Abstract
Neuropathic cancer pain is caused by tumors compressing the spinal nerve roots and is usually difficult to treat. The aim of current study was to determine the influence of NGF antibody on pain-related markers and behavior in a mouse model of neuropathic cancer pain. Twenty mice were used to model neuropathic cancer pain by applying murine sarcoma cells to their left sciatic nerve. Ten mice were sham operated. Two weeks after surgery, the murine sarcoma-affected mice were allocated randomly into treatment groups receiving either sterile saline (saline group) or an anti-nerve growth factor antibody (anti-NGF group). Three weeks after surgery (a week after treatment), the pain-related behavior of mice was evaluated using a CatWalk system. Subsequently, bilateral dorsal root ganglia (DRGs) from the L4–L6 levels and spinal cords at L4–L6 levels were resected. DRGs were immunostained for calcitonin gene-related peptide (CGRP) and activating transcription factor 3 (ATF-3), and spinal cords were immunostained for ionized calcium-binding adaptor molecule-1 (iba-1). Mechanical allodynia was observed in mice from the saline group and was improved in mice from the anti-NGF group. CGRP and ATF-3-immunoreactivity in DRGs and microglia expression in the spinal dorsal horn were upregulated in the saline group compared with the sham group, and they were suppressed in the anti-NGF group compared with the saline group (P<0.05). These findings suggest that anti-NGF therapy might be valuable for treating neuropathic cancer pain.
Keywords: CatWalk analysis, nerve growth factor antibody, neuropathic cancer pain
Introduction
Metastases to the spine occur frequently in patients with advanced cancer. An increase in the number of patients with bone metastases is observed because of the development of treatments such as surgery and radiotherapy, which extend the life expectancy of cancer patients. The occurrence of spinal metastases can cause significant morbidity, with pain and neurological deficits adversely affecting the patient’s quality of life. Neuropathic cancer pain is caused by compression of spinal nerve roots by tumors.
A treatment option for neuropathic cancer pain is conventional radiotherapy. The efficacy of radiotherapy depends on the sensitivity of tumor cells to ionizing radiation [4, 22]. Therefore, it is sometimes difficult to control neuropathic cancer pain by radiotherapy. Surgery is the most effective treatment for neuropathic cancer pain. However, surgery is invasive and difficult for advanced cancer patients having poor general conditions. Medication is another treatment option for the relief of neuropathic cancer pain.
We have focused on neurotrophins including nerve growth factor (NGF) as new targets for the treatment of neuropathic cancer pain. NGF is not only important for the maintenance and development of the sensory nervous system [15, 19] but is also a major contributor to inflammation and nociception [17]. Lewin et al. reported that systemic injection of NGF induced thermal and mechanical hyperalgesia [18]. In animal models of neuropathic pain, such as nerve trunk or spinal nerve ligation, systemic injection of anti-NGF antibody reduces allodynia and hyperalgesia [23, 24, 33].
We hypothesized that anti-NGF therapy may be effective for neuropathic cancer pain. The purpose of the current study was to investigate the efficacy of nerve growth factor antibody for reducing the mechanical allodynia and upregulated expression of pain markers seen in a mouse model of neuropathic cancer pain.
Materials and Methods
All protocols for animal procedures were approved by the Ethics Committee of Chiba University in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (1996 revision).
Model of neuropathic cancer pain
In this study, we used 6-week-old male C57 BL/6 mice. Mice were anesthetized with sodium pentobarbital (40 mg/kg, intraperitoneal) and treated aseptically throughout the experiments. The left sciatic nerves of all 30 mice used were exposed. Ten mice were used as sham controls. To model neuropathic cancer (Ca) pain, NCTC 2472 murine fibrosarcoma cells (DS Pharma Biomedical Co., Ltd., Osaka, Japan) were applied to the sciatic nerve of 20 mice using the same method as previously reported [26]. Two weeks after surgery, the 20 Ca mice were randomly assigned into treatment groups receiving either sterile saline (10 mg/kg, intraperitoneally (i.p.)) (Ca+saline group, n=10) or anti-NGF antibody (Exalpha Biologicals Inc., Shirley, MA, USA) (10 mg/kg, i.p.) (Ca+anti-NGF group, n=10).
