Abstract
While the cage refinement is a necessary step towards improving the welfare of research rats, increasing the complexity and surface area of the living space of an animal may have physiological impacts that need to be taken into consideration. In this study, ketamine (80 mg/kg) and xylazine (10 mg/kg) caused a short duration anesthesia that was significantly decreased in Sprague-Dawley rats housed in multilevel cages (MLC), compared to rats housed in standard cages (SDC). The withdrawal reflex, the palpebral reflexes and the time-to-sternal all occurred earlier in MLC housed rats, suggesting an effect of housing on the physiology of the rats. In addition, during anesthesia, cardiac frequencies were increased in animals housed in the smaller SDC. Respiratory frequencies, the blood oxygen saturation and rectal temperatures during anesthesia did not vary between conditions during the anesthesia. While xylazine pharmacokinetics were unchanged with caging conditions, the clearance and half-lives of ketamine and its metabolite, norketamine, were altered in the rats housed in MLC. Finally, while no difference was ultimately seen in rat body weights, isolated liver and adrenal gland weights were significantly lighter in rats housed in the MLC. Increasing cage sizes, while having a positive impact on wellbeing in rats, can alter anesthetic drug metabolism and thus modify anesthesia parameters and associated physiological processes.
Keywords: cage space, ketamine, pharmacokinetics, Sprague Dawley rats, xylazine
Introduction
Rats are known to be active creatures that can run, jump and stand up on their hind-legs. The current standard laboratory rat housing does not allow full expression of these activities. Recently, there has been an ever-increasing amount of scientific publications detailing the benefits of providing larger, more complex cages for rats. In fact, multilevel caging (MLC) systems are now commercially available, allowing rats to exhibit more natural behaviors, all the while maintaining an individual ventilated environment if required by the research study [9, 12, 33, 35].
While the cage refinement is a necessary step towards improving the welfare of research rats [10, 17], increasing the complexity and surface area of the living space of an animal may have physiological impacts that need to be taken into consideration. For instance, the standard rat cage does not provide the space nor the environmental complexity for adult rats to ensure minimal physical activity, contributing in part to obesity and other health issues associated with lack of exercise [14]. In fact, obesity is an overwhelming problem in laboratory rat colonies, introducing scientific biases that are often overlooked in the course of experimental research. Commercially available rat MLC systems provide rats with approximately twice the floor area of standard rat cages (on two levels) with an area to exhibit full bi-pedal postures at all ages, and enough space for chasing, jumping and sheltering. Thus, doubling the cage surface area could potentially decrease such confounding effect of obesity in rat colonies.
Studying drug metabolism gives researchers good indices on various physiological parameters, such as hepatic and renal functions. Many such factors have been seen to affect the pharmacokinetics of drugs such as sex, nutrition, environmental condition, disease and decreased exercise [16, 21, 22, 29, 30, 31]. In previous publications, we showed that the pharmacokinetics of ketamine and xylazine differed greatly between young and old Sprague Dawley rats [13, 34]. Ketamine and xylazine half-lives are 2 and 1 h, respectively, in 8- and 12-week-old rats. Both drug availability (area under the curve: AUC) and their elimination were seen to be greatly increased in old rats, with half lives increased to 8.5 and 13 h, respectively [13]. This change in drug pharmacokinetics was, in part, explained by alterations in the liver enzymes CYP3A in older rats. In addition, since both ketamine and xylazine are lipophilic drugs and have a high volume of tissue distribution, the degree of fat deposits could potentially act as a storage compartment and affect the drug release into the blood stream. Thus, as rats get older, and inevitably bigger, less floor space will be available for locomotor activity. This could very well be an important factor that would modify drug metabolism. The main objective of this study is to compare the potential physiological and pharmacokinetic changes associated with the administration of ketamine and xylazine in rats that have been housed in standard cages (SDC) or in commercial MLC.
Materials and Methods
Subjects
Sixteen 5- to 6-week-old, specific pathogen free, male Sprague-Dawley (CR1:CD (SD)) rats were purchased from Charles River (St-Constant, Qc, Canada) for this study [8]. At arrival, the animals were randomly divided into two housing groups: 1) open-top solid-bottomed cages (43 cm × 22 cm × 20.5 cm=946 cm2 total floor area on a single floor; Ancare, Bellmore, NY, USA) placed on a stainless steel rack representing the standard housing cage (SDC) and 2) individually ventilated two-tier cage (46.2 cm × 40.3 × m × 40.4 cm=1,862 cm2 total floor area on two floors; Tecniplast, West Chester, PA, USA), representing the multilevel cage (MLC). The rats were co-housed in pairs, resulting in a total of 4 cages per housing condition, for 12 weeks. All rats were housed in the same animal holding room with standard laboratory animal environment, under a 12:12 h light cycle in a controlled environment: temperature (21 ± 2°C) and humidity (50% ± 20%). The rats in the MLC received 75 fresh-filtered air changes an hour, while the SDC were open to the air. The room was ventilated with 100% fresh air at approximately 15 air changes an hour. The rats had ad libidum access to standard rodent chow (2920X Irradiated Teklad Global 19.1% Extruded Protein Rodent Diet, Harlan, Teklad, Bartonville, IL, USA) and tap water. The animals were housed on corncob bedding (7097 corncob, Harlan Teklad, Bartonville, IL, USA), had one polychloride vinyl (PVC) tube per cage for environmental enrichment. All procedures were approved by the Institutional Animal Care and Use Committee of McGill University and of the Faculty of Veterinary Medicine of the University of Montreal prior to animal use in compliance with the guidelines of the Canadian Council on Animal Care [7].
Treatment and physiological parameters
Following the 3 month housing period, twelve of the sixteen rats (n=6 per group) received ketamine (80 mg/kg IP; Ketalean Bimeda-MTC, Cambridge, Canada) and xylazine (10 mg/kg IP; Xylamax, Bimeda-MTC, Cambridge, Canada), which represents a standard anesthesia cocktail commonly used in laboratory rats [13]. Following the intraperitoneal (IP) ketamine-xylazine (KX) injection, different reflexes and physiological parameters were monitored at chosen time points (5, 15, 30, 45, and 60 min). The withdrawal reflex (WR) was evaluated by pressing the interdigital skin of a hind paw with hemostatic forceps and the palpebral reflex (PR) was evaluated by softly pressing a cotton-tip on outer corner of the eyes. Reflexes were evaluated by the same experimenter. A rodent-specific oximeter (CANL-425V, Med Associates, St-Alban, VT, USA) was used to monitor cardiac frequency and blood oxygen saturation (SaO2) by taping the probe on the right hind paw, as previously described [13]. Respiratory frequency was taken over 15 sec by direct visual observation and rectal temperature was taken with a rectal probe (Thermalert TH-8, Physitemp, Clifton, NJ, USA). The duration of anesthesia was evaluated as the time until the animal became sternal on its own following the KX injection.
