Abstract
Hepatitis B virus (HBV) is the leading cause of liver disease and hepatic carcinoma (HCC). Approximately 350 million people worldwide are infected with HBV and at risk of chronicity. An efficient HBV-tolerant murine model that mimics HBV infection in humans is desirable for HBV-related research. In this study, we investigated and established a murine model by hydrodynamic injection (HDI) of pAAV/HBV into the tail vein of AAVS1 site element-transgenic mice. In 80% of the injected mice, the serum level of HBsAg reached 103-4 IU/ml and persisted for more than half a year. Next, the model was used to evaluate RNA interference (RNAi)-based antiviral therapy. Data obtained using the model demonstrated that this model will facilitate the elucidation of the mechanisms underlying chronic HBV infection and will also be useful for evaluating new antiviral drugs.
Keywords: AAVS1 mice, chronic HBV-tolerant model, hydrodynamic injection, pAAV/HBV plasmid
Introduction
Hepatitis B virus (HBV) is a highly contagious virus that infects the liver. HBV is circular, partially double-stranded DNA genome. HBV infection is now a global public health problem [21]. Chronic HBV infection is the major cause of hepatic cirrhosis and hepatocellular carcinoma (HCC) [24], which are difficult to cure and have high death rates. Although a highly effective preventive vaccine has been adopted, the estimated 350 million people who have already been infected are at high risk of chronicity and developing end-stage liver disease and HCC [22].
HBV has a narrow host range, so effective small animal models are desirable and indispensable to the study of chronic HBV infection. A variety of animal models are available for HBV research. HBV-transgenic mice are congenitally tolerant to products of the HBV-encoding transgenes and are broadly used to evaluate the pathogenesis of HBV infection [2, 9, 19, 27]. Human liver chimeric murine models based on immunodeficient mice have great potential to model human liver injury and the process of hepatitis virus infection, but the construction of these models is costly and complex [17, 38]. An immunocompetent murine model based on hydrodynamic injection has been used to analyze acute HBV infection and to develop new antiviral drugs to treat HBV infection. However, this model is not suitable for chronic HBV infection because viral antigens disappear from the blood as early as 7 days after transfection [36]. A substantial advance in the development of a mouse model of HBV chronic infection was the development in 2006 of an immunocompetent, HBV-tolerant mouse model based on the hydrodynamic injection of pAAV/HBV into C57BL/6 mice. In this model, HBV surface antigenemia persisted for >6 months in 40% of the injected mice [16]. The characteristics of this mouse model imitate those of immunotolerant chronic HBV infection in humans [6, 25] and will facilitate the elucidation of the mechanisms of HBV chronicity. The mechanism by which C57BL/6 mice sustain long-term HBV expression is unclear, and other mice strains may be able to support HBV persistence.
Human AAVS1 site element-transgenic mice (AAVS1 mice) allow the site-specific integration of hybrid vectors containing the AAV rep protein and genes of interest flanked by AAV inverted terminal repeats. Strategies for gene therapy and functional gene research have been established that utilize site-specific integration into the AAVS1 site of AAVS1 mice [10, 13, 18, 23]. Therefore, this study sought to investigate the feasibility of using AAVS1 mice to create an HBV-tolerant mouse model and to evaluate the potential application of this model to support studies of chronic HBV infection. This model may be a useful tool for scientists to study the mechanisms of HBV chronicity and assist the millions of individuals suffering from HBV and related diseases.
Methods
Ethics statement
All animal experiments were carried out in accordance with the guidelines of the Xiamen university institutional committee for the care and use of laboratory animals and were approved by the Xiamen university laboratory animal management ethics committee. All of the animals were raised in a SPF facility, with no HBV in house.
Plasmid constructs
The pAAV/HBV were kindly provided by Pei-Jer Chen (National Taiwan University) 20. pcDNA3.1/HBV, pAAV-C4371, pAAV-Y1021, pAAV-Y10 were constructed in the State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, Xiamen University. The constructs and molecular and phenotypic characteristics were described in our previous report 52. pAAV/DJ (Cell Biolabs, Inc. San Diego, CA, USA), a cloning vector expressing the rep/cap of AAV, can mediate the site-specific integration of an exogenous gene when co-injected with the pAAV expression vector. All plasmids were amplified in DH5α E. coli and purified using a Qiagen plasmid Maxi kit (endo-free).
Animal studies
SPF C3;B6-Tg(AAVS1)A1Xob/J mice, referred to as AAVS1 (purchased from Jackson Laboratory); C3H/HeJ mice (purchased from Jackson Laboratory); BALB/c mice (purchased from Shanghai SLAC laboratory Animal Co., Ltd.); and C57BL/6 mice (purchased from Shanghai SLAC laboratory Animal Co., Ltd.) were housed in the animal facility at the School of Life Science, Xiamen University. Mice with 6~8 weeks old were used in this research. Blood were collected from orbital venous of mice.
The AAVS1 mice were originally hemizygous. Primers were designed and synthesized to confirm the genotypes of newborn AAVS1 mice by polymerase chain reaction (PCR) according to the standard protocol of Jackson Laboratory. The primers for AAVS1 site element detection were AAVS1-F: 5’GCA GTC TGC TAT TCA TCC CCT TTA CGC G3’ and AAVS1-R: 5’CCA GGG TGT GCT GGG CAG GTA GC3’. The offspring of the mice were generally called AAVS1 mice and were grouped into two types based on their PCR results; PCR-positive mice were referred to as AAVS1 TG mice, and PCR-negative mice were referred to as AAVS1 NT mice.
