Abstract
Objectives
The aim of the study was to investigate the organization and delivery of HIV and tuberculosis (TB) health care and to analyse potential differences between treatment centres in Eastern (EE) and Western Europe (WE).
Methods
Thirty-eight European HIV and TB treatment centres participating in the TB:HIV study within EuroCoord completed a survey on health care management for coinfected patients in 2013 (EE: 17 respondents; WE:21; 76% of all TB:HIV centres). Descriptive statistics were obtained for regional comparisons. The reported data on health care strategies were compared with actual clinical practice at patient level via data derived from the TB:HIV study.
Results
Respondent centres in EE comprised: Belarus (n = 3), Estonia (1), Georgia (1), Latvia (1), Lithuania (1), Poland (4), Romania (1), the Russian Federation (4) and Ukraine (1); those in WE comprised: Belgium (1), Denmark (1), France (1), Italy (7), Spain (2), Switzerland (1) and UK (8). Compared with WE, treatment of HIV and TB in EE are less often located at the same site (47% in EE versus 100% in WE; P < 0.001) and less often provided by the same doctors (41% versus 90%, respectively; P = 0.002), whereas regular screening of HIV-infected patients for TB (80% versus 40%, respectively; P = 0.037) and directly observed treatment (88% versus 20%, respectively; P < 0.001) were more common in EE. The reported availability of rifabutin and second- and third-line anti-TB drugs was lower, and opioid substitution therapy (OST) was available at fewer centres in EE compared with WE (53% versus 100%, respectively; P < 0.001).
Conclusions
Major differences exist between EE and WE in relation to the organization and delivery of health care for HIV/TB-coinfected patients and the availability of anti-TB drugs and OST. Significant discrepancies between reported and actual clinical practices were found in EE.
Keywords: delivery of health care, Europe, HIV, integrated, tuberculosis
Introduction
The HIV epidemic has spread rapidly in Eastern Europe (EE) since the 1990s, predominantly among people who inject drugs (PWID) [1]. The concurrent severe resurgence of a tuberculosis (TB) co-epidemic in the region further aggravated the situation, and today HIV/TB coinfection poses one of the most complex and fatal public health problems in EE [1–3]. Increasing incidence rates of HIV infection are being reported in EE together with decreasing rates of TB treatment success and alarming rates of anti-TB drug resistance [1,2,4–7]. According to the 2014 World Health Organization (WHO) global tuberculosis report, the WHO European region is currently the region with the highest burden of multi-drug resistant (MDR) and extensively drug resistant (XDR) TB globally, leading to a potential public health crisis [2]. Pronounced differences in the incidence of TB and MDR TB between EE and WE have been reported. Whereas TB incidence rates are among the world’s lowest in most WE countries (0–20/100 000 population per year), TB incidence rates in EE countries reach 50 – 150/100 000 per year. MDR TB rates are among world’s highest in EE countries and account for 6 to > 20% of all new TB cases. For previously treated TB cases, the MDR TB proportion increases to > 50% in some countries of the EE region. WE countries have some of the lowest proportions of MDR TB cases among all TB cases (< 3%) [2]. HIV infection, a potential risk factor for MDR TB [7,8], adds further complexity to the MDR TB public health emergency witnessed in EE today.
We have previously demonstrated 3–5-fold higher mortality rates among HIV/TB-coinfected patients in EE compared with WE in a European cohort of HIV-positive patients diagnosed with TB during 2004–2006 [3]. This difference was only partially explained by regional differences in patient demographics, traditional risk factors for TB and HIV acquisition, resistance patterns, localization of TB disease, anti-TB therapy and combination antiretroviral treatment (cART) [3].
Our earlier study did not collect information on the organization and delivery of health care, clinical management strategies, and access to specialist HIV/TB health care and medicines. Data on HIV/TB health care organization and management of coinfected patients in Europe are limited [9–12]. The objective of this study was therefore to survey the organization and delivery of HIV/TB health care at treatment centre level among centres participating in the TB:HIV study. We compared the organization of and access to HIV/TB health care between treatment centres in EE and WE, and the survey results to the actual management of patients enrolled in the TB:HIV study.