Pharmacokinetic study
The blood sampling method has previously been described [34]. Briefly, blood was collected via the jugular vein (0.2 ml/time point) immediately following the evaluation of the reflexes and physiological parameters. When needed (when withdrawal and palpebral reflexes were present), very brief (1–2 min) isoflurane anesthesia was used (0.5 ml/min oxygen by face mask). This procedure was used in a comparable percentage of animals, at all individual time points. Blood was collected at 15 min, 30 min, 1 h, 2 h, 4 h, and 8 h after KX injection. During blood collection, rats were kept on an electric heating pad; and after sampling they were kept on a heating disk, until sternally recumbent. Blood was collected in 1 ml microtainer tubes containing K2EDTA (Becton Dickenson, Franklin Lakes, NJ) and kept on ice until centrifuged (3,200 g for 8 min) within 30 min of collection. Plasma was collected and all samples were kept at −80°C pending analysis by high performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS).
Mass spectrometry and pharmacokinetics
The analysis of ketamine and xylazine was performed using a HPLC-MS/MS that has previously been described [13]. Pharmacokinetic parameters of ketamine and xylazine in plasma were calculated using noncompartmental methods [26]. The AUC from time 0 to the last measurable concentration (AUC0-t) was calculated using the linear trapezoidal rule. A terminal rate constant of elimination (Kel) was calculated using a minimum of three measured plasma concentrations and a terminal elimination half-life (T1/2) was calculated using (ln 2)/ Kel. The AUC extrapolated to infinity (AUC∞) was calculated using AUC0-t + Clast/Kel where Clast was the last measured plasma concentration. All pharmacokinetic parameters were calculated using WinNonLin 5.2 (Pharsight Corporation, Mountain View, CA, USA) and plasmatic drug profiles were modelized with WinNonLin.
Post-mortem examination
Immediately after the last blood collection time point (8 h post-KX injection), the animals were euthanized with CO2 weighed and necropsied. The heart, liver, kidneys, spleen and adrenal glands were examined, collected, weighed and preserved in a buffered 10% formalin solution, prior to histological preparation (paraffin embedding; hematoxylin-eosin staining). Specimens were sent to the Pathology Department of the Faculty of Veterinary Medicine (University of Montreal) for processing and evaluated by a board-certified veterinary pathologist).
Statistical analyses
To determine differences in reflexes prevalence between time points, a sign test was used (corneal and withdrawal reflex results were combined since both were affected the same way by the anesthesia). A t-test was performed to compare the cage effect on anesthesia duration and organ weights. As for the physiological (heart rate, respiratory frequency, oxygen saturation, and rectal temperature) and pharmacokinetic results (T 1/2. Kel, AUC0-t. AUC 0-∞), statistical analyses were performed for each group using an analysis of variance (ANOVA) linear model with repeated measures and post hoc Tukey test.
Results
Reflexes and physiological changes
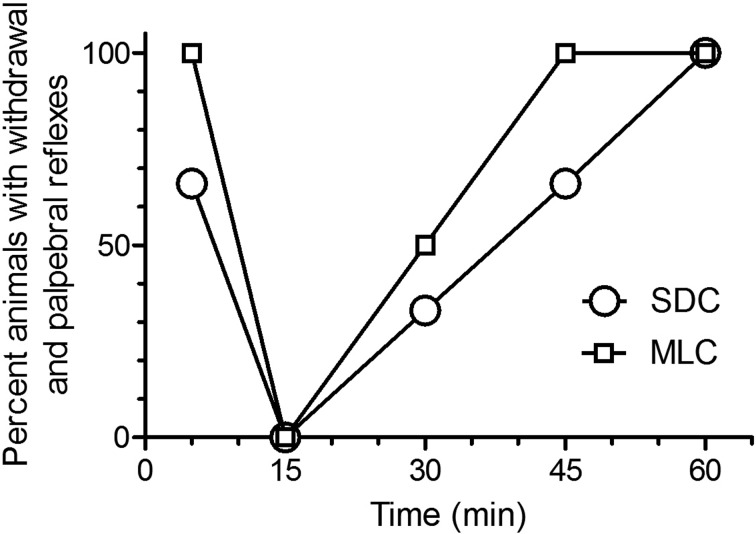
Based on the percentage of animals without WR and PR (Fig. 1) there was no significant difference in anesthesia induction between the two caging systems: animals in both groups were fully anesthetized at 15 min (P<0.001). While there was no significant difference in the percentage of animals without reflexes at 30 min post KX injection, 100% of the animals housed in MLC (multilevel cages) had reflexes at 45 min, while it took 60 min for 100% of the animals housed in standard cages (SDC) to recover their reflexes. In fact, the time to sternal recumbency was significantly shorter (P<0.02) in the rats housed in MLC (81.5 min ± 20.83), compared to rats from SDC (110 min ± 17.67). It should be noted that WR and PR were concomitant at all time points.
Fig. 1.
Percent of Sprague Dawley rats (n=6/group; standard housing cage (SDC), multilevel housing cage (MHC)) showing positive reflexes (withdrawal and palpebral reflexes) when evaluated at selected time points (5, 15, 30, 45, and 60 min) following the intraperitoneal administration of ketamine (80 mg/kg) and xylazine (10 mg/kg). The WR was evaluated by pressing the interdigital skin of a hind paw with hemostatic forceps, the PR was evaluated by softly pressing a cotton-tip on outer corner of the eyes. The reflexes were evaluated until animals became sternal.