The ends of the tails were collected from newborn AAVS1 mice. Next, the tissue genome was extracted using a DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) for use as the template for PCR. The results were visualized by gel electrophoresis and imaging. The reagents used for PCR were obtained from TAKARA, including dNTPs, buffer, and rTaq DNA polymerase. The PCR cycling conditions were pre-denaturation at 94°C for 5 min, followed by 30 cycles of denaturation at 94°C for 40 s, annealing at 56°C for 30 s, and extension at 72°C for 30 s.
Tail vein hydrodynamic injection
The plasmid DNA was injected into the tail veins of mice in 2 ml of physiological saline. The total volume was delivered within 5 s. The serum specimens were assayed for the presence of HBV antigen or antibody at the indicated times after injection. Livers of the sacrificed mice were separated by scissors and preserved in formalin for immunohistochemical analysis.
Detection of HBV antigen and HBV DNA
Serum levels of HBV antigen (HBsAg/HBeAg) in the mice were determined using the CLEIA system kit (Wantai, China). For all measurements, the obtained S/N ratio was converted to international units per milliliter (IU/ml) or nation health laboratory center units (NCU/ml) by reference to a standard curve of known concentrations. Serum levels of anti-HBs in the mice were determined using the ELISA system kit (Wantai, China). The HBV DNA levels in the mouse serum specimens were measured using a real-time qPCR assay from Premix Ex Taq™ (Takara, Dalian, China). The primer sequences were 5’-GTT CAA GCC TCC AAG CTG TG-3’ and 5’-TCA GAA GGC AAA AAA GAG AGT AAC TC-3’. The probe sequence was 5’-Hex- CCT TGG GTG GCT TTG GGG CAT GGA-BHQ-1-3’.
Immunohistochemistry
Liver tissues were collected from mice sacrificed at the indicated time points. Intrahepatic HBcAg or HBsAg was visualized by immunohistochemical staining of paraffin-embedded tissues using rabbit anti-HBc (DAKO, Carpinteria, CA), mice anti-HBs 13H10 and Envision System HRP (diaminobenzidine) (Maixin, China).
Antiviral siRNA
B245, the siRNA which inhibits hepatitis B virus of different genotypes in vitro and in vivo was constructed in previous studies and detailed in Zhang, Y. L. et al. BMC microbiology 10, 214, doi:10.1186/1471-2180-10-214 (2010).
Results
HBV persistence in AAVS1 is not dependent on the pAAV/DJ and the AAVS1 transgenic site
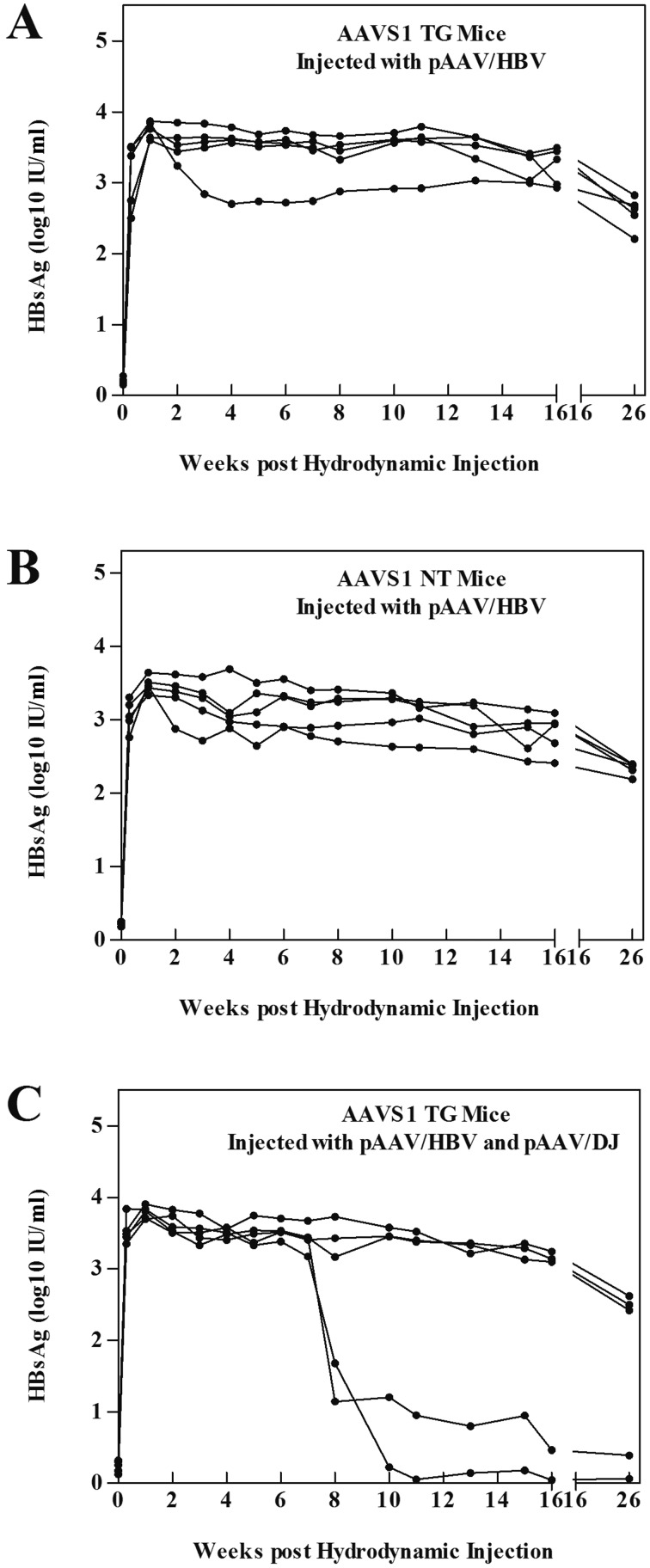
Ten μg of pAAV/HBV DNA was injected hydrodynamically into the tail veins of male AAVS1 TG (Fig. 1A) or AAVS1 NT mice (Fig. 1B). Then, 10 μg of pAAV/HBV and 20 μg of pAAV/DJ DNA were co-injected into male AAVS1 TG mice (Fig. 1C). After injection, the mice were regularly bled to monitor the serum levels of HBsAg. HBV persistence was observed over 26 weeks, with high expression in the three groups of mice and without significant disparity. The persistent expression of HBV in AAVS1 mice was not dependent on HBV genome integration into the AAVS1 site but was related to the genetic background of the mouse strain. The Rep protein does not enhance the persistence of HBV but may promote the clearance of transgenic plasmids for the high-dose injection.
