Abstract
Deep vein thrombosis (DVT) affects up to 2 million people in the United States, and worldwide incidence is 70 to 113 cases per 100,000 per year. Mortality from DVT is often due to subsequent pulmonary embolism (PE). Precise diagnosis and treatment is thereby essential for the management of DVT. DVT is diagnosed by a thorough history and physical examination followed by laboratory and diagnostic tests. The choice of laboratory and diagnostic test is dependent on clinical pretest probability. Available laboratory and diagnostic techniques mainly involve D-dimer test, ultrasound, venography, and magnetic resonance imaging. The latter two diagnostic tools require high doses of contrast agents including either radioactive or toxic materials. The available treatment options include lifestyle modifications, mechanical compression, anticoagulant therapy, inferior vena cava filter, and thrombolysis/thrombolectomy. All of these medical and surgical treatments have serious side effects including improper clot clearance and increased risk of hemorrhage occurrence. Therefore, research in this field has recently focused on the development of non-invasive and accurate diagnostics, such as ultrasound enhanced techniques and molecular imaging methods, to assess thrombus location and its treatment course. The frontier of nanomedicine also shows high prospects in tackling DVT with efficient targeted drug delivery. This review describes the pathology of DVT along with successive medical problems such as PE and features a detailed listing of various diagnostic and therapeutic modalities that have been in use and are under development.
Keywords: Thrombosis, Fibrin clot, Embolism, Nanoparticles
1. Introduction
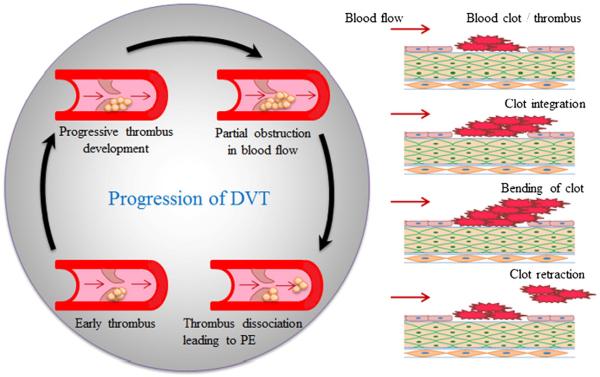
Deep vein thrombosis (DVT) is defined as thrombi formation in the deep venous system. Majority of the deep vein clots are subjected to thigh and lower leg veins that primarily include iliac vein, deep femoral vein, popliteal vein, and calf vein (Landefeld 2008; Tovey and Wyatt 2003). Thrombi or blood clots consist of coagulation factor, fibrin, and platelets. Most often, thrombus is developed due to blood vessel wall damage or pathological process that induces activation of coagulation factor, which causes accumulation of platelets and fibrin (Ruggeri 2003; Schreijer et al. 2010). The thrombi may break or disintegrate from their site of formation and form thromboemboli (Fig. 1) and travel to the lungs causing pulmonary embolism (PE), to the heart causing myocardial infarction, and to the brain causing stroke (Galson 2008). These events are life-threatening and deadly (Murray and Lopez 1997). In addition, DVT is a progressive condition that may also cause post thrombotic syndrome and recurrent venous thromboembolism (Vedantham 2009). Over 25% of DVT patients also suffer from `Varicose veins', a medical condition in which superficial veins abnormally elongate, dilate, and twist under high pressure (Goldman et al. 1994). There are several causes and symptoms of DVT, which can be useful factors to consider in taking preventive measures. This review briefly discusses the causes and effects of DVT and highlights the advances in the diagnostic and therapeutic modalities.
Fig. 1.
Development and progression of DVT adjacent to a vessel valve (left) and at the site of damaged endothelium (right). DVT clot bends in the direction of blood flow and disintegrates from the site, resulting in PE.
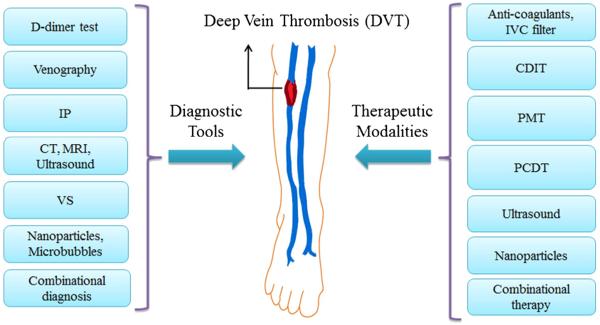
Understanding the physiology behind DVT, especially at the molecular level, is essential for designing an effective therapeutic strategy. Three major attributes involved in the development and progression of venous thrombosis are vessel wall injury, venous stasis, and elevated coagulability (Murray and Lopez 1997); together known as Virchow's Triad (Bates and Ginsberg 2004). Vessel wall injury is caused by numerous factors including direct trauma to the endothelium as a result of surgical procedures, low oxygen tension, and exposure to endotoxins, inflammatory toxins, or tumor necrosis factors (Murray and Lopez 1997). Venous stasis is characterized by the gradual protracted blood flow from the lower leg veins caused by long periods of immobility, high blood viscosity, and obstructions in the blood vessels, which in turn prevents the clearance and dilution of activated coagulation factors (Bates and Ginsberg 2004; Murray and Lopez 1997). Finally, elevated or hyper-coagulability accounts for age, malignancy, and myocardial infarction along with the two other major causes stated above (Goldhaber and Morrison 2002; Lowe 2002; Murray and Lopez 1997; Tovey and Wyatt 2003). Several diagnostic and therapeutic techniques, including invasive and non-invasive approaches, have been developed and used for many years for the management of progressed DVT. The following sections discus the diagnostic and therapeutic modalities that are in use, as well as those that are currently in research. The list of diagnostic and therapeutic modalities for DVT is outlined in Fig. 2.
Fig. 2.
Diagnostic and therapeutic modalities for deep vein thrombosis.
2. Diagnostic modalities for DVT
The detection and evaluation of thrombi involved in DVT is a critical step to determine appropriate treatment options. Both serology and imaging modalities can be used for diagnosis of DVT. Currently, serological markers play limited roles in prediction of DVT (Hou et al. 2012). However, discovery of sensitive and specific serological markers will be very useful for screening and diagnosis of DVT. The plasma molecules known as biomarkers of DVT include D-dimer, P-selectin, Factor VIII, thrombin generation, inflammatory cytokines, microparticles, fibrin monomer and leukocyte count (Hou et al. 2012). A study has demonstrated that diagnosis using a combination of D-dimer and venous ultrasonography is more efficient (Hirsh and Lee 2002). Most often algorithmic strategy is used in clinical setting to evaluate pretest probability. D-dimer test is the analysis of the degradation product of cross-linked fibrin in thrombi, where acute venous thrombosis has high levels (Adam et al. 2009; Scarvelis and Wells 2006). D-dimer analysis is a sensitive, but non-specific blood marker for DVT. A negative D-dimer assay is considered equivalent to negative ultrasound result and helpful in excluding DVT (Adam et al. 2009; Lowe 2002). In addition to ultrasound, several other imaging strategies have been developed to visualize thrombus formation, which are discussed in following sections. A brief description, advantages, and limitations of these diagnostic modalities are also listed in Table 1.
Table 1.