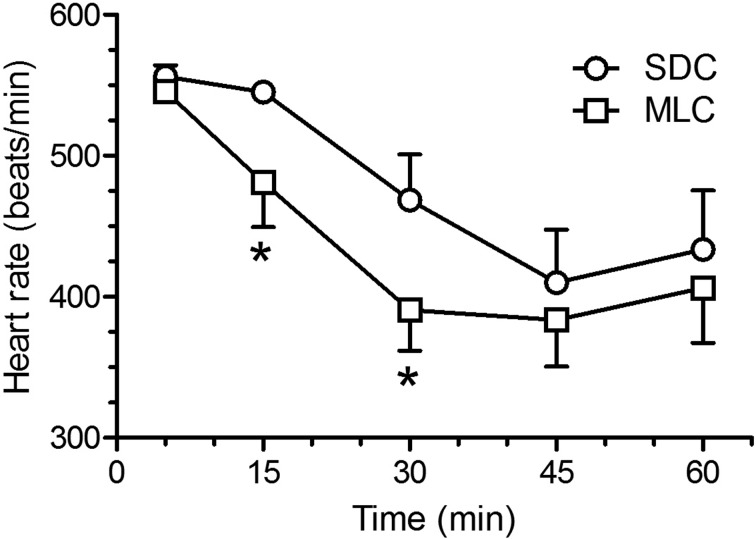
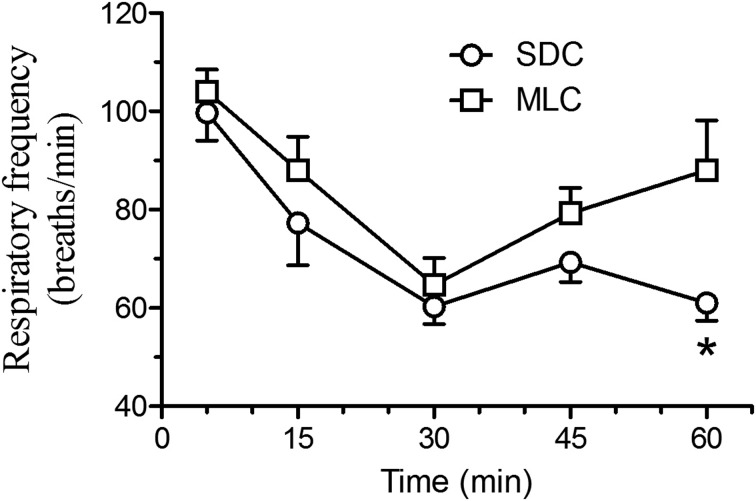
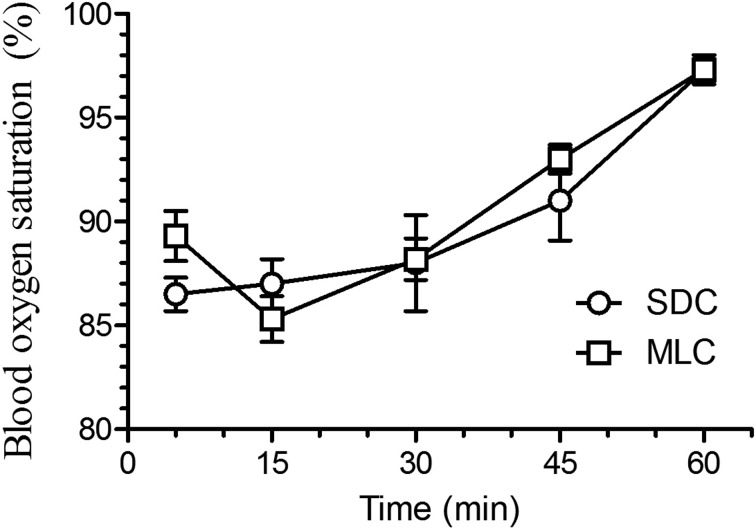
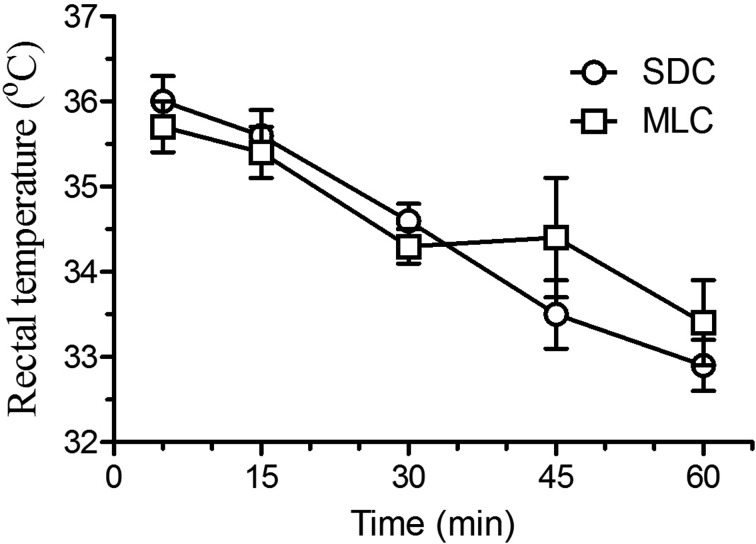
Cardiac frequency (Fig. 2) did show statistical differences between the two housing conditions, with lower heart rates at 15 and 30 min post-injection in the rats that were housed in the MLC rats (P<0.05). Respiratory frequency (Fig. 3) showed a similar decrease at 30 min post-injection for both housing conditions. However, the MLC rats showed a significant increase in respiratory frequency at 60 min, compared to the standard condition (P<0.05). Blood oxygen saturation (Fig. 4) and rectal temperatures (Fig. 5) did not vary significantly between the two groups.
Fig. 2.
Mean ± SE heart rate (beat/min) in Sprague Dawley rats (n=6/group; standard housing cage (SDC), multilevel housing cage (MLC)) when evaluated at selected time points (5, 15, 30, 45, and 60 min) following the intraperitoneal administration of ketamine (80 mg/kg) and xylazine (10 mg/kg). The cardiac frequency was evaluated with a small animal pulse oximeter by taping the probe on the right hind paw and ended when animals became sternal. Rats housed in MLC had a significant decrease in cardiac frequency at 15 and 30 min (*P<0.05).
Fig. 3.
