Abstract
We reared ICR mice during a growth period (3 to 10 weeks of age) and examined the effect of exercise induction, by enriching the rearing environment with obstacles such as ladders, compared to the standard environment. Environmental enrichment significantly increased the amount of exercise in both sexes (P<0.01). Enriched exercise mice had higher body weight than control mice at 6 to 9 weeks of age in males and 8 weeks of age in females (P<0.05). The sexual maturation of female enriched exercise mice was significantly advanced compared to the control (P<0.001). Enriched exercise mice showed decreased anxiety-like behavior in the open field test and lower plasma corticosterone levels in both sexes compared to the control, and differences were statistically significant in males (P<0.05). In both sexes, enriched exercise appeared to increase natural killer cells in blood compared to the control, but no statistical differences was detected. In conclusion, we confirmed that daily low-stress exercise could be induced using a three-dimensional rearing environment in growing mice. In addition, we suggest that exercise has beneficial effects on physical growth, sexual maturation and anxiety-like behavior. Furthermore, environmental enrichment might be more effective in male than female in group-housed mice.
Keywords: exercise induction, growth period, mice, rearing environment
Introduction
The growth period is an important time in the life cycle, because the majority of physical and mental growth, and reproductive and immune function development occurs during this period. For optimal health, growth and development, daily, low-stress exercise is recommended in the early years of the life cycle [33]. However, the basic effects of exercise on growing animals have not been fully clarified due to a lack of data. For this reason, the effects of exercise on physical growth and anxiety on growing animals are controversial issues. While some studies demonstrated the effects of exercise on increasing body weight and lowering anxiety-like behavior compared to control [19, 31, 32], others did not observe such effects [11, 21]. In addition, there are concerns about the effects of exercise on the development of reproductive and immune system functions. Few studies have examined sexual maturation, which is the advent of the animal’s reproductive period. Furthermore, the effect of chronic exercise on the number of natural killer (NK) cells during growth period has not been clarified. NK cells are a subpopulation of leucocytes involved in innate immunity and are known to recognize and attack a variety of tumor and virally infected cells. Thus, animal studies are necessary to clarify the basis of the effects of exercise during the growth period.
Previous studies have employed forced treadmill running and swimming or voluntary wheel running to induce exercise in animals. However, forced exercise might produce high stress levels [1, 24] and voluntary wheel exercise might be stereotypical stress responses [6]. To control for these issues and provide access to low-stress, natural exercise, we selected an enriched three-dimensional environment comprised of various obstacles in this study. An enriched environment is thought to increase the amount of exercise. However, this treatment has not been chosen for exercise studies because the effect of inducing exercise has not been demonstrated. In this study, we observed the amount of exercise undertaken in a rearing environment. In addition, we examined whether an enriched environment has an influence on stress levels. Stress levels are usually reflected in the activation of the hypothalamic–pituitary–adrenocortical (HPA) systems, and the activity of the HPA axis is typically measured by serum or plasma levels of cortisol or corticosterone [13, 14]. In this study, stress levels were assessed by measuring plasma corticosterone levels.
The purpose of the current study was to: (1) examine whether the amount of exercise can be increased by providing an enriched rearing environment and the resultant effect on plasma corticosterone levels, and (2) clarify the effects of exercise on growth parameters such as weight and sexual maturation, anxiety-like behavior assessed by the open field test, and NK cell levels in blood during active growth in an animal study.
Materials and Methods
Animals and housing
Eighty Jcl: ICR mice (20 days old) were obtained from CLEA Japan (Tokyo, Japan). Half of the mice were male, half were female. During the study, four mice were housed per cage. All mice were maintained in identical plastic cages measuring W282 × H157 × D451 mm with woodchip bedding and a wire-top lid. Half of the cages had three (ladder, cube, partition) aspen wooden obstacles (Tapvei, Kortteinen, Finland) as the three-dimensional complicated environment to induce exercise (Fig. 1). Mice were provided with food (MF, Oriental Yeast Co., Tokyo, Japan) and water ad libitum. Cages were maintained at a temperature of 24 ± 2°C and 40 ± 20% relative humidity and with a 12 h light/dark cycle, lights on at 6.00 h.
Fig. 1.
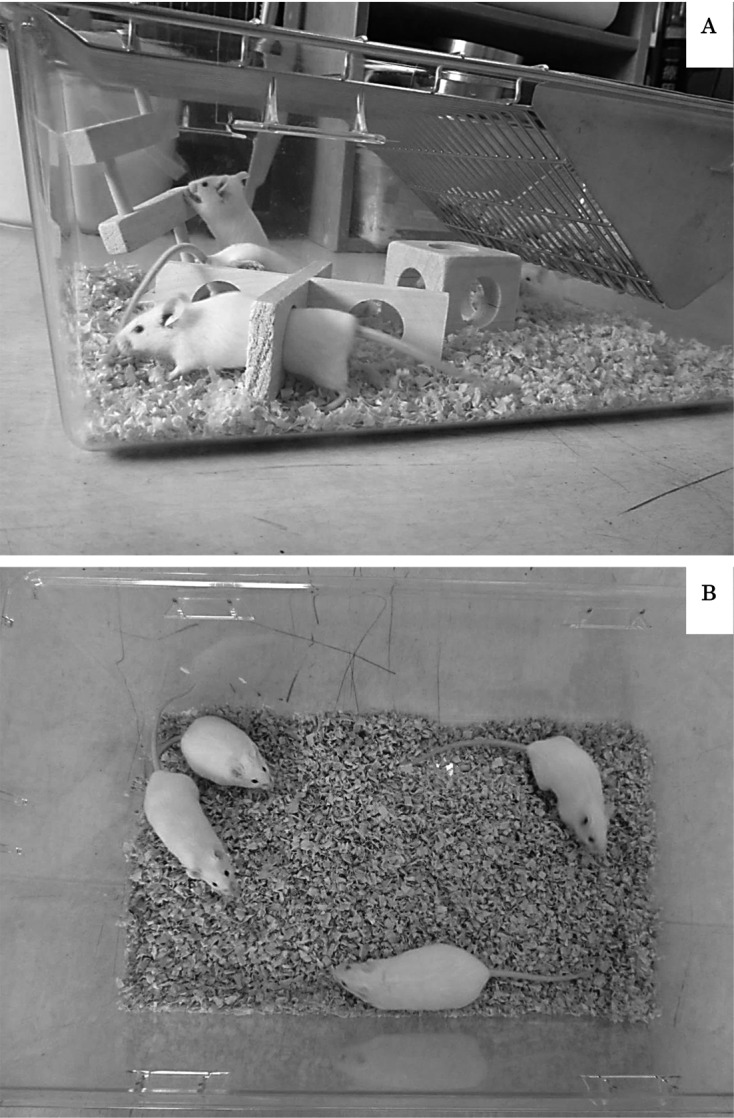
Housing conditions. (A) Enriched exercise group (male and female). (B) Control group (male and female). n=20 per group.