Behavioral evaluation (CatWalk analysis)
The CatWalk system (Noldus Information Technology, Wageningen, The Netherlands) was used to perform a detailed analysis of gait. This system has been described in detail elsewhere [11]. Briefly, in this system, rats or mice are placed on a glass plate located in a darkened room and are allowed to walk freely. A light beam is projected through the glass plate. The light is completely reflected internally. However, when a paw touches the glass plate, light is reflected downwards. This results in a sharp bright image of the paw print. The entire run is recorded by a video camera. The data are acquired and analyzed using CatWalk software.
Three weeks after the surgical procedure (a week after treatment), all mice walked on the glass plate three times. The gaits of all mice were recorded three times and analyzed using the CatWalk system. The ratio of the ipsi- and contralateral mean intensities from the CatWalk system could be determined. We compared the ratio of the ipsi- and contralateral mean intensities between the three groups.
Immunohistochemical analysis
Three weeks after surgery (a week after treatment), all mice were anesthetized with sodium pentobarbital (40 mg/kg, intraperitoneal) and perfused transcardially with 0.9% saline, followed by 30 ml of 4% paraformaldehyde in phosphate buffer (0.1 M, pH 7.4). Bilateral dorsal root ganglia (DRGs) from the L4 to L6 levels and the spinal cord at the epicomus level were resected, and the specimens were immersed in the paraformaldehyde fixative solution overnight at 4°C before being immersed in 0.01 M phosphate buffered saline (PBS) containing 20% sucrose for 20 h at 4°C for cryoprotection.
Each DRG was sectioned at a thickness of 10 µm on a cryostat, and sections were mounted on poly-l-lysine-coated slides. Endogenous tissue peroxidase activity was quenched by soaking sections in 0.3% hydrogen peroxide solution in 0.01 M PBS for 30 min. Specimens were then treated for 90 min at room temperature with a solution consisting of 0.01 M PBS containing 0.3% Triton X-100 and 3% skim milk to block nonspecific binding sites. To evaluate the expression of neuropeptides in DRGs, the sections were processed for calcitonin gene-related peptide (CGRP) immunohistochemistry using a rabbit antibody to CGRP (1:1,000; Chemicon, Temecula, CA, USA) diluted in blocking solution, and the sections were incubated for 20 h at 4°C. To detect CGRP immunoreactivity in the DRGs, sections were incubated for 3 h with goat anti-rabbit Alexa Fluor 488 fluorescent antibody conjugate (1:400; Molecular Probes, Eugene, OR, USA).
To evaluate nerve injury, we examined the expression of activating transcription factor 3 (ATF-3) in DRG neurons. The sections were processed for immunohistochemistry using a rabbit antibody to ATF-3 (1:800; Santa Cruz Biotechnology, Dallas, TX, USA) diluted in blocking solution, and they were then incubated for 20 h at 4°C. To detect ATF-3 immunoreactivity in the DRGs, sections were incubated for 3 h with Alexa Fluor 488 conjugated goat anti-rabbit IgG (1:1,000; Molecular Probes, Eugene, OR, USA). Labeled DRG sections were examined using a fluorescence microscope, and the total numbers of DRG neurons and the numbers of CGRP-immunoreactive (IR) or ATF3-IR neurons per 0.45 mm2 in 10 randomly selected fields were counted at ×400 magnification using a counting grid. The proportion of CGRP-IR or ATF-3-IR DRG neurons among all DRG neurons was then calculated.
Each spinal cord was sectioned at a thickness of 25 µm on a cryostat. Endogenous tissue peroxidase activity was quenched by soaking the sections in 0.3% hydrogen peroxide solution in 0.01 M PBS for 30 min. The sections were then incubated in blocking solution for 90 min. To identify microglia in the spinal cord, sections were processed immunohistochemically using a free-floating avidin–biotin complex technique and incubated with a rabbit antibody to ionized calcium-binding adaptor molecule-1 (iba1) (a marker for microglia; 1:1,000; Wako Pure Chemical Industries, Osaka, Japan) for 20 h at 4°C. To detect iba1 immunoreactivity in the spinal cord, sections were incubated with goat anti-rabbit Alexa Fluor 488 fluorescent antibody conjugate (1:1,000; Molecular Probes, Eugene, OR, USA). Subsequently, sections were floated in 0.01 M PBS and mounted on poly-l-lysine-coated slides. Sections were examined using a fluorescence microscope, and the numbers of iba1-IR neurons in the spinal dorsal horn were counted. Specifically, the area of the spinal dorsal horn was calculated by using the ImageJ software (Wayne Rasband, NIH), and IR spinal dorsal horn neurons were counted at ×400 magnification using a counting grid to determine the number of iba1-IR neurons per 1 mm2.
