Abstract
Intestinal mucositis is one of the major problems in the patients receiving cancer treatment. Nimesulide is a drug with antioxidant, antiinflammatory and antiulcer features. We aimed to investigate the effect of nimesulide on the small intestine mucositis induced by methotrexate (MTX) in rats. Experimental animals were divided into the control group, MTX group (MTXG) and nimesulide+MTX administered group (NMTXG) with eight rats per group. The control and MTXG groups were given distilled water by gavage and the NMTXG was given nimesulide 100 mg/kg orally. After one hour, the NMTXG and MTXG rat groups were administered oral MTX 5 mg/kg. This procedure was repeated once a day for 15 days and the rats were sacrificed. The duodenum and jejunum of each rat was removed for the assessment of biochemical markers and histopathological evaluation. Malondialdehyde (MDA) and myeloperoxidase (MPO) levels were significantly higher in the duodenal and jejunal tissues of the animals which received MTX, compared to the control and NMTXG (P<0.001). Also, the levels of total glutathione (tGSH), glutathione reductase (GSHRd), glutathione peroxidase (GSHPx), catalase (CAT) and superoxide dismutase (SOD) were significantly lower in the MTXG (P<0.001) compared to other groups. MTX led to villus and crypt epithelial damage and inflammation containing marked PMNL and eosinophils in the intestinal tissues histopathologically. Whereas, there was only mild irregularities in the villus structures of the NMTXG. Nimesulide protected the small intestines against damage by MTX. Intestinal mucositis caused by MTX may be preventable by co-administered nimesulide.
Keywords: Nimesulide, mucositis, oxidative stress, rat
Introduction
Methotrexate (MTX) is an anticancer drug classified as folic acid antimetabolite of folic acid and widely used in chemotherapy. Intestinal mucositis seen in 40% of patients receiving chemotherapy at standard doses, while this rate has been reported as almost 100% at high doses [2, 24]. This indicates that, intestinal mucositis is one of the major problems arising in the patients receiving cancer treatment. Hoekstra et al. demonstrated that MTX leads to gastrointestinal damage at high doses [10]. Also, MTX can cause fatal gastrointestinal damage even in low doses [30]. Reactive oxygen species (ROS) are known to have a role in the pathogenesis of tissue toxicity caused by MTX. MTX has been shown to increase myeloperoxidase (MPO), neutrophil infiltration and decrease antioxidant glutathione by increasing the production of ROS [11, 21]. Kolli et al. reported that, oxidants such as malondialdehyde (MDA) which is a product of lipid peroxidation and MPO which is the marker of neutrophil activation and infiltration increase, while the levels of non-enzymatic and enzymatic antioxidants such as glutathione (GSH), glutathione reductase (GSHRd), glutathione peroxidase (GSHPx), glutathione s-transferase (GST), catalase (CAT) and superoxide dismutase (SOD) decrease in MTX induced small intestine mucositis [13]. Elevation in the oxidant parameters and reduction in the antioxidant parameters in duodenal and jejunal tissues given MTX have also been emphasized in the experimental trials by Kaynar et al. and Gulgun et al. [9, 12].
It is understood from the literature that mucositis is a crucial pathology which begins with oxidative stress, continues with inflammatory reaction and leads to ulcer [15, 19]. This information from the literature suggests that the chemical agents which features antioxidant, antiinflammatory and antiulcer activities in association may be beneficial for the treatment of mucositis. Nimesulide, a COX selective inhibitor which we tried against MTX mucositis is a drug which has antiinflammatory, analgesic, antipyretic, antioxidant and antiulcer activities together [26,27,28]. This information indicates that nimesulide may be useful in the prevention of MTX mucositis. The objective of this study was to investigate the protective effect of nimesulide in duodenal and jejunal mucositis induced with MTX in rats.
Materials and Methods
Animals
Experimental animals were supplied from Recep Tayyip Erdogan University Medical Experimental Application and Research Center. A total of 24 male albino Wistar rats, each weighing 230–245 g, were randomly chosen, and divided into three groups with 8 rats in each group. The rats were kept and fed in the pharmacology laboratory at normal room temperature (22°C). Animal experiments were performed in accordance with the National Guidelines for the Use and Care of Laboratory Animals and were approved by the local animal ethics committee of Recep Tayyip Erdogan University, Rize, Turkey (Ethics Committee Number: 2015/30).
Chemical agents
Thiopental sodium used in the experiment was supplied from I.E. Ulagay-Turkey, nimesulide from Deva-Turkey and MTX from Med-ilac-Turkey.
Experimental procedure
Experimental animals were divided into the control group, MTX group (MTXG) and nimesulide+MTX administered group (NMTXG). The control and MTXG groups were given distilled water at the same volume by gavage and the NMTXG was given nimesulide 100 mg/kg orally. After one hour, the NMTXG and MTXG rat groups were administered oral MTX 5 mg/kg. This procedure was repeated once a day for 15 days. At the end of this period, all the animals were sacrificed using high dose of thiopental sodium anesthesia. The duodenum and jejunum of each rat was removed for the assessment of biochemical markers (MDA, MPO, tGSH, GSHRd, GSHPx, CAT and SOD). Histopathological features of the duodenal and jejunal tissues were also assessed. The results obtained in the NMTXG were compared to those of the healthy and MTXG groups.
