Abstract
Induction of hyperbilirubinemia in experimental rabbits by phenylhydrazine was optimized in terms of dose, dose interval and number of doses using response surface methodology. Central Composite Design was employed using five levels for each of the three input variables. Degree of hyperbilirubinemia was measured in terms of bilirubin level in serum of animals. A dose dependent significant elevation (P<0.05) of total serum bilirubin level was observed which was optimized by using eight factorial, six axial and six central points as suggested by experimental design. Optimum levels of phenylhydrazine dose, total number of doses and a dose interval to achieve maximum elevation (4.06 mg/dl−1) of total serum bilirubin were found to be 11.56 mg/kg−1 body weight, 8 and 24.65 h, respectively. The induction procedure was validated by performing five replicate experiments on a group of five animals which showed 3.56 ± 0.47 mg/kg−1 body weight elevation in total serum bilirubin level.
Keywords: hyperbilirubinemia, jaundice in rabbit, phenylhydrazine, response surface methodology, total serum bilirubin
Introduction
Bilirubin is a bile pigment produced in animal body through a natural hemolytic process. It is insoluble in its free form. It gets associated with albumin in blood and is transported to liver where it undergoes the process of conjugation with glucoronic acid catalyzed by UDP-glucoronosyl transferase. The conjugated bilirubin, generally known as direct bilirubin (DB), is soluble in water and is excreted through bile into small intestine and finally into feces [20, 29]. Excessive hemolysis, abnormal hepatic uptake of unconjugated bilirubin, abnormalities in conjugation process, such as in hepatitis and liver cirrhosis, and obstruction in biliary excretion of DB are responsible for elevation in serum bilirubin level, a clinical condition known as hyperbilirubinemia. Unconjugated bilirubin, being insoluble in water, deposits in adipose tissue resulting in a physiological condition known as jaundice [6, 20].
In human hyperbilirubinemia may result in irreversible bilirubin induced brain damage, termed as kernicterus, in newborns that must be managed immediately [8, 17]. Phototherapy involving photodegradation of bilirubin by ultra-violet (UV) radiation is the most commonly used method for treatment of this condition [2, 10, 30]. The recovery process is slow, requires a long exposure time and must be discontinued during feeding. There are also chances of rebinding of bilirubin after phototherapy [3]. It is, therefore, desirable to find rapid and more efficient methods for management of hyperbilirubinemia. An ever first study demonstrating degradation of bilirubin to biliverdin (an excretable product) in vitro by gamma irradiation was reported from our laboratory [12] followed by others [27, 28], which verified these results. This in vitro work could not be extended to in vivo experiments due to non-availability of suitable animal models. The only animal model available at that time was rat [31,32,33,34,35,36,37], which being too small to conduct sequential multiple experimentation could not be used. Therefore, the objective of this study was to develop a suitable animal model, which can be used to study the effect of gamma irradiation or any other agent to degrade bilirubin to excretable (water soluble) products. Rabbit being bigger, easy to handle and suitable for blood sampling spread over several weeks, was considered most suitable for the purpose.
Phenylhydrazine (PH) has been reported to induce hemolytic anemia and hyperbilirubinemia in animals due to its hemolytic activity [4, 31, 34]. Hemolytic anemia and hyperbilirubinemia are closely interlinked with each other as the breakdown of red cells leads to jaundice (hyperbilirubinemia) and hyperbilirubinemia in turn triggers anemia by inducing death of red cells [18]. Ursodeoxycholic acid is a bile acid which prevents bilirubin excretion by inhibiting UDP-glucoronosyl transferase in liver [33]. Thus we, hereby, report a method where PH was used in combination with ursodeoxycholic acid to induce hyperbilirubinemia in rabbit. Our preliminary experimentation indicated that we need to optimize conditions for induction in terms of i) dose level, ii) number of doses and iii) dose interval. The conventional method of varying one parameter and keeping others constant cannot be applied to optimize the procedure because this would neglect inter-parametric effect on optimal value. Therefore, a multivariate technique was thought to be more appropriate. In order to optimize a multivariate process, Response Surface Methodology (RSM) is considered to be the most suitable technique. RSM consists of a set of statistical and mathematical tools which are applied for designing, developing, improving and optimizing a process [24, 35]. This technique has been extensively used in various fields of research, which generates response-surface models for optimization and prediction of changes in response variables as a function of changes in input variables [22, 23].
Materials and Methods
Materials
All chemicals were of analytical grade. PH, ursodeoxycholic acid, bilirubin, and ethanol were obtained from Sigma Aldrich, USA; bilirubin assay kit from Randox Laboratories, UK and sample tubes containing gel and clot activator (Y330984 IMPROVACUTER) from Guangzhou Improve Medical Instruments Co., Ltd., China.
Preparation of solutions
PH solution (10 mg/ml−1) was freshly prepared in 20% aqueous ethanol. Ursodeoxycholic acid (10 mg/ml−1) solution was also prepared fresh in 20% aqueous ethanol and bilirubin standard solution was prepared in 80% methanol. Bilirubin assay kit (Randox, UK ) was used according to its instruction manual.
