Abstract
Objectives: Multiple lines of evidence from genetic linkage studies to animal models implicate aberrant cortical plasticity and metaplasticity in the pathophysiology of autism spectrum disorder (ASD) and fragile X syndrome (FXS). However, direct experimental evidence of these alterations in humans with these disorders is scarce. Transcranial magnetic stimulation (TMS) is a noninvasive tool for probing mechanisms of plasticity and metaplasticity in vivo, in humans. The aim of the current study was to examine mechanisms of plasticity and metaplasticity in humans with ASD and FXS. We employed a repetitive TMS protocol developed specifically to probe cortical plasticity, namely continuous theta burst stimulation (cTBS).
Methods: We applied a 40-second train of cTBS to primary motor cortex (M1) to healthy control participants and individuals with ASD or FXS, and we measured the cTBS-induced modulation in motor-evoked potentials (MEPs) in a contralateral intrinsic hand muscle. Each participant completed two sessions of the same protocol on two consecutive days. The degree of modulation in MEPs after cTBS on the first day was evaluated as a putative index of cortical plasticity. Examination of the changes in the effects of cTBS on the second day, as conditioned by the effects on the first day, provided an index of metaplasticity, or the propensity of a given cortical region to undergo plastic change based on its recent history.
Results: After a 40-second cTBS train, individuals with ASD show a significantly longer duration of suppression in MEP amplitude as compared with healthy controls, whereas individuals with FXS show a significantly shorter duration. After a second train of cTBS, 24 hours later, the ASD group was indistinguishable from the control group, and while in the FXS group MEPs were paradoxically facilitated by cTBS.
Conclusion: These findings offer insights into the pathophysiology of ASD and FXS, specifically providing direct experimental evidence that humans with these disorders show distinct alterations in plasticity and metaplasticity, consistent with the findings in animal models. If confirmed in larger test–retest studies, repeated TMS measures of plasticity and metaplasticity may provide a valuable physiologic phenotype for ASD and FXS.
Introduction
Autism spectrum disorder (ASD) is characterized by core social communication deficits as well as by restricted, repetitive, and stereotyped patterns of behaviors and interests (Diagnostic and Statistical Manual of Mental Disorders, 5th edition [DSM-5]; American Psychiatric Association 2013). ASD is diagnosed by clinical criteria, and the exact brain dysfunction that leads to the behavioral phenotype in nonsyndromic ASD remains unknown. However, altered mechanisms of synaptic, use-dependent plasticity and metaplasticity are likely essential components of the ASD pathophysiology (Dolen and Bear 2009; Markram and Markram 2010; Oberman et al. 2010, 2012; Oberman 2015).
Plasticity refers to the brain's ability to adapt to environmental stimuli through strengthening, weakening, pruning, or adding of synaptic connections, and by promoting neurogenesis (Pascual-Leone et al. 2005; Feldman 2009). The propensity of the brain to undergo plastic change relies on the influence of a number of innate molecular mechanisms (Kandel 2001) as well as on the current state of any given synapse. That is, the capacity for use-dependent modification of synaptic strength is, in part, contingent on whether a group of synapses have undergone a plastic change in the recent past (Abraham 2008; Mockett and Hulme 2008). This process is kept in check by feedback mechanisms, including homeostatic synaptic scaling, whereby uniform increases or decreases in network activity over hours or days lead to an opposing increase or decrease in excitatory synaptic strength (Turrigiano and Nelson 2004), and so-called metaplastic influences, whereby experience-dependent alterations in inhibitory tone, dendritic excitability, and receptor functioning alter the ability of future stimuli to induce plastic changes (Abraham and Bear 1996). These changes at the level of a single neuron or synapse lead to the development and maintenance of neural circuitry. We and others have suggested that abnormalities in regulation of these synaptic changes lead to larger-scale alterations in plasticity and metaplasticity that may account for the neurological and behavioral phenotype of ASD and fragile X syndrome (FXS) (Markram and Markram 2010; Oberman et al. 2015).
Notably, and relevant to our work with patients with ASD, some of the proteins involved in this regulation of plasticity are those affected in syndromic forms of ASD (e.g., FMRP, PTEN, and TSC) (Dolen and Bear 2009). Additionally, the more than 100 genes and recurrent genomic imbalances whose mutations confer increased risk of ASD (Betancur 2011) appear to converge on a relatively small set of molecular pathways, notably those critical for synaptic development and plasticity (Murdoch and State 2013). Several recent large-scale exome sequencing and association studies support this claim (Iossifov et al. 2012; Neale et al. 2012; O'Roak et al. 2012).
FXS is the most common genetic cause of autism, with 30% of children with FXS diagnosed with autism and 2%–5% of autistic children having FXS (Kaufmann et al. 2004). Evidence both from animal FXS models (Simonyi et al. 2005) (Huber et al. 2002) and from studies in patients with FXS conducted in our laboratory (Oberman et al. 2010) indicate that loss of Fragile X mental retardation protein FMRP (the physiological consequence of the genetic mutation that leads to FXS) leads to abnormalities in synaptic plasticity. Apart from FXS, other animal ASD models also suggest a causal role for abnormal plasticity mechanisms (reviewed in Tordjman et al. [2007]). However, metaplasticity has not been evaluated in these animal models.
The aim of the current study was to examine mechanisms of plasticity and metaplasticity in humans with ASD and FXS using transcranial magnetic stimulation (TMS), thus providing experimental evidence bridging data from animal models to human patients with ASD or FXS.