Mean (± SE) respiratory frequency (breaths/min) in Sprague Dawley rats (n=6/group; standard housing cage (SDC), multilevel housing cage (MLC)) when evaluated at selected time points (5, 15, 30, 45, and 60 min) following the intraperitoneal administration of ketamine (80 mg/kg) and xylazine (10 mg/kg). The respiratory frequency was evaluated by direct visual observation and ended when animals became sternal. Rats housed in MLC had a significant increase in respiratory frequency at 60 min, compared to the SDC condition (*P<0.05).
Fig. 4.
Mean (± SE) blood oxygen saturation (%) in Sprague Dawley rats (n=6/group; standard housing cage (SDC), multilevel housing cage (MLC)) when evaluated at selected time points (5, 15, 30, 45, and 60 min) following the intraperitoneal administration of ketamine (80 mg/kg) and xylazine (10 mg/kg). The SaO2 was evaluated with a small animal pulse oximeter by taping the probe on the right hind paw and ended when animals became sternal. No significant difference between groups.
Fig. 5.
Mean (± SE) rectal temperature (°C) in Sprague Dawley rats (n=6/group; standard housing cage (SDC), multilevel housing cage (MLC)) when evaluated at selected time points (5, 15, 30, 45, and 60 min) following the intraperitoneal administration of ketamine (80 mg/kg) and xylazine (10 mg/kg). The rectal temperature was evaluated with a rectal probe and ended when animals became sternal. No significant difference between groups.
Pharmacokinetics
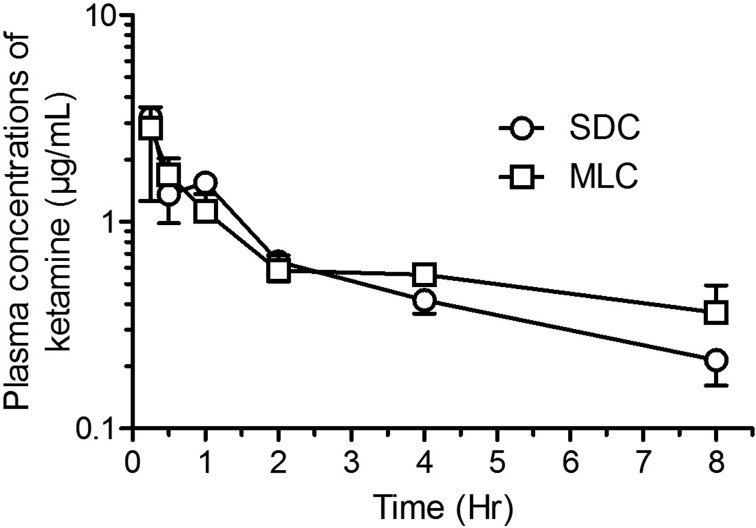
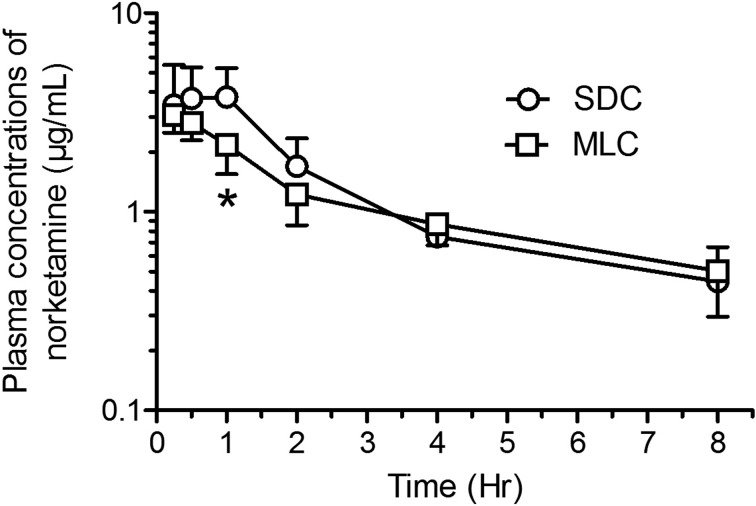
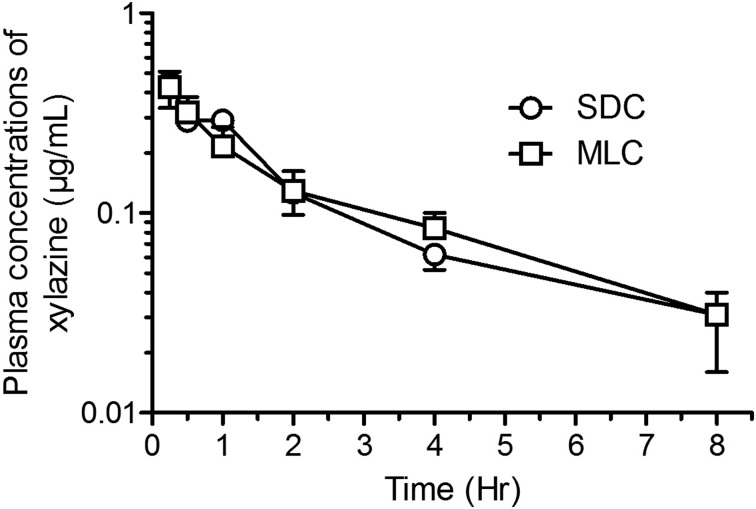
Mean pharmacokinetic parameters (± SD) are presented in Table 1 and mean concentration-time curves for ketamine, norketamine and xylazine are shown in Figs. 6, 7, 8 respectively. Although the ketamine exposure (AUC0-t and AUC0-∞) was not statistically different in the two housing conditions, the terminal elimination time increased (P<0.02) and the apparent clearance decreased (P<0.002) significantly in rats housed in MLC. The norketamine, a metabolite of ketamine, had a trend for lower overall drug exposure time in the rats housed in MLC, although these results were not statistically significant. In the plasma concentration-time curve for norketamine (Fig. 7), a peak at time point 1 h can be observed in the rats from SDC (P<0.05). Finally, for xylazine, the pharmacokinetic analyses and plasma concentration-time curves were strikingly similar for both rat housing groups (Fig. 8).