Diagnostic modalities for DVT.
| Modality | Description | Advantages | Limitations | Ref |
|---|---|---|---|---|
| D-dimer test | Analysis of degradation product levels of fibrin blood clot | Simple; direct combinational algorithm | Inaccurate; depends on patient condition | 10, 12–14 |
| Venography | Contrast agent with an external transducer; observation of preventive blood flow | Widely used; improved potential especially with combination methods; high accuracy | Invasive; high costs; risks – allergy, renal dysfunction, morbidity; inaccurate in low limb thrombosis; accessing difficulty in obesity, edema, cellulitis | 15 – 17 |
| IP | Electrical impedance detects blood volume changes due to cobstruction in flow | More sensitive compared to Venography | Not specific; false positive results due to variation in position, pregnancy, tumor; fails to detect calf thrombus | 15, 16 |
| CT | Contrast medium based technique | Potentially detects concurrent DVT as stand-alone technique | Rarely used due to intravenous administration of contrast medium | 17 |
| MRI | Enhanced contrast agents instead of gaseous substances; requires specific targeted nanomarkers | High spatial resolution and structural definition; can distinguish old and new clots | Molecular marker concentration dependent sensitivity, most expensive technique | 15, 17–19 |
| VS | Radioactive contrast agent (99mTc)-labeled peptides for thrombus targeting | High sensitivity; improved visualization | Toxicity due to radioactive materials; time consuming | 15, 16, 20–23 |
| Ultrasound | B-mode/doppler, compression, color duplex, and combined ultrasound | High spatial and temporal resolution; no pain | Rare use in new thrombi detection within post thrombotic limb | 24 – 29 |
2.1. Venography
Venography involves the use of a 4.5 MHz transducer over the suspected thrombus site on the leg and injection of a contrast agent. Detection of the clot is based on the observation of preventive blood flow within the vein. This technique has been in use for a while and also in combination with other diagnostic techniques. Dilution of the contrast material in the proximal lower limb, difficulties with venous access related to obesity, severe edema, or cellulitis are some of the obstacles in the use of contrast venography (Line 2001). Although venography provides a definite diagnosis, it is not commonly used due to several drawbacks such as its invasive nature, high technical demands, high costs, and clinical risks such as allergic reactions, renal toxicity, and morbidity (Hull et al. 1976). Computer tomography (CT) is used to diagnose venous thrombosis in pelvic and abdominal venous system. The administration of intravenous contrast agent involves its bioavailability to areas beyond the region of the thrombus, thus enhancing toxicity and reducing specificity. CT pulmonary angiography of the pulmonary arteries, prior to CT venography of the pelvic and lower extremity veins, has shown potential in detection of concurrent DVT (Kanne and Lalani 2004).
2.2. Impedance plethysmography (IP)
IP measures electrical impedance to detect the blood volume change induced by obstruction of the blood flow through veins. The blood volume changes are produced by the inflation and deflation of the cuff around the limb. These changes in turn produce deviations in electrical resistance, also known as impedance, around the areas containing thrombus. However, IP is not specific for thrombotic obstruction to venous flow and tends to provide false positive results. False positive results can be generated if the patient is positioned incorrectly, the vein is compressed by a tumor like mass, or as a result of raised central venous pressure (Line 2001). In comparison with venography, IP is more sensitive with thrombosis in the proximal vein; however, fails to detect thrombosis within the calf (Hull et al. 1976). Due to some of the limitations displayed by IP, Doppler ultrasound or duplex scanning may be considered as better options, which is discussed later in this section.
2.3. Magnetic resonance imaging (MRI)
MRI provides high spatial resolution and structural definition that aids in effective imaging. MRI with enhanced contrast agents further enhances effectiveness of the imaging in the absence of any gaseous substances (Line 2001; Wiethoff et al. 2010). Moreover, MRI can distinguish old and new clots in the presence of high signal, but only with subacute thrombosis (van Beek et al. 2003). However, this technique has low sensitivity in the case of low concentrations of molecular markers. Amplified signals may be obtained through the use of specific targeted nanoprobes that will be discussed in the section on nanoparticle-based diagnostic approaches. MRI is the most expensive technique and may not be available in emergency room settings (Line 2001). MR pulmonary angiography and MR venography have also shown improved diagnosis of thrombus (Kanne and Lalani 2004).
2.4. Venous scintigraphy (VS)
VS uses radioactive contrast agents, such as 99mTc, to label the peptides that target the molecular biology of thrombosis (Line 2001). The peptides, such as apcitide/P280, have the ability to bind to glycoprotein IIb/IIIa that is highly expressed in thrombi. In a clinical study, the effectiveness of VS against venography was evaluated and showed that VS in the presence of 99mTc-labeled peptide was effective in diagnosis of acute DVT with high sensitivity (Taillefer et al. 2000). This technique enhances the diagnosis of recurrent venous thrombosis with improved visualization and reduces the occurrence of false-positive results. However, the use of radioactive materials can be harmful to the human body and may cause serious genetic disorders, especially when it is associated with long-term and repeated exposure (Hosseinimehr 2009).
Another technique that uses radioactive material is radio-labeled fibrinogen scanning. 125I-labeled fibrinogen scanning takes about 72 hours post injection to appear in the patient with the established thrombosis, and yet may sometimes not appear at all (Atkins and Hawkins 1968; Hull et al. 1976). 131I-labeled fibrinogen has also been used in diagnosis of DVT located in the lower extremities (Prescott et al. 1978). This technique displays low sensitivity to thrombi present in the proximal femoral vein and iliac vein. Furthermore, it should not be used in post-operated patients for diagnosis of DVT, as they might face the outcome of labeled fibrinogen leaking into the surgical site (Hull et al. 1976).
2.5. Ultrasound
Ultrasound provides improved spatial and temporal resolution with 3D imaging and capability of evaluation of dynamic physiological processes (Kaufmann and Lindner 2007). Ultrasound techniques comprise a vast range of modalities that are used in diagnosis of thrombi. (a) B-mode ultrasound or Doppler ultrasound is the standard test for DVT diagnosis with a sensitivity and specificity of 91% and 85–100%, respectively (Mustafa et al. 2002). (b) Compression ultrasound involves the use of a real time transducer that has a frequency of 3–7 MHz, and diagnosis is based on the collapsing of the thin lumen of veins to see if lumen is collapsible. The portion of the lumen that prevents the collapse indicates the presence of thrombi. This technique has a high sensitivity and specificity of above 95%. However, the test is insensitive for calf vein thrombosis and needs repeated trials to have sufficient effect (Wicky et al. 1994). (c) Color duplex ultrasound or Duplex ultrasound is used as a conformational technique to verify the results obtained from compression ultrasound. Color flow Doppler helps evaluate the flow patterns within the blood vessels (Richards et al. 1976; Unger et al. 1998). (d) Combined ultrasound is combination of B-mode ultrasound and color flow Doppler imaging. It is the most popularly used diagnosis technique for DVT as it involves no pain and is very sensitive to the thrombus located above the popliteal vein (Orbell et al. 2008). Ultrasound or ultrasonography is of minimum use in detection of new thrombi within a post thrombotic limb.
Every diagnostic technique evaluated so far is associated with some limitations including reduced detection accuracy with the increased depth, the inability to distinguish between acute and chronic DVT, and occurrence of false-negative results when a patent vein parallels an occluded one. Another undesirable outcome associated with compression technique is that some freshly formed clots may emboli and travel into the bloodstream creating occlusions in smaller blood vessels in lung, heart, and brain. Although several diagnostic modalities have been developed, their advantages as well as limitations have been noted. Research on more advances and cutting edge diagnostics is ongoing with a hope to provide a better solution with improved and early diagnosis for those suffering from DVT.