Statistical analysis
The ratio of the ipsi- and contralateral mean intensities from the CatWalk data, the proportion of CGRP-IR neurons among all DRG neurons, the proportion of ATF-3-IR neurons among all DRG neurons, and the density of microglia in the spinal dorsal horn were compared between the 3 groups using a nonrepeated measures analysis of variance (ANOVA) with Bonferroni post hoc correction. P<0.05 was considered significant.
Results
Behavioral analysis
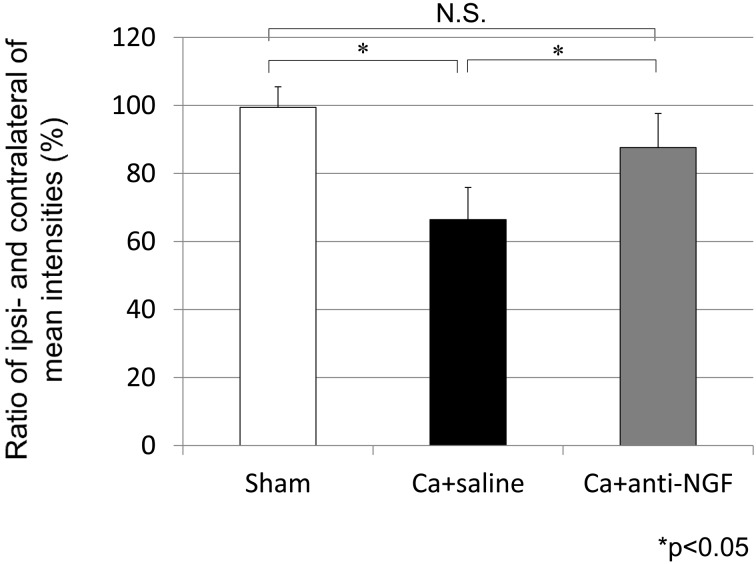
In the Ca+saline group, the ratio of the ipsi- and contralateral mean intensities from the CatWalk data at 3 weeks after surgery (66.4%) was significantly lower than that in the sham group (99.5%) (P<0.05). The ratio of the ipsi- and contralateral mean intensities was significantly higher in the Ca+anti-NGF group (87.6%) compared with the Ca+saline group (P<0.05). There were no significant differences between the sham group and the Ca+anti-NGF group (P>0.05) (Fig. 1).
Fig. 1.
The ratio of the ipsi- and contralateral mean intensities from the CatWalk system (*P<0.05, N.S.: nonsignificant, compared between the 3 groups using a nonrepeated measures ANOVA with Bonferroni post hoc correction).
Immunohistochemical analysis
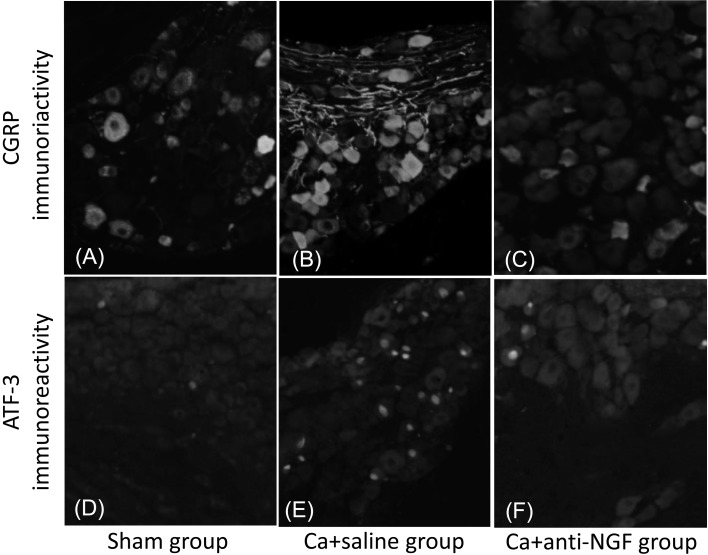
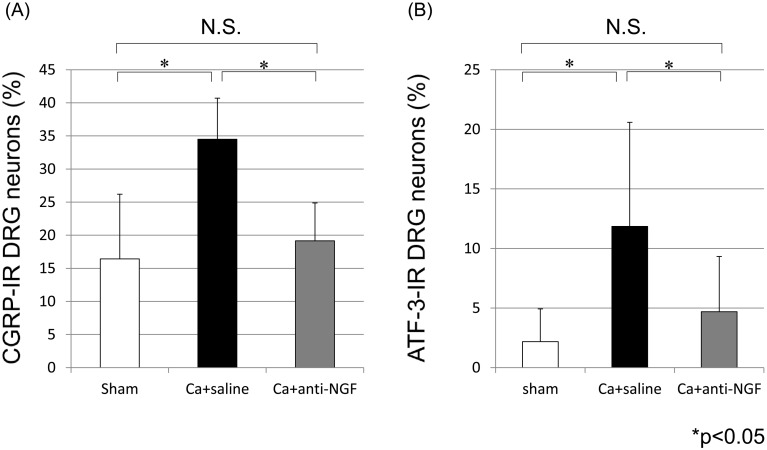
CGRP-IR DRG neurons (Figs. 2A–2C) and ATF-3-IR DRG neurons (Figs. 2D–2F) were present in the bilateral DRGs from L4 through L6. The proportion of CGRP-IR neurons among all DRG neurons in the Ca+saline group (34.5%) was significantly greater than the corresponding proportion in the sham group (16.4%) (P<0.05). The proportion of CGRP-IR neurons among all