJ, Louis GM. Association of endometriosis with body size and figure. Fertil Steril 2005;84;1366–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stunkard AJ, Sorenson T, Schulsinger F. In: Kety SS, Rowland LP, Sidman RL, Matthysse SW, eds. Use of the Danish adoption register for the study of obesity and thinness: The genetics of neurological and psychiatric disorders. New York City: Raven Press, 1983:115–120 [PubMed] [Google Scholar]
- 29.Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometris, and lifestyle factors. Am J Epidemiol 2004;160:784–796 [DOI] [PubMed] [Google Scholar]
- 30.Cooney MA, Buck Louis GM, Hediger ML, Vexler A, Kostyniak PJ. Organochlorine pesticides and endometriosis. Reprod Toxicol 2010;30:365–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol 2009;2:236–248 [DOI] [PubMed] [Google Scholar]
- 32.The Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility. Fertil Steril 2006;86:S156–S160 [DOI] [PubMed] [Google Scholar]
- 33.Kennedy S, Bergqvist A, Chapron C, et al. ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. Hum Reprod 2005;20:2698–2704 [DOI] [PubMed] [Google Scholar]
- 34.American Society for Reproductive Medicine. Revised American Society for Reproductive Medicine classification of endometriosis. Fertil Steril 1997;67:817–821 [DOI] [PubMed] [Google Scholar]
- 35.Zeng C, Xu JN, Zhou Y, et al. Reproductive performance after surgery for endometriosis: Predictive value of the revised American Fertility Society classification and the endometriosis fertility index. Gynecol Obstet Invest 2014;77:180–185 [DOI] [PubMed] [Google Scholar]
- 36.Vercellini P, Fedele L, Aimi G, De Giorgi O, Consonni D, Crosignani PG. Reproductive performance, pain recurrence and disease relapse after conservative surgical treatment for endometriosis: The predictive value of the current classification system. Hum Reprod 2006;21:2679–2685 [DOI] [PubMed] [Google Scholar]
- 37.Cox DR. Regression models and life tables. J Royal Stat Soc Series 1972;20:187–220 [Google Scholar]
- 38.Liang KY, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13–22 [Google Scholar]
- 39.Guo S-W, Wang Y. Sources of heterogeneities in estimating the prevalence endometriosis in infertile and previously fertile women. Fertil Steril 2006;86:1584–1595 [DOI] [PubMed] [Google Scholar]
- 40.Brosens I, Brosens JJ, Fusi L, Al-Sabbagh M, Kuroda K, Benagiano G. Risks of adverse pregnancy outcome in endometriosis. Fertil Steril 2012;98:30–35 [DOI] [PubMed] [Google Scholar]
- 41.Matorras R, Corcóstegui B, Esteban J, et al. Fertility in women with minimal endometriosis compared with normal women was assessed by means of a donor insemination program in unstimulated cycles. Am J Obstet Gynecol 2010;203;345e:1–6 [DOI] [PubMed] [Google Scholar]
- 42.Barbosa MAP, Teixeira DM, Navarro PAAS, Ferriani RA, Nastri CO, Martins WP. Impact of endometriosis and its staging on assisted reproduction outcomes: A systematic review and meta-analysis. Ultrasound Obstet Gynecol 2014;44:261–278 [DOI] [PubMed] [Google Scholar]
- 43.Leone Roberti Maggiore U, Ferrero S, Mangili G, et al. A systematic review on endometriosis during pregnancy: Diagnosis, misdiagnosis, complications and outcomes. Hum Reprod Update 2016;22:70–103 [DOI] [PubMed] [Google Scholar]
- 44.Saraswat L, Ayansina DT, Cooper KG, et al. Pregnancy outcomes in women with endometriosis: An national record linkage study. BJOG 2016:1–9. DOI: 10.1111/1471-0528.13920 [DOI] [PubMed] [Google Scholar]
- 45.Schliep KC, Stanford JB, Zhang B, et al. Inter- and intra-reliability in the diagnosis and staging of endometriosis: The ENDO Study. Obstet Gynecol 2012;120:104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cooney MA, Buck Louis GM, Sundaram R, McGuiness BM, Lynch CD. Validity of self-reported time to pregnancy. Epidemiology 2009;26:56–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nisenblat V, Bossuyt PMM, Farquhar C, Johnson N, Hull ML. Imaging modalities for non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev 2016;2:CD009591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schliep KC, Chen Z, Stanford JB, et al. Endometriosis diagnosis and staging by operating surgeon and expert review using multiple diagnostic tools: An inter-rater agreement study. BJOG 2015. DOI: 10.1111/1471-0528.13711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stratton P, Winkel C, Premkumar A, et al. Diagnostic accuracy of laparoscopy, magnetic resonance imaging, and histopathologic examination for the detection of endometriosis. Fertil Steril 2003;79:1078–1085 [DOI] [PubMed] [Google Scholar]
- 50.Brosens I, Puttemans P, Benagiano G. Endometriosis: A life cycle approach? Am J Obstet Gynecol 2013;209:307–314 [DOI] [PubMed] [Google Scholar]
- 51.Kvaskoff M, Mu F, Terry KL, et al. Endometriosis: A high-risk population for major chronic diseases? Hum Reprod Update 2015;21:500–5016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buck Louis GM, Cooney MA, Peterson CM. The ovarian dysgenesis syndrome. J Dev Orig Health Dis 2011;2:25–35 [Google Scholar]