Biochemical analyses
We checked weight of samples, and after those cutting samples, rapidly frozen with liquid nitrogen and homogenized by pestle and mortar; maintained samples at 2–8°C after melting. We added PBS (pH 7.4), 1/10 (w/v), after that vortex for 10 s, centrifuged 20 min at 10,000 × g and collected the supernatants carefully. The solution was then aliquoted, kept one for examination and frozen the others for later use.
The levels of MDA and tGSH were measured using according to a commercial kit supplied by Eastbiopharm Co., Ltd., ELISA kit, China.
For determination of MPO in the small intestine tissue homogenates, pH=6 potassium phosphate buffer containing 0.5% HDTMAB (0.5% hexadecyl-trimethyl ammonium bromide) was prepared. The mixture then was centrifuged at 10,000 rpm at 4°C for 15 min. Supernatant part was used as analysis sample. In determination of MPO enzyme activity, oxidation reaction with MPO mediated H2O2 which included 4-amino antipyrine phenol solution as substrate was used [5].
Glutathione peroxidase (GSHPx) was determined by monitoring NADP+ production at 340 nm and 25°C. The assay mixture contained 10 mm magnesium chloride, 0.2 mM NADPH, 0.1 U/ml GSHRd and 0.1 m GSH in 100 mm tris-hydrochlorid buffer solution at pH 8.0. Assays were carried out in triplicate and the activities were followed up for 60 s. One unit of activity (U) is defined as the amount of enzyme required to reduce 1 µmol/min of NADPH under the assay conditions. The activity of GSHPx was calculated using the extinction coefficient of 6.22 mm cm−1 [4].
GSHRd enzyme activity was measured by Beutler’s method [4]. One enzyme unit is defined as the oxidation of 1 mmol NADPH per min under the assay condition (25°C, pH 8.0). Final concentrations of reaction mix are 0.68 mM EDTA, 20 mM K-Phosphate, pH: 7.6, 0.2 mM NADPH and 2 mM GSSG.
We used the methodology of the Aebi for measuring CAT activity [1]. In this method, 20 ml enzyme solution was added to the 1 ml 10 mM H2O2 in 20 mM potassium phosphate buffer (pH 7.0) and incubated at 25°C for 1 min. Initial reaction rate was measured from the decrease in absorbance at 240 nm [1].
Superoxide dismutase (SOD) activity was based on the generation of superoxide radicals produced by xanthine and xanthine oxidase, which reacts with nitro blue tetrazolium to form formazan dye. SOD activity was then measured at 560 nm by the degree of inhibition of this reaction [29].
Histopathological study
The duodenal and jejunal tissues taken from the rats were fixed in 10% formalin for 24 h. Following the routine procedures, 4 µm-sections obtained from paraffin blocks were stained with haematoxylin and eosin stain. Villus epithelial cells, villus structure, polymorphonuclear leukocytes (PMNL), mixed cellular infiltration, crypt and dilated congested capillaries were examined under light microscope (Olympus BX 52, Tokyo, Japan) by two pathologists who were blinded to the treatment protocols. Histological findings were evaluated based on a three-point scoring in order to compare the severity of the damage: 1 point was accepted as mild damage while 2 points were considered as moderate damage, and 3 points as severe damage. Healthy tissue was accepted as 0 point.
Statistical analysis
Statistical analyses were carried out using the Statistical Package for Social Sciences, Windows version 18.0 (SPSS, Chicago, IL, USA). Descriptive statistics for each variable were determined. Normality of the data distribution was assessed with the Kolmogorov-Smirnov test. Results for continuous variables were demonstrated as mean ± standard deviation (mean ± SD). The significance of differences between the groups was determined using the one-way ANOVA test followed by Fisher’s post-hoc LSD (least significant differences) analysis. A P value less than 0.05 was considered significant.
Results
Biochemical results
1) Duodenal tissues
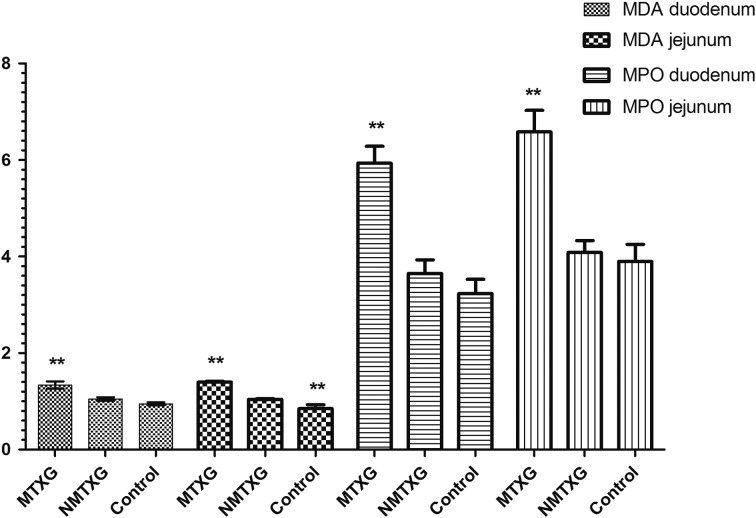
As shown in Fig. 1, the amount of MDA in the duodenal tissue was 0.95 ± 0.03 nmol/ml in the control group, while these values were increased to 1.34 ± 0.07 nmol/ml in those administred MTX. However, nimesulide decreased the elevation of MDA with MTX to 1.05 ± 0.04 nmol/ml.