Experimental design
After mice were ear-punctured for individual identification at 20 days of age, they were separated by sex and randomly assigned to two groups so that half of the mice were reared in conventional cages (control group) and the other half in cages that contained wooden obstacles (exercise group) until mice were sacrificed at 10 weeks old. These four groups (control males, exercise males, control females and exercise females) were replicated five times with four mice per cage. The wooden obstacles were changed every week to maintain cleanliness. Experiments were initiated when mice were 3 weeks old. Behavior in the home cage was captured on video every week. The body growth of Jcl: ICR mice reaches its maximum by 9–10 weeks after birth according to Ibaraki and Nomura [16]. Therefore, body weight, food intake, and testis size were also measured weekly until 9 weeks of age. Taking of video and measuring physical parameters were performed on different days in order to avoid any influencing the parameters. After opening of the vaginal cavity, stages of estrous cycle were observed for 5 days per week until estrous cycles were stable. At 9 weeks of age, the open field test was performed. Blood samples were collected at 10 weeks of age to determine the number of NK cells and plasma corticosterone concentration. Permission for this study was obtained from the Tokyo University of Agriculture and Technology Laboratory Animal Care and Use Committee.
Observation of exercise performance
Video capture of mice in cages was used to measure the time of exercise. All videos were taken for 10 min during the light period between 08:00 and 09:00, with each group recorded at the same time each week. The composition of exercising was distributed into two patterns to confirm that the obstacle was actually used for exercise. Exercising with obstacles included climbing, passing, and overstepping obstacles. Exercising without obstacles included locomotion on the bedding and climbing the cage lid. These behaviors were manually counted from the video recording. The data of the exercise time of 3–10 weeks were averaged.
Body growth and food intake
Individual body weight and food intake per group were measured between 11:00 and 12:00 weekly throughout the study using an electronic balance (GX-12; A&D, Tokyo, Japan). In order to measure feed intake, animals were provided weekly with 300 g of pellets, and the amount of food ingested was calculated after weighing the food remaining at the end of each week.
Sexual maturation
Sexual maturation of males was determined by testes size. After both testes descended into the scrotum, the minor and longer axes of testis were measured by vernier caliper (VC-15; Niigataseiki, Niigata, Japan) and assumed to be ellipsoid to allow calculation of testis volume. We calculated the relative value of the testis volume by normalizing body weight (BW) to 100 g in order to control for the effect of body growth on testis volume.
This study examined the onset of one complete estrous cycle lasting 4 days, which was used to indicate female sexual maturation. After the vaginal opening was visually observed, vaginal smears were collected. Subsequent smear tests were performed until stabilization of the estrous cycle, as previously described [29].
Open field test
Mice were tested with a single 10-min trial during the light period between 8:00 and 14:00. The open field was a square arena (W50×H41×D50 cm divided into 25 equal squares) on a white plastic floor and white plastic walls. The animals were placed in the center of the box; the number of lines crossed with all four paws, defecation, duration of grooming (including washing or mouthing of forelimbs, hind-paws, face, body and genitals), frequency of freezing and starting latency (length of time in the open field before moving from the center) were manually counted from the video recording. The arena was cleaned with 70% ethyl alcohol after each test.
Blood sampling
After mice were anesthetized with diazepam (Teva Pharma Japan Inc., Nagoya, Japan) and ketamine hydrochloride (Ketalar; Daiichi Sankyo Co. Limited, Tokyo, Japan), blood samples were collected by cardiac puncture. Blood volumes of 0.6–1.5 ml were obtained upon euthanization by exsanguination under anesthesia by means of a heparinized syringe (Heparin Lithium Salt; Nacalai Tesque, Tokyo, Japan). Blood (150 µl) was used for NK cell counts immediately after collection. Plasma was separated from the remaining blood by centrifugation (3,000 × g for 10 min) and frozen (−20°C) until assay for corticosterone concentrations.