3. Therapeutic modalities for DVT
Effective treatment is critical for DVT patients to enhance blood circulation and prevent further progressive problems. Studies have shown that untreated or improper exclusion of thrombus leads to progressive and recurrent DVT and other pathology conditions including chronic symptoms of pain, swelling, and pain-associated mobility (Landefeld 2008). Several treatment approaches have been developed towards reduction of venous thrombosis including anticoagulation therapy with therapeutic agents such as low molecular weight heparin (LMWH) and vitamin K antagonists (warfarin) (Labropoulos et al. 2008). Delivery of therapeutic agents to the thrombus can be achieved in three different ways including systemic delivery, local regional administration, and catheter-directed delivery (Alesh et al. 2007). Anticoagulant therapy with thrombolytic drugs is normally prescribed as a preventive measure of DVT. These drugs have lower allergenicity and greater fibrin specificity and are commonly known as blood thinners. However, they cause late post-thrombotic or post-phlebitic syndrome that leads to low quality of patient life and eventual morbidity (Vedantham 2009). LMWH is usually continued for a period of three months and thereafter with dosage depending on the extent of thrombosis (Hirsh and Lee 2002). A study has showed that long-term anticoagulant therapy with LMWH is more effective in cancer patients suffering from DVT, resulting in few recurrent events (Lee et al. 2003).
Anticoagulation therapy, though capable of reducing thrombosis, is associated with limitations of high cost, improper clot clearance (Landefeld 2008), and increased risk of intracranial hemorrhage occurrence (Scarvelis and Wells 2006). Anticoagulant therapy is often associated with critical contraindications such as severe bleeding as a result of low platelet count, brain metastasis, and severe hypertension (Bates and Ginsberg 2004). The absolute contraindications to anticoagulant therapy are hemorrhagic stroke, intracranial hemorrhage, and gastrointestinal hemorrhage (Kaufman et al. 2006). The relative contraindications include recent major surgery or trauma, uncontrolled hypertension, renal or hepatic disease, and positive guaiac stool test (Liu et al. 2012). The contraindication to warfarin also includes pregnancy due to teratogenicity (Liu et al. 2012). Furthermore, in some instances cancer has been considered as contraindication (Kaufman et al. 2006). Anticoagulants such as fondaparinux with enoxaparin have also been tested, but these medications did not show comparable results with heparin and warfarin (Bates and Ginsberg 2004; Buller et al. 2004). In patients with absolute contraindication or failure of anticoagulant therapy, inferior vena cava (IVC) filter is used to prevent life threatening events such as PE. Although IVC filter is effective in reducing DVT complications, there is a 3–5% recurrence rate (Kaufman et al. 2006). Furthermore, there are some complications associated with insertion of IVC filter such as post-insertion migration and IVC thrombosis and perforation.
To improve the therapeutic efficacies, local delivery of individual or combined therapeutic agents has been prescribed for efficient and effective thrombolysis. Systemic administration of therapeutic agents is associated with prolonged infusion times and high incidence of partial thrombolysis (Alesh et al. 2007). To overcome the limitations of the traditional oral drug therapies, endovascular catheter techniques to deliver the thrombolytic drugs directly into the thrombus have been used successfully. The main advantage of these techniques is minimizing the dosage requirement, thus reducing the side effects caused by the drugs, if any. The different catheter and ultrasound-based therapeutic techniques are discussed in following sections. A brief description, advantages, and limitations of these therapeutic modalities are also listed in Table 2.
Table 2.
Therapeutic modalities for DVT.
| Modality | Description | Advantages | Limitations | Ref |
|---|---|---|---|---|
| Anti-coagulant medication | Blood thinners; heparin, warfarin, LMWH, combinational drugs | Low allergenicity and high fibrin specificity | Late post-thrombotic syndrome; risks – hemorrhage, severe bleeding, severe hypertension; eventual morbidity; high cost | 1, 7, 9, 12, 13, 30–35 |
| IVC filter | Implantable device to prevent PE | Effective in reducing DVT complications such as PE | Post-insertion migration complications; IVC thrombosis and perforation | 33 |
| CDIT | Fibrinolytic drug infused into the thrombus by a multi-side-hole catheter using imaging guidance | High lysis rate; long term improved outcomes | Safety problems | 7, 36–39 |
| PMT | Percutaneous catheter to remove thrombus by fine thrombus fragmentation, maceration, aspiration, or in combination | Low concentration of lyric drugs; minimum risk of hemorrhages compared to CDIT; short treatment durations | Unable to safely remove enough thrombus; risks – stroke, gastrointestinal bleeding, primary or metasratic CNS malignancy and coagulation | 7, 40, 41 |
| PCDT | Combined use of CDIT and PMT | Advantages of both CDIT and PMT | limitations of both CDIT and PMT | 40, 41 |
| Ultrasound | Catheter-based clot removal; emits low power ultrasound energy to loosen fibrin strands along with drug infusion | Reduced time; minimal drug use; reduced mechanical perturbation of vein | Includes drawbacks of catheter methods | 42 |
3.1. Catheter-directed intrathrombus thrombolysis (CDIT)
In CDIT, a fibrinolytic drug is directly infused into the venous thrombus via a multi-side-hole catheter using imaging guidance. Delivery of drug in this manner may result in long-term improved outcomes for DVT patients (Liu et al., 2011). One of the clinical studies used urokinase as the therapeutic drug that was delivered to thrombus in the illiofemoral vein (Semba and Dake, 1994). An efficient delivery of urokinase via CDIT showed significant thrombus elimination with thrombus lysis rate of 72%. Another clinical trial used CDIT with a 5-F straight catheter with ten side-holes (Verhaeghe et al., 1997). This probe was introduced via contralateral femoral vein and over the caval bifurcation. The tip was finally positioned at the thrombus and the Alteplase drug was infused by an infusion pump. However, stand-alone CDIT is not a user friendly treatment method and is perceived to have safety limitations that precluded its use as the first-line DVT therapy (Day, 2003; Vedantham, 2009).
3.2. Percutaneous mechanical thrombectomy (PMT)
A percutaneous catheter-based device is used in PMT, which contributes to thrombus removal via fine thrombus fragmentation, maceration, aspiration, or a combination of these methods (Vedantham et al., 2009). Thrombolysis using PMT is associated with advantages such as short treatment durations, and minimum risk of hemorrhages compared to CDIT (Kim et al., 2006). Unfortunately, currently available PMT devices cannot safely remove enough thrombus (Kim et al., 2006; Vedantham, 2009).
3.3. Pharmacomechanical catheter-directed thrombolysis (PCDT)
In PCDT, the thrombus is dissolved via combined use of CDIT and PMT (Vedantham et al., 2009). Fibrinolytic drugs administered via CDIT render thrombus more susceptible to mechanical fragmentation and removal, thereby dissolving clot fragments that could otherwise embolize to the lungs. A clinical study compared the efficiency of CDIT and combinational therapy, in which the thrombus in the popliteal vein was accessed by a 5-F hydrophilic catheter along with a guide wire (Kim et al., 2006). Venograms were taken with the use of iodine as a contrast agent. PMT was performed with a 6-F AngioJet rheolytic thrombectomy catheter and was then followed by CDIT procedure that used a multi side-hole infusion catheter. However, this technique involves limitations of both CDIT and PMT techniques as described earlier such as stroke, gastrointestinal bleeding, primary or metastatic central nervous system malignancy and coagulation (Kim et al., 2006; Vedantham et al., 2009).