Fig. 1.
The effects of nimesulide on MDA and MPO levels in the duodenum and jejunum tissues of rats given methotrexate. Bars are mean ± SD. The NMTXG is compared with the MTXG and control groups. **: P<0.001.
MPO activity was found as 3.23 ± 0.29 U/ml in the control group, 5.93 ± 0.35 U/ml in MTX administered group and 3.65 ± 0.28 U/ml in the NMTXG (Fig. 1).
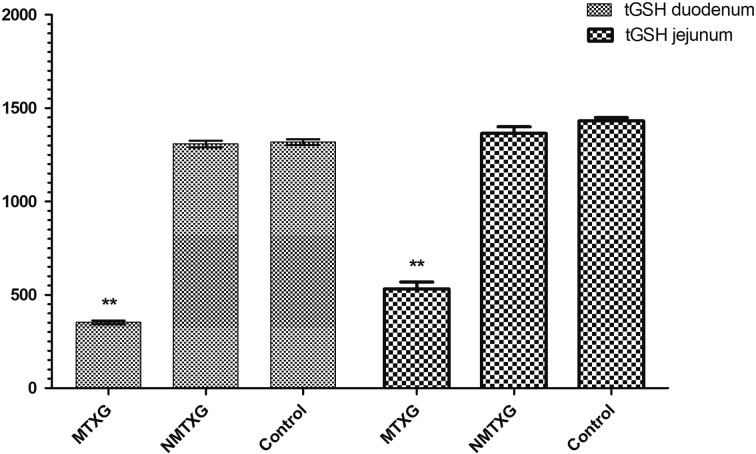
While the amount of tGSH was 1317 ± 15 mg/l in the control group, MTX decreased tGSH down to 352 ± 9 mg/l and nimesulide increased tGSH up to 1,307 ± 17 mg/l (Fig. 2).
Fig. 2.
The effects of nimesulide on tGSH levels in the duodenum and jejunum tissues of rats given methotrexate. Bars are mean ± SD. The NMTXG is compared with the MTXG and control groups. **: P<0.001.
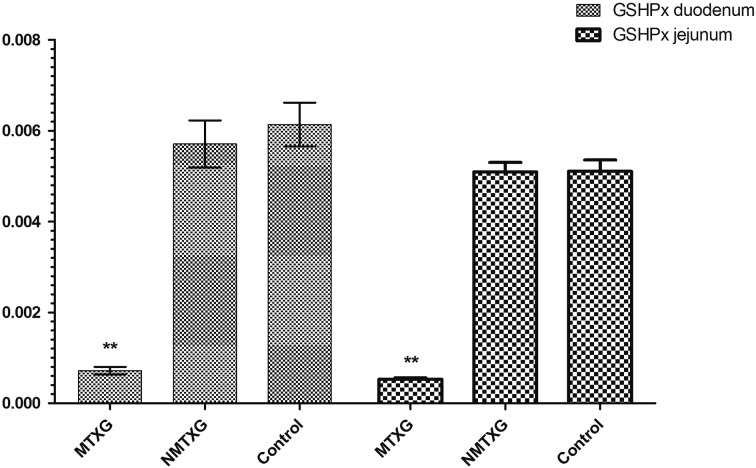
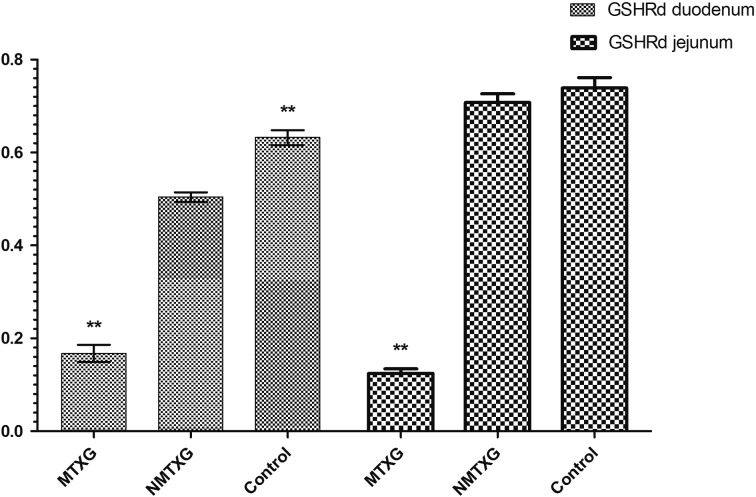
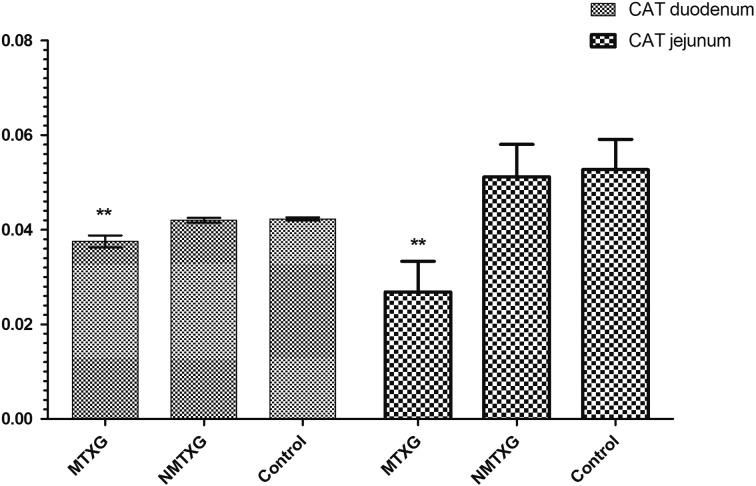
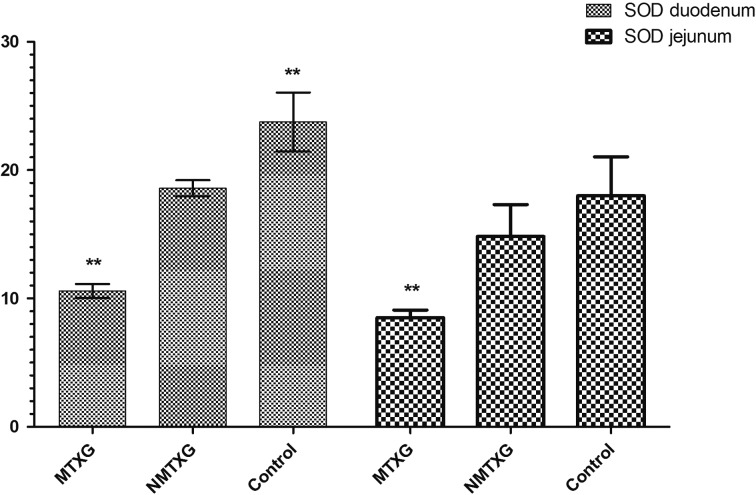
In addition; GSHPx, GSHRd, CAT and SOD activities were respectively found as 0.00613 ± 0.00048, 0.632 ± 0.016, 0.0421 ± 0.0007 and 23.7 ± 2.3 U/ml in the control group. These parameters were found as 0.000719 ± 0.00008, 0.168 ± 0.018, 0.037 ± 0.003 and 10.6 ± 0.5 U/ml in the MTXG. Nimesulide prevented the reduction of GSHPx, GSHRd, CAT and SOD activities that were decreased with MTX, to 0.00571 ± 0.00051, 0.504 ± 0.01, 0.0420 ± 0.001, 18.6 ± 0.6 U/ml (Figs. 3, 4, 5, 6).
Fig. 3.