Methods
The study design was cross-sectional and data were collected through a self-administered structured online questionnaire. All European HIV and TB treatment centres participating in the international TB:HIV study collaboration (for details of the study, see http://www.cphiv.dk/TBHIV) as per Spring 2013 were invited to complete an online questionnaire (n = 41). Data were collected and managed through Research Electronic Data Capture (REDcap; http://project-redcap.org) hosted at Rigshospitalet, University of Copenhagen. The questionnaire (available at at http://www.cphiv.dk/Ongoing-Studies/TBHIV/TBHIV-Study-Group) included 40 questions related to the organization and availability of HIV/TB health care and medicines and clinical management strategies for coinfected patients. Persons invited to respond on behalf of each participating centre were principal investigators in the TB:HIV study who were also medical doctors in charge of the treatment of HIV/TB-infected patients at the treatment centre in question.
Content, construct and face validity of the questionnaire were ensured by performing pre-tests and consulting experts in the field. Descriptive statistics were obtained for comparisons with regard to health care arrangements and delivery and management strategies between EE and WE.
In order to study the discrepancy between reported and actual clinical practices, we used the TB:HIV health care index (HCI) previously developed by our group [13] – a scale developed to assess clinical performance in terms of HIV/TB care [based on TB drug susceptibility testing (DST), standardized anti-TB treatment and cART] (Table 1). HCI scores were calculated for centres in EE and WE using survey data and compared with the HCI scores derived from the baseline data from the TB:HIV study (AM Werlinrud Efsen, personal communication). The chosen key clinical indicators were related to (1) DST of anti-TB drugs; (2) initial anti-TB treatment for HIV/TB-coinfected patients; (3) timing of cART initiation for HIV/TB-coinfected patients. For example, individuals were given a score of 1 for the indicator ‘DST of anti-TB drugs’ if they had a DST performed and a score of 0 if they did not (Table 1). This allowed us to determine the discrepancy between reported clinical management strategies in the survey and the actual clinical practice that had taken place. HCI scores were calculated on an individual centre level for the survey, and the mean reported HCI score for each region was calculated based on these centre-level scores. TB:HIV study baseline data from centres that participated in the survey were used to calculate individual patient-level scores for each centre, and the mean actual HCI score for each region was then derived from these individual-level scores. In total, data from 1041 individuals contributed to the actual patient-level HCI scores.
Table 1.
Method of calculating the health care index (HCI) score
| Podlekareva et al. 2013 [13]
|
Survey (reported)
|
Baseline (actual)
|
|||
|---|---|---|---|---|---|
| Criteria | Score | Criteria | Score | Criteria | Score |
| Performance of DST for M. tuberculosis | 1 | DST routinely performed | 1 | Had a resistance result at baseline (within 1 month of the TB diagnosis date) | 1 |
| Inclusion of rifamycin (R), isoniazid (H) and pyrazinamide (Z) in the initial anti-tuberculosis treatment regimen | 2 | Standard initial anti-TB treatment for HIV/TB-coinfected patients contains RHZE | 2 | Initial treatment (drugs started on the first date of starting treatment) contained at least RHZE | 2 |
| Initiation of cART (a combination of at least three antiretroviral drugs from any class) before or up to 1 month after TB diagnosis | 2 | Start as soon as possible and within 2 months after TB diagnosis irrespective of CD4 count | 2 | cART (3 or more antiretroviral drugs from any class) was initiated before or up to 2 months after the TB diagnosis date | 2 |
CI: DST, drug susceptibility testing; cART, combination antiretroviral therapy; TB, tuberculosis.
Two-sided Fisher’s exact test was used for calculation of p-values and corresponding confidence intervals to explore associations. All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
Results
Of the 41 European centres treating approximately 1300 HIV/TB-coinfected patients per year, 38 (93%) completed the survey. Respondent centres in EE (n = 17) were located in: Belarus (n = 3), Estonia (1), Georgia (1), Latvia (1), Lithuania (1), Poland (4), Romania (1), the Russian Federation (4) and Ukraine (1), and those in WE (n = 21) were located in: Belgium (1), Denmark (1), France (1), Italy (7), Spain (2), Switzerland (1) and the UK (8). Four centres (one in the Russian Federation, two in Spain and one in the UK) did not reply. Treatment centres were in general larger hospital departments or clinics [the median number of beds was 160; interquartile range (IQR) 35–700] with a median of 10 (IQR 3–26) new TB cases in HIV-positive persons diagnosed per year. There were no significant differences in basic patient characteristics (gender, age, and HIV and TB risk factors) between respondent and nonrespondent centres (data not shown).