DRG neurons in the Ca+anti-NGF group (19.2%) was significantly smaller than the corresponding proportion in the Ca+saline group (P<0.05). There were no significant differences between the sham group and the Ca+anti-NGF group (P>0.05) (Fig. 3A). The proportion of ATF-3-IR neurons among all DRG neurons in the Ca+saline group (11.9%) was significantly greater than the corresponding proportion in the sham group (2.2%) (P<0.05). The proportion of ATF-3-IR neurons among all DRG neurons in the Ca+anti-NGF group (4.7%) was significantly smaller than the corresponding proportion in the Ca+saline group (P<0.05). There were no significant differences between the sham group and the Ca+anti-NGF group (P>0.05) (Fig. 3B).
Fig. 2.
Representative CGRP-IR (A, B, and C) and ATF-3-IR (D, E, and F) DRG neurons. A and D are from the sham group, B and E are from the Ca+saline group, and C and F are from the Ca+anti-NGF group.
Fig. 3.
The proportions of CGRP-IR neurons (A) or ATF-3-IR neurons (B) among all DRG neurons (*P<0.05, N.S.: nonsignificant, compared between the 3 groups using a nonrepeated measures ANOVA with Bonferroni post hoc correction).
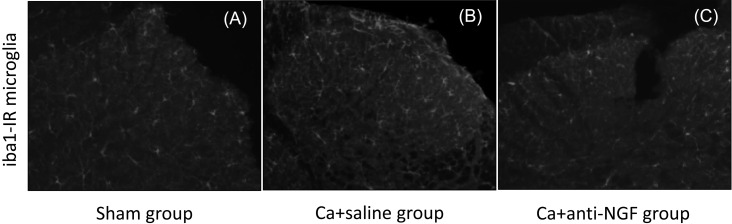
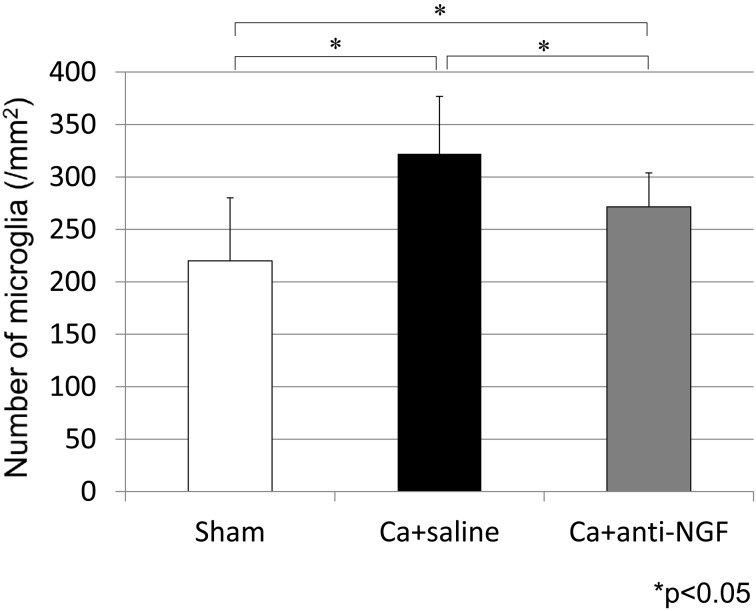
In the spinal dorsal horn, iba1-IR microglia were present in the 3 groups (Fig. 4). The densities of iba1-IR microglia in the Ca+saline group (322.2/mm2) and Ca+anti-NGF group (271.5/mm2) were significantly higher than the corresponding density in the sham group (220.0/mm2) (P<0.05). The density of iba1-IR microglia in the Ca+anti-NGF group was significantly lower than the corresponding density in the Ca+saline group (P<0.05) (Fig. 5).