The effects of nimesulide on GSHPx levels in the duodenum and jejunum tissues of rats given methotrexate. Bars are mean ± SD. The NMTXG is compared with the MTXG and control groups. **: P<0.001.
Fig. 4.
The effects of nimesulide on GSHRd levels in the duodenum and jejunum tissues of rats given methotrexate. Bars are mean ± SD. The NMTXG is compared with the MTXG and control groups. **: P<0.001.
Fig. 5.
The effects of nimesulide on CAT levels in the duodenum and jejunum tissues of rats given methotrexate. Bars are mean ± SD. The NMTXG is compared with the MTXG and control groups. **: P<0.001.
Fig. 6.
The effects of nimesulide on SOD levels in the duodenum and jejunum tissues of rats given methotrexate. Bars are mean ± SD. The NMTXG is compared with MTXG and control groups. **: P<0.001.
2) Jejunal tissues
As shown in Fig. 1, MTX significantly increased the amount of MDA (1.399 ± 0.018 nmol/ml) also in jejunal tissue compared to the control (0.850 ± 0.084 nmol/ml) and NMTXG (1.041 ± 0.015 nmol/ml) groups. While MPO activity was increased also in the group administered MTX (6.6 ± 0.4 U/ml), no statistically significant was observed between the NMTXG (4.1 ± 0.2 U/ml) and control (3.9 ± 0.4 U/ml) groups in MPO values.
Similarly, MTX decreased tGSH also in the jejunal tissue (532 ± 36 mg/l). Nimesulide prevented reduction of tGSH with MTX in the jejunal tissue. The tGSH values were found as 1,364 ± 36 mg/l in the NMTXG and 1,431 ± 17 mg/l in the control group (Fig. 2).