Integration of care
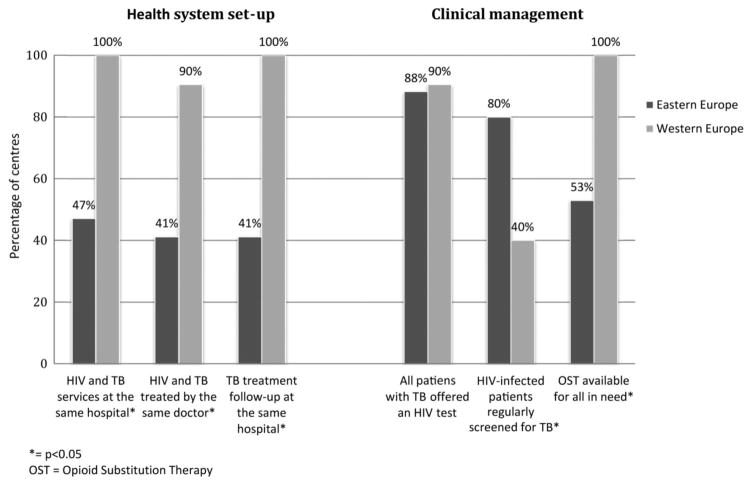
Compared with WE, treatment of HIV and TB in EE were less often provided at the same location (47% in EE versus 100% in WE; P < 0.001) and by the same doctors (41% versus 90%, respectively; P = 0.002) (Fig. 1). All WE centres reported that patients were followed up at the same facility for the entire course of anti-TB treatment, whereas only 41% of EE centres reported that patients were followed up at the same facility after the anti-TB treatment initiation phase (P < 0.001) (Fig. 1). Opioid substitution therapy (OST) was reported to be available for all HIV/TB-coinfected patients in need at all treatment centres in WE but at significantly fewer centres in EE (53%; P < 0.001) (Fig. 1). Similar percentages of centres in the two regions reported that they offer all patients with TB an HIV test (88% in EE versus 90% in WE; P = 1.00), whereas regular screening of HIV-positive patients for active TB was more widespread in EE (80% versus 40% in WE; P = 0.037) (Fig. 1) and more often carried out as screening of all HIV-infected patients once per year (53% in EE versus 0% in WE; P < 0.001).
Fig. 1.
Integration of care for patients with HIV/tuberculosis (TB) in Eastern (EE) and Western Europe (WE). *P < 0.05. OST, opioid substitution therapy.
TB diagnostics including anti-TB drug susceptibility testing
In both regions, centres generally reported that performing sputum cultures was standard procedure (94% in EE and 85% in WE; P = 0.609). Rapid diagnostics were widely available in both regions, somewhat more so in WE (53% in EE versus 70% in WE; P = 0.328), and were reported to be available only at TB centres in EE, not at HIV clinics. Eighty-eight per cent of all treatment centres in EE and 95% in WE reported routinely performing DST on all positive TB cultures (P = 0.574). Whereas there was no significant difference in terms of reported DST of second-line drugs (71% in EE and 60% in WE; P = 0.731), routine DST of third-line drugs was lower in EE (12% versus 40% in WE; P = 0.073).
Anti-TB treatment strategies and drug availability
The vast majority of centres in both regions, although more in WE (69% in EE and 95% in WE; P = 0.069), reported the standard anti-TB treatment regimen for HIV/TB-coinfected patients as 2 months of rifampicin (R), isoniazid (H), pyrazinamide (Z) and ethambutol (E) for the intensive phase, followed by 4 months of RH in the continuation phase. Three EE centres reported a 7-month continuation phase of RH, one EE centre reported a 7-month continuation phase of HZ, and another EE centre reported an intensive phase of 4–6 months’ duration as standard. A test of cure (culture of sputum/other material) at the end of anti-TB treatment course was reported as not routinely performed by 13% of centres in EE and 30% in WE (P = 0.419).
Directly observed treatment (DOT) was more frequently applied in EE, where 88% of centres reported using DOT for all patients with TB at least for the intensive phase, compared with 20% in WE (P < 0.001).