3.4. Ultrasound catheter based thrombolysis
Ultrasound-based technology has been used in catheter-based clot removal devices. A catheter that emits low power ultrasound energy during the drug infusion, loosen fibrin strands and thereby enhance fibrinolytic drug dispersion, has shown potential to speed up the thrombolytic therapy with minimal additional mechanical perturbation of the vein (Parikh et al., 2008). However, this technique also has disadvantages of catheter-based thrombolytic techniques.
Based on the overview of the advantages and drawbacks of current diagnostic and therapeutic systems, the need of modalities that provide more specific and accurate diagnosis and local treatments without systemic side effects, is of critical importance. In specific, nano- and microsystems have been developed and used in recent years. These systems have not only shown precise targeting at the cellular level, but also overcome limitations and reduce side effects of conventionally used modalities. The development of nano- and microtechnology in this area is highlighted in the following section.
4. Nanoparticle and microbubble-based systems for DVT
Current detection and treatment methods for DVT have mixed results due to their ineffectiveness as stand-alone systems. To overcome the limitations of conventional modalities, recent developments on nano- and microparticles have been investigated and provide some hope on the potential breakthrough of DVT detection and treatment (McCarthy et al. 2009). Natural microparticles known as “cellular dust” released during cellular activation or apoptosis should not be confused with artificial microparticles (Campello et al. 2011; Hou et al. 2012; VanWijk et al. 2003). In this section, artificial micro/nanoparticles will be discussed in detail.
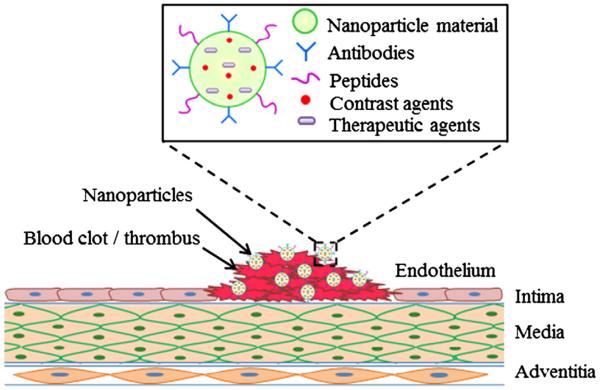
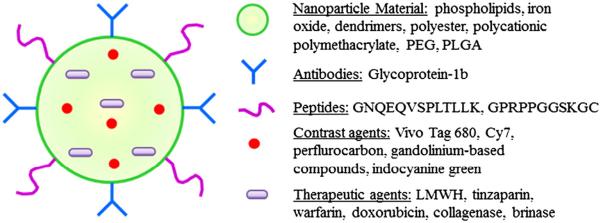
Immense research advances have taken place in the field of micro/nanotechnology, especially related to the diagnosis and therapy of cardiovascular diseases. Nanoparticle systems are concomitant with several advantages such as: (a) nanoparticles have the ability to both diagnose and treat the thrombus. (b) Owing to the small size that ranges from 50 to 500 nm, particles are capable of travelling through small diameter blood vessels located in the lower leg, as well as bypassing needless obstructions and thus reaching the targeted thrombus site. (c) Nano-diagnostic systems can also travel to a wider area within the leg, thus enabling visualization onto a larger area unlike the localized focus obtained by use of traditional ultrasound. (d) Functionalized particles with target specific ligands may be synthesized that provide enhanced specificity over the targeted clot formation in the blood vessel. (e) Properties of nanoparticle coating can be altered to achieve a desired drug release profile for efficient and timely dissolution of clots. (f) A multifunctional particle system can be designed to serve several tasks including targeting, visualization/imaging, dissolving thrombi, as well as detecting inflammation and injury of the blood vessel caused by clot detachment. The outline of multifunctional nanoparticles for DVT management has been represented in Figs. 3 and 4. Moreover, the types of nanoparticles and microparticles along with their applications, advantages, and limitations are listed in Table 3.
Fig. 3.
Multifunctional nanoparticle system with target specific, diagnostic, and therapeutic capabilities for DVT management.
Fig. 4.
Multifunctional nanoparticles depicting various components used in nano based diagnosis and therapy.
Table 3.
Nanoparticle- and microbubble-based systems for DVT.
| System | Application | Advantages | Limitations | Ref |
|---|---|---|---|---|
| GNQEQVSPLTLLK and GPRPPGGSKGC conjugated iron oxide nanoparticles | FXIII and fibrin targeting, MRI via Vivo Tag 680 and optical imaging Cy7 | Multifunctional capability and improved binding to thrombi | Complicated imagery systems; some level of in vivo toxicity | 43 |
| Lipid based perfluorocarbon and Gd-DTPA complex conjugated with anti-fibrin antibodies | Targeting of cell aggregates expressing fibrin | Improved signal contrast, ion and particle relaxivity, and enhanced detectability | – | 46 – 48 |
| Biotinylated phospholipid perfluorocarbon nanoparticles | Molecular imaging of thrombi | Enhanced echogenicity, acoustic contrast and target specificity | – | 49 |
| ICAM-1 and VCAM-1 conjugated microbubbles | Ultrasound imaging of thrombi | Successful targeting of thrombi | – | 24, 27 |
| Abciximab conjugated phospholipid microbubbles | Molecular imaging of clots by steady binding to platelets | Specific contrast administration and targeting of platelets | – | 51, 52 |
| MRX-408 incorporated aerosome microbubble | Ultrasound imaging of thrombi | Efficient targeting and improved enhancement | Microbubble concentration dependent contrast enhancement | 27, 53 |
| PEGylated polyamidoamine dendrimeric nanocarriers | Thrombi clearance with increased half-life of LMWH | Improved pulmonary absorption and bioavailability of heparin | – | 54, 55 |
| Polyester/polycationic polymethacrylate nanoparticles | Oral delivery of LMWH and Tinzaparin for thrombi treatment | Enhanced availability and overcomes drug–drug interaction | Rapid clearance of small sized and no functionalized nanoparticles | 56, 57 |
4.1. Nanoparticles for DVT diagnosis
Although nanoparticles have been used to diagnose and target the vascular endothelial dysfunction (Ikuta et al. 2008; Simone et al. 2009), their use in DVT detection and therapy is not much studied and has only been reported in a few studies. For instance, two multimodal thrombus-targeted nanoparticles, exhibiting either covalent or non-covalent binding to thrombi, were developed to monitor and detect the thrombogenesis and fibrinolysis (McCarthy et al. 2009). To formulate these nanoparticles, cross-linked iron oxide nanoparticles were synthesized and conjugated with GNQEQVSPLTLLK and GPRPPGGSKGC peptides that specifically target factor XIII (FXIII) and fibrin, respectively. Prior to peptide conjugation, nanoparticles were functionalized with Vivo Tag 680 and Cy7 separately. These composite functionalized particles can be detected by both MRI and optical imaging modalities. In vitro imaging efficiencies, in the presence of blood clots, were analyzed via fluorescence and MRI. Testing of imaging efficiency was extended to in vivo studies within injured jugular veins in mice. Both studies displayed improved binding tendencies of the nanoparticles onto the thrombus. However, these agents utilized two spectrally distinct fluorescence channels, which make the system complicated. Moreover, the iron oxide nanoparticles have shown some degree of toxicity in vivo.