Enzymatic antioxidant parameters; GSHPx, GSHRd, CAT and SOD activities were respectively found as 0.00511 ± 0.0002, 0.739 ± 0.022, 0.0527 ± 0.006 and 18.0 ± 3 U/ml in the control group. These parameters were found as 0.00509 ± 0.0002, 0.707 ± 0.019, 0.0512 ± 0.006 and 14.8 ± 2.5 U/ml in the NMTXG and 0.000531 ± 0.00003, 0.124 ± 0.009, 0.0268 ± 0.006 and 8.5 ± 0.6 U/ml in the MTXG (Figs. 3, 4, 5, 6).
Histopathological findings
1) Duodenal tissues
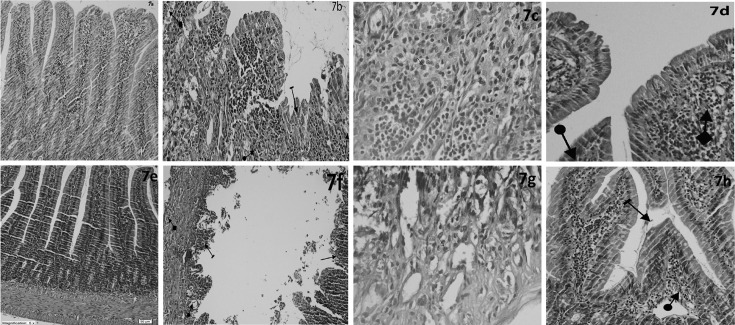
Figure 7a shows normal histopathological appearance of the duodenal tissue in control group. Figure 7b shows villus epithelial damage, mucosal crypt damage, mixed inflammatory cell infiltration containing PMNL and dilated congested capillaries in the duodenal tissue of MTXG. Also, Fig. 7c shows mixed inflammatory cell infiltration containing PMNL and eosinophils in the duodenal tissue of MTXG. In Fig. 7d, mild irregularities in the villus structures and localized areas which contains dilated congested capillaries are seen in the duodenal tissue of NMTXG which was treated with nimesulide. As seen in Table 1, severe damage in the crypt structures, villus and villus epithelial cells, moderate PMNL infiltration, mixed inflammatory cells and dilated congested vessels were observed in the mucosa of MTXG. However, there were only mild dilated congested vessels and mild damage in the villi in NMTXG. There was a statistically significant difference between the damage observed in MTXG and that of NMTXG (P<0.001).
Fig. 7.
Sections of the duodenum (a–d) and jejunum tissue (e–h). (a) Normal duodenal tissue of the control group, (b) Surface villus epithelial damage (line arrow), mucosal crypt damage (straight arrow), mixed inflammatory cell infiltration containing PMNL (circle arrow), and dilated congested capillaries (square arrow) of the MTXG, (c) mixed inflammatory cell infiltration containing PMNL and eosinophils of the MTXG, (d) Mild irregularities in the villus structures (circle arrow) and localized areas which contains dilated congested capillaries (square arrow) of the NMTXG, (e) Normal jejunal tissue of the control group, (f) Near-total necrosis in villus structures (line arrow), mixed inflammatory cell infiltration containing PMNL (square arrow), mucosal crypt damage (circle arrow), surface epithelial and villus damage (long straight arrow), and dilated congested capillaries (short straight arrow) of the MTXG, (g) mixed inflammatory cell infiltration containing PMNL and eosinophils of the MTXG, (h) Mild irregularities in the villus structures (line arrow) and localized areas which contains dilated congested capillaries (square arrow) of the NMTXG. HE. Original magnification. a,b,d,e,f,h ×100; c,g ×400.
Table 1. Results of histopathologic scorings in duodenum tissues of MTXG, NMTXG and control groups.
| Pathological findings | MTXG | NMTXG | Control |
|---|---|---|---|
| Number of animals examined | 8 | 8 | 8 |
| Villus epithelial damage | 3a | 0b | 0 |
| Damage in the villus structures | 3a | 1b | 0 |
| Mucosal crypt damage | 3a | 0b | 0 |
| PMNL infiltration | 2a | 0b | 0 |
| Mixed inflammatory cells | 2a | 0b | 0 |
| Dilated congested capillaries | 2a | 1b | 0 |
aSignificantly different from control group (P<0.001). bSignificantly different from MTXG (P<0.001).
2) Jejunal tissues
Figure 7e shows no pathological finding in the jejunal tissue of control group. However, near-total necrosis in villus structures, damage in villus epithelial cells, mucosal crypt damage, mixed inflammatory cell infiltration containing PMNL and dilated congested capillaries are monitored in the jejunal tissue of MTXG (Fig. 7f). Figure 7 g shows mixed inflammatory cell infiltration containing PMNL and eosinophils in the jejunal tissue of MTXG. In Fig. 7h, mild irregularities in the villus structures and dilated congested capillaries in the mucosa are seen in the jejunal tissue of NMTXG which was treated with nimesulide. As seen in Table 2, severe damage in the villus, crypt structures and villus epithelial cells, severe PMNL, mixed inflammatory cellular infiltration and moderate dilated congested blood vessels in the jejunum of MTXG. Whereas, mild dilated congested vessels were seen in NMTXG administered nimesulide. Additionally, mild damage was observed in the villi of NMTX group. The damage in the jejunal tissue of MTXG was significantly higher compared to that of NMTXG (P<0.001).