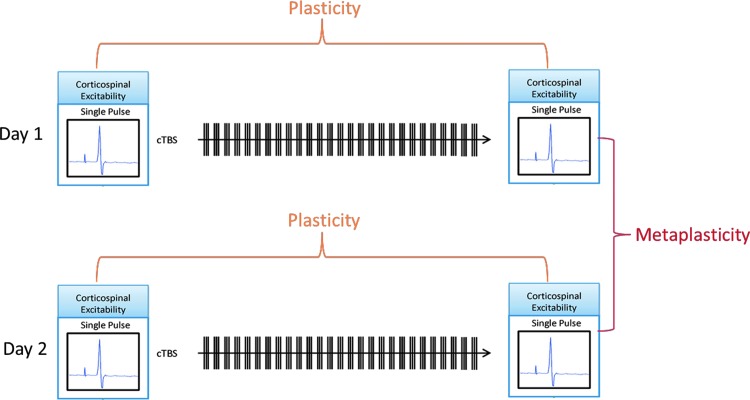
TMS is a method for noninvasive focal brain stimulation based on Faraday's principle of electromagnetic induction, where localized intracranial electrical currents are generated by rapidly changing extracranial magnetic fields (Wagner et al. 2007). When single-pulse TMS is applied in the primary motor cortex (M1) at suprathreshold intensities, it activates corticospinal outputs, producing a twitch in a peripheral muscle (a motor-evoked potential [MEP]), which can be used as an index of corticospinal excitability (Barker et al. 1985). Trains of repeated TMS (rTMS) pulses at various stimulation frequencies and patterns can induce a lasting modulation of excitability in the targeted brain region. The aftereffects of rTMS are believed to relate to activity-dependent changes in the effectiveness of synaptic connections between cortical neurons, reflecting cortical plasticity mechanisms (Fitzgerald et al. 2006; Thickbroom 2007; Ziemann et al. 2008; Hoogendam et al. 2010). If the targeted brain region is M1, comparison of the peak-to-peak amplitude of the TMS-induced MEP recorded after the rTMS train with baseline amplitude can provide an index of cortical plasticity (Fig. 1).
FIG. 1.
Schematic depiction of protocol. Cortical excitability based on electromyography recordings of single-pulse transcranial magnetic stimulation–induced MEPs was measured at baseline and immediately after cTBS. Duration of response (extent of time until MEPs returned to baseline values) was used as a measure of plasticity. This protocol was repeated 24 hours later with the difference in duration between the first and second session being used as a measure of metaplasticity. cTBS, continuous theta burst stimulation; MEPs, motor-evoked potentials.
In the current study, we applied a specific rTMS protocol called continuous theta burst stimulation (cTBS). cTBS was specifically developed to probe plasticity mechanisms (Huang et al. 2005, 2007; Cardenas-Morales et al. 2010) and involves application of three pulses of 50-Hz rTMS repeated every 200 ms for a total of 40 seconds (for total of 600 pulses) at an intensity of 80% of the active motor threshold (AMT) (Huang et al. 2005, 2008). After cTBS is applied to the motor cortex, TMS-induced MEPs typically show decreased amplitude for a period of 20–30 minutes in healthy adult brains.
Evaluation of rTMS-mediated plasticity on two consecutive days enables the characterization of metaplasticity mechanisms by examining the changes in the effects of the rTMS on the second day, as conditioned by the effects on the first day. Studies aimed at exploring metaplastic effects of rTMS sessions on sequential days have found that in neurotypical individuals the degree of modulation seen after the second rTMS session can be greater, but in the same direction as the first (Baumer et al. 2003). We applied this strategy to examine modulation of corticospinal excitability on consecutive days of cTBS as a putative index of metaplasticity in individuals with ASD and FXS.
Methods
Participants
Twenty-eight individuals participated in this study. There were three groups: (1) high functioning individuals with ASD (n = 10); (2) neurotypical age- and gender-matched control participants (n = 12); and (3) individuals with full mutation FXS and no comorbid ASD diagnosis (n = 6). Participants were recruited through the National Fragile X Society, the Fragile X Research Foundation (FRAXA), local community advertisements, and local Asperger's associations and clinics. All participants with FXS had a diagnosis with molecular confirmation of full mutation status. All participants in the ASD group met Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision (DSM-IV-TR) (American Psychiatric Association 2000) criteria for Asperger's syndrome and were independently assessed with the Autism Diagnostic Observation Schedule (mean score = 10.78, SD = 5.24). The participants in the typically developing group had no neurological or psychological disorder (see Table 1 for detailed demographic information). All participants underwent a neurological exam to ensure normal sensorimotor functioning. All were confirmed to have normal tone, bulk, and strength in all major muscle groups of all four extremities; normal fine and gross motor skills; absence of involuntary movements; normal balance and gait; and normal epicritic and proprioceptive sensory function. All participants signed an informed consent/assent form for the study, which had been approved by the Committee on Clinical Investigations (Institutional Review Board) at Beth Israel Deaconess Medical Center.
Table 1.