NK cell count
The NK cell numbers were obtained by multiplying the total white blood cell (WBC) counts by percentages of CD49b-positive cells with a flow cytometer. Briefly, 64 samples were counted with an automated cell counter (CountessTM Automated Cell Counter; Invitrogen, Carlsbad, USA) and 16 were with an another type of counter (Sysmex XT-2000iv; LSI Medience Corporation, Tokyo, Japan). On the other hand, the percentages of NK cells in individual WBCs were simultaneously determined by flow cytometry. Cells were stained with phycoerythrin-conjugated anti-Ly-6G antibody (granulocytes; clone 1A8), and allophycocianin-cyanine7- conjugated anti-F4/80 antibody (monocytes; clone BM8), to eliminate granulocytes and monocytes from positively-stained cells with allophycocianin-conjugated anti-CD49b antibody (NK cells; clone DX5) at 4°C for 30 min. And, allophycocianin-cyanine7-conjugated anti-CD11b antibody (clone M1/70) was used for the compensation of anti-F4/80. All antibodies were provided by BioLegends Co. (San Diego, CA, USA). Stained cells were analyzed with a flow cytometer (BD FACSArray; BD Biosciences, Franklin Lakes, NJ, USA). 20,000 events were collected for analysis. Namely, NK cells were defined as CD49b-positive and Ly-6g- or F4/80-negative cells.
The following formula was, in turn, used for calculations: total NK cells (cells/µl)=WBC (cells/µl) × percentage of NK cells (%).
Corticosterone assay
After thawing, the plasma corticosterone concentrations (ng/ml) were measured. The corticosterone concentrations were determined by an enzyme-linked immunoassay (ELISA) kit (AssayMax; AssayPro, Saint Charles, USA) according to the manufacturer’s instructions. Details of the assay are described in Zhang et al. [44].
Data analysis
All values are expressed as means ± SEM. Male and female mice were separately analyzed. Unpaired Student’s t-tests were used to make comparisons between the exercise group and the control for the amount of exercise, the period of female sexual maturation and open field behavior as parametric tests. The Mann-Whitney U test was used for plasma corticosterone concentrations and NK counts as nonparametric tests, after valid outliers were identified with Smirnov-Grubbs outlier test and excluded from the analyses, because few mice might have too small amount of blood to analysis. The effect on body weight, food intake and testis size, was determined by analysis of variance (ANOVA) with repeated measures using environment × weight or volume as factors, and unpaired Student’s t-tests were subsequently performed to compare weekly parameters of the exercise groups and control groups. In all statistical tests, differences were considered significant at a probability level of 0.05 (5%).
Results
Exercise performance
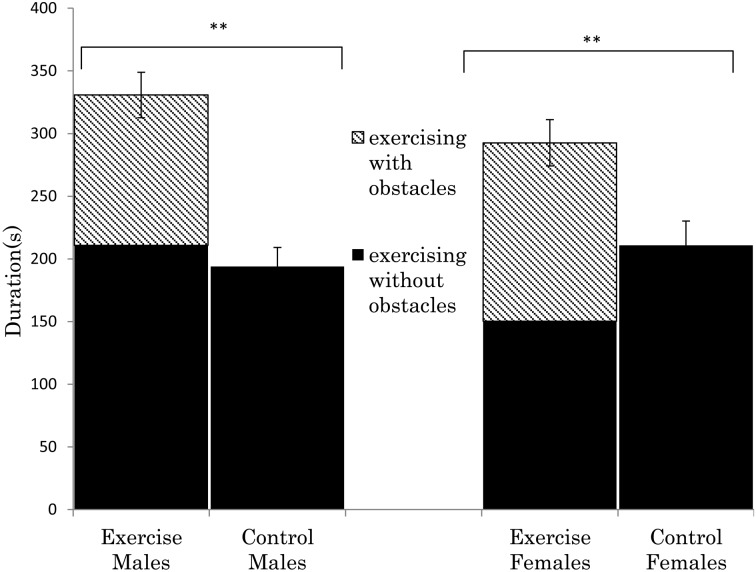
Figure 2 shows the total time spent exercise, divided according to exercise with obstacles and without obstacles. In the enriched groups, 1/3–1/2 of the total time exercising was with obstacles. In both sexes, there was a significant difference in the total exercise time between the enriched groups and controls (males, t (238)=5.76, P<0.01; females, t (238)=3.04, P<0.01, respectively).
Fig. 2.
Average of exercise time (seconds) per 10 min during 3 to 10 weeks of age in ICR mice. Mice were housed in 4 groups and the exercise data represents 4 cages of mice. Enriched exercise includes climbing, passing, and overstepping the three types of obstacles. Other exercise included locomotion and climbing the cage lid without touching the obstacles. In both sexes, there was a significant difference between the enriched exercise group and control (t238=5.76; P<0.01, t238=3.04; P<0.01), according to unpaired Student’s t-tests. **P<0.01, n=20 per group.