Table 2. Results of histopathologic scorings in jejunum tissues of MTXG, NMTXG and control groups.
| Pathological findings | MTXG | NMTXG | Control |
|---|---|---|---|
| Number of animals examined | 8 | 8 | 8 |
| Villus epithelial damage | 3a | 0b | 0 |
| Damage in the villus structures | 3a | 1b | 0 |
| Necrosis in villus structures | 3a | 0b | 0 |
| Mucosal crypt damage | 3a | 0b | 0 |
| PMNL infiltration | 3a | 0b | 0 |
| Mixed inflammatory cells | 3a | 0b | 0 |
| Dilated congested capillaries | 2a | 1b | 0 |
aSignificantly different from control group (P<0.001). bSignificantly different from MTXG (P<0.001).
Discussion
This study investigated the protective effect of nimesulide on duodenal and jejunal mucositis induced by MTX in rats. Our biochemical results indicate that MTX increased the levels of oxidants such as MDA and MPO and decreased the levels of endogenous antioxidants such as tGSH, GSHPx, GSHRd, CAT and SOD in the duodenal and jejunal tissues of rats. In addition, MTX caused damage to the villus, villus epithelial cells and crypt structures, mixed inflammatory cellular infiltration and dilated congested blood vessels in the intestinal tissue. We demonstrated that nimesulide prevented the increase of oxidants, decrease of antioxidants and histopathologic damage by MTX in the intestinal tissue. Kolli et al. demonstrated that higher dose of MTX then we used led to oxidative mucosal damage in the rat small intestines [14]. However, it was also reported that even lower doses of MTX (2.5 mg/kg) leads to marked oxidative damage in rat small intestines [3]. Oxidative stress is a change in oxidant/antioxidant balance in any tissue in favour of oxidants [31]. The increased levels of MDA and MPO, and decreased levels of tGSH, GSHRd, GSHPx, CAT and SOD in the rats given MTX in the present study confirm the results of Kolli et al. [13]. Moghadam et al. reported that MTX leads to oxidative stress in rat small intestine by increasing the amount of MDA and decreasing activities of some enzymatic antioxidants such as SOD, GSHPx and CAT [18].
In our study, nimesulide which was used against MTX damage prevented impairment of oxidant/antioxidant balance in small intestines tissue of rats. In addition, it was found that nimesulide largely restored histopathological disorders in the small intestines caused by MTX. As far as we know, there is no study in the literature on the protective effect of nimesulide against small intestinal mucositis induced by MTX. However, there are some studies demonstrating that nimesulide protects gastric and hepatic tissues against oxidative stress at a dose of 100 mg/kg [8, 28]. It has been reported that nimesulide and its metabolites may prove useful in prevention of acute and chronic free radicals mediated tissue damage [16].
Marked villus and crypt epithelial damage and mixed inflammatory cell infiltration containing PMNL and eosinophil leukocytes and hemorrhage which reflect mucositis were monitored in MTXG group in which oxidant parameters were increased and antioxidant parameters decreased. Kaynar et al. demonstrated that oxidant parameters were increased and antioxidant parameters decreased in small intestines of the rats administered MTX, in addition reported marked villus and crypt epithelial damage, mixed inflammatory cell infiltration containing PMNL and eosinophil leukocytes [12]. MTX was reported to cause hyperemia, inflammatory cell infiltration and loss of villus epithelial cells in the small intestines of rats [6]. In another study, MTX has been reported to lead to the cellular loss, severe villus atrophy and PMNL leukocyte infiltration [7]. In addition to these histopathological findings, MTX is known to cause intestinal hemorrhage [18]. Some studies emphasize that MTX creates more severe damage in the jejunum than in the duodenum [17, 25]. However, in our study histological findings in the duodenal and jejunal tissues administered MTX were almost similar. Mild irregularities in the villus structures and dilated congested capillaries in the mucosa are seen in the duodenal and jejunal tissues of the animals treated with nimesulide. In addition, nimesulide was found to decrease severity of mixed inflammatory cell infiltration and shrink hemorrhage areas that were increased with MTX. It has been reported that nimesulide prevents intestinal pathology induced with acetic acid and leukotriene in rats and decreases the activity of MPO which is a proinflammatory parameter [22]. Inflammation and increased MPO expression are important markers of intestinal mucositis [23]. Nimesulide has also been found to suppress MPO and significantly prevent the reduction of GSH that were increased in intestinal tissue created with burn [20].
In conclusion; MTX leads to oxidative stress and mucosal damage in the duodenal and jejunal tissues of rats. Nimesulide prevents duodenal and jejunal damage induced with MTX. Beneficial effects of nimesulide on intestinal mucositis might be resulted from its antioxidant, antiinflammatory and antiulcer activities. Nimesulide may be useful in the prevention of intestinal mucositis caused by MTX.
SOURCE (S): This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of Interests
The authors have no conflicts of interest.