Demographic Characteristics of Participants
| Group | Participant ID | Age | Gender | IQ | Handedness | Neuroactive medication | Comorbid symptoms |
|---|---|---|---|---|---|---|---|
| ASD (n = 10) | M = 28.7 (SD = 13.6) | 7 M; 3 F | M = 113 (SD = 18.1) | 9 Right; 1 Left | 7 Yes; 3 None | 7 Yes; 3 None | |
| A1 | 19 | M | 82 | R | None | None | |
| A2 | 26 | M | 123 | R | Venlafaxine HCL | Depression | |
| A3 | 27 | F | 128 | R | Mirtazapine | Depression | |
| A4 | 21 | F | 120 | R | Adderall | ADHD | |
| A5 | 62 | M | 123 | L | Buproprion; escitalopram | Depression | |
| A6 | 21 | M | 117 | R | None | None | |
| A7 | 16 | M | 81 | R | Aripiprazole | Irritability | |
| A8 | 30 | M | — | R | None | None | |
| A9 | 41 | F | 121 | R | Ativan; nefazadone | Anxiety; Depression | |
| A10 | 24 | M | 122 | R | Fluvoxamine; clonidine; risperdal | OCD; ADHD; Irritability |
| HC (n = 12) | M = 28.3 (SD = 11.9) | 7 M; 5 F | 111.5 (SD = 12.4) | 12 Right; 0 Left | 0 Yes; 12 None | 0 Yes; 12 None | |
|---|---|---|---|---|---|---|---|
| HC1 | 20 | F | 97 | R | None | None | |
| HC2 | 22 | M | 134 | R | None | None | |
| HC3 | 32 | F | 114 | R | None | None | |
| HC4 | 21 | M | 109 | R | None | None | |
| HC5 | 22 | M | 125 | R | None | None | |
| HC6 | 19 | M | 115 | R | None | None | |
| HC7 | 21 | F | — | R | None | None | |
| HC8 | 41 | F | 95 | R | None | None | |
| HC9 | 61 | M | 125 | R | None | None | |
| HC10 | 30 | M | 101 | R | None | None | |
| HC11 | 22 | M | 106 | R | None | None | |
| HC12 | 29 | F | 106 | R | None | None |
| FXS (n = 6) | M = 23.5 (SD = 5.9) | 2 M; 4 F | 91.0 (SD = 23.9) | 6 Right; 0 Left | 3 Yes; 3 None | 3 Yes; 3 None | |
|---|---|---|---|---|---|---|---|
| FX1 | 16 | F | 101 | R | None | None | |
| FX2 | 20 | F | 126 | R | None | None | |
| FX3 | 21 | M | 76 | R | Fluoxetine | Depression | |
| FX4 | 33 | F | 107 | R | Citalopram | Depression | |
| FX5 | 26 | F | 67 | R | Methylphenidate | ADHD | |
| FX6 | 25 | M | 69 | R | None | None |
Group averages are presented as means and standard deviations (in parentheses). One participant in the ASD group and one in the HC group did not complete an IQ assessment.
ASD, autism spectrum disorder; HC, Healthy Control; FXS, fragile X syndrome; ADHD, attention-deficit/hyperactivity disorder; OCD, Obsessive Compulsive Disorder.
Stimulation and recording
cTBS was applied according to the standard protocol that was consistent with our previous studies in ASD and FXS (i.e., three pulses of 50-Hz rTMS repeated every 200 ms for a total of 40 seconds [for a total of 600 pulses]) at an intensity of 80% of AMT (Huang et al. 2005, 2008; Oberman et al. 2010, 2012, 2015). Corticospinal excitability was assessed both before and after cTBS by measuring peak-to-peak amplitude of MEPs induced in the contralateral first dorsal interosseus (FDI) muscle in response to supra threshold (120% of resting motor threshold [RMT] intensity) single-pulse TMS. Ag-AgCl electromyography (EMG) surface electrodes were used to measure MEPs and placed in a belly-tendon montage over the FDI muscle of participants’ right hands. Raw signals were amplified and band pass-filtered between 20 and 2000 Hz. EMG signals were sampled at 5000 Hz.
RMT and AMT were defined following recommendation from the International Federation of Clinical Neurophysiology (Rossini et al. 2015). RMT was defined as the minimum single-pulse TMS intensity required to induce an MEP in the contralateral FDI of >50 μV peak-to-peak amplitude on more than 5 out of 10 consecutive trials while the target muscle was at rest. AMT was defined as the minimum single-pulse TMS intensity required to induce an MEP in the contralateral FDI of >200 μV peak to-peak amplitude on more than 5 out of 10 consecutive trials while the target muscle was held at ∼20% of the maximal contraction.
Single TMS pulses were applied at ∼0.1 Hz (with a random jitter of ±1 second) while avoiding any train effects. Three batches of 10 MEPs were recorded before cTBS and used as a baseline. After cTBS, batches of 10 MEPs were measured at periodic intervals for a total of 120 minutes to track changes in MEP amplitude over time.
All stimulation was delivered using a hand-held figure-of-eight coil attached to a Magstim Super Rapid (Magstim Co. Ltd, Whitland, UK) stimulator for single pulses and a MagproX100 (Magventure, Inc., Ferum, Denmark) stimulator for the cTBS protocol. Both coils were placed tangentially to the scalp with the handle pointing posteriorly and rotated ∼45° from the mid-sagittal plane. All stimulation was applied over the hand area of the left motor cortex. The stimulation site was the individually defined optimal position for eliciting MEPs of maximal amplitude in the right FDI. To precisely target the stimulation site (primary motor cortex) and keep the brain target constant throughout each and across both stimulation sessions, we used a frameless stereotactic neuronavigation system (Brainsight; Rogue Research, Inc., Montreal Quebec, Canada).