Body growth and food intake
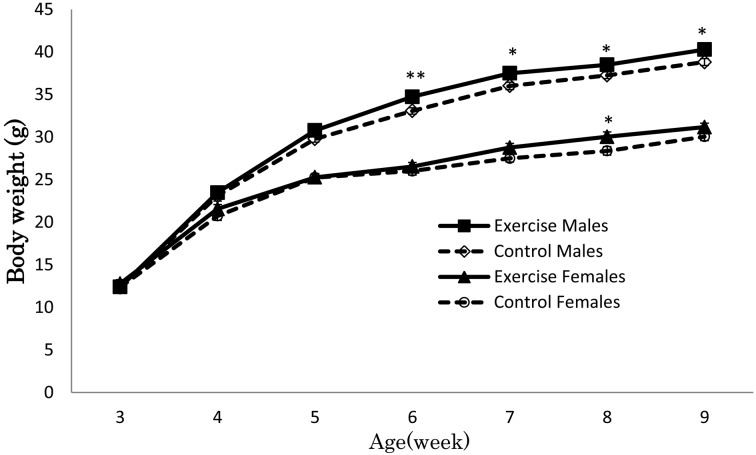
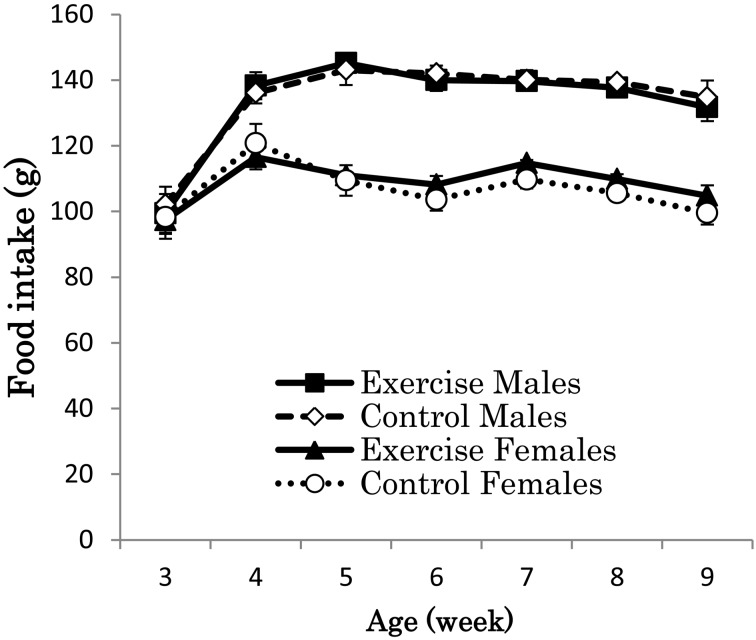
Figure 3 illustrates the increase in body weight of the four groups. Body weight was higher in the enriched exercise group than in the control, but the statistical significances were not detected between the exercise and control groups (male, ANOVA, F (1, 38)=3.12, P=0.09; female, ANOVA, F (1, 38)=1.82, P=0.18). Body weights did not differ among the groups at the beginning of the experiment and increased every week. When body weights were analyzed according to week, significant differences between exercise group and control group were observed at several weeks in males (6 weeks old, t (38)=3.11, P<0.01; 7 weeks old, t (38)=2.31, P<0.05; 8 weeks old, t (38)=2.11, P<0.05; 9 weeks old, t (38)=2.38, P<0.05) and in females (8 weeks old, t (38)=2.11, P<0.05). Food intake did not differ significantly between exercise and control groups in male (ANOVA, F (1, 8)=0.05, P=0.82) and female (ANOVA, F (1, 8)=0.05, P=0.84) through this experiment (Fig. 4).
Fig. 3.
Average body weight (g) of ICR mice. Body weight increased in both the enriched exercise and control groups. The male enriched exercise group had heavier body weight at 6 to 9 weeks of age, and the female enriched exercise group had heavier body weight at 8 weeks of age compared to control, according to unpaired Student’s t-tests. **P<0.01 and *P<0.05, n=20 per group.
Fig. 4.
Average food intake (g) of ICR mice (n=20 per group). Food intake did not differ significantly between exercise and control groups in male and female through this experiment, according to ANOVA.
Sexual maturation
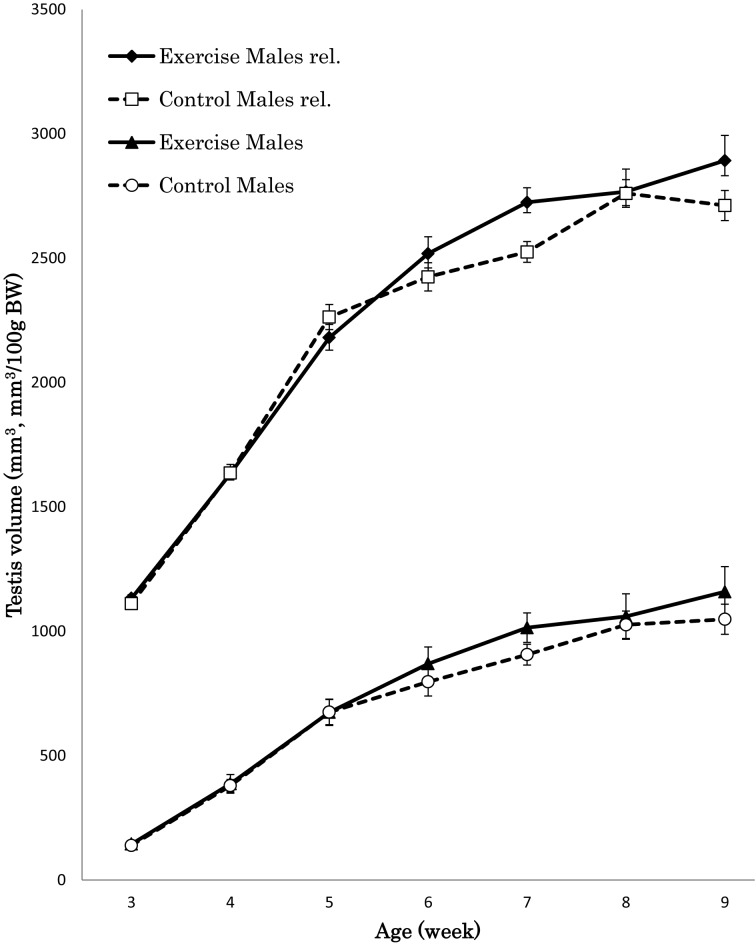
There were no significant differences in male testis size between groups throughout the experiment. However, enriched exercise male mice tended to have greater testis size than control male mice from 6 weeks old until the final measurement (Fig. 5). In addition, enriched exercise male mice also tended to have larger relative testis volume than control male mice from 6 weeks old until the final measurement.
Fig. 5.