References
- 1.Aebi H.1984. Catalase in vitro. Methods Enzymol. 105: 121–126. doi: 10.1016/S0076-6879(84)05016-3 [DOI] [PubMed] [Google Scholar]
- 2.Alamir I., Boukhettala N., Aziz M., Breuillé D., Déchelotte P., Coëffier M.2010. Beneficial effects of cathepsin inhibition to prevent chemotherapy-induced intestinal mucositis. Clin. Exp. Immunol. 162: 298–305. doi: 10.1111/j.1365-2249.2010.04220.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beutheu S., Ouelaa W., Guérin C., Belmonte L., Aziz M., Tennoune N., Bôle-Feysot C., Galas L., Déchelotte P., Coëffier M.2014. Glutamine supplementation, but not combined glutamine and arginine supplementation, improves gut barrier function during chemotherapy-induced intestinal mucositis in rats. Clin. Nutr. 33: 694–701. doi: 10.1016/j.clnu.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 4.Beutler E.1971. Red cell metabolism: a manual of biochemical methods. Academic Press, London. [Google Scholar]
- 5.Bradley P.P., Priebat D.A., Christensen R.D., Rothstein G.1982. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. 78: 206–209. doi: 10.1111/1523-1747.ep12506462 [DOI] [PubMed] [Google Scholar]
- 6.Chen C., Tian L., Zhang M., Sun Q., Zhang X., Li X., Cao X., Liu Q., Li X., Hao L.2013. Protective effect of amifostine on high-dose methotrexate-induced small intestinal mucositis in mice. Dig. Dis. Sci. 58: 3134–3143. doi: 10.1007/s10620-013-2826-3 [DOI] [PubMed] [Google Scholar]
- 7.de Araújo A.A., Borba P.B., de Souza F.H., Nogueira A.C., Saldanha T.S., Araújo T.E., da Silva A.I., de Araújo Júnior R.F.2015. In a methotrexate-induced model of intestinal mucositis, olmesartan reduced inflammation and induced enteropathy characterized by severe diarrhea, weight loss, and reduced sucrose activity. Biol. Pharm. Bull. 38: 746–752. doi: 10.1248/bpb.b14-00847 [DOI] [PubMed] [Google Scholar]
- 8.Demiryilmaz I., Turan M.I., Kisaoglu A., Gulapoglu M., Yilmaz I., Suleyman H.2014. Protective effect of nimesulide against hepatic ischemia/reperfusion injury in rats: effects on oxidant/antioxidants, DNA mutation and COX-1/COX-2 levels. Pharmacol. Rep. 66: 647–652. doi: 10.1016/j.pharep.2014.02.015 [DOI] [PubMed] [Google Scholar]
- 9.Gulgun M., Erdem O., Oztas E., Kesik V., Balamtekin N., Vurucu S., Kul M., Kismet E., Koseoglu V.2010. Proanthocyanidin prevents methotrexate-induced intestinal damage and oxidative stress. Exp. Toxicol. Pathol. 62: 109–115. doi: 10.1016/j.etp.2009.02.120 [DOI] [PubMed] [Google Scholar]
- 10.Hoekstra M., van Ede A.E., Haagsma C.J., van de Laar M.A., Huizinga T.W., Kruijsen M.W., Laan R.F.2003. Factors associated with toxicity, final dose, and efficacy of methotrexate in patients with rheumatoid arthritis. Ann. Rheum. Dis. 62: 423–426. doi: 10.1136/ard.62.5.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C., Hsu P., Hung Y., Liao Y., Liu C., Hour C., Kao M., Tsay G.J., Hung H., Liu G.Y.2005. Ornithine decarboxylase prevents methotrexate-induced apoptosis by reducing intracellular reactive oxygen species production. Apoptosis 10: 895–907. doi: 10.1007/s10495-005-2947-z [DOI] [PubMed] [Google Scholar]
- 12.Kaynar L., Cetin A., Hacioglu S.K., Eser B., Koçyigit İ., Canöz Ö., Tasdemir A., Karadag C., Kurnaz F., Saraymen R., Silici S.2012. Efficacy of royal jelly on methotrexate-induced systemic oxidative stress and damage to small intestine in rats. Afr. J. Tradit. Complement. Altern. Medicines 9: 412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolli V.K., Abraham P., Isaac B., Kasthuri N.2013. Preclinical efficacy of melatonin to reduce methotrexate-induced oxidative stress and small intestinal damage in rats. Dig. Dis. Sci. 58: 959–969. doi: 10.1007/s10620-012-2437-4 [DOI] [PubMed] [Google Scholar]
- 14.Kolli V.K., Kanakasabapathy I., Faith M., Ramamoorthy H., Isaac B., Natarajan K., Abraham P.2013. A preclinical study on the protective effect of melatonin against methotrexate-induced small intestinal damage: effect mediated by attenuation of nitrosative stress, protein tyrosine nitration, and PARP activation. Cancer Chemother. Pharmacol. 71: 1209–1218. doi: 10.1007/s00280-013-2115-z [DOI] [PubMed] [Google Scholar]