Each participant underwent the same procedures twice with exactly 24 hours between the two sessions. Before both cTBS sessions, participants were told to get a good night sleep and avoid caffeine (the day of the session) and alcohol the night before each session. There were no specific instructions for activity level for the period between the cTBS sessions. All groups received the same instructions.
Statistical analyses
Data analysis followed the methods described and applied previously in (Oberman et al. 2010, 2012). Briefly, data were analyzed using SPSS version 19 by an experimenter who was blinded to the identities of the participants. MEP amplitude at a given time point was defined as the mean amplitude of the 10 MEPs to single TMS pulses recorded in a given 2-minute time window. Post-cTBS, MEP amplitudes were then normalized to the participant's baseline MEP amplitude, forming a ratio of MEP amplitudes after TBS relative to average baseline MEP amplitude for each individual.
The Shapiro–Wilk test demonstrated a non-Gaussian distribution; thus, a natural log transformation was applied to the data, and the log-transformed data were used for analysis. Consistent with previous studies in our laboratory suggesting that enhanced duration of modulation after cTBS may be a reliable endophenotype in ASD (Oberman et al. 2012), our primary outcome measure was empirically defined as the number of minutes after cTBS until the amplitude of the MEPs returned to baseline excitability levels (“time to baseline”). Two between-subjects one-way ANOVAs with post hoc t-tests were conducted. The first, aimed at evaluating plasticity between the three groups, compared the “time to baseline” in the first session in the ASD, matched control, and FXS groups. The second analysis evaluated the degree of metaplasticity as defined as the difference in the “time to baseline” between session one and session two for the three groups.
Results
Plasticity
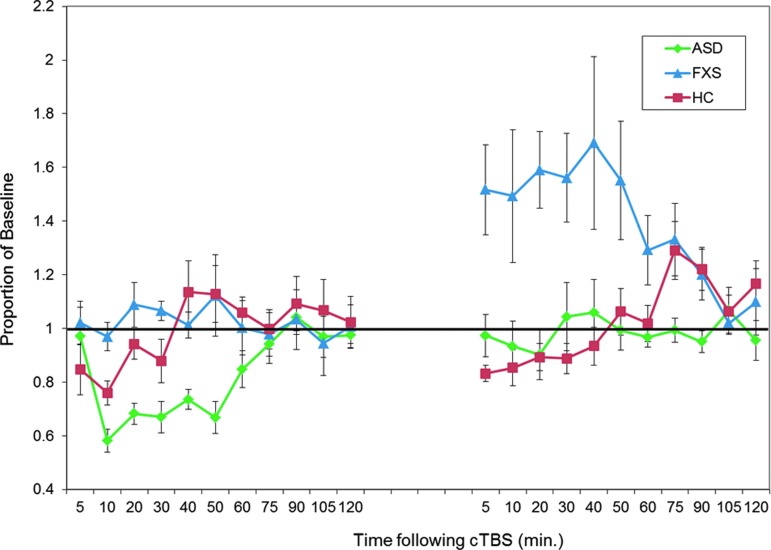
The effect of diagnosis on the “time to baseline” in the first session was significant, F(2, 25) = 32.73, p < 0.001 (ASD: M = 80, SD = 22.73; FXS: M = 16.67, SD = 5.16; HC: M = 29.17, SD = 15.64). Post hoc t-tests confirmed that the times to baseline for both clinical groups were significantly different from the healthy control group and from each other, with the FXS group showing a significantly shorter duration of MEP modulation after cTBS (on average no discernible response) and the ASD group showing a significantly greater duration of MEP modulation after cTBS as compared with the control group (all p's < 0.01) (Fig. 2).
FIG. 2.
Baseline-corrected MEP amplitude after cTBS. Average baseline-corrected MEP amplitude for the ASD group (in green), the FXS group (in blue), and the healthy control group (in red) at 11 time points from 5 to 120 minutes post-cTBS. Error bars indicate SEM for each time point. Values are represented as a proportion of baseline amplitude with a line at 1.0 (representing baseline amplitude). ASD, autism spectrum disorder; cTBS, continuous theta burst stimulation; FXS, fragile X syndrome.
Metaplasticity
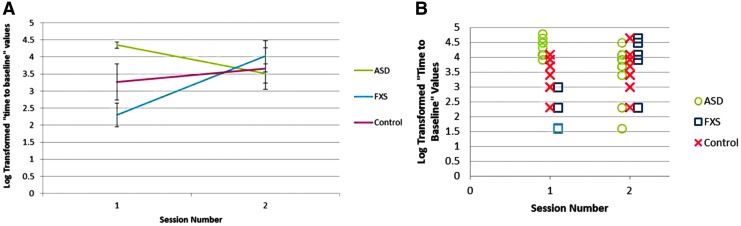
The effect of diagnosis on the difference in the “time to baseline” between the two sessions was also significantly different between the groups, F(2, 25) = 17.22, p < 0.001. Post hoc t-tests confirmed that for this measure also both the ASD and FXS groups were significantly different from the healthy control group and from each other (all p's < 0.01). Specifically, the control group had a nonsignificant (t(11) = 1.53, p = 0.16) increase in their “time to baseline” values between the two sessions (mean difference = 0.39 [SD = 0.88]), whereas the ASD group showed a significant reduction in their “time to baseline” (t(9) = 3.11, p < 0.05) and the FXS group showed a significant increase in their “time to baseline” (t(5) = 5.30, p < 0.01) (Figs. 2 and 3).