Fig. 4.
Representative iba1-IR microglia in the spinal dorsal horn A, sham group; B, Ca+saline group, and C, Ca+anti-NGF group.
Fig. 5.
The density of iba1-IR microglia in the spinal dorsal horn (*P<0.05, N.S.: nonsignificant, compared between the 3 groups using a nonrepeated measures ANOVA with Bonferroni post hoc correction).
Discussion
In the present study, application of the murine sarcoma cells to the sciatic nerve produced mild gait disturbance, an increase of CGRP and ATF-3 immunoreactivity in DRGs, and an increased density of microglia in the spinal dorsal horn that could be suppressed by inhibiting NGF.
The CatWalk system was initially developed to evaluate motor function, such as that modified in models of spinal cord injury, Parkinson disease, or olivocerebellar degeneration [6, 7, 12, 16]. It has been applied to various models to evaluate behavior associated with pain, such as mechanical allodynia [9, 29]. In a rat model of neuropathic pain involving chronic sciatic nerve constriction injury, there was a high correlation between thresholds in a Von Frey test and the mean intensity from a CatWalk system [29]. It is well known that the Von Frey test can be used to evaluate mechanical allodynia. These findings suggest that the mild gait disturbance in the present study reflects mechanical allodynia. Therefore, application of the murine sarcoma cells to the sciatic nerve could induce mechanical allodynia.
In the current study, application of the murine sarcoma cells to the sciatic nerve induced an increase of CGRP- and ATF-3 immunoreativity in DRGs and an increased density of microglia in the spinal dorsal horn. CGRP has been identified in small NGF-dependent DRG neurons involved in the perception of inflammatory pain [2], suggesting that the upregulation of CGRP indicates a pain state. By contrast, ATF-3 is a transcription factor and a marker of nerve injury induced by stress [11, 20]. ATF-3 is not thought to be expressed during inflammation; therefore, upregulation of ATF-3 in DRG neurons suggests gradually progressive nerve injury [2, 13, 20]. Glial cells including microglia are activated by nerve injury [8, 27, 31]. Early microglial activation in the spinal dorsal horn follows various nerve injuries [8]. Microglia may be responsible for the initiation of neuropathic pain induced by peripheral nerve injury [5]. Therefore, our findings suggest that application of murine sarcoma cells to the sciatic nerve induced not only a neuropathic pain state but also an inflammatory pain state.
The increase in mechanical allodynia seen in the behavioral study, the CGRP and ATF-3 immunoreactivity in the DRGs, and activation of microglia in the spinal dorsal horn can be suppressed by inhibiting NGF. NGF was first discovered by Rita Levi-Montalcini in the early 1950s [1]. It is thought that NGF is essential for neuronal survival, proliferation, and differentiation in the peripheral and central nervous systems [30]. Averill et al. reported that intrathecal injection of NGF ameliorates the increase in ATF-3 expression [3]. However, by contrast, NGF is considered a mediator of chronic pain [32]. Anti-NGF therapy may be effective for reducing pain in models of low back pain [21], neck pain [25], and wrist pain [14]. NGF is thought to be new target for pain therapy. Therefore, anti-NGF therapy might be valuable for the treatment of neuropathic cancer pain. Regarding the possible mechanism of anti-NGF therapy for cancer neuropathic pain, it has been reported that NGF was produced by cells involved in local inflammation including macrophage, keratinocyte, and mast cells and that inflammatory cytokines enhanced NGF production [10, 28]. Anti-NGF therapy might suppress inflammation, and then inhibit nerve sensitization.