Animals
White male rabbits (n=67, age: 4–6 month, weight: 1.00 ± 0.15 kg) were acclimated in natural underground environment for one week at 24 ± 5°C, 12 h (in light and dark) duration and relative humidity 40–60%. The animals were allowed to drink fresh water de labitum and fed three times a day on a diet containing blend of different cereals and legumes including wheat, maize, barley, chickpea and small pea throughout the study period. Use of animals in this research was approved by Institutional Ethical Committee of Bahauddin Zakariya University, Multan.
Determination of tolerable PH dose
In order to determine maximum tolerable dose of PH, the animals were randomly allocated to 9 groups each group consisting of 3 animals and were administered orally varying amounts of PH solution (10% in 20% ethanol) as detailed in Table 1. The animals were examined physically after each dose for any change in eye and skin color and activity. Based on this experiment, tolerable doses, at which the animals survived, were selected for optimization process.
Table 1. Preliminary selection of maximum tolerable dose of PH for experimental animals.
| Animal groups | Dose (mg/kg-1 bw) | Dose interval (h) | Total number of doses (n) | Total dose (mg/kg-1 bw) | Total duration of treatment (h) | Physical signs | Tolerance by animals |
|---|---|---|---|---|---|---|---|
| 1 | 5 | 12 | 2 | 10 | 24 | No physical change | Survived |
| 5 | 12 | 4 | 20 | 48 | Slight appearance of yellow color of skin and eyes | Survived | |
| 5 | 12 | 8 | 40 | 96 | Slight change in skin and eye color | Survived | |
| 2 | 5 | 24 | 2 | 10 | 48 | No physical change | Survived |
| 5 | 24 | 4 | 20 | 96 | Slight appearance of yellow color of skin and eyes | Survived | |
| 5 | 24 | 8 | 40 | 192 | Slight appearance of yellow color of skin and eyes | Survived | |
| 3 | 5 | 48 | 2 | 10 | 96 | No physical change | Survived |
| 5 | 48 | 4 | 20 | 192 | No physical change | Survived | |
| 5 | 48 | 8 | 40 | 382 | Slight appearance of yellow color of skin and eyes | Survived | |
| 4 | 25 | 12 | 2 | 50 | 24 | Appearance of deep yellow color of skin and eyes | Survived |
| 25 | 12 | 4 | 100 | 48 | Anemic and lethargic response, gray color of skin and eyes due to excess hemolysis | Survived | |
| 25 | 12 | 8 | 200 | 96 | Swear anemic response, dark gray color of skin and eyes due to almost complete hemolysis | Died after 8th dose | |
| 5 | 25 | 24 | 2 | 50 | 48 | Appearance of deep yellow color of skin and eyes | Survived |
| 25 | 24 | 4 | 100 | 96 | Appearance of deep yellow color of skin and eyes | Survived | |
| 25 | 24 | 8 | 200 | 192 | Anemic and lethargic response, gray color of skin and eyes due to excess hemolysis | Survived | |
| 6 | 25 | 48 | 2 | 50 | 96 | No physical change | Survived |
| 25 | 48 | 4 | 100 | 192 | Appearance of deep yellow color of skin and eyes | Survived | |
| 25 | 48 | 8 | 200 | 382 | Anemic and lethargic response, gray color of skin and eyes due to excess hemolysis | Survived | |
| 7 | 50 | 12 | 2 | 100 | 24 | Anemic and lethargic response, gray color of skin and eyes due to excess hemolysis | Survived |
| 50 | 12 | 4 | 200 | 48 | Swear anemic response, dark gray color of skin and eyes due to almost complete hemolysis | Died after 4th dose | |
| 50 | 12 | 8 | 400 | Not treated | |||
| 8 | 50 | 24 | 2 | 100 | 48 | Anemic and lethargic response, gray color of skin and eyes due to excess hemolysis | Survived |
| 50 | 24 | 4 | 200 | 96 | Anemic and lethargic response, gray color of skin and eyes due to excess hemolysis | Survived | |
| 50 | 24 | 8 | 400 | 192 | Swear anemic response, dark gray color of skin and eyes due to almost complete hemolysis | Died after 7th dose | |
| 9 | 50 | 48 | 2 | 100 | 96 | Appearance of deep yellow color of skin and eyes | Survived |
| 50 | 48 | 4 | 200 | 192 | Anemic and lethargic response, gray color of skin and eyes due to excess hemolysis | Survived | |
| 50 | 48 | 8 | 400 | 382 | Swear anemic response, dark gray color of skin and eyes due to almost complete hemolysis | Died after 8th dose | |
Induction of hyperbilirubinemia
Hyperbilirubinemia was induced by administration of an oral dose of PH solution (10% in 20% ethanol) immediately followed by an intraperitoneal injection of ursodeoxycholic acid solution (1 mg/kg−1 body weight (bw)) as an inhibitor of bilirubin-UDP glucoronyl transferase to prevent conjugation of bilirubin with glucoronic acid. Serum bilirubin level was determined after administration of PH doses of 5, 10, 15, 20 and 25 mg/kg−1 bw to a group of five animals. The dose was repeated three times after an interval of 24 h in each case.