Table 1. Mean (± SD) plasmatic pharmacokinetic parameters following a single intraperitoneal (IP) injection of ketamine (80 mg/kg) and xylazine (10 mg/kg) administered to male Sprague-Dawley rats (n=6/group) housed in two different caging systems (standard cages (SDC) and multilevel cages (MLC).
| Units | SDC | MLC | ||
|---|---|---|---|---|
| Ketamine | ||||
| AUC0-t | μg.h/ml | 5.11 ± 0.95 | 5.45 ± 0.20 | |
| AUC0-∞ | μg.h/ml | 6.63 ± 0.29 | 8.37± 1.33 | |
| Kel | h-1 | 0.183 ± 0.057 | 0.140 ± 0.044 | |
| T1/2 | h | 4.13 ± 1.35 | 8.5 ± 5.2* | |
| Cmax | μg/ml | 3.19 ± 1.93 | 2.84 ± 0.74 | |
| CL/F | ml/h | 12.42 ± 0.57 | 9.77 ± 1.64* | |
| Norketamine | ||||
| AUC0-t | μg.h/ml | 10.78 ± 2.99 | 8.88 ± 1.68 | |
| AUC0-∞ | μg.h/ml | 13.25 ± 0.062 | 12.85 ± 3.49 | |
| Kel | h-1 | 2.18 ± 0.062 | 0.165 ± 0.075 | |
| T1/2 | h | 3.46 ± 1.19 | 4.92 ± 1.99 | |
| Cmax | μg/ml | 4.13 ± 1.62 | 3.17 ± 0.48 | |
| Xylazine | ||||
| AUC0-t | μg.h/ml | 0.877 ± 0.062 | 0.896 ± 0.079 | |
| AUC0-∞ | μg.h/ml | 1.032 ± 0.183 | 1.023 ± 0.055 | |
| Kel | h-1 | 0.257 ± 0.090 | 0.267 ± 0.066 | |
| T1/2 | h | 3.04 ±1.24 | 2.72 ± 0.61 | |
| Cmax | μg/ml | 0.452 ± 0.113 | 0.438 ± 0.069 | |
| CL/F | ml/h | 9.91 ± 1.54 | 9.80 ± 9.76 | |
AUC0-t (Area Under Curve from time zero to the last measurement concentration); T1/2 (terminal elimination half-life); Cmax (maximum plasmatic concentration); CL/F (relative clearance rate) . Variance (ANOVA) linear model with repeated measures and Post hoc Tukey statistical analyses were used to compare both groups. *Values significantly different (P<0.02).
Fig. 6.
Semi-logarithmic graph of the concentration-time profiles (Mean ± SD) of ketamine in Sprague-Dawley rats (n=6/group; standard housing cage (SDC), multilevel housing cage (MLC)) following the intraperitoneal administration of ketamine (80 mg/kg) and xylazine (10 mg/kg). No significant difference between groups.
Fig. 7.
Semi-logarithmic graph of the concentration-time profiles (Mean ± SD) of norketamine in Sprague-Dawley rats (n=6/group; standard housing cage (SDC), multilevel housing cage (MLC)) following the intraperitoneal administration of ketamine (80 mg/kg) and xylazine (10 mg/kg). A peak at time point 1 hr can be observed in the rats housed in SDC, compared to rats housed in MLC (*P<0.05).
Fig. 8.
Semi-logarithmic graph of the concentration-time profiles (Mean ± SD) of xylazine in Sprague-Dawley rats (n=6/group; standard housing cage (SDC), multilevel housing cage (MLC)) following the intraperitoneal administration of ketamine (80 mg/kg) and xylazine (10 mg/kg). No significant difference between groups.
Body weights, organs weights and histopathology
The body weights of the animals were taken at the time of euthanasia. No differences were found between housing conditions, with the average weight of 720.4 ± 79.2 g for rats from SDC, compared to 695.8 ± 63.1 g in rats housed in MLC. Heart, kidney and spleen weights were similar in both conditions. The average in liver:body weight ratio was significantly heavier in rats housed in SDC compared to the livers of the MLC housed rats (39.9 ± 6.5 mg compared to 32.5 ± 2.5 mg, P<0.05). Interestingly, a similar finding was observed for the adrenal glands:body weights ratios (0.13 ± 0.02 mg compared to 0.11 ± 0.02 mg, P<0.05). No lesions were observed in the selected tissues from animals of either housing condition.
Discussion
In this study, ketamine (80 mg/kg) and xylazine (10 mg/kg) caused a short duration anesthesia that was significantly decreased in rats housed in MLC, compared to rats from SDC. The recovery of the withdrawal and palpebral reflexes, and the time-to-sternal all occurred earlier in MLC housed rats, suggesting an effect of housing on the physiology of the rats. Cardiac frequency decreases at 15 and 30 min after the ketamine-xylazine (KX) injections. Respiratory frequency, blood oxygen saturation and rectal temperatures during anesthesia did not vary significantly between conditions. While xylazine and norketamine pharmacokinetics were unchanged with caging conditions, the clearance and half-lives of ketamine were altered in the rats housed in MLC. Interestingly the plasma concentration of norketamine was decreased at 1 h post-KX administration for the animals housed in the MLC. Finally, while no difference was ultimately seen in rat weights and histopathology of filter organs (liver and kidneys), liver and adrenal gland weights were significantly lighter in rats housed in MLC. These changes suggest a noticeable effect of housing on metabolic processes and possible stress response, resulting in differences in anesthetic outcomes.
Anesthesia durations in rats housed in SDC were similar to those previously reported for age-matched individuals (5- to 6-month-old rats) [13]. Interestingly, the rats housed in MLC had anesthesia depths more similar to 3-month-old rats from the same previous study. In addition, while the initial heart rates were similar in both conditions, the rats housed in MLC had lower heart rates during the anesthesia than the rats housed in SDC. This could potentially be explained by an increase in activity level, benefiting overall the cardiovascular system [19, 36]. While the potential activity levels of the caged rats was not directly observed in this study, Wheeler et al. recorded the activity levels and location of rats in identical MLC and established that the rats housed in MLC had significantly increased active behaviours, and this difference was mostly found during the light cycle [35].
Respiratory frequency showed a similar decrease during anesthesia for both housing conditions, except for the notable increase at 60 min for the MLC housed rats, justified by their speedy recovery. Surprisingly, the blood oxygen saturation was similar for both conditions, reaching its maximum at 60 min for both conditions, indicating that the recovery phase for SDC rats was prolonged despite near normal awake values (>95%).