Nanoparticulate MRI contrast agents were also synthesized by several groups to enhance the specificity and sensitivity of MRI in diagnosis and detection of thrombus at the molecular level. Fibrin targeted nanoparticle system was synthesized, which was comprised of a lipid shell encapsulating perfluorocarbon and gadolinium-diethylenetriaminepentaacetic acid (Gd-DTPA) complexes embedded in the shell (Winter et al. 2003). They utilized anti-fibrin antibodies that are capable of attaching themselves over cell aggregates expressing fibrin. Electron microscopy analysis on in vitro clot studies showed aggregation of nanoparticles over the clot surface, thus suggesting enhanced detectability. MRI testing further proved the capability of these nanoparticles to enhance the signal contrast over the clot surface (Vymazal et al. 2009; Yu et al. 2000). Moreover, Gd-DTPA-bisoleate and Gd-DTPA-phosphatidyethanolamine were synthesized and their relaxivities were studied. The latter batch of nanoparticles showed improved ion and particle relaxivity and also represented strong binding to the thrombus surface via anti-fibrin antibodies (Winter et al. 2003). Furthermore, targeted perfluorocarbon nanoparticles were also prepared in a study to aid in molecular imaging of thrombi (Marsh et al. 2007). These nanoparticles were composed of biotinylated phospholipid. The target-specific acoustic nanoparticles were evaluated for their imaging efficiency, based on the concentration at the target. Cell studies performed to test the targeting efficiency show enhanced echogenicity for the cell surface. These particles provided enhanced acoustic contrast, thus providing better visualization of the clot along with the ultrasound.
4.2. Microbubbles for DVT diagnosis
Microbubbles have long been considered as interesting contrast agents for ultrasound. Microbubbles are comprised of gas or air medium in the core. Their potential as ultrasound contrast agents is due to the fact that they oscillate on receiving an ultrasound input. Bubble compression occurs during the pressure peaks while expansion takes place during the nadirs of the ultrasound wave. Stability of the bubble is very essential for it to be used as a contrast agent. Bubble stability depends on several factors that may be broadly divided into gas characteristics and polymer shell properties. The gas characteristics include the stability, solubility, and diffusion tendency of the gas, while the polymer shell properties include the type of the polymer, thickness of the shell, porosity characteristics, and surface modification (Plesset and Sadhal 1982; Unger et al. 1998).
Microbubbles, while traveling through the blood vessels, can be imaged using ultrasound, leading to their use in imaging of the diseased endothelium or thrombus formation. Imaging of thrombosis can be enhanced by either conjugating specific targeting ligands over the shell surface or modifying the shell components to enhance its specificity to the abnormal cells in the blood vessel or damaged endothelium. Successful targeting of damaged endothelium and thrombi has been shown in the presence of receptors such as intra-cellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), as demonstrated by in vitro and in vivo studies (Kaufmann and Lindner 2007; Unger et al. 1998). In addition, Abciximab, a monoclonal antibody against glycoprotein IIb/IIIa, conjugated immunobubbles have been synthesized by activating the phospholipid shell of the microbubbles (Alonso et al. 2007). This antibody has long been used as a platelet inhibitor and is proved to be an effective targeting agent for thrombi. The conjugated microbubbles, along with ultrasound imaging, have shown potential for molecular imaging of clots with steady binding to platelet aggregates observed during in vitro studies. In vivo studies, within the carotid arteries of rats, demonstrated clear visualization of platelets in thrombi compared with a control group. The main advantage of conjugated microbubbles is that they provide more specific targeting of platelets expressing activated glycoprotein IIb/IIIa receptors (Alonso et al. 2007; Schumann et al. 2002).
Another interesting thrombus specific microbubble describes the incorporation of an ultrasound contrast agent (MRX-408) covalently bound over a lipid composite (dipalmitoyl glycerol succinate), forming a resultant aerosome microbubble (Unger et al. 1998). Significant in vitro studies were performed within an acoustic flow chamber that revealed efficient targeting of the composite microsystems over the blood clot. The study showed an improved enhancement of the clot area compared to that of unconjugated particles. MRX-408-conjugated particles provided over an approximately 9-fold increase in clot visualization. However, this system had a limitation of decreased contrast enhancement in the older thrombi. The degree of contrast enhancement depends primarily on the concentration of the microbubbles attached to the clot, which eventually reduces with time. Fortunately, this contrast enhancement dependence characteristic is useful in differentiation of thrombi based on the time of occurrence (Takeuchi et al. 1999; Unger et al. 1998).
4.3. Nanoparticles for DVT treatment
Nanoparticles have also been used to treat the vascular endothelial dysfunctions (Ikuta et al. 2008); however, their use in DVT therapy is rarely studied. In a study, PEGylated polyamidoamine dendrimeric nanocarriers were synthesized for DVT treatment with increased half-life of LMWH (Bai and Ahsan 2009; Bai et al. 2007). The increased half-life further improved the pulmonary absorption of heparin. Dendrimers have useful physical and chemical properties for the encapsulation and delivery of therapeutic agents based on electrostatic interactions, hydrophobic attractions, or hydrogen and covalent bonding. Furthermore, increased bioavailability of a therapeutic agent is essential for enhanced and effective clearance of thrombi. Few studies used polymeric nanoparticles to enhance the availability of heparin within the system. For oral drug delivery of LMWH, Tinzaparin encapsulated polyester/polycationic polymethacrylate nanoparticles were prepared by a double emulsion technique (Hoffart et al. 2006). The release and pharmacokinetic studies on these nanoparticles showed their potential in enhancing the availability of the therapeutic agents. Moreover, oral delivery of these particles helped overcome the drug–drug interaction caused by administration of warfarin. Another study involved the synthesis of biomimetic solid lipid nanoparticles for oral bioavailability enhancement of LMWH (Paliwal et al. 2011).
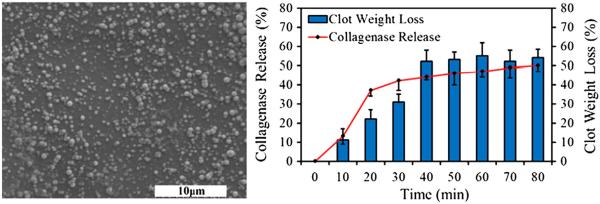
In our laboratory, we had synthesized poly(lactic-co-glycolic acid) (PLGA) nanoparticles aiming at an increased therapeutic advantage towards thrombi reduction. In brief, biocompatible, biodegradable PLGA nanoparticles were synthesized by a standard double emulsion technique (Shah et al. 2011). These nanoparticles were about 150–200 nm in diameter with polydispersity index of 0.054 (Fig. 5). Nanoparticles were then loaded with collagenase as a thrombolytic drug model with a loading efficiency of 63%. From drug release studies, it was observed that there was a burst release of the collagenase followed by a sustained release. Moreover, the bioavailability of the collagenase was studied by quantifying the degree of thrombus reduction in vitro. A 54% weight loss of the clots was observed after 80 minutes of incubation with the therapeutic particles. The preliminary studies performed in our lab led to the conclusion that these nanoparticles may have potential applications in DVT management.
Fig. 5.
PLGA nanoparticles for a potential DVT therapy. SEM of PLGA nanoparticles (left). Collagenase release from PLGA nanoparticles and bioavailability of collagenase showing loss in the clot weights (right).