Fig. 1.
The influence of the human AAVS1 integration site, pAAV/DJ on the expression and positive rate of pAAV-HBV in male AAVS1 mice. (A) (B) (C) Titer of serum HBsAg in mice with or without the AAVS1 integration gene element after pAAV/HBV injection or pAAV/HBV and pAAV/DJ co-injection. Percentages of serum HBsAg-positive mice were observed in three groups (group size: n=5) of AAVS1 mice at various time points after injection.
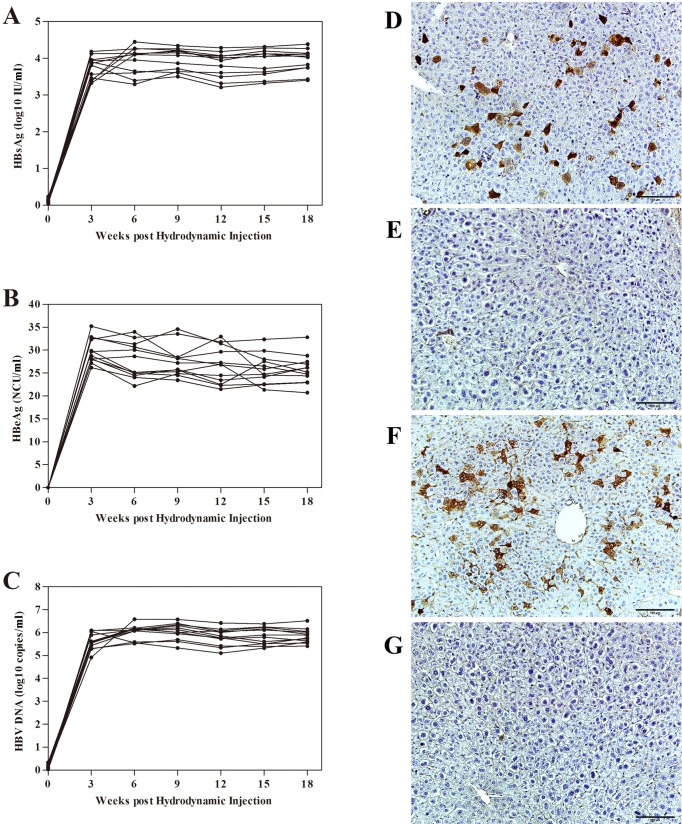
As a supplement, detection of HBsAg, HBeAg and HBV DNA in serum of 12 male AAVS1 mice injected with pAAV/HBV (10 μg/mice) has been done. In these mice, the level of HBsAg increased to 103–4 IU/ml (Fig. 2A), the level of HBeAg increased to 20–40 NCU/ml (Fig. 2B), the level of HBV DNA increased to 105–7 IU/ml (Fig. 2C) after the injection of pAAV/HBV, and maintaining over 18 weeks. At the same time, immuno-histochemistry assay of HBsAg and HBcAg in liver tissue of a male AAVS1 mice injected with pAAV/HBV or pAAV/DJ (10 μg/mice) at 180 days post injection (dpi) has been supplied to confirm the existence of HBV in vivo. The expression of HBsAg and HBcAg could only be observed in mice injected with pAAV/HBV (Figs. 2D and 2F), while mice injected with pAAV/DJ have a negative result (Figs. 2E and 2G). Moreever, plasmid DNA remained in serum of HDI AAVS1 mice is lower than 105 copies/ml in 24 hr post injection (Fig. S3).
Fig. 2.
Detction of HBV antigens and DNA in AAVS1 mice injected with pAAV/HBV. (A,B,C) Serum HBsAg/HBeAg/HBV DNA expression over 18 weeks (group size: n=12, male). (D,E) Immuno-histochemistry assay of HBcAg. (F,G)Immuno- histochemistry assay of HBsAg. (D,F)Liver tissue of injected with pAAV/HBV (10 μg/mice) at 180 days post injection. (E,G) Liver tissue of injected with pAAV/DJ (10 μg/mice) at 180 days post injection.