There are some limitations of this study. First, we did not evaluate the histology of the sciatic nerve. Although the relationship between NGF and cancer growth or metastasis has been investigated [26], the role of NGF in cancer growth remains unclear. In the present study, we investigated the efficacy of NGF inhibition for neuropathic pain induced only by cancer. Second, we did not evaluate side effects of anti-NGF therapy. Third, we did not investigate whether there was a dose–response effect of the anti-NGF antibody. Finally, we evaluated pain behavior in this study using only the CatWalk system, so we could not distinguish between pain-related behavior and sciatic nerve dysfunction without pain. Therefore, we also need to evaluate pain-related behavior using another behavioral test such as the Von Frey test. Further study is needed to clarify these points.
In conclusion, application of murine sarcoma cells onto the sciatic nerve induced mechanical allodynia, an increase in CGRP and ATF-3 immunoreactivity in DRGs, and activation of microglia in the spinal dorsal horn that could be suppressed by inhibiting NGF. These findings suggest that anti-NGF therapy might be valuable for treating neuropathic cancer pain.
References
- 1.Aloe L.2011. Rita Levi-Montalcini and the discovery of NGF, the first nerve cell growth factor. Arch. Ital. Biol. 149: 175–181. [DOI] [PubMed] [Google Scholar]
- 2.Averill S., McMahon S.B., Clary D.O., Reichardt L.F., Priestley J.V.1995. Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur. J. Neurosci. 7: 1484–1494. doi: 10.1111/j.1460-9568.1995.tb01143.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Averill S., Michael G.J., Shortland P.J., Leavesley R.C., King V.R., Bradbury E.J., McMahon S.B., Priestley J.V.2004. NGF and GDNF ameliorate the increase in ATF3 expression which occurs in dorsal root ganglion cells in response to peripheral nerve injury. Eur. J. Neurosci. 19: 1437–1445. doi: 10.1111/j.1460-9568.2004.03241.x [DOI] [PubMed] [Google Scholar]
- 4.Bartels R.H., van der Linden Y.M., van der Graaf W.T.2008. Spinal extradural metastasis: review of current treatment options. CA Cancer J. Clin. 58: 245–259. doi: 10.3322/CA.2007.0016 [DOI] [PubMed] [Google Scholar]
- 5.Cao H., Zhang Y.Q.2008. Spinal glial activation contributes to pathological pain states. Neurosci. Biobehav. Rev. 32: 972–983. doi: 10.1016/j.neubiorev.2008.03.009 [DOI] [PubMed] [Google Scholar]
- 6.Cendelín J., Voller J., Vozeh F.2010. Ataxic gait analysis in a mouse model of the olivocerebellar degeneration. Behav. Brain Res. 210: 8–15. doi: 10.1016/j.bbr.2010.01.035 [DOI] [PubMed] [Google Scholar]
- 7.Chuang C.S., Su H.L., Cheng F.C., Hsu S.H., Chuang C.F., Liu C.S.2010. Quantitative evaluation of motor function before and after engraftment of dopaminergic neurons in a rat model of Parkinson’s disease. J. Biomed. Sci. 17: 9. doi: 10.1186/1423-0127-17-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colburn R.W., DeLeo J.A., Rickman A.J., Yeager M.P., Kwon P., Hickey W.F.1997. Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. J. Neuroimmunol. 79: 163–175. doi: 10.1016/S0165-5728(97)00119-7 [DOI] [PubMed] [Google Scholar]
- 9.Deumens R., Jaken R.J., Marcus M.A., Joosten E.A.2007. The CatWalk gait analysis in assessment of both dynamic and static gait changes after adult rat sciatic nerve resection. J. Neurosci. Methods 164: 120–130. doi: 10.1016/j.jneumeth.2007.04.009 [DOI] [PubMed] [Google Scholar]