5. Future outlook and conclusion
Polymeric nanoparticles containing a fluorescent agent within its core along with thrombi reducing therapeutics and dual targeting potential (targeting both the thrombus and the damaged endothelium) may be a prospective development in this research milieu. For DVT management, multifunctional PLGA nanoparticles loaded with indocyanine green (a near infrared dye) for fluorescence imaging and brinase molecules (anti-thrombolytic enzymes) for therapy have been proposed (Saxena et al. 2004). Nanoparticles could also be conjugated with thrombi-specific GPRPPGGSKGC peptides for targeting the activated factor XIII (FXIIIa) (McCarthy et al. 2009) and GP1b for targeting damaged endothelium with specific binding to P-selectin (Burgess et al. 2000). GPRPPGGSKGC is a fibrin–avidin peptide that inhibits fibrin thrombin clotting, thus increasing resistance to proteolysis. Another area that might show prospective treatment efficiency is in the use of gene therapy and stem cell therapy. Mutations in prothrombin and factor V gene are associated with high risk of DVT (Simioni et al. 2000), which might be treated using an effective gene delivery system. Several modes of diagnosis and treatment techniques have been developed and are still under investigation. Of those, nanotechnology has shown improved diagnostic potential and therapeutic ability for not only cardiovascular disorders, but also the biomedical field as a whole. Due to high incidence rate of DVT worldwide and the morbidity and mortality associated with it (Chandra et al. 2009; Silverstein et al. 1998), highly advanced methods to avoid the occurrence of DVT, as well as improved and efficient techniques for diagnosis and treatment of DVT, are of critical importance. The US Food and Drug Administration (FDA) has approved some drug delivery nanoparticles such as PEGylated liposomal doxorubicin (Doxil), liposomal daunorubicin (DaunoXome), and albumin bound paclitaxel nanoparticles (Abraxane) (Bharali and Mousa 2010). However, their use in DVT management has not yet been studied extensively. Moreover, several therapeutic agents have gone through or are currently in clinical trials (Table 4) (NIH 2012), which can be incorporated within particulate systems for target specific and efficient DVT diagnosis and therapy. If done so, this will be a paradigm shift in the DVT management.
Table 4.
Clinical trials of therapeutic and diagnostic modalities for DVT management.
| Therapeutic / diagnostic modality | Description | Possible outcomes | Status |
|---|---|---|---|
| Low molecular weight heparin | Anticoagulant for DVT in cancer patients | Recurrent DVT/PE, bleeding | Phase IV recruiting |
| Fondaparinux sodium and Un-fractionated heparin | Evaluate the efficacy in subjects with acute symptomatic DVT | – | Phase III completed |
| Warfarin | Oral anticoagulant for idiopathic DVT | Recurrent venous thromboembolism, hemorrhage | Phase IV completed |
| Dalteparin and Warfarin | Anticoagulant for effective catheter preservation | Recurrent DVT/PE, bleeding | Phase II completed |
| Dalteparin sodium injection | Anticoagulant for long-term treatment of DVT | – | Phase IV completed |
| Clopidogrel | Inhibit platelet aggregation in coronary artery stent thrombosis | Prevalence of genetic polymorphisms influencing pharmacokinetics | Phase IV completed |
| Tinzaparin sodium | Anticoagulant for long-term treatment of proximal venous thrombosis | Recurrent venous thromboembolism, bleeding | Phase IV completed |
| Rosuvasratin and Enoxaparin | Prevention of DVT occurrence based on inhibition of HMO-co-A reductase (statin) | Development of DVT | Phase IV recruiting |
| Catheter-directed thrombolysis | DVT treatment | Bleeding, prevalence of vein anomalies, underlying thrombophilia | Ongoing, not recruiting |
| D-dimer and Ultrasound | Diagnosis of DVT | – | Completed |
| Doxil, DaunoXome, or Abraxane | Liposomal nanoparticle formulations for possible use for DVT management | – | FDA approved |
Acknowledgements
The authors thank Nidhi Singh, a former member of their laboratory, for her preliminary work on collagenase-loaded PLGA nanoparticles. The authors also thank the financial support from American Heart Association (Predoctoral Fellowship award, A.S.W., and Grant-in-Aid award, K.T.N.)
References
- Adam SS, Key NS, Greenberg CS. D-dimer antigen: current concepts and future prospects. Blood. 2009;113:2878–87. doi: 10.1182/blood-2008-06-165845. [DOI] [PubMed] [Google Scholar]
- Alesh I, Kayali F, Stein PD. Catheter-directed thrombolysis (intrathrombus injection) in treatment of deep venous thrombosis: a systematic review. Catheter Cardiovasc Interv. 2007;70:143–8. doi: 10.1002/ccd.21079. [DOI] [PubMed] [Google Scholar]
- Alonso A, Della Martina A, Stroick M, Fatar M, Griebe M, Pochon S, et al. Molecular imaging of human thrombus with novel abciximab immunobubbles and ultrasound. Stroke. 2007;38:1508–14. doi: 10.1161/STROKEAHA.106.471391. [DOI] [PubMed] [Google Scholar]
- Atkins P, Hawkins LA. The diagnosis of deep-vein thrombosis in the leg using 125I-fibrinogen. Br J Surg. 1968;55:825–30. doi: 10.1002/bjs.1800551106. [DOI] [PubMed] [Google Scholar]
- Bai S, Ahsan F. Synthesis and evaluation of pegylated dendrimeric nanocarrier for pulmonary delivery of low molecular weight heparin. Pharm Res. 2009;26:539–48. doi: 10.1007/s11095-008-9769-y. [DOI] [PubMed] [Google Scholar]
- Bai S, Thomas C, Ahsan F. Dendrimers as a carrier for pulmonary delivery of enoxaparin, a low-molecular weight heparin. J Pharm Sci. 2007;96:2090–106. doi: 10.1002/jps.20849. [DOI] [PubMed] [Google Scholar]
- Bates SM, Ginsberg JS. Treatment of deep-vein thrombosis. N Engl J Med. 2004;351:268–77. doi: 10.1056/NEJMcp031676. [DOI] [PubMed] [Google Scholar]
- Bharali DJ, Mousa SA. Emerging nanomedicines for early cancer detection and improved treatment: current perspective and future promise. Pharmacol Ther. 2010;128:324–35. doi: 10.1016/j.pharmthera.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Buller HR, Davidson BL, Decousus H, Gallus A, Gent M, Piovella F, et al. Fondaparinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: a randomized trial. Ann Intern Med. 2004;140:867–73. doi: 10.7326/0003-4819-140-11-200406010-00007. [DOI] [PubMed] [Google Scholar]
- Burgess JK, Hotchkiss KA, Suter C, Dudman NP, Szollosi J, Chesterman CN, et al. Physical proximity and functional association of glycoprotein 1balpha and protein-disulfide isomerase on the platelet plasma membrane. J Biol Chem. 2000;275:9758–66. doi: 10.1074/jbc.275.13.9758. [DOI] [PubMed] [Google Scholar]