Gender has an impact on the expression and persistence of HBV in AAVS1 mice
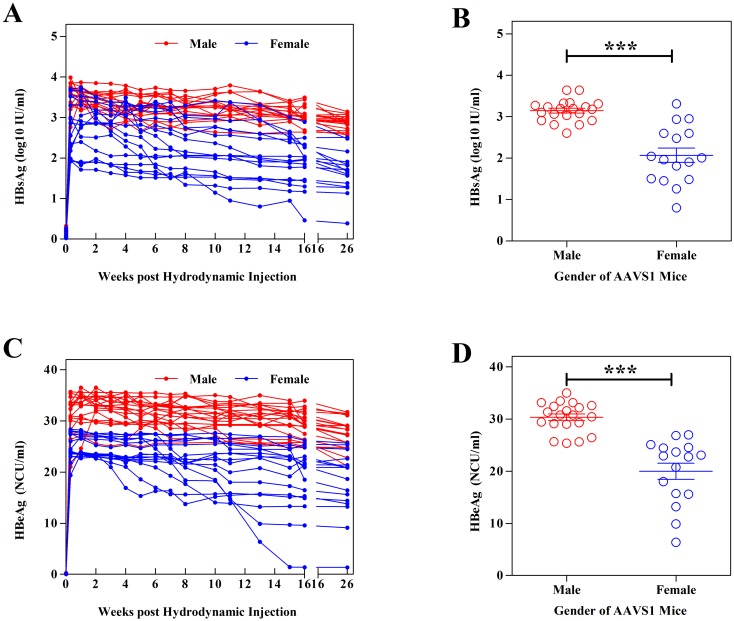
Previous research has elucidated that the development of chronic hepatitis B is associated with gender [5, 28, 37]; male HBV carriers usually have higher viral loads [34]. A series of experiments was conducted using AAVS1 mice to validate this phenomenon. 10 μg of pAAV/HBV DNA was injected hydrodynamically into the tail veins of 20 male and 12 female AAVS1 mice. After injection, the mice were regularly bled to monitor the serum levels of HBsAg. In male AAVS1 mice, the level of HBsAg increased to 103–4 IU/ml, and the level of HBeAg increased to 20–40 NCU/ml, higher than the increase in female mice (Figs. 3A and 3C). All male AAVS1 mice sustained HBV expression over 26 weeks, while some female ones have a drop of HBsAg. Comparison of HBsAg and HBeAg level at the section of 13 weeks have shown a notable difference between male and female AAVS1 mice injected with pAAV/HBV (10 μg/mice) (Figs. 3B and 3D). Obviously, male have great advantage on expression and persistence of HBV.
Fig. 3.
Comparison of serum HBsAg and HBeAg expression between male and female after injection of pAAV/HBV into AAVS1 mice (group size: male=20, female=16). (A) (C) Titer of serum HBsAg and HBeAg in female and male AAVS1 mice after pAAV/HBV injection for over 26 weeks. (B) (D) Comparison of HBsAg and HBeAg level at the section of 13 weeks between male and female AAVS1 mice. The data were analyzed using paired t test (P<0.0001).
HBV persistence is related to genetic background
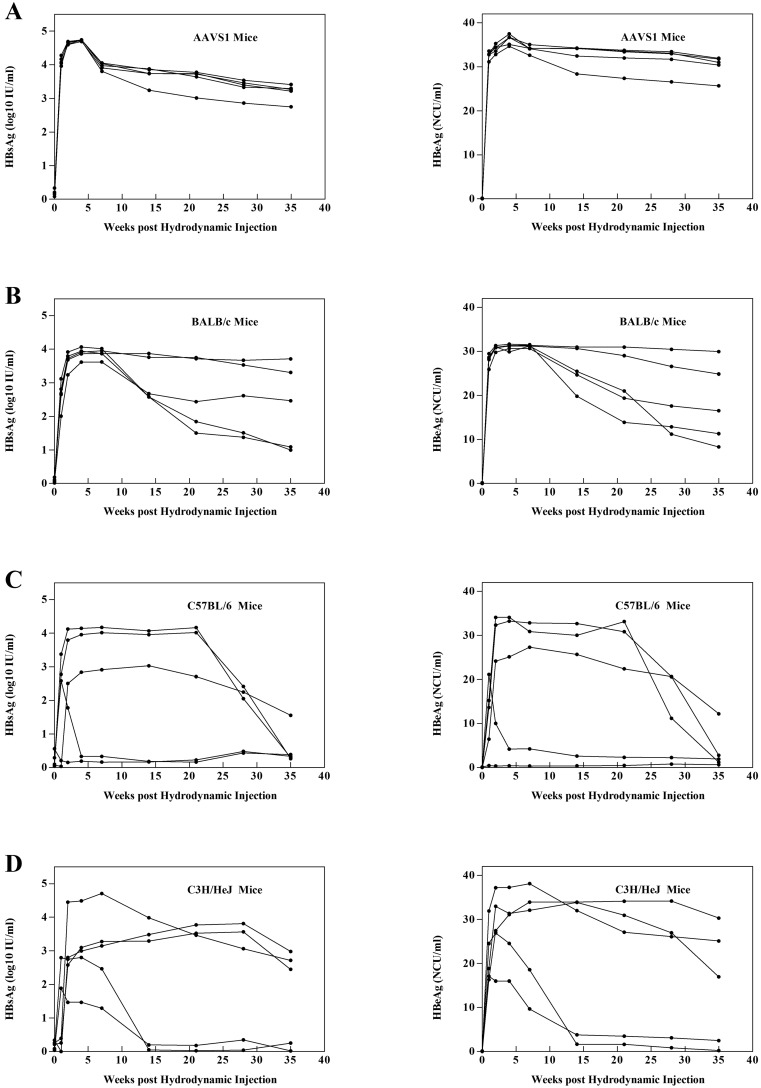
Ten μg of pAAV/HBV DNA was injected hydrodynamically into the tail veins of male AAVS1, BALB/c,C57BL/6, and C3H/HeJ mice. After injection, all mice were regularly bled to monitor the serum levels of HBsAg and HBeAg (Fig. 4). Among these four mouse strains, HBV persistence was highest in AAVS1 mice (Fig. 4A). Serum HBsAg and HBeAg persisted in these mice at a high level for longer than one month, and the mice also had the highest positive rate. The level of HBsAg and HBeAg in the serum of the other three mouse strains decreased significantly, and the HBsAg-positive and HBeAg-positive rate was also worse than that of the AAVS1 mice (Figs. 4B–4D). Further more, anti-HBs level in serum of the four strains were in a relatively low level, especially in AAVS1 mice, at 30 days post injection (Fig. S2).