- 10.Gelincik A., Aydın F., Ozerman B., Ergüven M., Aydın S., Bilir A., Genç S., Eroğlu H., Colakoğlu B., Erden S., Büyüköztürk S.2012. Enhanced nerve growth factor expression by mast cells does not differ significantly between idiopathic and allergic rhinitis. Ann. Allergy Asthma Immunol. 108: 396–401. doi: 10.1016/j.anai.2012.04.006 [DOI] [PubMed] [Google Scholar]
- 11.Hai T.W., Liu F., Coukos W.J., Green M.R.1989. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes Dev. 3: 2083–2090. doi: 10.1101/gad.3.12b.2083 [DOI] [PubMed] [Google Scholar]
- 12.Hamers F.P., Lankhorst A.J., van Laar T.J., Veldhuis W.B., Gispen W.H.2001. Automated quantitative gait analysis during overground locomotion in the rat: its application to spinal cord contusion and transection injuries. J. Neurotrauma 18: 187–201. doi: 10.1089/08977150150502613 [DOI] [PubMed] [Google Scholar]
- 13.Inoue G., Ohtori S., Aoki Y., Ozawa T., Doya H., Saito T., Ito T., Akazawa T., Moriya H., Takahashi K.2006. Exposure of the nucleus pulposus to the outside of the anulus fibrosus induces nerve injury and regeneration of the afferent fibers innervating the lumbar intervertebral discs in rats. Spine 31: 1433–1438. doi: 10.1097/01.brs.0000219946.25103.db [DOI] [PubMed] [Google Scholar]
- 14.Iwakura N., Ohtori S., Orita S., Yamashita M., Takahashi K., Kuniyoshi K.2010. Role of low-affinity nerve growth factor receptor inhibitory antibody in reducing pain behavior and calcitonin gene-related Peptide expression in a rat model of wrist joint inflammatory pain. J. Hand Surg. Am. 35: 267–273. doi: 10.1016/j.jhsa.2009.10.030 [DOI] [PubMed] [Google Scholar]
- 15.Johnson E.M., Jr, Rich K.M., Yip H.K.1986. The role of NGF in sensory neurons in vivo. Trends Neurosci. 9: 33–37. doi: 10.1016/0166-2236(86)90012-3 [DOI] [Google Scholar]
- 16.Koopmans G.C., Deumens R., Honig W.M., Hamers F.P., Steinbusch H.W., Joosten E.A.2005. The assessment of locomotor function in spinal cord injured rats: the importance of objective analysis of coordination. J. Neurotrauma 22: 214–225. doi: 10.1089/neu.2005.22.214 [DOI] [PubMed] [Google Scholar]
- 17.Lewin G.R., Mendell L.M.1993. Nerve growth factor and nociception. Trends Neurosci. 16: 353–359. doi: 10.1016/0166-2236(93)90092-Z [DOI] [PubMed] [Google Scholar]
- 18.Lewin G.R., Rueff A., Mendell L.M.1994. Peripheral and central mechanisms of NGF-induced hyperalgesia. Eur. J. Neurosci. 6: 1903–1912. doi: 10.1111/j.1460-9568.1994.tb00581.x [DOI] [PubMed] [Google Scholar]
- 19.Mendell L.M.1996. Neurotrophins and sensory neurons: role in development, maintenance and injury. A thematic summary. Philos. Trans. R. Soc. Lond. B Biol. Sci. 351: 463–467. doi: 10.1098/rstb.1996.0043 [DOI] [PubMed] [Google Scholar]
- 20.Obata K., Yamanaka H., Fukuoka T., Yi D., Tokunaga A., Hashimoto N., Yoshikawa H., Noguchi K.2003. Contribution of injured and uninjured dorsal root ganglion neurons to pain behavior and the changes in gene expression following chronic constriction injury of the sciatic nerve in rats. Pain 101: 65–77. doi: 10.1016/S0304-3959(02)00296-8 [DOI] [PubMed] [Google Scholar]