Blood samples (5 ml) were collected in gel sample tubes 24 h after the last dose by punching the middle vein of ear with a syringe needle (gauge: 22, length: 1.5 in.). The samples were centrifuged at 5,000 rpm for 20 min at 25 ± 2°C. Sera were collected in serum tubes and stored in dark at −4°C for determination of total serum bilirubin (TSB).
Determination of serum bilirubin
TSB and serum DB were determined by diazosulfanilic acid method according to a reported method [11] using the bilirubin kit. Results were reported as mg dl−1 and the degree of hyperbilirubinemia induced was expressed in terms of elevation in TSB level. Erythrocyte count and hemoglobin level were determined by use of hematology analyzer (Abbot Hematology, CELL-DYN Emerald). Graphs were plotted between PH dose, TSB, DB, erythrocyte count and hemoglobin level to verify inhibition of bilirubin conjugation and to determine the correlation between hyperbilirubinemia and hemolytic anemia. TSB was used as response for optimization process.
Experimental design for optimization of induction of hyperbilirubinemia
Optimization of minimal dose required for maximal hyperbilirubinemia was carried out by using three-factorial central composite design (CCD) in RSM as it is suitable for fitting a quadratic surface, which is considered to work well for process optimization. It consists of a core factorial that forms a cube with sides that are two coded units in length from −2 to +2. The three variables (factors) were χ1: dose of PH (mg/kg-1 bw), χ2: number of doses and χ3 dose interval (h) and the response was measured as TSB (mg/dl-1). Each variable was input at five levels in the tolerable range; thus χ1 was set at 5, 10, 15, 20 and 25 mg kg-1 bw, χ2 at 2, 4, 6, 8 and 10, and χ3 at 12, 24, 36, 48 and 60 h.
Coded levels of these variables were calculated by use of expression 1.
 (1) (1)
|
where  and
Si are suitable location and scale factors respectively and
χ1 is the coded value of variable
ξ1 (i = 1,2,…,k). The
specific codes of the selected variables were calculated using expressions 2–4 [19]. The coded values of the variables without
replicates with six
and
Si are suitable location and scale factors respectively and
χ1 is the coded value of variable
ξ1 (i = 1,2,…,k). The
specific codes of the selected variables were calculated using expressions 2–4 [19]. The coded values of the variables without
replicates with six
 (2) (2)
|
 (3) (3)
|
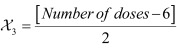 (4) (4)
|
center points were used in the RSM (CCD) routine of Design Expert® (DX9, Stat-Ease, Inc., USA) to obtain the design matrix as shown in Table 2. Optimization of response was carried out after studying the analysis of variance (ANOVA), which suggested significance of model on the basis of P<0.05 and F (lack of fit)>3.21. The design suggested 20 experiments having 8 factorial, 6 axial and 6 center points as shown in Table 2. These experiments performed under the suggested conditions and results were included in the respective column. The data fitted best in the polynomial quadratic response surface model and following generalized equation was obtained.
 (5) (5)
|
where Yi is predicted response; βo is a constant; β1, β2 and β3 are regression coefficients for main variable effects; β11, β22 and β33 are quadratic effects and β12, β13 and β23 are interaction effects of variables.
Table 2. Coded and actual levels of independent variables as per design by central composite design.
| Standards | Experimental runs | Coded levels of variables | Actual levels of variables | |||||
|---|---|---|---|---|---|---|---|---|
| χ1 | χ2 | χ3 | ξ1: Dose of PH (mg/kg-1 bw) | ξ2: Total number of doses (n) | ξ3: Dose interval (h) | |||
| 1 | 10 | –1 | –1 | –1 | 10 | 4 | 24 | |
| 2 | 1 | 1 | –1 | –1 | 20 | 4 | 24 | |
| 3 | 17 | –1 | 1 | –1 | 10 | 8 | 24 | |
| 4 | 20 | 1 | 1 | –1 | 20 | 8 | 24 | |
| 5 | 14 | –1 | –1 | 1 | 10 | 4 | 48 | |
| 6 | 12 | 1 | –1 | 1 | 20 | 4 | 48 | |
| 7 | 9 | –1 | 1 | 1 | 10 | 8 | 48 | |
| 8 | 8 | 1 | 1 | 1 | 20 | 8 | 48 | |
| 9 | 2 | –2 | 0 | 0 | 5 | 6 | 36 | |
| 10 | 3 | 2 | 0 | 0 | 25 | 6 | 36 | |
| 11 | 6 | 0 | –2 | 0 | 15 | 2 | 36 | |
| 12 | 18 | 0 | 2 | 0 | 15 | 10 | 36 | |
| 13 | 4 | 0 | 0 | –2 | 15 | 6 | 12 | |
| 14 | 15 | 0 | 0 | 2 | 15 | 6 | 60 | |
| 15* | 5 | 0 | 0 | 0 | 15 | 6 | 36 | |
| 16* | 16 | 0 | 0 | 0 | 15 | 6 | 36 | |
| 17* | 19 | 0 | 0 | 0 | 15 | 6 | 36 | |
| 18* | 13 | 0 | 0 | 0 | 15 | 6 | 36 | |
| 19* | 11 | 0 | 0 | 0 | 15 | 6 | 36 | |
| 20* | 7 | 0 | 0 | 0 | 15 | 6 | 36 | |
| Coded level of variables | –2 | –1 | 0 | 1 | 2 | |||
| Actual levels of variables | ||||||||
| ξ1: Dose of PH (mg/kg-1 bw) | 5 | 10 | 15 | 20 | 25 | |||
| ξ2: Total number of doses | 2 | 4 | 6 | 8 | 10 | |||
| ξ3: Dose Interval (h) | 12 | 24 | 36 | 48 | 60 | |||
*Center points.