Average testis volume (mm3) and relative testis volume (mm3 /100g body weight) of ICR mice (n=20 per group). Testis volume increased in both the enriched exercise and control groups. The male enriched exercise group tended to have larger testis volume and relative volume by normalizing body weight to 100 g at 6 to 9 weeks of age (BW=body weight, rel.=relative volume).
The estrous cycle of enriched exercise female mice stabilized significantly earlier than control female mice (35.85 ± 6.24 vs. 46.8 ± 7.85 days, t (38)=4.76, P<0.001).
Open field behavior
Table 1 shows the behavioral scores of the open field test during treatment with enriched exercise or control. Females did not exhibit significant differences, but showed the same tendency as males for all parameters assessed. The male enriched exercise group showed significantly higher line crossing (t (38)=3.44, P<0.01) and lower starting latency (t (38)=2.06, P<0.05) and defecation (t (38)=2.15, P<0.05) compared to control males. In addition, the enriched exercise group tended to have less freezing frequency and longer duration of grooming compared to the control; however, differences were not significant.
Table 1. Summary of results in open field test.
| Measurement/Housing difference | Male | Female | |||
|---|---|---|---|---|---|
| Exercise | Control | Exercise | Control | ||
| Line Crossings (frequency/10 min) | 543 ± 30.9 | 413 ± 21.9 | 473 ± 33.6 | 428 ± 28.6 | |
| **P=0.0014 | P=0.33 | ||||
| Starting Latency (s) | 0.7 ± 0.17 | 1.5 ± 0.34 | 0.8 ± 0.24 | 1.6 ± 0.35 | |
| *P=0.046 | P=0.093 | ||||
| Defecation (frequency/10 min) | 2.2 ± 0.37 | 3.5 ± 0.44 | 3.1 ± 0.57 | 3.8 ± 0.47 | |
| *P=0.038 | P=0.36 | ||||
| Freezing (frequency/10 min) | 2.2 ± 0.95 | 2.8 ± 0.82 | 1.4 ± 0.44 | 3.3 ± 0.89 | |
| P=0.64 | P=0.069 | ||||
| Grooming (s) | 18.8 ± 2.61 | 16.2 ± 2.40 | 24.7 ± 8.39 | 15.8 ± 3.46 | |
| P=0.48 | P=0.35 | ||||
Values showed the means ± SEM, n=20 per group.**P<0.01 and *P<0.05.
NK cell counts
Table 2 shows the number of NK cells in blood, the percentage of NK cells in WBC and WBC count of enriched exercise or control.
Table 2. NK cells count obtained by multiplying the total WBC by percentage of NK cells.
| Male | Female | ||||
|---|---|---|---|---|---|
| Exercise | Control | Exercise | Control | ||
| WBC count (cells/μl) | 3,840 ± 333.5 | 3,742 ± 387.3 | 4,203 ± 414.4 | 3,607 ± 335.4 | |
| NK cells in WBC (%) | 8.84 ± 1.46 | 6.93 ± 1.33 | 7.57 ± 1.11 | 6.22 ± 0.87 | |
| NK cells count (cells/μl) | 339 ± 4.87 | 259 ± 5.17 | 318 ± 4.59 | 224 ± 2.93 | |
| P=0.34 | P=0.44 | ||||
NK=natural killer, WBC=white blood cells. Values showed the means ± SEM, n=20 per group.
Enriched exercise mice were inclined to have more NK cells in blood than control mice did, but the differences were not significant in sex matched groups.
Corticosterone
Plasma corticosterone concentrations of both sexes were lower in the enriched exercise groups than in the respective controls (Table 3). In particular, a significant difference was observed in males (P<0.05).
Table 3. Corticosterone (ng/ml) in mice with or without environmental enrichment.
| Male | Female | ||||
|---|---|---|---|---|---|
| Exercise | Control | Exercise | Control | ||
| CORT (ng/ml) | 63.0 ± 13.5 | 148.4 ± 29.4 | 142.2 ± 23.4 | 187.0 ± 33.4 | |
| *P=0.013 | P=0.29 | ||||
CORT=corticosterone, Values showed the means ± SEM, n=20 per group. *P<0.05.
Discussion
During active growth, the exercise groups had a higher body weight than the control in both sexes. Previous studies in rearing rodents showed that running exercise decreased body weight and increased food intake due to caloric demands [25, 36]. In another report, mice housed in a three-dimensional environment had significant heavier body weights compared to the control; however, as in our study, food consumption was not impacted [38]. This contradiction suggests that the effects of exercise may depend on the types of exercise and food. The present enriched environment might not have induced a sufficient level of exercise to decrease body weight, like running and swimming. When growing rats were provided a high-fat diet, exercise reduced body weight by burning fat [19]. Kimura also reported that a high-protein diet (22.9%) increased the body weight of exercising rats during active growth; additionally, the high-protein diet promoted energy utilization efficiency [19]. In this study, we provided a high-protein diet (23.1%) to mice, which have exceeded protein requirements for mice (18–20%) that were estimated by NRC (National Research Council) [7]. Because of the moderate exercise and improved energy efficiency by high-protein diet intake, enriched exercise mice showed higher growth and unchanged food intake compared to the control.
Testicular volume tended to be greater in enriched exercise males after 6 weeks of age, even when values were corrected for the increased body weight. Christian reported that testis weight decreased upon stress [5]. In addition, he claimed that this indicated increased corticosteroid levels and involved the suppression of gonadotrophic function by adrenocorticotrophic activity. In this study, males housed in an enriched environment showed lower corticosterone than the control. Therefore, stress might suppress testicular growth in our control. Toelle and Robinson suggested that larger testicular size would lead to an improvement in reproduction [35]. This suggests that exercise has beneficial effects on reproductive development in growing animals. Sexual maturation was advanced in the female exercise group. Advanced sexual maturation in response to exercise has been described in humans [23]. Viru et al. also demonstrated that hormone responses in sexual maturation were induced by exercise during puberty [41].