Fig. 4.
HBV persistence in mice after hydrodynamic injection of HBV plasmids is related to the genetic background of the recipients. Male (A) AAVS1, (B) BALB/c, (C) C57BL/6, and (D) C3H/HeJ mice (group size: n=5) were injected hydrodynamically with 10 μg of HBV plasmid. HBsAg and HBeAg titer in the mouse serum was detected using the chemiluminescent enzyme immunoassay (CLEIA) system kit (Wantai, China).
HBV persistence is related to the pAAV vector
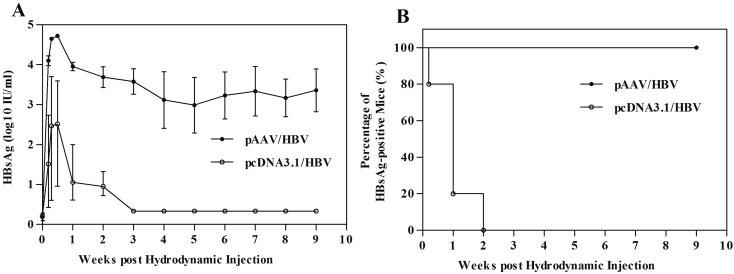
In addition to the genetic background of the mice, the selected vector influences the persistence of HBV in mice. Ten μg of pAAV/HBV or pcDNA3.1/HBV DNA was injected hydrodynamically into male AAVS1 mice. The mice were regularly bled to monitor the serum level of HBsAg. In the mice injected with pcDNA3.1/HBV, the level of HBsAg increased promptly within 3 days post injection (dpi) but decreased rapidly thereafter. HBsAg was cleared completely at 7 dpi (Fig. 5A). In the mice injected with pAAV/HBV, the level of HBsAg was much higher and declined more slowly. The number of HBsAg-positive mice in the two groups presented a sharp contrast. All mice of the group injected with pAAV/HBV maintained a high HBV expression level (Fig. 5B). Further more, there exist difference of HBsAg level in mice serum, when pAAV plasmid carried HBV gene with different genotypes were injected (Fig. S1).
Fig. 5.
The AAV vector enhances HBV persistence in the AAVS1 mouse liver. (A) Titer of serum HBsAg in the male AAVS1 mice after pAAV/HBV or pcDNA3.1/HBV injection (group size: n=5). (B) Number of serum HBsAg-positive mice in two groups of AAVS1 mice injected with different cloning vectors, at various time points after injection. The data were analyzed with the Log-rank/Mantel-Cox test, and the difference was significant (P<0.01).
Evaluation of HBV-targeted siRNA antiviral therapy using HBV carrier AAVS1 mice
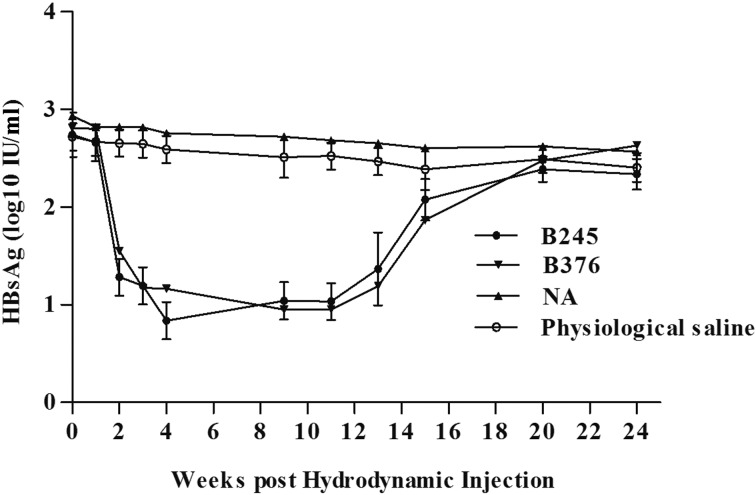
The HBsAg-positive AAVS1 mice were assigned to two groups. The experimental group was treated with the siRNA, B245 and B376, which were constructed in a previous study [39]. The control group was treated with physiological saline and control siRNA. All treated mice were bled to monitor the serum HBsAg level at the indicated time points. The level of HBsAg in mice treated with B245 and B376 declined rapidly and was undetectable after 7 days. In the control group, HBV was sustained at a high level without a significant decline (Fig. 6). This result demonstrates that this siRNA is highly effective at clearing serum HBV. Furthermore, the data demonstrate that the HBV-tolerant AAVS1 mouse model can effectively imitate human HBV chronicity and can therefore be used to study new antiviral drugs against chronic HBV infection.
Fig. 6.
Dynamic changes in HBsAg in the HBV-tolerant AAVS1 mouse model after B245 and B376 treatment. The titer of serum HBsAg in the HBV carrier AAVS1 mouse model (group size: n=5) was determined at various time points after B245 and B376 injection. physical saline and control siRNA (NA) as the negative control.
Discussion
HBV only infects humans and primates. Owing to its limited host range, in vivo HBV studies are restricted by the available animal models. Transgenic and chimeric mouse models have been developed to gain better tools to understand viral infection and replication and disease pathogenesis and to facilitate the development of new drugs. However, these models do not accurately mimic chronic HBV infection in humans. Hydrodynamic injection is a simple and rapid method of delivering exogenous genes into immunocompetent mice and is a powerful tool for establishing mouse models. An in vivo model based on hydrodynamic injection in which an exogenous gene was expressed long-term was first constructed for non-HBV research [15, 35]. Models were then established using the same method to study HBV infection [4, 7, 12, 16, 20, 29, 33, 36]. In this study, we introduced HBV DNA into mouse livers by hydrodynamic injection. Using this method, HBsAg was maintained in the sera of AAVS1 mice at 103–4 IU/ml for more than half a year (Fig. S4). This approach may also be valuable for developing new model systems of infection with other viral pathogens.