Significance of the estimated regression coefficient for each response variable was assessed by lack of fit test (F-ratio) at a probability (P) of 0.05. The lack of fit measures the failure of a model to fit the data in experimental domain particularly for reduced points in a randomized experiment. The reduced model contained only those terms, which were found statistically significant (P<0.05). Corresponding variables with larger F-values and smaller P-values were considered more significant. Coefficient of determination (R2) and adjusted coefficient of determination (R2adj) were also determined to check adequacy of response surface models and to measure fairness of fit of regression equation, respectively. Precision and reliability of experiments were checked by determining coefficient of variation (CV). A low value of CV suggests a better precision and reliability of experiments. Adequate precision measures signal to noise ratio in an experiment. A ratio greater than 4 indicates an adequate signal.
Results
Tolerable dose of PH
Results for determination of maximum tolerable dose of PH in the experimental animals are given in Table 1. In twenty-seven experiments performed the animals showed tolerance in the PH dose range of 50–200 mg/kg−1 bw distributed into 2–8 doses repeated at an interval of 24–48 h; they continued to survive with anemic and lethargic responses and some changes in skin and eye color (Table 1). Based on this observation a range of 20–200 mg/kg−1 bw distributed in 4–8 doses and repeated after 24–48 h was selected to generate reasonable data for optimization.
Induction of hyperbilirubinemia
In order to assess the progress of hyperbilirubinemia a dose dependent behavior was studied by plotting TSB and DB separately against dose administered and the results are shown in Fig. 1 (a). An exponential rise (R2=0.9584) in TSB was observed against PH dose, whereas an almost horizontal line was obtained in case of DB. In line with this the erythrocyte count and hemoglobin level decreased with an increase in PH dose as shown in Fig. 1 (b).
Fig. 1.
Dose dependent response of (a) Total serum bilirubin (TSB) and direct bilirubin (DB) level, and (b) Erythrocyte count and hemoglobin level of phenylhydrazine treated animals.
Response surface analysis and optimization
The experimental values of TSB were used to optimize the procedure for induction of
hyperbilirubinemia. The values as suggested by the design under specific conditions are
listed in Table 3. These data were analyzed using ANOVA; the results are given in Table 4. This analysis indicated that the quadratic model selected in this study is
significant with F and P values of 7.35 (>3.21) and
0.0022 (<0.05), respectively. Similarly dose and number of doses have significant
linear effect on TSB level. No significant interaction was indicated between the
variables, whereas the number of doses and dose interval has significant quadratic effect
on TSB. The model best fitting the experimental data was represented by following
equation.
Table 3. Experimental values of serum total bilirubin (STB) before and after treatment with PH at various levels of independent variables as per design by CCD.
| Standards | Experimental runs | Actual levels of variables | STB (mg/dl-1) | |||||
|---|---|---|---|---|---|---|---|---|
| ξ1: Dose of PH (mg/kg-1 bw) | ξ2: Total number of doses | ξ3: Dose interval (h) | Control | PH treated | Elevation | |||
| 1 | 10 | 10 | 4 | 24 | 0.21 | 1.99 | 1.78 | |
| 2 | 1 | 20 | 4 | 24 | 0.33 | 3.32 | 2.99 | |
| 3 | 17 | 10 | 8 | 24 | 0.39 | 4.84 | 4.45 | |
| 4 | 20 | 20 | 8 | 24 | 0.37 | 5.2 | 4.83 | |
| 5 | 14 | 10 | 4 | 48 | 0.3 | 2.97 | 2.67 | |
| 6 | 12 | 20 | 4 | 48 | 0.3 | 3.21 | 2.91 | |
| 7 | 9 | 10 | 8 | 48 | 0.24 | 3.37 | 3.13 | |
| 8 | 8 | 20 | 8 | 48 | 0.31 | 4.22 | 3.91 | |
| 9 | 2 | 5 | 6 | 36 | 0.25 | 2.11 | 1.86 | |
| 10 | 3 | 25 | 6 | 36 | 0.29 | 5.19 | 4.9 | |
| 11 | 6 | 15 | 2 | 36 | 0.31 | 2.21 | 1.9 | |
| 12 | 18 | 15 | 10 | 36 | 0.21 | 3.31 | 3.1 | |
| 13 | 4 | 15 | 6 | 12 | 0.3 | 3.6 | 3.3 | |
| 14 | 15 | 15 | 6 | 60 | 0.38 | 3.41 | 3.03 | |
| 15 | 5 | 15 | 6 | 36 | 0.29 | 4.24 | 3.95 | |
| 16 | 16 | 15 | 6 | 36 | 0.25 | 3.98 | 3.73 | |
| 17 | 19 | 15 | 6 | 36 | 0.35 | 4.84 | 4.49 | |
| 18 | 13 | 15 | 6 | 36 | 0.32 | 4.93 | 4.61 | |
| 19 | 11 | 15 | 6 | 36 | 0.3 | 4.36 | 4.06 | |
| 20 | 7 | 15 | 6 | 36 | 0.31 | 4.65 | 4.34 | |
Table 4. ANOVA in total serum bilirubin (TSB) in response to three variables by use of quadratic model.