FIG. 3.
Group average (A) and individual subjects’ (B) duration of modulation of corticospinal excitability after cTBS on days 1 and 2. The ASD group had a reduction in duration of modulation on day 2 as compared with that on day 1. The FXS group had a greater duration of modulation on day 2 as compared with that on day 1. Healthy control participants had no significant difference in the duration of modulation on day 2 as compared with that on day 1. ASD, autism spectrum disorder; cTBS, continuous theta burst stimulation; FXS, fragile X syndrome.
Of note, the FXS group response to the second cTBS session, in addition to being longer in duration, was also in a paradoxical, facilitatory direction. In other words, in this group, the second cTBS session led to an increase in corticospinal excitability rather than the expected suppression as was induced after the first cTBS session and as was seen in both the control and ASD groups across both sessions (Fig. 2).
Though participants ranged in age and both genders were included in the study, there was no significant correlation between age and time to baseline on either the first or second visit, nor was there any difference in time to baseline by gender either between or across diagnostic groups.
Discussion
Our results show significant and distinct alterations of plasticity and metaplasticity in ASD and FXS. Specifically, and consistent with our previous studies (Oberman et al. 2010, 2012), compared with the control group on day 1, the ASD group showed a significantly greater duration of response to cTBS, whereas the FXS group showed a significantly shorter duration (on average no discernable response). Our results confirm and extend those findings. In addition, in the current study, the FXS group was completely separable from the ASD group on day 1, as the longest duration to return to baseline in the FXS group was 20 minutes and the shortest duration in the ASD group was 50 minutes.
The second cTBS session also produced notable alterations in response in both clinical groups, with the ASD group showing a reduced duration of suppression compared with their response on day 1 and the FXS group displaying a paradoxical facilitation of corticospinal excitability. Thus, the modulation of corticospinal excitability induced by cTBS was different across the two consecutive sessions in the ASD and FXS groups, whereas the control group showed a similar pattern on both days. These findings are novel and represent a major new insight of the present study.
Previous studies have shown a metaplastic effect with noninvasive brain stimulation protocols applied over M1 in healthy controls when the two protocols are applied within minutes of each other. Specifically, when cTBS is preceded by a facilitatory, intermittent (i)TBS protocol, there is a longer duration of suppression in corticospinal excitability measured by TMS-induced MEPs than with cTBS alone (Todd et al. 2009; Doeltgen and Ridding 2011). On the other hand, when another form of suppressive noninvasive brain stimulation, cathodal transcranial direct current stimulation, is applied before a suppressive, 1 Hz, rTMS train, there is a paradoxical facilitation in MEPs (Siebner et al. 2004). When the delay between sessions in healthy controls is between 12 and 24 hours, with a period of sleep between the sessions, there appears to be an additive metaplastic effect whereby the modulation in corticospinal excitability as measured by TMS-induced MEPs of the second session lasts longer, but is in the expected direction, as compared with the first. This additive effect appears to gradually disappear over the course of a week (Maeda et al. 2000; Baumer et al. 2003; Cohen et al. 2010).
The metaplastic effect of multiple sessions of rTMS has primarily been studied in M1, and it has only been studied with two consecutive sessions. In contrast, therapeutic rTMS protocols, often applied outside of M1, are reliant on the cumulative effects of daily stimulation over several weeks This long-lasting behavioral effect is also believed to be a result of plastic and metaplastic effects (Hoogendam et al. 2010). If rTMS is applied to ASD and FXS with therapeutic intent, then such protocols ought to consider the specific metaplasticity of these syndromes. Alternatively, the metaplasticity profile for each subject should be measured to develop personalized rTMS protocols.
The basic cellular and molecular mechanisms underlying rTMS-induced plasticity (and metaplasticity) remain poorly understood. rTMS affects a relatively large area of the cortex (∼0.5–1 cm3) that contains hundreds of millions of neurons and other glial and endothelial cells. Thus, the modulation in corticospinal excitability by rTMS is likely a result of both classic synaptic LTP-like and LTD-like mechanisms as well as nonsynaptic plasticity mechanisms, including biochemical and genetic changes (see Tang et al. 2015 for a review). Future studies involving advanced techniques such as optogenetics or combining TMS with real-time in vivo calcium imaging or patch clamp electrophysiology in animal models may elucidate the cellular- and circuit-level neurobiological mechanisms underlying both these and other human rTMS studies.
Though TMS indexes plasticity at the level of cortical circuits, our findings are consistent with theories proposed to explain induction of these processes at the synaptic level. The findings from both the ASD and FXS groups are consistent with a shifting of threshold whereby stimulation leads to either facilitation or suppression of excitability depending on the current state of the synapse, which is consistent with the Bienenstock Cooper Munroe model of metaplasticity (Bienenstock et al. 1982). In the current study, we only evaluated one point in this timeline (24 hours); however, further studies are planned to explore the response to two sessions of cTBS separated by variable delays in these three clinical populations to further elucidate this state-dependent effect.