Observations during the construction of this HBV-tolerant AAVS1 mouse model indicated that the support of persistent HBV expression is correlated with the genetic background of the AAVS1 mice. HBsAg levels were lower in the sera of BALB/c, C57BL/6, and C3H/HeJ mice than in AAVS1 mice and were cleared rapidly from these mice. The transgenic AAVS1 gene site has no obvious effect on HBV expression in AAVS1 mice. It is unclear why AAVS1 mice support long-term HBV expression, but sex hormones [11], various cytokines [3, 31, 40], and other factors may play a role. We successfully established an HBV carrier model using hydrodynamic tail vein injection of pAAV/HBV in AAVS1 mice, which will facilitate the elucidation of the mechanism of HBV chronicity and the development of new drugs and vaccines.
HBV persistence in the AAVS1 mouse model was closely related to the vector used. The pAAV vector but not the pcDNA3.1 and pGEM4Z vectors efficiently mediated persistent HBV expression in the liver [7, 16]. This phenomenon has been described previously and has been attributed to the suppression of exogenous gene expression in the mouse liver by the covalent linkage of bacterial DNA in episomal vectors [8]. The maintenance and persistence of the exogenous gene in vivo is related to the expression cassette [30]. A hepatic control region from the apolipoprotein E locus can induce persistent, high-level expression of an exogenous gene in the liver [26]. These mechanisms may explain, at least in part, why the pAAV vector can support long-term HBV expression in the mouse liver, but further confirmation is needed.
HBV DNA levels are typically higher in male than female HBV carriers [32]. This phenomenon was conformed in transgenic mice in 2009. We also observed this phenomenon in a nontransgenic immunocompetent mouse model in the present study. The level of serum HBsAg was more than tenfold higher in male mice than in female mice. Related research has demonstrated that androgens increase HBV mRNA synthesis, while estrogen decreases HBV mRNA levels [1]. Notably, the HBx antigen of HBV inhibits the transcriptional activity of the estrogen receptor [14]. The mechanisms by which male and female hormones affect HBV infection remain to be elucidated, although initial work has been performed. The model constructed in this study will be a powerful tool for further studies. In this study, the HBV-tolerant AAVS1 mouse model was used for research on siRNA-based anti-HBV therapies. After treatment with B245 and B376, the HBsAg level in the carrier mice decreased rapidly and was undetectable within 1 week. After 10 weeks, serum HBsAg was again detectable and increased gradually. This pattern is typical of chronic HBV infection and is similar to the drug therapy response of human HBV carriers. Therefore, the HBV-tolerant AAVS1 mouse model provides a useful tool with which to investigate the mechanisms of HBV chronicity.
In conclusions, an HBV-tolerant immunocompetent model that effectively simulates chronic hepatitis B virus infection in AAVS1 mice was successfully built. As results showed the characteristic that AAVS1 mice can sustain HBV long-term expression is related to the genetic background and the expression vector but not the AAVS1 transgenic site. And the gender has an impact on the persistent time and expression level of HBV in AAVS1 mice. This new model has already been used for research on siRNA-based anti-HBV therapies and will further help medical researchers develop and screen effective drugs for HBV therapy. Moreover, additional models for the study of other hepatitis viruses based on AAVS1 mice can be established by hydrodynamic injection. This model will be used broadly in future immunology and virology studies.
Conflict of Interests
The authors declare that they have no competing financial interests.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Science and Technology Major Project of Infectious Diseases (No.2012ZX10004503-005, and No.2012ZX10002005 -001), the National Natural Science Foundation of China (No.30925030). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Almog Y., Klein A., Adler R., Laub O., Tur-Kaspa R.1992. Estrogen suppresses hepatitis B virus expression in male athymic mice transplanted with HBV transfected Hep G-2 cells. Antiviral Res. 19: 285–293. doi: 10.1016/0166-3542(92)90010-3 [DOI] [PubMed] [Google Scholar]
- 2.Araki K., Miyazaki J., Hino O., Tomita N., Chisaka O., Matsubara K., Yamamura K.1989. Expression and replication of hepatitis B virus genome in transgenic mice. Proc. Natl. Acad. Sci. USA 86: 207–211. doi: 10.1073/pnas.86.1.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Ari Z., Mor E., Papo O., Kfir B., Sulkes J., Tambur A.R., Tur-Kaspa R., Klein T.2003. Cytokine gene polymorphisms in patients infected with hepatitis B virus. Am. J. Gastroenterol. 98: 144–150. doi: 10.1111/j.1572-0241.2003.07179.x [DOI] [PubMed] [Google Scholar]