| Source | Sum of squares | df | Mean square | F-value | P-value |
|---|---|---|---|---|---|
| β0: Model | 15.94 | 9 | 1.77 | 7.35 | 0.0022 |
| β1: PH Dose | 4.73 | 1 | 4.73 | 19.65 | 0.0013 |
| β2: No. of doses | 4.38 | 1 | 4.38 | 18.18 | 0.0017 |
| β3: Dose interval | 0.24 | 1 | 0.24 | 1.01 | 0.3396 |
| β12 | 0.011 | 1 | 0.011 | 0.045 | 0.8369 |
| β13 | 0.04 | 1 | 0.04 | 0.17 | 0.6917 |
| β23 | 1.16 | 1 | 1.16 | 4.81 | 0.0531 |
| β11 | 1.02 | 1 | 1.02 | 4.24 | 0.0666 |
| β22 | 4.48 | 1 | 4.48 | 18.59 | 0.0015 |
| β33 | 1.64 | 1 | 1.64 | 6.82 | 0.026 |
| Residual | 2.41 | 10 | 0.24 | ||
| Lack of Fit | 1.84 | 5 | 0.37 | 3.21 | 0.1134 |
| Pure Error | 0.57 | 5 | 0.11 | ||
| Cor Total | 18.35 | 19 | |||
| CV | 14.3 | ||||
| R2 | 0.8687 | ||||
| Adjusted R2 | 0.7506 | ||||
| Predicted R2 | 0.1355 | ||||
| Adequate precision | 9.97 |
A summary statistics of the model is given in Table 4.
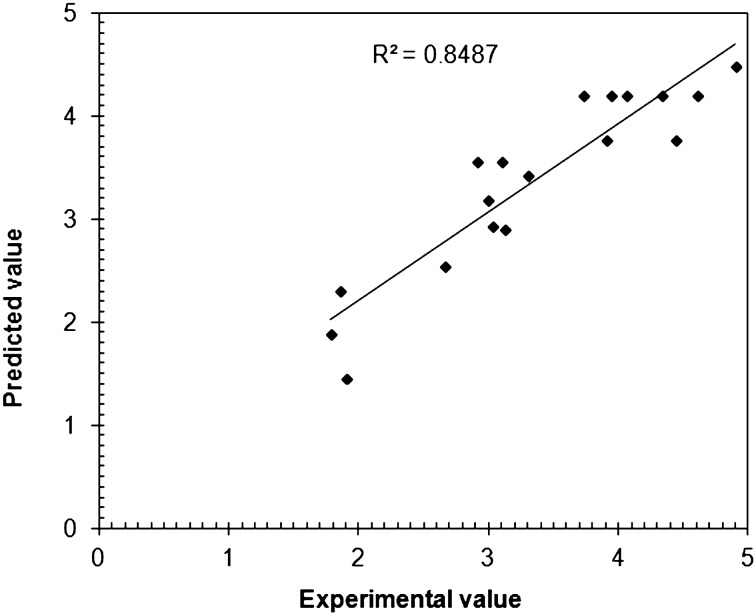
Variations in TSB in response to a change in dose, number of doses and dose interval are presented in three-dimensional (3D) response surface plots (Figs. 2a–2c). The model was validated by comparing the predicted values of TSB with experimental values (Fig. 3). A good agreement (R2=0.8487, R2adj=0.7506) was found to exist between them with a lower value of CV (14.01%) indicating reliability of the model. A higher value of adequate precision (9.97) provided an adequate signal which indicates that the suggested model can be used to navigate the design space for optimization of hyperbilirubinemia in animals.
Fig. 2.
3D response surface plots of elevation in total serum bilirubin level (a-c) in response to variation in phenylhydrazine dose, total number of doses and dose interval.
Fig. 3.
Correlation between the experimental and predicted values of total serum bilirubin levels (mg/dl−1) at selected levels of phenylhydrazine dose, number of doses and dose interval.
Optimization results are presented in Table 5. The optimum conditions thus suggested by the model were found to be: dose approximately 11.5 mg/kg−1 bw, number of equally distributed doses 8 and dose interval of 24 h to achieve a TSB level of 4.06 ± 0.49 mg/dl−1. An experiment was performed under the suggested optimum conditions to validate the optimization process by use of a group of 5 animals. The variation in TSB was found to be 3.58 ± 0.47 mg/dl−1 which was close to that optimized by CCD.