The combination of ketamine and xylazine is the first choice of injectable anesthetic drugs for rodent species, and results in an effective and safe plane of anesthesia [1, 5, 16]. Ketamine induces immobilization, analgesia, and hypotension, while xylazine complements with muscle relaxation and further analgesia properties. Both drugs are metabolised by hepatic CYP3A enzymes and excreted by the kidneys. More specifically, ketamine is first biotransformed into norketamine by CYP3A4, followed by further demethylation and hydroxylation steps into dehydronorketamine and hydroxynorketamine by CYP2A6 and CYP2B6. Norketamine, the major metabolite, is potent anesthetically, acting similarly as an NMDA receptor antagonist, while hydroxynorketamine is a nicotinic acetylcholine receptor antagonist and thus has no anesthetic properties [18, 20]. Xylazine, on the other hand, has been shown to be metabolised into at least 20 different metabolites [23, 24] and could be differently affected. We have previously shown that an increase in the depth and duration of KX anesthesia is observed with aging (12- and 18-month-old vs. 3- and 6-month-old rats). This change in drug metabolism was, in part, explained by the rapid norketamine saturation of the CYP3A enzyme active sites in liver S9 fractions in older rats, compared to younger rats, rendering ketamine metabolism less effective, thus increasing its drug exposure (AUC) and decreasing its elimination [34]. In our pharmacokinetic evaluation, we noted a peak in norketamine at 60 min in the SDC rats, possibly justified by a possible saturation of the hepatic enzymes and most likely associated with a greater depth of anesthesia. Cytochrome P450 expression and function are influenced by obesity levels, such as CYP3A hepatic expression levels are increased in high-fat fed Sprague-Dawley rats [14], whereas decreased activity has previously been linked to obesity in humans [4]. In addition, liver transcriptomics analysis of mouse induced obesity models revealed a global dysregulation of Cyp genes, most notably in the Cyp3a and Cyp2c families [11]. Similar changes were also observed in diet-induced metabolic syndrome in guinea pigs, where decreases CYP3A expression and activity were observed in high-fat high-sucrose and high-fat high-fructose diets [25]. Downstream enzymes that metabolize norketamine, notably CYP2B6 and CYP2A6 could also be affected. Studies have shown that increasing activity can increase muscular blood flow and decrease hepatic and renal blood flow, thus in turn affecting pharmacokinetics of that possesses high hepatic extraction and renal clearance [3, 18]. This in turn, could at least in part explain the increase in half-life and decrease in clearance of ketamine in the rats housed in MLC, compared to the SDC. In addition, since ketamine and xylazine are lipophilic drugs and have a high volume of tissue distribution, the degree of fat deposits could also act as a storage compartment and affect the drug release into the blood stream. Altogether the depth of anesthesia is decreased in MLC cages rats and this seems to be associated with a decrease of the norketamine seen at 1 h post KX injection, although ketamine plasmatic concentration is similar in animals from both caging system. In the elimination phase, terminal elimination half-life (T1/2) of ketamine was increased and relative clearance rate (CL/F) was decreased significantly in rats housed in MLC which suggests a decreased ketamine metabolism or a modification in the distribution of the drug. Both of these observations are related to different phase of the drug metabolism and both ketamine and norketamine seems to be differently affected in early and late phases of their metabolism.
In this study, while we did not verify for differences in hepatic liver enzymes, a notable difference was seen in liver sizes between conditions, with the SDC having notably heavier livers. No histological differences were noted between animal. This macroscopic change suggests a change in the liver function, thus affecting the ketamine and norketamine metabolism, which in turn led to a significant increase in drug exposures (AUC) and impedance in their clearance.
An important desired feature of the MLC system for rats is the potential ability to decrease overall stress of the animal. It is generally accepted that environmental enrichment is critical for the wellbeing of the animal and a way to reduce anxiety and stereotypic behaviors [27, 28, 37]. Cage size can be considered an environmental enrichment as rats showed a significant preference for larger cages [28]. Many published articles have previously studied the impacts of environmental enriched housing systems. When given vertical space, laboratory rats spent between 5.5 and 8.7% of their daily locomotor activity in bi-pedal posture, suggesting that upright standings is an integral part of the rat’s natural ethogram [6]. In a study where rats were required to be bi-pedal in order to reach their food and water for a period of 12 weeks, the rats had improved hind limb mass and decreased bone loss in 6-month-old orchidectomized rats [38]. In fact, provision of complex and enhanced vertical space can result in a positive emotional affective state in rats [35]. Various environmental enrichments can also have an impact on the pharmacological effects of drugs. Male rats housed in larger cages (2,052 cm2) in larger groups (4 /cage) for 4 weeks showed a decreased effect of diazepam in the elevated plus-size maze test, and an increased sensitivity to amphetamine on the home cage activity test, compared to isolated standard caged rats. These larger cages also affected food consumption, as the animals were significantly lighter than the isolated control rats [29]. In the study herein, it should be noted that food consumption (data not shown) and body weights between groups did not vary between conditions. This finding could vary according to specific strain used, as Sprague-Dawley rats were seen to lose the least amount of weight when given a running wheel, compared to other strains [15]. Alternative sources of environmental enrichment (crinkled paper, wooden blocks), in exception to the PVC tube, was not added to the cages to mimic standard rat housing practices in North America.
The use of MLC have yielded positive alterations in the animal’s behavior, such that rats housed in similar MLC with post-sciatic nerve injuries showed more explorative behaviours and reduced anxiety [33]. In a recent article, Gaskill et al .have observed more rat press postures, in which male or female rats press their ventrum into the cage floor or against a vertical surface [12]. This behavior has been associated with possible stress or crowding, as the frequency of press postures was seen to increase with litter age in smaller commercial rat cages. In another report, neutrophil:lymphocyte ratio was significantly increased in rats with restricted access to the bottom floor of MLC after being allowed full access, compared to control rats in SDC, suggesting a chronic distress response after environmental change [35]. In the present study, we measured the relative adrenal gland: body weight ratios and were found to be greater for rats housed in the smaller, SDC, compared to rats housed in the MLC, suggesting a chronic increase in adrenocortical activity and thus, environmental-associated stress [2, 32].