- 4.Chang W.W., Su I.J., Lai M.D., Chang W.T., Huang W., Lei H.Y.2003. The role of inducible nitric oxide synthase in a murine acute hepatitis B virus (HBV) infection model induced by hydrodynamics-based in vivo transfection of HBV-DNA. J. Hepatol. 39: 834–842. doi: 10.1016/S0168-8278(03)00389-1 [DOI] [PubMed] [Google Scholar]
- 5.Chen C.J., Yang H.I., Su J., Jen C.L., You S.L., Lu S.N., Huang G.T., Iloeje U.H., REVEAL-HBV Study Group2006. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 295: 65–73. doi: 10.1001/jama.295.1.65 [DOI] [PubMed] [Google Scholar]
- 6.Chen D.S.1993. Natural history of chronic hepatitis B virus infection: new light on an old story. J. Gastroenterol. Hepatol. 8: 470–475. doi: 10.1111/j.1440-1746.1993.tb01551.x [DOI] [PubMed] [Google Scholar]
- 7.Chen S.H., Wu H.L., Kao J.H., Hwang L.H.2012. Persistent hepatitis B viral replication in a FVB/N mouse model: impact of host and viral factors. PLoS ONE 7: e36984. doi: 10.1371/journal.pone.0036984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Z.Y., He C.Y., Meuse L., Kay M.A.2004. Silencing of episomal transgene expression by plasmid bacterial DNA elements in vivo. Gene Ther. 11: 856–864. doi: 10.1038/sj.gt.3302231 [DOI] [PubMed] [Google Scholar]
- 9.Chisari F.V.1995. Hepatitis B virus transgenic mice: insights into the virus and the disease. Hepatology 22: 1316–1325. [DOI] [PubMed] [Google Scholar]
- 10.Cortés M.L., Oehmig A., Saydam O., Sanford J.D., Perry K.F., Fraefel C., Breakefield X.O.2008. Targeted integration of functional human ATM cDNA into genome mediated by HSV/AAV hybrid amplicon vector. Mol. Ther. 16: 81–88. doi: 10.1038/sj.mt.6300338 [DOI] [PubMed] [Google Scholar]
- 11.Deng G., Zhou G., Zhai Y., Li S., Li X., Li Y., Zhang R., Yao Z., Shen Y., Qiang B., Wang Y., He F.2004. Association of estrogen receptor alpha polymorphisms with susceptibility to chronic hepatitis B virus infection. Hepatology 40: 318–326. doi: 10.1002/hep.20318 [DOI] [PubMed] [Google Scholar]
- 12.Giladi H., Ketzinel-Gilad M., Rivkin L., Felig Y., Nussbaum O., Galun E.2003. Small interfering RNA inhibits hepatitis B virus replication in mice. Mol. Ther. 8: 769–776. doi: 10.1016/S1525-0016(03)00244-2 [DOI] [PubMed] [Google Scholar]
- 13.Glover D.J., Lipps H.J., Jans D.A.2005. Towards safe, non-viral therapeutic gene expression in humans. Nat. Rev. Genet. 6: 299–310. doi: 10.1038/nrg1577 [DOI] [PubMed] [Google Scholar]
- 14.Han J., Ding L., Yuan B., Yang X., Wang X., Li J., Lu Q., Huang C., Ye Q.2006. Hepatitis B virus X protein and the estrogen receptor variant lacking exon 5 inhibit estrogen receptor signaling in hepatoma cells. Nucleic Acids Res. 34: 3095–3106. doi: 10.1093/nar/gkl389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hibbitt O.C., Harbottle R.P., Waddington S.N., Bursill C.A., Coutelle C., Channon K.M., Wade-Martins R.2007. Delivery and long-term expression of a 135 kb LDLR genomic DNA locus in vivo by hydrodynamic tail vein injection. J. Gene Med. 9: 488–497. doi: 10.1002/jgm.1041 [DOI] [PubMed] [Google Scholar]
- 16.Huang L.R., Wu H.L., Chen P.J., Chen D.S.2006. An immunocompetent mouse model for the tolerance of human chronic hepatitis B virus infection. Proc. Natl. Acad. Sci. USA 103: 17862–17867. doi: 10.1073/pnas.0608578103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ince W.L., Zhang L., Jiang Q., Arrildt K., Su L., Swanstrom R.2010. Evolution of the HIV-1 env gene in the Rag2-/- gammaC-/- humanized mouse model. J. Virol. 84: 2740–2752. doi: 10.1128/JVI.02180-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakowska J.C., Di Maria M.V., Camp S.M., Wang Y., Allen P.D., Breakefield X.O.2003. Targeted transgene integration into transgenic mouse fibroblasts carrying the full-length human AAVS1 locus mediated by HSV/AAV rep(+) hybrid amplicon vector. Gene Ther. 10: 1691–1702. doi: 10.1038/sj.gt.3302061 [DOI] [PubMed] [Google Scholar]
- 19.Jin Z., Sun R., Wei H., Gao X., Chen Y., Tian Z.2011. Accelerated liver fibrosis in hepatitis B virus transgenic mice: involvement of natural killer T cells. Hepatology 53: 219–229. doi: 10.1002/hep.23983 [DOI] [PubMed] [Google Scholar]
- 20.Ketzinel-Gilad M., Zauberman A., Nussbaum O., Shoshany Y., Ben-Moshe O., Pappo O., Felig Y., Ilan E., Wald H., Dagan S., Galun E.2006. The use of the hydrodynamic HBV animal model to study HBV biology and anti-viral therapy. Hepatol. Res. 34: 228–237. doi: 10.1016/j.hepres.2006.01.008 [DOI] [PubMed] [Google Scholar]
- 21.Lavanchy D.2005. Worldwide epidemiology of HBV infection, disease burden, and vaccine prevention. J. Clin. Virol. 34:(Suppl 1): S1–S3. doi: 10.1016/S1386-6532(05)00384-7 [DOI] [PubMed] [Google Scholar]
- 22.Lavanchy D.2004. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J. Viral Hepat. 11: 97–107. doi: 10.1046/j.1365-2893.2003.00487.x [DOI] [PubMed] [Google Scholar]