In the open field test, the high number of line crossings in the enriched exercise group might be due to the increased activity in the home cage [9]. However, open-field activity has not shown to be correlated to the rearing environment [8, 34]. The results of the open field test demonstrated reduced anxiety-like behavior in the enriched exercise mice. In the open field test, high anxiety is generally associated with lower overall levels of activity (line crossing), longer starting latency and durations of grooming, increased defecation and freezing [15, 18, 28]. Lower anxiety was reported in a previous study of effect of exercise on anxiety [10], but was not observed in another study [11]. This contradiction may be attributed to the variable impact of exercise type and animal strain employed [4, 37, 40]. The changes in anxiety-like behavior in the open field test may reflect changes in the limbic organs. The limbic system is recognized as a major player in the control of fear and anxiety [2]. Moreover, the limbic system is most probably modified by exercise [20]. It is likely that mental modifications of mice in the enriched environment occurred through the limbic system.
In this study, NK cells appeared to be increased by exercise, but no statistical significance was detected. NK cell counts was influenced by both the number of WBC and the percentage of NK cells in WBC. In a previous study, WBC levels did not significantly increase in mice housed in an enriched environment [37], which supports our results. It has been proven that physical activity enhances natural immunity in both humans and animals, probably through interactions between the central nervous and endocrine systems [17, 22]. According to Benaroya-Milshtein et al., “lymphocytes have receptors for endocrine hormones and there is an anatomical connection between the lymphoid and nervous systems. Thus, pathways of communication exist among the immune, nervous and endocrine systems [2]”. Previous studies reported exercise could stimulate alterations in the immune system, including NK cells [2, 3, 27]. This suggests that exercise induced in an enriched environment affected NK cell levels but we could not show significant increase in NK cell counts in this study. Therefore, the enhancement of immune function was not suggested in this study. In addition, Pedersen and Hoffman-Goetz suggested that the intensity of exercise is responsible for the degree of increment in the number of NK cells [27]. If the exercise has lasted for a long period and has been very intense such as a triathlon race, a increase in NK cells is found after exercise [30]. In this study, mice could select the intensity of exercise, which might have been low. Further studies are needed in order to clarify the intensity of exercise that is induced in environmental enrichment and whether it is enough to increase NK cell counts.
The lower corticosterone concentrations of mice housed in an enriched environment might reflect HPA axis modulation as a result of increased exercise. Provision of an enriched environment increased corticosterone concentrations in a previous study [2]. Benaroya-Milshtein et al. suggested that repeated mild stress caused by weekly exposure to novel obstacles elevated corticosterone concentrations [2]. It was also implied that repeated mild environmental stress might lead to emotional stability and risk-taking behavior, indicating reduced anxiety. However, our result showed that the enriched environment decreased corticosterone concentrations and anxiety-like behavior. In addition, the corticosterone concentrations observed in the control mice were higher value for normal ICR, compared to control ICR in other study [12]. An explanation for the high corticosterone levels in the control mice is as follows. It is possible that the absence of obstacles is psychologically stressful for animals. Compared to animals provided an enriched environment, control animals demonstrated higher frequencies of biting and sniffing, representing an increase in stereotypical behaviors normally observed in rodents under stress [42, 43]. In this study, we observed that only control mice showed stereotypical and highly aggressive behaviors. On the other hand, the increased activity in an enriched environment could also increase corticosterone levels [26]. In the present study, the enriched exercise groups showed increased activity in the cages. Thus, the higher corticosterone levels and anxiety-like behavior in the control mice could be caused by their higher stress levels as opposed to mild stress resulting from exercise. Therefore, we successfully controlled for stress in mice by providing an enriched rearing environment. Finally, in this study, aggressive behavior in control mice was more obvious in male than female, which was less observed in enriched exercise mice. A means of organizing social hierarchy vary according to sexes, and male are generally more aggressive than female [39]. Hence, the decrease of corticosterone levels and anxiety-like behavior that is affected by stress in enriched exercise might have reached statistical significance in male group, but not in female.
In conclusion, the results showed that the enriched rearing environment induced low-stress exercise in both male and female mice. In addition, we demonstrated some basic effects of exercise using a three-dimensional environment; exercise increased body weight, advanced sexual maturation, reduced anxiety-like behaviors as assessed by the open field test, decreased corticosterone. These effects are potentially beneficial to physical growth, anxiety, reproductive functions during active growth.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number 25340054.