Despite the constant need of improving laboratory animal welfare, researchers are often reluctant to change the environment of their research animals in fear that it will impact the animal’s biological and physiological parameters, thus potentially changing the experimental results. As this report demonstrates, changing housing conditions to a MLC system may affect physiology and pharmacokinetic, requiring adaptations to current procedures, such as anesthesia, and validation of drug metabolism, distribution and elimination. For instance, an increase in anesthesia dosages would be required for longer procedures. On the other hand, earlier recovery times can also have its benefits when performing shorter procedures. Nonetheless, housing rats in MLC may very well be a better representation of physiological processes, as opposed to rats housed in small standard cages that limit locomotion and natural posture. Validation and proper controls are thus important to fully compare the experimental variable in question, as opposed to rely on historical controls which can incorporate a multitude of biases. In conclusion, long term housing of laboratory rats in commercial MLC systems, compared to SDC systems, while having a positive impact on wellbeing, can alter anesthetic drug metabolism and thus modify certain anesthesia parameters and associated physiological processes.
Acknowledgments
The authors would like to thank Dr. Guy Beauchamp, statistician at the Faculty of Veterinary Medicine, University of Montreal, for the statistical analyses. This study was funded by the Fond du Centenaire de la Faculté de Médecine Vétérinaire (Aurore Dodelet-Devillers) and the Fond pour le développement des animaux de laboratoire (Pascal Vachon). We would like to thank Thermo Fisher Scientific for providing a generous access to a Q-Exactive Orbitrap Mass Spectrometer.
References
- 1.Arras M., Autenried P., Rettich A., Spaeni D., Rülicke T.2001. Optimization of intraperitoneal injection anesthesia in mice: drugs, dosages, adverse effects, and anesthesia depth. Comp. Med. 51: 443–456. [PubMed] [Google Scholar]
- 2.Belz E.E., Kennell J.S., Czambel R.K., Rubin R.T., Rhodes M.E.2003. Environmental enrichment lowers stress-responsive hormones in singly housed male and female rats. Pharmacol. Biochem. Behav. 76: 481–486. doi: 10.1016/j.pbb.2003.09.005 [DOI] [PubMed] [Google Scholar]
- 3.Björkman S., Redke F.2000. Clearance of fentanyl, alfentanil, methohexitone, thiopentone and ketamine in relation to estimated hepatic blood flow in several animal species: application to prediction of clearance in man. J. Pharm. Pharmacol. 52: 1065–1074. doi: 10.1211/0022357001774985 [DOI] [PubMed] [Google Scholar]
- 4.Brill M.J., van Rongen A., van Dongen E.P., van Ramshorst B., Hazebroek E.J., Darwich A.S., Rostami-Hodjegan A., Knibbe C.A.2015. The pharmacokinetics of the CYP3A substrate midazolam in morbidly obese patients before and one year after bariatric surgery. Pharm. Res. 32: 3927–3936. doi: 10.1007/s11095-015-1752-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buitrago S., Martin T.E., Tetens-Woodring J., Belicha-Villanueva A., Wilding G.E.2008. Safety and efficacy of various combinations of injectable anesthetics in BALB/c mice. J. Am. Assoc. Lab. Anim. Sci. 47: 11–17. [PMC free article] [PubMed] [Google Scholar]
- 6.Büttner D.1993. Upright standing in the laboratory rat--time expenditure and its relation to locomotor activity. J. Exp. Anim. Sci. 36: 19–26. [PubMed] [Google Scholar]
- 7.Canadian Council on Animal Care1993. Guide to the care and use of experimental animals. Vol 1, 2nd ed. Ottawa (Canada): Canadian Council on Animal Care. [Google Scholar]
- 8.Charles River Sprague-Dawley Growth Curve[Internet]. 2015. Available at: http://www.criver.com/products-services/basic-research/find-a-model/sprague-dawley-rat.
- 9.Cloutier S., Newberry R.C.2013. Do rat moms need a break? Abstract presented at the AALAS National Meeting, Baltimore, MD. J. Am. Assoc. Lab. Anim. Sci. 52: 673. [Google Scholar]
- 10.Council of Europe[Internet]. 2006. Appendix A of the European Convention for the Protection of Vertebrate Animals Used for Experimental and other Scientific Purposes (ETS no. 123): Guidelines for accommodation and care of animals (Article 5 of the Convention), p 21–22. [Cited 15 June 2014]. Available at: http:// conventions.coe.int/Treaty/EN/Treaties/PDF/123-Arev.pdf.