- 23.Liu R., Li Y., Hu R., Jin T., Deng S., Liang W., Zhang N., Chen J., Prud’homme G.J., Jia W.W., Ma D., Wang Q.2010. A site-specific genomic integration strategy for sustained expression of glucagon-like peptide-1 in mouse muscle for controlling energy homeostasis. Biochem. Biophys. Res. Commun. 403: 172–177. doi: 10.1016/j.bbrc.2010.10.131 [DOI] [PubMed] [Google Scholar]
- 24.Llovet J.M., Burroughs A., Bruix J.2003. Hepatocellular carcinoma. Lancet 362: 1907–1917. doi: 10.1016/S0140-6736(03)14964-1 [DOI] [PubMed] [Google Scholar]
- 25.McMahon B.J.2009. The natural history of chronic hepatitis B virus infection. Hepatology 49:(Suppl): S45–S55. doi: 10.1002/hep.22898 [DOI] [PubMed] [Google Scholar]
- 26.Miao C.H., Thompson A.R., Loeb K., Ye X.2001. Long-term and therapeutic-level hepatic gene expression of human factor IX after naked plasmid transfer in vivo. Mol. Ther. 3: 947–957. doi: 10.1006/mthe.2001.0333 [DOI] [PubMed] [Google Scholar]
- 27.Moriyama T., Guilhot S., Klopchin K., Moss B., Pinkert C.A., Palmiter R.D., Brinster R.L., Kanagawa O., Chisari F.V.1990. Immunobiology and pathogenesis of hepatocellular injury in hepatitis B virus transgenic mice. Science 248: 361–364. doi: 10.1126/science.1691527 [DOI] [PubMed] [Google Scholar]
- 28.Naugler W.E., Sakurai T., Kim S., Maeda S., Kim K., Elsharkawy A.M., Karin M.2007. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 317: 121–124. doi: 10.1126/science.1140485 [DOI] [PubMed] [Google Scholar]
- 29.Qi Z., Li G., Hu H., Yang C., Zhang X., Leng Q., Xie Y., Yu D., Zhang X., Gao Y., Lan K., Deng Q.2014. Recombinant covalently closed circular hepatitis B virus DNA induces prolonged viral persistence in immunocompetent mice. J. Virol. 88: 8045–8056. doi: 10.1128/JVI.01024-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riu E., Grimm D., Huang Z., Kay M.A.2005. Increased maintenance and persistence of transgenes by excision of expression cassettes from plasmid sequences in vivo. Hum. Gene Ther. 16: 558–570. doi: 10.1089/hum.2005.16.558 [DOI] [PubMed] [Google Scholar]
- 31.Thursz M.2001. Genetic susceptibility in chronic viral hepatitis. Antiviral Res. 52: 113–116. doi: 10.1016/S0166-3542(01)00175-9 [DOI] [PubMed] [Google Scholar]
- 32.Tian Y., Kuo C.F., Chen W.L., Ou J.H.2012. Enhancement of hepatitis B virus replication by androgen and its receptor in mice. J. Virol. 86: 1904–1910. doi: 10.1128/JVI.06707-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J., Wang B., Huang S., Song Z., Wu J., Zhang E., Zhu Z., Zhu B., Yin Y., Lin Y., Xu Y., Zheng X., Lu M., Yang D.2014. Immunosuppressive drugs modulate the replication of hepatitis B virus (HBV) in a hydrodynamic injection mouse model. PLOS ONE 9: e85832. doi: 10.1371/journal.pone.0085832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang S.H., Yeh S.H., Lin W.H., Wang H.Y., Chen D.S., Chen P.J.2009. Identification of androgen response elements in the enhancer I of hepatitis B virus: a mechanism for sex disparity in chronic hepatitis B. Hepatology 50: 1392–1402. doi: 10.1002/hep.23163 [DOI] [PubMed] [Google Scholar]
- 35.Wooddell C.I., Reppen T., Wolff J.A., Herweijer H.2008. Sustained liver-specific transgene expression from the albumin promoter in mice following hydrodynamic plasmid DNA delivery. J. Gene Med. 10: 551–563. doi: 10.1002/jgm.1179 [DOI] [PubMed] [Google Scholar]
- 36.Yang P.L., Althage A., Chung J., Chisari F.V.2002. Hydrodynamic injection of viral DNA: a mouse model of acute hepatitis B virus infection. Proc. Natl. Acad. Sci. USA 99: 13825–13830. doi: 10.1073/pnas.202398599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu M.W., Chang H.C., Liaw Y.F., Lin S.M., Lee S.D., Liu C.J., Chen P.J., Hsiao T.J., Lee P.H., Chen C.J.2000. Familial risk of hepatocellular carcinoma among chronic hepatitis B carriers and their relatives. J. Natl. Cancer Inst. 92: 1159–1164. doi: 10.1093/jnci/92.14.1159 [DOI] [PubMed] [Google Scholar]
- 38.Zhang L., Su L.2012. HIV-1 immunopathogenesis in humanized mouse models. Cell. Mol. Immunol. 9: 237–244. doi: 10.1038/cmi.2012.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y.L., Cheng T., Cai Y.J., Yuan Q., Liu C., Zhang T., Xia D.Z., Li R.Y., Yang L.W., Wang Y.B., Yeo A.E., Shih J.W., Zhang J., Xia N.S.2010. RNA Interference inhibits hepatitis B virus of different genotypes in vitro and in vivo. BMC Microbiol. 10: 214. doi: 10.1186/1471-2180-10-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhi-ming L., Yu-lian J., Zhao-lei F., Chun-xiao W., Zhen-fang D., Bing-chang Z., Yue-ran Z.2007. Polymorphisms of killer cell immunoglobulin-like receptor gene: possible association with susceptibility to or clearance of hepatitis B virus infection in Chinese Han population. Croat. Med. J. 48: 800–806. doi: 10.3325/cmj.2007.6.800 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.