In line with the previous literature investigating plasticity mechanisms with rTMS, we focused on the primary motor cortex in the left hemisphere. Whether and to what extent these findings will translate to nonmotor cortical regions is uncertain. The left primary motor cortex was chosen in this study for two reasons. First, MEPs are the standard index used to quantify the effect of TBS protocols. Other indices of cortical excitability outside the motor cortex (e.g., based on electroencephalographic measures) have not yet been well validated for this application. We chose the left hemisphere, as it is typically the dominant hemisphere for both right- and left-handed individuals. Second, although motor abnormalities are not considered core symptoms of ASD, many studies have reported motor deficits in individuals with ASD. Additionally, though motor and social-cognitive abilities in ASD have historically been studied separately, there is a great deal of evidence to suggest that abilities in the motor domain may precede and set the stage for higher-level social and communicative skills, including understanding of intention, social communicative behaviors (such as gesturing), and imitation (Mostofsky and Ewen 2011; Casartelli et al. 2016). Thus, developmental abnormalities in this region of the cortex may both underlie the behavioral deficits mentioned earlier and play a role in the core deficits of ASD. Though participants in this study did not show any fine or gross motor abnormalities on the neurological exam, a more detailed motor evaluation assessing more subtle motor skills was not performed. Given the results of the current study, suggesting aberrant plasticity and metaplasticity mechanisms, participants may have had more subtle impairments or differences related to motor learning. Consistent with this, a recent study conducted in our laboratory in a different sample of individuals with ASD found differences between individuals with ASD and healthy controls on a motor learning task (Sharer et al. 2016).
The present study should be considered preliminary in nature, providing directions for future investigations into the neurophysiology of ASD and FXS. There were a number of limitations in this study that deserve mention. Despite having a large age range and both genders included in this study, the sample sizes were small, leading to reduced power for any subgroup or correlation analyses. Given the behavioral and physiological heterogeneity in the ASD population, future large-scale studies are warranted to investigate whether these measures of plasticity and metaplasticity may serve as potential endophenotypes that may serve as biomarkers for subject stratification or outcome measures in future clinical trials.
For the protection of participants, individuals with a history of seizure or other neurological conditions were excluded from participation. This specifically impacted the FXS sample, leading to the smaller number of participants in this group. With such small samples, the groups were not matched on IQ; however, there was no significant difference in IQ between the groups. Additionally, due to the exclusion of individuals with seizures or other neurological conditions, the generalizability of the findings to these individuals is unclear. Participants on medications that may lower seizure threshold were excluded; however, participants were on a number of neurotropic medications for comorbid symptoms that may have impacted the results of the study. A full neuropsychological evaluation and detailed sensorimotor exam was not conducted, and, therefore, the relationship between the degree of plasticity/metaplasticity and severity or quality of behavioral or cognitive or specific sensorimotor symptoms cannot be determined. However, despite these limitations, this study provides preliminary support for distinct alterations in plasticity and metaplasticity in ASD and FXS, offering insights into the pathophysiology of these disorders.
Conclusions
Our findings provide novel experimental evidence of abnormal plastic and metaplastic regulation in ASD and FXS. Future studies are needed to replicate the findings in a larger, well-characterized sample. In addition, real-time integration of TMS with elecroencephalography (EEG) will allow investigators to apply these measures to cortical brain regions other than the motor cortex (Thut et al. 2005; Thut and Pascual-Leone 2010a, 2010b). Future studies are also needed to clarify the role of plasticity and metaplasticity mechanisms in the neurological and behavioral ASD and FXS phenotypes. As more evidence is garnered about aberrant responses to the modulatory effects of TBS in different neurodevelopmental disorders, extensions of the current experiment should facilitate the assessment of the full diagnostic and clinical utility of such tests.
Clinical Significance
If responses to the TBS protocol prove to be unique across different neurodevelopmental disorders, future studies might represent a measurable endophenotype that may be used to establish valuable neurophysiological biomarkers in clinical populations. Furthermore, if it is determined that there is a relationship between TBS response and behavioral symptoms, this protocol would facilitate the development of therapeutic interventions that are aimed at regulating plasticity and metaplasticity mechanisms.
Acknowledgments
L.M.O. is further supported by grants from the National Institutes of Health (1 R01 MH100186-01A1), Simons Foundation, and the Nancy Lurie Marks Family Foundation. A.P.L. is further supported by grants from the National Institutes of Health (R01HD069776, R01NS073601, R21 MH099196, R21 NS082870, R21 NS085491, R21 HD07616, and UL1 RR025758), Michael J. Fox Foundation, and Sidney R. Baer Foundation. J.G.H. is further supported by the Tommy Fuss Fund and the Al Rashed Family Fund. A.R. is further supported by the Center for Integration of Medicine and Innovative Technology (CIMIT), Department of Defense PR121509, Autism Speaks Grant #8702, and grants from Eisai, Inc. and the Epilepsy Research Foundation and epilepsy therapy project. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or any of the listed granting agencies.
Disclosures
A.P.L. serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Neuroelectrics, and Neosync; and is listed as an inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation (TMS) with electroencephalography (EEG) and magnetic resonance imaging (MRI). J.G.H. has received grant support from Glaxo-SmithKline, and Johnson & Johnson and has served as a consultant to Abbott Laboratories, Pfizer, Inc., Johnson & Johnson (Janssen, McNeil Consumer Health), Novartis, Parke-Davis, Glaxo-SmithKline, AstraZeneca, and Seaside Therapeutics; has been a speaker for Abbott Laboratories, Pfizer, Inc., Novartis, Bristol-Meyers Squibb; and has received grant support from Abbott Laboratories, Pfizer, Inc., Johnson & Johnson (Janssen, McNeil Consumer Health), Akzo-Nobel/Organon, and the NIMH. A.R. has served as an advisor for Nexstim, Inc., Sage Therapeutics, Inc., and NeuroRex, Inc., and has joint grants with Brainsway, Inc., and Vivonics, Inc. He is a founder and consultant to Neuro'motion Labs and is an inventor on a patent pending for technologies to enhance the development of emotional regulation. For all other authors, no conflicts of interest exist.