- 15.Lalla R.V., Peterson D.E.2006. Treatment of mucositis, including new medications. Cancer J. 12: 348–354. doi: 10.1097/00130404-200609000-00004 [DOI] [PubMed] [Google Scholar]
- 16.Maffei Facino R., Carini M., Aldini G., Saibene L., Morelli R.1995. Differential inhibition of superoxide, hydroxyl and peroxyl radicals by nimesulide and its main metabolite 4-hydroxynimesulide. Arzneimittelforschung 45: 1102–1109. [PubMed] [Google Scholar]
- 17.Miyazono Y., Gao F., Horie T.2004. Oxidative stress contributes to methotrexate-induced small intestinal toxicity in rats. Scand. J. Gastroenterol. 39: 1119–1127. doi: 10.1080/00365520410003605 [DOI] [PubMed] [Google Scholar]
- 18.Moghadam A.R., Mohajeri D., Namvaran-Abbas-Abad A., Manafi H., Shahi D., Mazani M.2013. Protective effect of turmeric extract on ethotrexate-induced intestinal damage and oxidative stress. Chin. J. Nat. Med. 11: 477–483. [DOI] [PubMed] [Google Scholar]
- 19.Niscola P., Romani C., Cupelli L., Scaramucci L., Tendas A., Dentamaro T., Amadori S., de Fabritiis P.2007. Mucositis in patients with hematologic malignancies: an overview. Haematologica 92: 222–231. doi: 10.3324/haematol.10232 [DOI] [PubMed] [Google Scholar]
- 20.Oktar B.K., Cakir B., Mutlu N., Celikel C., Alican I.2002. Protective role of cyclooxygenase (COX) inhibitors in burn-induced intestinal and liver damage. Burns 28: 209–214. doi: 10.1016/S0305-4179(02)00004-9 [DOI] [PubMed] [Google Scholar]
- 21.Phillips D.C., Woollard K.J., Griffiths H.R.2003. The anti-inflammatory actions of methotrexate are critically dependent upon the production of reactive oxygen species. Br. J. Pharmacol. 138: 501–511. doi: 10.1038/sj.bjp.0705054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh V.P., Patil C.S., Jain N.K., Singh A., Kulkarni S.K.2003. Effect of nimesulide on acetic acid- and leukotriene-induced inflammatory bowel disease in rats. Prostaglandins Other Lipid Mediat. 71: 163–175. doi: 10.1016/S1098-8823(03)00038-8 [DOI] [PubMed] [Google Scholar]
- 23.Soares P.M., Lopes L.O., Mota J.M., Belarmino-Filho J.N., Ribeiro R.A., Souza M.H.2011. Methotrexate-induced intestinal mucositis delays gastric emptying and gastrointestinal transit of liquids in awake rats. Arq. Gastroenterol. 48: 80–85. [DOI] [PubMed] [Google Scholar]
- 24.Sonis S.T., Elting L.S., Keefe D., Peterson D.E., Schubert M., Hauer-Jensen M., Bekele B.N., Raber-Durlacher J., Donnelly J.P., Rubenstein E.B., Mucositis Study Section of the Multinational Association for Supportive Care in Cancer, andInternational Society for Oral Oncology2004. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 100: 1995–2025. doi: 10.1002/cncr.20162 [DOI] [PubMed] [Google Scholar]
- 25.Southcott E., Tooley K.L., Howarth G.S., Davidson G.P., Butler R.N.2008. Yoghurts containing probiotics reduce disruption of the small intestinal barrier in methotrexate-treated rats. Dig. Dis. Sci. 53: 1837–1841. doi: 10.1007/s10620-008-0275-1 [DOI] [PubMed] [Google Scholar]
- 26.Suleyman H., Cadirci E., Albayrak A., Halici Z.2008. Nimesulide is a selective COX-2 inhibitory, atypical non-steroidal anti-inflammatory drug. Curr. Med. Chem. 15: 278–283. doi: 10.2174/092986708783497247 [DOI] [PubMed] [Google Scholar]
- 27.Süleyman H., Demircan B., Karagöz Y.2007. Anti-inflammatory and side effects of cyclooxygenase inhibitors. Pharmacol. Rep. 59: 247–258. [PubMed] [Google Scholar]
- 28.Suleyman H., Halici Z., Cadirci E., Hacimuftuoglu A., Keles S., Gocer F.2007. Indirect role of alpha2-adrenoreceptors in anti-ulcer effect mechanism of nimesulide in rats. Naunyn Schmiedebergs Arch. Pharmacol. 375: 189–198. doi: 10.1007/s00210-007-0151-0 [DOI] [PubMed] [Google Scholar]
- 29.Sun Y., Oberley L.W., Li Y.1988. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 34: 497–500. [PubMed] [Google Scholar]
- 30.Tsukada T., Nakano T., Miyata T., Sasaki S.2013. Life-Threatening Gastrointestinal Mucosal Necrosis during Methotrexate Treatment for Rheumatoid Arthritis. Case Rep. Gastroenterol. 7: 470–475. doi: 10.1159/000356817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yapca O.E., Borekci B., Suleyman H.2013. Ischemia-reperfusion damage. Eurasian J. Med. 45: 126–127. doi: 10.5152/eajm.2013.24 [DOI] [PMC free article] [PubMed] [Google Scholar]