Table 5. Optimum levels of independent variables where the desired level of the response variables is predicted to be achieved.
| Variables | Goal | Lower limit | Upper limit | Optimum levels | Desirability | |||
|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Y | |||||
| Dose of PH (mg/kg-1 bw) | minimize | 5 | 25 | |||||
| Total number of doses (n) | in range | 2 | 10 | |||||
| Dose interval (h) | in range | 12 | 60 | |||||
| Elevation in total serum bilirubin (mg/dl-1) | maximize | 1.78 | 4.9 | 11.56 | 8 | 24.56 | 4.06 | 0.7 |
| Reproducibility | ||||||||
| Elevation in total serum bilirubin (mg/dl-1) | 3.58 ± 0.47 | |||||||
Discussion
PH is known to cause hemolytic anemia in experimental animals by disrupting the cytoskeleton of erythrocytes. The proposed mechanism of PH induced hemolysis involves increased oxidative stress in erythrocyte membrane caused by i) peroxidation of membrane lipids as a result of auto-oxidation of PH and interaction of oxygen radicals with membrane lipids, ii) generation of superoxide anion radical by PH and formation of Heinz bodies, iii) enhanced levels of hydrogen peroxide in erythrocytes resulting in glutathione depletion and iv) production of aryl, hydroxyl, superoxide, and phenyl radicals by PH [4, 14, 15, 21, 34 ]. The mechanism of PH induced hemolysis also involves rupturing of erythrocyte membrane due to binding of PH with the membrane proteins such as spectrin with a ligand derived from its aryl portion [1]. PH is also known to degrade hemoglobin by direct oxidation of oxyhemoglobin to methemoglobin [26]. Degradation of hemoglobin leads to excess production of unconjugated bilirubin [5]. PH has been used for induction of haemolytic anaemia and hyperbilirubinemia to study various hematological and physiological mechanisms in experimental animal models [7, 13, 16, 31, 32, 36, 37]. Previously reported animal models of hyperbilirubinemia induced by PH include Sprague-Dawley rat [9], Gunn rat [31] and Wistar rat [37]. Enzymatic activity of red blood cell in rabbit has been used as a model for humans [38], therefore, rabbit was considered as the most appropriate model of hyperbilirubinemia in the present work.
Tolerable dose of PH
PH induced excess hemolysis causes swear anemia which finally leads to death [4]. It was, therefore, necessary to treat experimental animals with a tolerable dose of PH while working on such animal models. The dose in the range 20–200 mg/kg−1 bw distributed in 4–8 doses and repeated after 24–48 h was found to be tolerable and was subsequently optimized by RSM.
Induction of hyperbilirubinemia
Hyperbilirubinemia was successfully induced and found to be dose dependent (Fig. 1 (a)) after administration of repeated doses (3 times) of PH varying between 5 and 25 mg/kg−1 bw. The results clearly indicate that induction is dose dependent and free from effect of UDP glucoronyl transferase. Erythrocyte count and hemoglobin level reduced accordingly (Fig. 1 (b)), which established a link between hyperbilirubinemia and hemolytic anemia. The elevation in TSB may be attributed to excessive release of bilirubin due to PH induced hemolysis. It may also be attributed to slow rate of bilirubin conjugation due to partial inhibition of UDP-glucoronosyl transferase by ursodeoxicholic acid in liver. A slight elevation in DB level even after the partial inhibition of UDP-glucoronosyl transferase may be correlated with an increase in UDP-glucoronosyl transferase activity due to comparatively high substrate (unconjugated bilirubin) concentration than that of inhibitor (ursodeoxycholic acid).
Response surface analysis and optimization
RSM was successfully used to optimize the conditions for induction of hyperbilirubinemia. Initially, the variables were screened by use of CCD; thereby, non-significant variables were dropped and the experimental data were fitted only to the significant variables to obtain a final reduced model. However, non-significant linear terms were included in the final reduced model where the quadratic or interaction terms containing these variables were found to be significant. As a result of this the optimized dose, number of doses and dose interval were determined as 11.5 mg/kg−1 bw, 8 and 24 h respectively by use of the validated model.
In conclusion, a dose dependent significant elevation in TSB level of experimental rabbits was observed in response to an increase in PH dose. Hyperbilirubinemia may be induced in experimental rabbits up to 4.06 mg/dl−1 elevation in TSB level using 11.56 mg/kg−1 bw PH dose repeated for 8 times at a dose interval of 24 h. The study provides a reliable method for induction of hyperbilirubinemia in experimental rabbits using suggested optimum levels of process variables.