References
- 1.Armario A., Gavaldà A., Martí J.1995. Comparison of the behavioural and endocrine response to forced swimming stress in five inbred strains of rats. Psychoneuroendocrinology 20: 879–890. doi: 10.1016/0306-4530(95)00018-6 [DOI] [PubMed] [Google Scholar]
- 2.Benaroya-Milshtein N., Hollander N., Apter A., Kukulansky T., Raz N., Wilf A., Yaniv I., Pick C.G.2004. Environmental enrichment in mice decreases anxiety, attenuates stress responses and enhances natural killer cell activity. Eur. J. Neurosci. 20: 1341–1347. doi: 10.1111/j.1460-9568.2004.03587.x [DOI] [PubMed] [Google Scholar]
- 3.Ben-Eliyahu S., Page G.G., Schleifer S.J.2007. Stress, NK cells, and cancer: Still a promissory note. Brain Behav. Immun. 21: 881–887. doi: 10.1016/j.bbi.2007.06.008 [DOI] [PubMed] [Google Scholar]
- 4.Chapillon P., Manneché C., Belzung C., Caston J.1999. Rearing environmental enrichment in two inbred strains of mice: 1. Effects on emotional reactivity. Behav. Genet. 29: 41–46. doi: 10.1023/A:1021437905913 [DOI] [PubMed] [Google Scholar]
- 5.Christian J.J.1955. Effect of population size on the adrenal glands and reproductive organs of male mice in populations of fixed size. Am. J. Physiol. 182: 292–300. [DOI] [PubMed] [Google Scholar]
- 6.Ciarleglio C.M., Gamble K.L., Axley J.C., Strauss B.R., Cohen J.Y., Colwell C.S., McMahon D.G.2009. Population encoding by circadian clock neurons organizes circadian behavior. J. Neurosci. 29: 1670–1676. doi: 10.1523/JNEUROSCI.3801-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Council N.R.1995. Nutrient requirements of the mouse. Nutrient Requirements of Laboratory Animals pp. 80–102. In: National Academy Press Washington, DC. [Google Scholar]
- 8.Dewsbury D.A.1980. Wheel-running behavior in 12 species of muroid rodents. Behav. Processes 5: 271–280. doi: 10.1016/0376-6357(80)90007-8 [DOI] [PubMed] [Google Scholar]
- 9.Elliott B.M., Grunberg N.E.2005. Effects of social and physical enrichment on open field activity differ in male and female Sprague-Dawley rats. Behav. Brain Res. 165: 187–196. doi: 10.1016/j.bbr.2005.06.025 [DOI] [PubMed] [Google Scholar]
- 10.Fulk L.J., Stock H.S., Lynn A., Marshall J., Wilson M.A., Hand G.A.2004. Chronic physical exercise reduces anxiety-like behavior in rats. Int. J. Sports Med. 25: 78–82. doi: 10.1055/s-2003-45235 [DOI] [PubMed] [Google Scholar]
- 11.Fuss J., Ben Abdallah N.M., Vogt M.A., Touma C., Pacifici P.G., Palme R., Witzemann V., Hellweg R., Gass P.2010. Voluntary exercise induces anxiety-like behavior in adult C57BL/6J mice correlating with hippocampal neurogenesis. Hippocampus 20: 364–376. [DOI] [PubMed] [Google Scholar]
- 12.Girard I., Garland T., Jr2002. Plasma corticosterone response to acute and chronic voluntary exercise in female house mice. J. Appl. Physiol. 92: 1553–1561. doi: 10.1152/japplphysiol.00465.2001 [DOI] [PubMed] [Google Scholar]
- 13.Givner M.L., Rochefort J.G.1965. An improved assay of corticosterone in rat serum and adrenal tissue. Steroids 6: 485–489. doi: 10.1016/0039-128X(65)90061-9 [DOI] [Google Scholar]
- 14.Henning S.J.1978. Plasma concentrations of total and free corticosterone during development in the rat. Am. J. Physiol. 235: E451–E456. [DOI] [PubMed] [Google Scholar]
- 15.Hirsjärvi P., Väliaho T.1995. Effects of gentling on open-field behaviour of Wistar rats in fear-evoking test situation. Lab. Anim. 29: 380–384. doi: 10.1258/002367795780739953 [DOI] [PubMed] [Google Scholar]
- 16.Ibaraki T., Nomura S.1967. Studies of the growth in ICR-Jcl mice. Exp. Anim. 16: 1–11(In Japanese). [Google Scholar]
- 17.Jonsdottir I.H.2000. Exercise immunology: neuroendocrine regulation of NK-cells. Int. J. Sports Med. 21:(Suppl 1): S20–S23. doi: 10.1055/s-2000-1447 [DOI] [PubMed] [Google Scholar]
- 18.Kalueff A.V., Tuohimaa P.2004. Contrasting grooming phenotypes in C57Bl/6 and 129S1/SvImJ mice. Brain Res. 1028: 75–82. doi: 10.1016/j.brainres.2004.09.001 [DOI] [PubMed] [Google Scholar]
- 19.Kimura M.1982. Effects of exercise and diet on growth of rats —comparison of high carbohydrate, high protein, and high fat diet—. Jpn. J. Phys. Fit. Sports Med. 31: 103–111(In Japanese). doi: 10.7600/jspfsm1949.31.103 [DOI] [Google Scholar]
- 20.Kitamura T., Mishina M., Sugiyama H.2003. Enhancement of neurogenesis by running wheel exercises is suppressed in mice lacking NMDA receptor ε 1 subunit. Neurosci. Res. 47: 55–63. doi: 10.1016/S0168-0102(03)00171-8 [DOI] [PubMed] [Google Scholar]
- 21.Leasure J.L., Jones M.2008. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience 156: 456–465. doi: 10.1016/j.neuroscience.2008.07.041 [DOI] [PubMed] [Google Scholar]
- 22.MacNeil B., Hoffman-Goetz L.1993. Chronic exercise enhances in vivo and in vitro cytotoxic mechanisms of natural immunity in mice. J. Appl. Physiol. 74: 388–395. [DOI] [PubMed] [Google Scholar]