References
- Abraham WC: Metaplasticity: Tuning synapses and networks for plasticity. Nat Rev Neurosci 9:387, 2008 [DOI] [PubMed] [Google Scholar]
- Abraham WC, Bear MF: Metaplasticity: The plasticity of synaptic plasticity. Trends Neurosci 19:126–130, 1996 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Text Revision. (DSM-IV-TR). Arlington, VA: American Psychiatric Association, 2000 [Google Scholar]
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 5th ed. (DSM-5). Arlington, VA: American Psychiatric Publishing, 2013 [Google Scholar]
- Barker AT, Jalinous R, Freeston IL: Non-invasive magnetic stimulation of human motor cortex. Lancet 325:1106–1107, 1985 [DOI] [PubMed] [Google Scholar]
- Baumer T, Lange R, Liepert J, Weiller C, Siebner HR, Rothwell JC, Munchau A: Repeated premotor rTMS leads to cumulative plastic changes of motor cortex excitability in humans. Neuroimage 20:550–560, 2003 [DOI] [PubMed] [Google Scholar]
- Betancur C: Etiological heterogeneity in autism spectrum disorders: More than 100 genetic and genomic disorders and still counting. Brain Res 1380:42–77, 2011 [DOI] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW: Theory for the development of neuron selectivity: Orientation specificity and binocular interaction in visual cortex. J Neurosci 2:32–48, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas-Morales L, Nowak DA, Kammer T, Wolf RC, Schonfeldt-Lecuona C: Mechanisms and applications of theta-burst rTMS on the human motor cortex. Brain Topogr 22:294–306, 2010 [DOI] [PubMed] [Google Scholar]
- Casartelli L, Molteni M, Ronconi L: So close yet so far: Motor anomalies impacting on social functioning in autism spectrum disorder. Neurosci Biobehav Rev 63:98–105, 2016 [DOI] [PubMed] [Google Scholar]
- Cohen DA, Freitas C, Tormos JM, Oberman L, Eldaief M, Pascual-Leone A: Enhancing plasticity through repeated rTMS sessions: The benefits of a night of sleep. Clin Neurophysiol 121:2159–2164, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeltgen SH, Ridding MC: Modulation of cortical motor networks following primed theta burst transcranial magnetic stimulation. Exp Brain Res 215:199–206, 2011 [DOI] [PubMed] [Google Scholar]
- Dolen G, Bear MF: Fragile x syndrome and autism: From disease model to therapeutic targets. J Neurodev Disord 1:133–140, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE: Synaptic mechanisms for plasticity in neocortex. Annu Rev Neurosci 32:33–55, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Fountain S, Daskalakis ZJ: A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol 117:2584–2596, 2006 [DOI] [PubMed] [Google Scholar]
- Hoogendam JM, Ramakers GM, Di Lazzaro V: Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul 3:95–118, 2010 [DOI] [PubMed] [Google Scholar]
- Huang YZ, Chen RS, Rothwell JC, Wen HY: The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol 118:1028–1032, 2007 [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC: Theta burst stimulation of the human motor cortex. Neuron 45:201–206, 2005 [DOI] [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC, Edwards MJ, Chen RS: Effect of physiological activity on an NMDA-dependent form of cortical plasticity in human. Cereb Cortex 18:563–570, 2008 [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF: Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A 99:7746–7750, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iossifov I, Ronemus M, Levy D, Wang Z, Hakker I, Rosenbaum J, Yamrom B, Lee YH, Narzisi G, Leotta A, Kendall J, Grabowska E, Ma B, Marks S, Rodgers L, Stepansky A, Troge J, Andrews P, Bekritsky M, Pradhan K, Ghiban E, Kramer M, Parla J, Demeter R, Fulton LL, Fulton RS, Magrini VJ, Ye K, Darnell JC, Darnell RB, Mardis ER, Wilson RK, Schatz MC, McCombie WR, Wigler M: De novo gene disruptions in children on the autistic spectrum. Neuron 74:285–299, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER: The molecular biology of memory storage: A dialogue between genes and synapses. Science 294:1030–1038, 2001 [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Cortell R, Kau AS, Bukelis I, Tierney E, Gray RM, Cox C, Capone GT, Stanard P: Autism spectrum disorder in fragile X syndrome: Communication, social interaction, and specific behaviors. Am J Med Genet A 129A:225–234, 2004 [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A: Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol 111:800–805, 2000 [DOI] [PubMed] [Google Scholar]
- Markram K, Markram H: The intense world theory—A unifying theory of the neurobiology of autism. Front Hum Neurosci 4:224, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockett BG, Hulme SR: Metaplasticity: New insights through electrophysiological investigations. J Integr Neurosci 7:315–336, 2008 [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Ewen JB: Altered connectivity and action model formation in autism is autism. Neuroscientist 17:437–448, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch JD, State MW: Recent developments in the genetics of autism spectrum disorders. Curr Opin Genet Dev 23:310–315, 2013 [DOI] [PubMed] [Google Scholar]