References
- 1.Arduini A., Storto S., Belfiglio M., Scurti R., Mancinelli G., Federici G.1989. Mechanism of spectrin degradation induced by phenylhydrazine in intact human erythrocytes. Biochim. Biophys. Acta 979: 1–6. doi: 10.1016/0005-2736(89)90515-4 [DOI] [PubMed] [Google Scholar]
- 2.Atkinson L.R., Escobar G.J., Takayama J.I., Newman T.B.2003. Phototherapy use in jaundiced newborns in a large managed care organization: do clinicians adhere to the guideline? Pediatrics 111: e555–e561. doi: 10.1542/peds.111.5.e555 [DOI] [PubMed] [Google Scholar]
- 3.Bansal A., Jain S., Parmar V.R., Chawla D.2010. Bilirubin rebound after intensive phototherapy for neonatal jaundice. Indian Pediatr. 47: 607–609. doi: 10.1007/s13312-010-0133-z [DOI] [PubMed] [Google Scholar]
- 4.Berger J.2007. Phenylhydrazine haematotoxicity. J. Appl. Biomed. 5: 125–130. [Google Scholar]
- 5.Bloom J.C., Brandt J.T.2001. Toxic responses of the blood. pp. 389–417. In: Casarett and Doull’s Toxicology 6th edition Klaassen, C.D. (ed). McGraw-Hill Medical Publishing Division New York. [Google Scholar]
- 6.Brumbaugh D., Mack C.2012. Conjugated hyperbilirubinemia in children. Pediatr. Rev. 33: 291–302. doi: 10.1542/pir.33-7-291 [DOI] [PubMed] [Google Scholar]
- 7.Chakrabarti S., Naik A.A., Reddy G.R.1990. Phenylhydrazine mediated degradation of bovine serum albumin and membrane proteins of human erythrocytes. Biochim. Biophys. Acta 1028: 89–94. doi: 10.1016/0005-2736(90)90269-T [DOI] [PubMed] [Google Scholar]
- 8.Dani C., Martelli E., Tronchin M., Buonocore G., Longini M., Di Filippo A., Giossi M., Rubaltelli F.F.2004. Bilirubin influence on oxidative lung damage and surfactant surface tension properties. Pediatr. Pulmonol. 38: 179–185. doi: 10.1002/ppul.20045 [DOI] [PubMed] [Google Scholar]
- 9.Fareed K.N., Arthur E.W., Ebenezer, Terlabi O., Larbie C. 2012. Bilirubin Lowering Potential of Annona muricata (Linn.) in Temporary Jaundiced Rats. Am. J. Pharmacol. Toxicol. 7: 33–40. doi: 10.3844/ajptsp.2012.33.40 [DOI] [Google Scholar]
- 10.Hohenauer L., Haschke F., Gerstl J.W.1976. [Fototherapy of neonatal hyperbilirubinemia. Results of its clinical application (author’s transl)]. Klin. Padiatr. 188: 314–319. [PubMed] [Google Scholar]
- 11.Ichida T., Nobuoka M.1968. Ultramicro method for determination of total and direct bilirubin in serum by modified “alkaline azobilirubin blue” reaction. Clin. Chim. Acta 19: 249–255. doi: 10.1016/0009-8981(68)90333-1 [DOI] [PubMed] [Google Scholar]
- 12.Iqbal M.S., Shad M.A., Akhtar M.I.2001. The effect of gamma radiation on bilirunbin. J. Radioanal. Nucl. Chem. 250: 397–398. doi: 10.1023/A:1017940906191 [DOI] [Google Scholar]
- 13.Itano H.A., Hosokawa K., Hirota K.1976. Induction of haemolytic anaemia by substituted phenylhydrazines. Br. J. Haematol. 32: 99–104. doi: 10.1111/j.1365-2141.1976.tb01879.x [DOI] [PubMed] [Google Scholar]
- 14.Jain S.K., Hochstein P.1979. Generation of superoxide radicals by hydrazine: Its role in phenylhydarzine − induced Hemolytic anaemia. Biochim. Biophys. Acta 586: 128–136. doi: 10.1016/0304-4165(79)90411-2 [DOI] [Google Scholar]
- 15.Jain S.K., Hochstein P.1980. Membrane alterations in phenylhydrazine-induced reticulocytes. Arch. Biochem. Biophys. 201: 683–687. doi: 10.1016/0003-9861(80)90560-3 [DOI] [PubMed] [Google Scholar]
- 16.Kamisah Y., Lim J.J., Lim C.L., Asmadi A.Y.2014. Inhibitory effects of palm tocotrienol-rich fraction supplementation on bilirubin-metabolizing enzymes in hyperbilirubinemic adult rats. PLOS ONE 9: e89248. doi: 10.1371/journal.pone.0089248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan M., Hammerman C.2005. Understanding severe hyperbilirubinemia and preventing kernicterus: adjuncts in the interpretation of neonatal serum bilirubin. Clin. Chim. Acta 356: 9–21. doi: 10.1016/j.cccn.2005.01.008 [DOI] [PubMed] [Google Scholar]
- 18.Lang E., Gatidis S., Freise N.F., Bock H., Kubitz R., Lauermann C., Orth H.M., Klindt C., Schuier M., Keitel V., Reich M., Liu G., Schmidt S., Xu H.C., Qadri S.M., Herebian D., Pandyra A.A., Mayatepek E., Gulbins E., Lang F., Häussinger D., Lang K.S., Föller M., Lang P.A.2015. Conjugated bilirubin triggers anemia by inducing erythrocyte death. Hepatology 61: 275–284. doi: 10.1002/hep.27338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lenth R.V.2009. Response-Surface Methods in R, Using RSM. J. Stat. Softw. 32: 1–16. doi: 10.18637/jss.v032.i07 [DOI] [Google Scholar]