- 23.McKay H.A., Petit M.A., Schutz R.W., Prior J.C., Barr S.I., Khan K.M.2000. Augmented trochanteric bone mineral density after modified physical education classes: a randomized school-based exercise intervention study in prepubescent and early pubescent children. J. Pediatr. 136: 156–162. doi: 10.1016/S0022-3476(00)70095-3 [DOI] [PubMed] [Google Scholar]
- 24.Nakamura S., Kitayama I., Murase S.1991. Electrophysiological evidence for axonal degeneration of locus coeruleus neurons following long-term forced running stress. Brain Res. Bull. 26: 759–763. doi: 10.1016/0361-9230(91)90172-G [DOI] [PubMed] [Google Scholar]
- 25.Nance D.M., Bromley B., Barnard R.J., Gorski R.A.1977. Sexually dimorphic effects of forced exercise on food intake and body weight in the rat. Physiol. Behav. 19: 155–158. doi: 10.1016/0031-9384(77)90173-1 [DOI] [PubMed] [Google Scholar]
- 26.Olsson I.A., Dahlborn K.2002. Improving housing conditions for laboratory mice: a review of “environmental enrichment”. Lab. Anim. 36: 243–270. doi: 10.1258/002367702320162379 [DOI] [PubMed] [Google Scholar]
- 27.Pedersen B.K., Hoffman-Goetz L.2000. Exercise and the immune system: regulation, integration, and adaptation. Physiol. Rev. 80: 1055–1081. [DOI] [PubMed] [Google Scholar]
- 28.Prut L., Belzung C.2003. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur. J. Pharmacol. 463: 3–33. doi: 10.1016/S0014-2999(03)01272-X [DOI] [PubMed] [Google Scholar]
- 29.Rhodes M.E., Balestreire E.M., Czambel R.K., Rubin R.T.2002. Estrous cycle influences on sexual diergism of HPA axis responses to cholinergic stimulation in rats. Brain Res. Bull. 59: 217–225. doi: 10.1016/S0361-9230(02)00868-7 [DOI] [PubMed] [Google Scholar]
- 30.Rohde T., MacLean D.A., Hartkopp A., Pedersen B.K.1996. The immune system and serum glutamine during a triathlon. Eur. J. Appl. Physiol. Occup. Physiol. 74: 428–434. doi: 10.1007/BF02337723 [DOI] [PubMed] [Google Scholar]
- 31.Salam J.N., Fox J.H., Detroy E.M., Guignon M.H., Wohl D.F., Falls W.A.2009. Voluntary exercise in C57 mice is anxiolytic across several measures of anxiety. Behav. Brain Res. 197: 31–40. doi: 10.1016/j.bbr.2008.07.036 [DOI] [PubMed] [Google Scholar]
- 32.Salim S., Sarraj N., Taneja M., Saha K., Tejada-Simon M.V., Chugh G.2010. Moderate treadmill exercise prevents oxidative stress-induced anxiety-like behavior in rats. Behav. Brain Res. 208: 545–552. doi: 10.1016/j.bbr.2009.12.039 [DOI] [PubMed] [Google Scholar]
- 33.Sothern M.S., Loftin M., Suskind R.M., Udall J.N., Blecker U.1999. The health benefits of physical activity in children and adolescents: implications for chronic disease prevention. Eur. J. Pediatr. 158: 271–274. doi: 10.1007/s004310051070 [DOI] [PubMed] [Google Scholar]
- 34.Swallow J.G., Carter P.A., Garland T., Jr1998. Artificial selection for increased wheel-running behavior in house mice. Behav. Genet. 28: 227–237. doi: 10.1023/A:1021479331779 [DOI] [PubMed] [Google Scholar]
- 35.Toelle V.D., Robison O.W.1985. Estimates of genetic correlations between testicular measurements and female reproductive traits in cattle. J. Anim. Sci. 60: 89–100. [DOI] [PubMed] [Google Scholar]
- 36.Tokuyama K., Saito M., Okuda H.1982. Effects of wheel running on food intake and weight gain of male and female rats. Physiol. Behav. 28: 899–903. doi: 10.1016/0031-9384(82)90211-6 [DOI] [PubMed] [Google Scholar]
- 37.Tsai P.P., Pachowsky U., Stelzer H.D., Hackbarth H.2002. Impact of environmental enrichment in mice. 1: effect of housing conditions on body weight, organ weights and haematology in different strains. Lab. Anim. 36: 411–419. doi: 10.1258/002367702320389071 [DOI] [PubMed] [Google Scholar]
- 38.Tsai P.P., Stelzer H.D., Hedrich H.J., Hackbarth H.2003. Are the effects of different enrichment designs on the physiology and behaviour of DBA/2 mice consistent? Lab. Anim. 37: 314–327. doi: 10.1258/002367703322389889 [DOI] [PubMed] [Google Scholar]
- 39.Uhrich J.1938. The social hierarchy in albino mice. J. Comp. Psychol. 25: 373. doi: 10.1037/h0056350 [DOI] [Google Scholar]
- 40.van de Weerd H.A., Baumans V., Koolhaas J.M., van Zutphen L.F.M.1994. Strain specific behavioural response to environmental enrichment in the mouse. J. Exp. Anim. Sci. 36: 117–127. [PubMed] [Google Scholar]
- 41.Viru A., Laaneots L., Karelson K., Smirnova T., Viru M.1998. Exercise-induced hormone responses in girls at different stages of sexual maturation. Eur. J. Appl. Physiol. Occup. Physiol. 77: 401–408. doi: 10.1007/s004210050351 [DOI] [PubMed] [Google Scholar]
- 42.Wolfensohn S., Lloyd M.1994. Recognition of pain and stress in laboratory animals. Handbook of Laboratory Animal Management and Welfare, 174–180. [Google Scholar]
- 43.Würbel H., Stauffacher M.1997. Age and weight at weaning affect corticosterone level and development of stereotypies in ICR-mice. Anim. Behav. 53: 891–900. doi: 10.1006/anbe.1996.0424 [DOI] [Google Scholar]
- 44.Zhang H.Y., Zhao Y.N., Wang Z.L., Huang Y.F.2015. Chronic corticosterone exposure reduces hippocampal glycogen level and induces depression-like behavior in mice. J. Zhejiang Univ. Sci. B 16: 62–69. doi: 10.1631/jzus.B1400166 [DOI] [PMC free article] [PubMed] [Google Scholar]