- 11.Deol P., Evans J.R., Dhahbi J., Chellappa K., Han D.S., Spindler S., Sladek F.M.2015. Soybean Oil Is More Obesogenic and Diabetogenic than Coconut Oil and Fructose in Mouse: Potential Role for the Liver. PLOS ONE 10: e0132672. doi: 10.1371/journal.pone.0132672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaskill B.N., Pritchett-Corning K.R.2015. Effect of Cage Space on Behavior and Reproduction in Crl:CD(SD) and BN/Crl Laboratory Rats. J. Am. Assoc. Lab. Anim. Sci. 54: 497–506. [PMC free article] [PubMed] [Google Scholar]
- 13.Giroux M.C., Santamaria R., Hélie P., Burns P., Beaudry F., Vachon P.2016. Physiological, pharmacokinetic and liver metabolism comparisons between 3-, 6-, 12- and 18-month-old male Sprague Dawley rats under ketamine-xylazine anesthesia. Exp. Anim. 65: 63–75. doi: 10.1538/expanim.15-0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghoneim R.H., Ngo Sock E.T., Lavoie J.M., Piquette-Miller M.2015. Effect of a high-fat diet on the hepatic expression of nuclear receptors and their target genes: relevance to drug disposition. Br. J. Nutr. 113: 507–516. doi: 10.1017/S0007114514003717 [DOI] [PubMed] [Google Scholar]
- 15.Gordon C.J., Phillips P.M., Johnstone A.F.2016. Impact of genetic strain on body fat loss, food consumption, metabolism, ventilation, and motor activity in free running female rats. Physiol. Behav. 153: 56–63. doi: 10.1016/j.physbeh.2015.10.025 [DOI] [PubMed] [Google Scholar]
- 16.He S., Atkinson C., Qiao F., Chen X., Tomlinson S.2010. Ketamine-xylazine-acepromazine compared with isoflurane for anesthesia during liver transplantation in rodents. J. Am. Assoc. Lab. Anim. Sci. 49: 45–51. [PMC free article] [PubMed] [Google Scholar]
- 17.Institute for Laboratory Animal Research2011. Guide for the care and use of laboratory animals, 8th ed., National Academy Press, Washington. [Google Scholar]
- 18.Jenkins A.J. Toxicokinetics and factors affecting pharmacokinetic parameters. pp. 21–24. In: Pharmacokinetics and pharmacodynamics of abused drugs. CRC Press: Boca Raton. [Google Scholar]
- 19.Khazaeinia T., Ramsey A.A., Tam Y.K.2000. The effects of exercise on the pharmacokinetics of drugs. J. Pharm. Pharm. Sci. 3: 292–302. [PubMed] [Google Scholar]
- 20.Lin H.C.2007. Dissociative Anesthetics. pp. 301–303. In: Lumb and Jones’ Veterinary anesthesia and analgesia. 4th ed., Blackwell Publishing. Ames. [Google Scholar]
- 21.Majewski-Tiedeken C.R., Rabin C.R., Siegel S.J.2008. Ketamine exposure in adult mice leads to increased cell death in C3H, DBA2 and FVB inbred mouse strains. Drug Alcohol Depend. 92: 217–227. doi: 10.1016/j.drugalcdep.2007.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer R.E., Fish R.E.2008. Pharmacology of injectable anesthetics, sedatives, and tranquilizers. pp. 27–82. In: Anesthesia and analgesia in laboratory animals. Academic Press. San Diego. [Google Scholar]
- 23.Mutlib A.E., Chui Y.C., Young L.M., Abbott F.S.1992. Characterization of metabolites of xylazine produced in vivo and in vitro by LC/MS/MS and by GC/MS. Drug Metab. Dispos. 20: 840–848. [PubMed] [Google Scholar]
- 24.Park Choo H.Y., Choi S.O.1991. The metabolism of xylazine in rats. Arch. Pharm. Res. 14: 346–351. doi: 10.1007/BF02876882 [DOI] [Google Scholar]
- 25.Patoine D., Levac X., Pilote S., Drolet B., Simard C.2013. Decreased CYP3A expression and activity in guinea pig models of diet-induced metabolic syndrome: is fatty liver infiltration involved? Drug Metab. Dispos. 41: 952–957. doi: 10.1124/dmd.112.050641 [DOI] [PubMed] [Google Scholar]
- 26.Rowland M., Towzer T.N.1995. Clinical pharmacokinetics: concepts and application, pp. 367–389, Lippincott, Williams and Wilkins, Philadelphia. [Google Scholar]
- 27.Sampedro-Piquero P., Begega A., Arias J.L.2014. Increase of glucocorticoid receptor expression after environmental enrichment: relations to spatial memory, exploration and anxiety-related behaviors. Physiol. Behav. 129: 118–129. doi: 10.1016/j.physbeh.2014.02.048 [DOI] [PubMed] [Google Scholar]
- 28.Simpson J., Kelly J.P.2011. The impact of environmental enrichment in laboratory rats--behavioural and neurochemical aspects. Behav. Brain Res. 222: 246–264. doi: 10.1016/j.bbr.2011.04.002 [DOI] [PubMed] [Google Scholar]
- 29.Simpson J., Kelly J.P.2012. The effects of isolated and enriched housing conditions on baseline and drug-induced behavioural responses in the male rat. Behav. Brain Res. 234: 175–183. doi: 10.1016/j.bbr.2012.06.015 [DOI] [PubMed] [Google Scholar]
- 30.Song G., Wu H., Yoshino K., Zamboni W.C.2012. Factors affecting the pharmacokinetics and pharmacodynamics of liposomal drugs. J. Liposome Res. 22: 177–192. doi: 10.3109/08982104.2012.655285 [DOI] [PubMed] [Google Scholar]
- 31.Struck M.B., Andrutis K.A., Ramirez H.E., Battles A.H.2011. Effect of a short-term fast on ketamine-xylazine anesthesia in rats. J. Am. Assoc. Lab. Anim. Sci. 50: 344–348. [PMC free article] [PubMed] [Google Scholar]
- 32.Turner P.V., Sunohara-Neilson J., Ovari J., Healy A., Leri F.2014. Effects of single compared with pair housing on hypothalamic-pituitary-adrenal axis activity and low-dose heroin place conditioning in adult male Sprague-Dawley rats. J. Am. Assoc. Lab. Anim. Sci. 53: 161–167. [PMC free article] [PubMed] [Google Scholar]
- 33.Vachon P.2014. Double Decker Enrichment cages have no effect on long term nociception in neuropathic rats but increase exploration while decreasing anxiety-like behaviors. Scand. J. Lab. Anim. Sci. 40: 1–6. [Google Scholar]
- 34.Veilleux-Lemieux D., Castel A., Carrier D., Beaudry F., Vachon P.2013. Pharmacokinetics of ketamine and xylazine in young and old Sprague-Dawley rats. J. Am. Assoc. Lab. Anim. Sci. 52: 567–570. [PMC free article] [PubMed] [Google Scholar]
- 35.Wheeler R.R., Swan M.P., Hickman D.L.2015. Effect of multilevel laboratory rat caging system on the well-being of the singly-housed Sprague Dawley rat. Lab. Anim. 49: 10–19. doi: 10.1177/0023677214547404 [DOI] [PubMed] [Google Scholar]
- 36.Wilson M.G., Ellison G.M., Cable N.T.2016. Basic science behind the cardiovascular benefits of exercise. Br. J. Sports Med. 50: 93–99. doi: 10.1136/bjsports-2014-306596rep [DOI] [PubMed] [Google Scholar]
- 37.Würbel H.2001. Ideal homes? Housing effects on rodent brain and behaviour. Trends Neurosci. 24: 207–211. doi: 10.1016/S0166-2236(00)01718-5 [DOI] [PubMed] [Google Scholar]
- 38.Yao W., Jee W.S., Chen J.L., Li C.Y., Frost H.M.2001. A novel method to ‘exercise’ rats: making rats rise to erect bipedal stance for feeding - raised cage model. J. Musculoskelet. Neuronal Interact. 1: 241–247. [PubMed] [Google Scholar]