- Neale BM, Kou Y, Liu L, Ma'ayan A, Samocha KE, Sabo A, Lin CF, Stevens C, Wang LS, Makarov V, Polak P, Yoon S, Maguire J, Crawford EL, Campbell NG, Geller ET, Valladares O, Schafer C, Liu H, Zhao T, Cai G, Lihm J, Dannenfelser R, Jabado O, Peralta Z, Nagaswamy U, Muzny D, Reid JG, Newsham I, Wu Y, Lewis L, Han Y, Voight BF, Lim E, Rossin E, Kirby A, Flannick J, Fromer M, Shakir K, Fennell T, Garimella K, Banks E, Poplin R, Gabriel S, DePristo M, Wimbish JR, Boone BE, Levy SE, Betancur C, Sunyaev S, Boerwinkle E, Buxbaum JD, Cook EH, Jr, Devlin B, Gibbs RA, Roeder K, Schellenberg GD, Sutcliffe JS, Daly MJ: Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature 485:242–245, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, Levy R, Ko A, Lee C, Smith JD, Turner EH, Stanaway IB, Vernot B, Malig M, Baker C, Reilly B, Akey JM, Borenstein E, Rieder MJ, Nickerson DA, Bernier R, Shendure J, Eichler EE: Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 485:246–250, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman LM, Eldaief M, Fecteau S, Ifert-Miller F, Tormos JM, Pascual-Leone A: Abnormal modulation of corticospinal excitability in adults with Asperger's syndrome. Eur J Neurosci 36:2782–2788, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman LM, Ifert-Miller F, Najib U, Bashir S, Woollacott I, Gonzalez-Heydrich J, Picker J, Rotenberg A, Pascual-Leone A: Transcranial magnetic stimulation provides means to assess cortical plasticity and excitability in humans with fragile x syndrome and autism spectrum disorder. Front Synaptic Neurosci 2:26, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman LM, Rotenberg A, Pascual-Leone A: Aberrant brain plasticity in autism spectrum disorders. In: Plasticity of Cognition in Neurologic Disorders. Edited by Tracy JI, Hampstead BM, Sathian K. New York, Oxford University Press, 2015, pp.119–145 [Google Scholar]
- Pascual-Leone A, Amedi A, Fregni F, Merabet LB: The plastic human brain cortex. Annu Rev Neurosci 28:377–401, 2005 [DOI] [PubMed] [Google Scholar]
- Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, Di Lazzaro V, Ferreri F, Fitzgerald PB, George MS, Hallett M, Lefaucheur JP, Langguth B, Matsumoto H, Miniussi C, Nitsche MA, Pascual-Leone A, Paulus W, Rossi S, Rothwell JC, Siebner HR, Ugawa Y, Walsh V, Ziemann U: Noninvasive electrical and magnetic stimulation of the brain, spinal cord roots, and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol 126:1071–1107, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharer EA, Mostofsky SH, Pascual-Leone A, Oberman LM: Isolating visual and proprioceptive components of motor sequence learning in ASD. Autism Res 9:563–569, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, Rothwell JC: Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: Evidence for homeostatic plasticity in the human motor cortex. J Neurosci 24:3379–3385, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyi A, Schachtman TR, Christoffersen GR: The role of metabotropic glutamate receptor 5 in learning and memory processes. Drug News Perspect 18:353–361, 2005 [DOI] [PubMed] [Google Scholar]
- Tang A, Thickbroom GW, Rodger J: Repetitive transcranial magnetic stimulation of the brain: Mechanisms from animal and experimental models. Neuroscientist 2015. [Epub ahead of print]; DOI: 10.1177/1073858415618897 [DOI] [PubMed] [Google Scholar]
- Thickbroom GW: Transcranial magnetic stimulation and synaptic plasticity: Experimental framework and human models. Exp Brain Res 180:583–593, 2007 [DOI] [PubMed] [Google Scholar]
- Thut G, Ives JR, Kampmann F, Pastor MA, Pascual-Leone A: A new device and protocol for combining TMS and online recordings of EEG and evoked potentials. J Neurosci Methods 141:207–217, 2005 [DOI] [PubMed] [Google Scholar]
- Thut G, Pascual-Leone A: Integrating TMS with EEG: How and what for? Brain Topogr 22:215–218, 2010a [DOI] [PubMed] [Google Scholar]
- Thut G, Pascual-Leone A: A review of combined TMS-EEG studies to characterize lasting effects of repetitive TMS and assess their usefulness in cognitive and clinical neuroscience. Brain Topogr 22:219–232, 2010b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd G, Flavel SC, Ridding MC: Priming theta-burst repetitive transcranial magnetic stimulation with low- and high-frequency stimulation. Exp Brain Res 195:307–315, 2009 [DOI] [PubMed] [Google Scholar]
- Tordjman S, Drapier D, Bonnot O, Graignic R, Fortes S, Cohen D, Millet B, Laurent C, Roubertoux PL: Animal models relevant to schizophrenia and autism: Validity and limitations. Behav Genet 37:61–78, 2007 [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB: Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci 5:97–107, 2004 [DOI] [PubMed] [Google Scholar]
- Wagner T, Valero-Cabre A, Pascual-Leone A: Noninvasive human brain stimulation. Annu Rev Biomed Eng 9:527–565, 2007 [DOI] [PubMed] [Google Scholar]
- Ziemann U, Paulus W, Nitsche MA, Pascual-Leone A, Byblow WD, Berardelli A, Siebner HR, Classen J, Cohen LG, Rothwell JC: Consensus: Motor cortex plasticity protocols. Brain Stimul 1:164–182, 2008 [DOI] [PubMed] [Google Scholar]