- Campello E, Spiezia L, Radu CM, Bulato C, Castelli M, Gavasso S, et al. Endothelial, platelet, and tissue factor-bearing microparticles in cancer patients with and without venous thromboembolism. Thromb Res. 2011;127:473–7. doi: 10.1016/j.thromres.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Chandra D, E.P., Mozaffarian D. Meta-analysis: travel and risk for venous thromboembolism. Ann Intern Med. 2009;151:180–90. doi: 10.7326/0003-4819-151-3-200908040-00129. [DOI] [PubMed] [Google Scholar]
- Day MW. Recognizing and managing deep vein thrombosis. Nursing. 2003;33:36–41. doi: 10.1097/00152193-200305000-00044. quiz 42. [DOI] [PubMed] [Google Scholar]
- Galson SK. Prevention of deep vein thrombosis and pulmonary embolism. Public Health Rep. 2008;123:420–1. doi: 10.1177/003335490812300402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldhaber SZ, Morrison RB. Cardiology patient pages. Pulmonary embolism and deep vein thrombosis. Circulation. 2002;106:1436–8. doi: 10.1161/01.cir.0000031167.64088.f6. [DOI] [PubMed] [Google Scholar]
- Goldman MP, Weiss RA, Bergan JJ. Diagnosis and treatment of varicose veins: a review. J Am Acad Dermatol. 1994;31:393–413. doi: 10.1016/s0190-9622(94)70202-0. [DOI] [PubMed] [Google Scholar]
- Hirsh J, Lee AY. How we diagnose and treat deep vein thrombosis. Blood. 2002;99:3102–10. doi: 10.1182/blood.v99.9.3102. [DOI] [PubMed] [Google Scholar]
- Hoffart Vr, Lamprecht A, Maincent P, Lecompte T, Vigneron C, Ubrich N. Oral bioavailability of a low molecular weight heparin using a polymeric delivery system. J Control Release. 2006;113:38–42. doi: 10.1016/j.jconrel.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Hosseinimehr SJ. Potential utility of radioprotective agents in the practice of nuclear medicine. Cancer Biother Radiopharm. 2009;24:723–31. doi: 10.1089/cbr.2009.0635. [DOI] [PubMed] [Google Scholar]
- Hou H, Ge Z, Ying P, Dai J, Shi D, Xu Z, et al. Biomarkers of deep venous thrombosis. J Thromb Thrombolysis. 2012 doi: 10.1007/s11239-012-0721-y. [DOI] [PubMed] [Google Scholar]
- Hull R, van Aken WG, Hirsh J, Gallus AS, Hoicka G, Turpie AG, et al. Impedance plethysmography using the occlusive cuff technique in the diagnosis of venous thrombosis. Circulation. 1976;53:696–700. doi: 10.1161/01.cir.53.4.696. [DOI] [PubMed] [Google Scholar]
- Ikuta K, Mori T, Yamamoto T, Niidome T, Shimokawa H, Katayama Y. Development of polymeric drug delivery system for recognizing vascular endothelial dysfunction. Bioorg Med Chem. 2008;16:2811–8. doi: 10.1016/j.bmc.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Kanne JP, Lalani TA. Role of computed tomography and magnetic resonance imaging for deep venous thrombosis and pulmonary embolism. Circulation. 2004;109:I15–21. doi: 10.1161/01.CIR.0000122871.86662.72. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Kinney T, Streiff M, Sing R, Proctor M, Becker D, et al. Guidelines for the use of retrievable and convertible vena cava filters: report from the Society of Interventional Radiology multidisciplinary consensus conference. J Vasc Interv Radiol. 2006;17:449–59. doi: 10.1097/01.rvi.0000203418-39769.0d. [DOI] [PubMed] [Google Scholar]
- Kaufmann BA, Lindner JR. Molecular imaging with targeted contrast ultrasound. Curr Opin Biotechnol. 2007;18:11–6. doi: 10.1016/j.copbio.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Kim HS, Patra A, Paxton BE, Khan J, Streiff MB. Adjunctive percutaneous mechanical thrombectomy for lower-extremity deep vein thrombosis: clinical and economic outcomes. J Vasc Interv Radiol. 2006;17:1099–104. doi: 10.1097/01.RVI.0000228334.47073.C4. [DOI] [PubMed] [Google Scholar]
- Labropoulos N, Waggoner T, Sammis W, Samali S, Pappas PJ. The effect of venous thrombus location and extent on the development of post-thrombotic signs and symptoms. J Vasc Surg. 2008;48:407–12. doi: 10.1016/j.jvs.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Landefeld CS. Noninvasive diagnosis of deep vein thrombosis. JAMA. 2008;300:1696–7. doi: 10.1001/jama.300.14.1696. [DOI] [PubMed] [Google Scholar]
- Lee AY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146–53. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- Line BR. Pathophysiology and diagnosis of deep venous thrombosis. Semin Nucl Med. 2001;31:90–101. doi: 10.1053/snuc.2001.21406. [DOI] [PubMed] [Google Scholar]
- Liu F, Lu P, Jin B. Catheter-directed thrombolysis for acute iliofemoral deep venous thrombosis. Ann Vasc Surg. 2011;25:707–15. doi: 10.1016/j.avsg.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Liu Y, Sun Y, Zhang S, Jin X. Placement of a retrievable inferior vena cava filter for deep venous thrombosis in term pregnancy. J Vasc Surg. 2012;55:1042–7. doi: 10.1016/j.jvs.2011.10.107. [DOI] [PubMed] [Google Scholar]
- Lowe GDO. Measurement of thrombosis and its prevention. Br J Clin Pharmacol. 2002;54:96–100. doi: 10.1046/j.1365-2125.2002.01626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh JN, Partlow KC, Abendschein DR, Scott MJ, Lanza GM, Wickline SA. Molecular imaging with targeted perfluorocarbon nanoparticles: quantification of the concentration dependence of contrast enhancement for binding to sparse cellular epitopes. Ultrasound Med Biol. 2007;33:950–8. doi: 10.1016/j.ultrasmedbio.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy JR, Patel P, Botnaru I, Haghayeghi P, Weissleder R, Jaffer FA. Multimodal nanoagents for the detection of intravascular thrombi. Bioconjug Chem. 2009;20:1251–5. doi: 10.1021/bc9001163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- Mustafa BO, Rathbun SW, Whitsett TL, Raskob GE. Sensitivity and specificity of ultrasonography in the diagnosis of upper extremity deep vein thrombosis: a systematic review. Arch Intern Med. 2002;162:401–4. doi: 10.1001/archinte.162.4.401. [DOI] [PubMed] [Google Scholar]
- NIH . Clinical Trials. 2012. [Google Scholar]
- Orbell JH, Smith A, Burnand KG, Waltham M. Imaging of deep vein thrombosis. Br J Surg. 2008;95:137–46. doi: 10.1002/bjs.6077. [DOI] [PubMed] [Google Scholar]
- Paliwal R, Paliwal SR, Agrawal GP, Vyas SP. Biomimetic solid lipid nanoparticles for oral bioavailability enhancement of low molecular weight heparin and its lipid conjugates: in vitro and in vivo evaluation. Mol Pharm. 2011;8:1314–21. doi: 10.1021/mp200109m. [DOI] [PubMed] [Google Scholar]