- 21.Orita S., Ohtori S., Nagata M., Horii M., Yamashita M., Yamauchi K., Inoue G., Suzuki M., Eguchi Y., Kamoda H., Arai G., Ishikawa T., Miyagi M., Ochiai N., Kishida S., Takaso M., Aoki Y., Takahashi K.2010. Inhibiting nerve growth factor or its receptors downregulates calcitonin gene-related peptide expression in rat lumbar dorsal root ganglia innervating injured intervertebral discs. J. Orthop. Res. 28: 1614–1620. doi: 10.1002/jor.21170 [DOI] [PubMed] [Google Scholar]
- 22.Peters L.J.1990. The ESTRO Regaud lecture. Inherent radiosensitivity of tumor and normal tissue cells as a predictor of human tumor response. Radiother. Oncol. 17: 177–190. doi: 10.1016/0167-8140(90)90202-8 [DOI] [PubMed] [Google Scholar]
- 23.Ramer M.S., Bisby M.A.1999. Adrenergic innervation of rat sensory ganglia following proximal or distal painful sciatic neuropathy: distinct mechanisms revealed by anti-NGF treatment. Eur. J. Neurosci. 11: 837–846. doi: 10.1046/j.1460-9568.1999.00491.x [DOI] [PubMed] [Google Scholar]
- 24.Ro L.S., Chen S.T., Tang L.M., Jacobs J.M.1999. Effect of NGF and anti-NGF on neuropathic pain in rats following chronic constriction injury of the sciatic nerve. Pain 79: 265–274. doi: 10.1016/S0304-3959(98)00164-X [DOI] [PubMed] [Google Scholar]
- 25.Sainoh T., Sakuma Y., Miyagi M., Orita S., Yamauchi K., Inoue G., Kamoda H., Ishikawa T., Suzuki M., Kubota G., Oikawa Y., Inage K., Sato J., Nakamura J., Aoki Y., Takaso M., Toyone T., Takahashi K., Ohtori S.2014. Efficacy of anti-nerve growth factor therapy for discogenic neck pain in rats. Spine 39: E757–E762. doi: 10.1097/BRS.0000000000000340 [DOI] [PubMed] [Google Scholar]
- 26.Shimoyama M., Tanaka K., Hasue F., Shimoyama N.2002. A mouse model of neuropathic cancer pain. Pain 99: 167–174. doi: 10.1016/S0304-3959(02)00073-8 [DOI] [PubMed] [Google Scholar]
- 27.Stoll G., Jander S.1999. The role of microglia and macrophages in the pathophysiology of the CNS. Prog. Neurobiol. 58: 233–247. doi: 10.1016/S0301-0082(98)00083-5 [DOI] [PubMed] [Google Scholar]
- 28.Takaoka K., Shirai Y., Saito N.2009. Inflammatory cytokine tumor necrosis factor-alpha enhances nerve growth factor production in human keratinocytes, HaCaT cells. J. Pharmacol. Sci. 111: 381–391. doi: 10.1254/jphs.09143FP [DOI] [PubMed] [Google Scholar]
- 29.Vrinten D.H., Hamers F.F.2003. ‘CatWalk’ automated quantitative gait analysis as a novel method to assess mechanical allodynia in the rat; a comparison with von Frey testing. Pain 102: 203–209. doi: 10.1016/s0304-3959(02)00382-2 [DOI] [PubMed] [Google Scholar]
- 30.Wang W., Chen J., Guo X.2014. The role of nerve growth factor and its receptors in tumorigenesis and cancer pain. Biosci. Trends 8: 68–74. doi: 10.5582/bst.8.68 [DOI] [PubMed] [Google Scholar]
- 31.Watkins L.R., Martin D., Ulrich P., Tracey K.J., Maier S.F.1997. Evidence for the involvement of spinal cord glia in subcutaneous formalin induced hyperalgesia in the rat. Pain 71: 225–235. doi: 10.1016/S0304-3959(97)03369-1 [DOI] [PubMed] [Google Scholar]
- 32.Watson J.J., Allen S.J., Dawbarn D.2008. Targeting nerve growth factor in pain: what is the therapeutic potential? BioDrugs 22: 349–359. doi: 10.2165/0063030-200822060-00002 [DOI] [PubMed] [Google Scholar]
- 33.Wild K.D., Bian D., Zhu D., Davis J., Bannon A.W., Zhang T.J., Louis J.C.2007. Antibodies to nerve growth factor reverse established tactile allodynia in rodent models of neuropathic pain without tolerance. J. Pharmacol. Exp. Ther. 322: 282–287. doi: 10.1124/jpet.106.116236 [DOI] [PubMed] [Google Scholar]