- 20.Levitt D.G., Levitt M.D.2014. Quantitative assessment of the multiple processes responsible for bilirubin homeostasis in health and disease. Clin. Exp. Gastroenterol. 7: 307–328. doi: 10.2147/CEG.S64283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magnani M., Stocchi V., Cucchiarini L., Chiarantini L., Fornaini G.1986. Red blood cell phagocytosis and lysis following oxidative damage by phenylhydrazine. Cell Biochem. Funct. 4: 263–269. doi: 10.1002/cbf.290040406 [DOI] [PubMed] [Google Scholar]
- 22.Mirhosseini H., Tan C.P.2009. Response surface methodology and multivariate analysis of equilibrium head space concentration of orange beverage emulsion composition and structure. Food Chem. 115: 324–333. doi: 10.1016/j.foodchem.2008.11.090 [DOI] [Google Scholar]
- 23.Montgomery D.C. 2009. Design and analysis of experiments: Response surface method and designs (Hoboken, N.J. ed.) John Wiley and Sons, New York. [Google Scholar]
- 24.Myer R., Montgomery D.C.2002. Response surface Methodology. Wiley, New York. [Google Scholar]
- 25.Nag N., Halder S., Chaudhuri R., Adhikary S., Mazumder S.2009. Role of bilirubin as antioxidant in neonatal jaundice and effect of ethanolic extract of sweet lime peel on experimentally induced jaundice in rat. Indian J. Biochem. Biophys. 46: 73–78. [PubMed] [Google Scholar]
- 26.Nakanishi A., Kinuta K., Abe T., Araki K., Yoshida Y., Liang S., Li S.A., Takei K., Kinuta M.2003. Formation of meso, N-diphenylprotoporphyrin IX by an aerobic reaction of phenylhydrazine with oxyhemoglobins. Acta Med. Okayama 57: 249–256. [DOI] [PubMed] [Google Scholar]
- 27.Pillay A.E., Salih F.M.2003. A comparative study on gamma irradiation of unconjugated bilirubin in aqueous and non-aqueous solutions. Anal. Bioanal. Chem. 375: 751–755. [DOI] [PubMed] [Google Scholar]
- 28.Pillay A.E., Salih F.M.2004. Gamma radiolysis and solar photolysis of bilirubin: Similarities and differences. J. Radioanal. Nucl. Chem. 261: 211–214. doi: 10.1023/B:JRNC.0000030959.94570.d5 [DOI] [Google Scholar]
- 29.Porter M.L., Dennis B.L.2002. Hyperbilirubinemia in the term newborn. Am. Fam. Physician 65: 599–606. [PubMed] [Google Scholar]
- 30.Rehak N.N., Cecco S.A., Hortin G.L.2008. Photolysis of bilirubin in serum specimens exposed to room lighting. Clin. Chim. Acta 387: 181–183. doi: 10.1016/j.cca.2007.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rice A.C., Shapiro S.M.2008. A new animal model of hemolytic hyperbilirubinemia-induced bilirubin encephalopathy (kernicterus). Pediatr. Res. 64: 265–269. doi: 10.1203/PDR.0b013e31817d9be0 [DOI] [PubMed] [Google Scholar]
- 32.Roque M., D’Anna C., Gatti C., Veuthey T.2008. Hematological and Morphological Analysis of the Erythropoietic Regenerative Response in Phenylhydrazine-induced Hemolytic Anemia in Mice. Scand. J. Lab. Anim. Sci. 35: 181–190. [Google Scholar]
- 33.Sánchez Pozzi E.J., Luquita M.G., Catania V.A., Rodríguez Garay E.A., Mottino A.D.1994. Inhibition of rat liver microsomal bilirubin UDP-glucuronosyltransferase by ursodeoxycholic acid. Life Sci. 55: 111–120. doi: 10.1016/0024-3205(94)90102-3 [DOI] [PubMed] [Google Scholar]
- 34.Shukla P., Yadav N.K., Singh P., Bansode F.W., Singh R.K.2012. Phenylhydrazine induced toxicity: A review on its haematotoxicity. Int. J. Basic Appl. Med. Sci 2: 86–91. [Google Scholar]
- 35.Taavitsainen V.T. 2012. Experimental Optimization and Response Surfaces, Chemometrics in Practical Applications (Varmuza, K. ed.). ISBN: 978–953-51–0438-4, InTech. [Google Scholar]
- 36.Li-de X., Li G., Zong-Yi Y., Wei-Juan Y., Da-Gong S., Zong-Yao W.2003. The microrheological changes in the course of erythrocyte senescence after phenylhydrazine injection. Clin. Hemorheol. Microcirc. 28: 5–11. [PubMed] [Google Scholar]
- 37.Zhang L., Yuan B., Wang H., Gao Y.2015. Therapeutic Effect of Agaricus brasiliensis on Phenylhydrazine-Induced Neonatal Jaundice in Rats. Biomed Res. Int. 1–6, article ID 651218. [DOI] [PMC free article] [PubMed]
- 38.Friedman M.M., Kahn B.S., Lapan B.1962. Dehydrogenase of regenerating red blood cells in phenylhydrazine anemia. Clin. Chem. 8: 486–496. [PubMed] [Google Scholar]