- Parikh S, Motarjeme A, McNamara T, Raabe R, Hagspiel K, Benenati JF, et al. Ultrasound-accelerated thrombolysis for the treatment of deep vein thrombosis: initial clinical experience. J Vasc Interv Radiol. 2008;19:521–8. doi: 10.1016/j.jvir.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Plesset MS, Sadhal SS. On the stability of gas bubbles in liquid–gas solutions. Appl Sci Res. 1982;38:133–41. [Google Scholar]
- Prescott SM, Tikoff G, Coleman RE, Richards KL, Armstrong JD, Jr, Hershgold EL, et al. 131I-labeled fibrinogen in the diagnosis of deep vein thrombosis of the lower extremities. AJR Am J Roentgenol. 1978;131:451–3. doi: 10.2214/ajr.131.3.451. [DOI] [PubMed] [Google Scholar]
- Richards KL, Armstrong JD, Jr, Tikoff G, Hershgold EJ, Booth JL, Rampton JB. Noninvasive diagnosis of deep venous thrombosis. Arch Intern Med. 1976;136:1091–6. [PubMed] [Google Scholar]
- Ruggeri ZM. Von Willebrand factor, platelets and endothelial cell interactions. J Thromb Haemost. 2003;1:1335–42. doi: 10.1046/j.1538-7836.2003.00260.x. [DOI] [PubMed] [Google Scholar]
- Saxena V, Sadoqi M, Shao J. Indocyanine green-loaded biodegradable nanoparticles: preparation, physicochemical characterization and in vitro release. Int J Pharm. 2004;278:293–301. doi: 10.1016/j.ijpharm.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Scarvelis D, Wells PS. Diagnosis and treatment of deep-vein thrombosis. Cmaj. 2006;175:1087–92. doi: 10.1503/cmaj.060366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreijer AJ, Reitsma PH, Cannegieter SC. High hematocrit as a risk factor for venous thrombosis. Cause or innocent bystander? Haematologica. 2010;95:182–4. doi: 10.3324/haematol.2009.017285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann PA, Christiansen JP, Quigley RM, McCreery TP, Sweitzer RH, Unger EC, et al. Targeted-microbubble binding selectively to GPIIb IIIa receptors of platelet thrombi. Invest Radiol. 2002;37:587–93. doi: 10.1097/00004424-200211000-00001. [DOI] [PubMed] [Google Scholar]
- Semba CP, Dake MD. Iliofemoral deep venous thrombosis: aggressive therapy with catheter-directed thrombolysis. Radiology. 1994;191:487–94. doi: 10.1148/radiology.191.2.8153327. [DOI] [PubMed] [Google Scholar]
- Shah B, Kona S, Gilbertson TA, Nguyen KT. Effects of poly-(lactide-co-glycolide) nanoparticles on electrophysiological properties of enteroendocrine cells. J Nanosci Nanotechnol. 2011;11:3533–42. doi: 10.1166/jnn.2011.3802. [DOI] [PubMed] [Google Scholar]
- Silverstein M, Heit J, Mohr D, Petterson T, O'Fallon W., III M.L. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158:585–93. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- Simioni P, Prandoni P, Lensing AW, Manfrin D, Tormene D, Gavasso S, et al. Risk for subsequent venous thromboembolic complications in carriers of the prothrombin or the factor V gene mutation with a first episode of deep-vein thrombosis. Blood. 2000;96:3329–33. [PubMed] [Google Scholar]
- Simone E, Ding BS, Muzykantov V. Targeted delivery of therapeutics to endothelium. Cell Tissue Res. 2009;335:283–300. doi: 10.1007/s00441-008-0676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taillefer R, Edell S, Innes G, Lister-James J. Acute thromboscintigraphy with (99m) Tc-apcitide: results of the phase 3 multicenter clinical trial comparing 99mTc-apcitide scintigraphy with contrast venography for imaging acute DVT. Multicenter Trial Investigators. J Nucl Med. 2000;41:1214–23. [PubMed] [Google Scholar]
- Takeuchi M, Ogunyankin K, Pandian NG, McCreery TP, Sweitzer RH, Caldwell VE, et al. Enhanced visualization of intravascular and left atrial appendage thrombus with the use of a thrombus-targeting ultrasonographic contrast agent (MRX-408A1): in vivo experimental echocardiographic studies. J Am Soc Echocardiogr. 1999;12:1015–21. doi: 10.1016/s0894-7317(99)70096-9. [DOI] [PubMed] [Google Scholar]
- Tovey C, Wyatt S. Diagnosis, investigation, and management of deep vein thrombosis. BMJ. 2003;326:1180–4. doi: 10.1136/bmj.326.7400.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger EC, McCreery TP, Sweitzer RH, Shen D, Wu G. In vitro studies of a new thrombus-specific ultrasound contrast agent. Am J Cardiol. 1998;81:58G–61G. doi: 10.1016/s0002-9149(98)00055-1. [DOI] [PubMed] [Google Scholar]
- van Beek EJ, Wild JM, Fink C, Moody AR, Kauczor HU, Oudkerk M. MRI for the diagnosis of pulmonary embolism. J Magn Reson Imaging. 2003;18:627–40. doi: 10.1002/jmri.10421. [DOI] [PubMed] [Google Scholar]
- VanWijk MJ, VanBavel E, Sturk A, Nieuwland R. Microparticles in cardiovascular diseases. Cardiovasc Res. 2003;59:277–87. doi: 10.1016/s0008-6363(03)00367-5. [DOI] [PubMed] [Google Scholar]
- Vedantham S. Deep venous thrombosis: the opportunity at hand. AJR Am J Roentgenol. 2009;193:922–7. doi: 10.2214/AJR.09.3214. [DOI] [PubMed] [Google Scholar]
- Vedantham S, Grassi CJ, Ferral H, Patel NH, Thorpe PE, Antonacci VP, et al. Reporting standards for endovascular treatment of lower extremity deep vein thrombosis. J Vasc Interv Radiol. 2009;20:S391–408. doi: 10.1016/j.jvir.2009.04.034. [DOI] [PubMed] [Google Scholar]
- Verhaeghe R, Stockx L, Lacroix H, Vermylen J, Baert AL. Catheter-directed lysis of iliofemoral vein thrombosis with use of rt-PA. Eur Radiol. 1997;7:996–1001. doi: 10.1007/s003300050239. [DOI] [PubMed] [Google Scholar]
- Vymazal J, Spuentrup E, Cardenas-Molina G, Wiethoff AJ, Hartmann MG, Caravan P, et al. Thrombus imaging with fibrin-specific gadolinium-based MR contrast agent EP-2104R: results of a phase II clinical study of feasibility. Investig Radiol. 2009;44:697–704. doi: 10.1097/RLI.0b013e3181b092a7. [DOI] [PubMed] [Google Scholar]
- Wicky J, Bongard O, Peter R, Simonovska S, Bounameaux H. Screening for proximal deep venous thrombosis using B-mode venous ultrasonography following major hip surgery: implications for clinical management. Vasa. 1994;23:330–6. [PubMed] [Google Scholar]
- Wiethoff A, Makowski M, Katoh M, Spuentrup E, Botnar R. Molecular imaging of thrombosis. Curr Cardiovasc Imaging Reports. 2010;3:34–41. [Google Scholar]
- Winter PM, Caruthers SD, Yu X, Song SK, Chen J, Miller B, et al. Improved molecular imaging contrast agent for detection of human thrombus. Magn Reson Med. 2003;50:411–6. doi: 10.1002/mrm.10532. [DOI] [PubMed] [Google Scholar]
- Yu X, Song SK, Chen J, Scott MJ, Fuhrhop RJ, Hall CS, et al. High-resolution MRI characterization of human thrombus using a novel fibrin-targeted paramagnetic nanoparticle contrast agent. Magn Reson Med. 2000;44:867–72. doi: 10.1002/1522-2594(200012)44:6<867::aid-mrm7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]