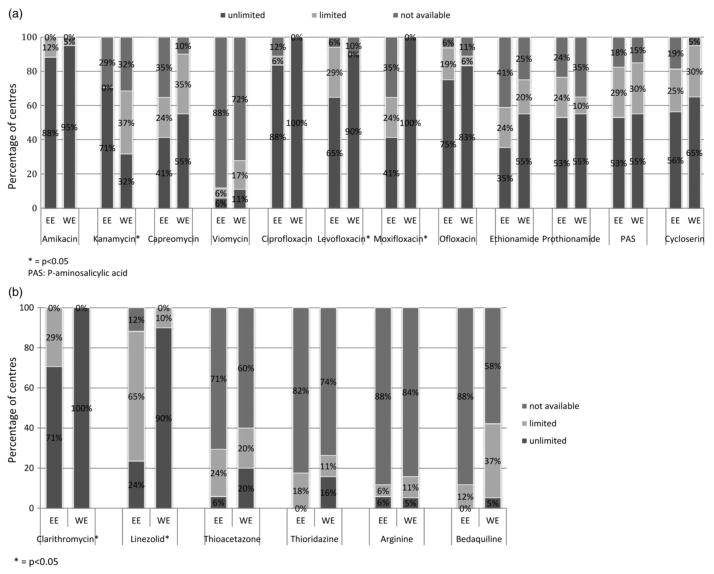
The availability of rifabutin was low in EE, with only 18% reporting unlimited availability, 59% reporting limited availability and 24% reporting no availability, compared with 95%, 5% and 0%, respectively, in WE (P < 0.001). The availability of second-line drugs (moxifloxacin: P < 0.001; levofloxacin: P = 0.029) and third-line drugs (linezolid: P < 0.001; clarithromycin: P = 0.014) was lower in EE than in WE, with the exception of kanamycin, which was more commonly available in EE (P = 0.01) (Fig. 2a,b). Bedaquiline was unavailable in the majority of treatment centres in both regions (89% in EE and 58% in WE; P = 0.087). We found no regional differences in the availability of the following drugs: amikacin, capreomycin, viomycin, ciprofloxacin, ofloxacin, ethionamide, prothionamide, P-aminosalicylic acid, cycloserin, thioacetazone, thioridazine, arginine and bedaquiline (Fig. 2a,b).
Fig. 2.
Availability of (a) second-line and (b) third-line anti-TB drugs in Eastern (EE) and Western Europe (WE). *P < 0.05. PAS, P-aminosalicylic acid.
cART in HIV/TB-coinfected patients
None of the treatment centres in EE and WE required user fees for cART. However, one centre in EE and one in WE required user fees for medical consultations. About half of all respondents in both regions (47% and 48% in EE and WE, respectively) reported starting cART as soon as possible after TB diagnosis irrespective of the CD4 cell count. The remaining centres responded that the timing of cART initiation was dependent on CD4 cell count, with no regional differences (P = 0.166). The most commonly reported cART regimen in both regions was two nucleoside reverse transcriptase inhibitors (NRTIs) plus one nonnucleoside reverse transcriptase inhibitor (NNRTI) (100% in EE; 85% in WE), with efavirenz the preferred NNRTI at all centres. Tenofovir/emtricitabine, abacavir/lamivudine and zidovudine/lamivudine were the preferred backbones in 95%, 5% and 0% of sites in WE and 41%, 29% and 24% of sites in EE, respectively (P < 0.001).
Comparison of reported and provided health care
In order to study the discrepancy between reported and actual health care provisions, we used the TB:HIV health care index (HCI) score, a scale developed to measure clinical performance [13]. Sixteen treatment centres in EE responded to the survey, enrolled patients and submitted clinical data to the TB:HIV study, of which 14 reported in the survey routinely performing DST of anti-TB drugs, 14 reported initiating RHZE and eight reported starting cART within 2 months of the TB diagnosis, irrespective of CD4 cell count. In WE, 17 centres both responded to the survey and enrolled patients to the TB:HIV study. Of these, 16 reported routinely performing DST, 17 reported initiating RHZE, and nine reported starting cART within 2 months of the TB diagnosis, irrespective of CD4 cell count. The HCI scores based on (1) data reported in the survey and (2) observed baseline data from clinical records of the TB:HIV study are shown in Table 2, and the relative score distribution in Table 3.
Table 2.
Comparison between ‘reported’ and ‘actual’ health care index (HCI) scores in Eastern Europe (EE) and Western Europe (WE)
| Indicator (mean) | EE
|
WE
|
||||
|---|---|---|---|---|---|---|
| Reported | Actual | P-value | Reported | Actual | P-value | |
| Routine DST | 0.88 (0.72–1.04) | 0.34 (0.31–0.37) | 0.03 | 0.94 (0.82–1.06) | 0.62 (0.55–0.68) | 0.18 |
| RHZE initiated | 1.75 (1.43–2.07) | 0.99 (0.92–1.08) | 0.03 | 2.00 (−) | 1.52 (1.40–1.63) | NA |
| cART ≤ 2 months | 1.00 (0.51–1.49) | 0.90 (0.82–0.96) | 0.78 | 1.00 (0.57–1.55) | 1.43 (1.31–1.55) | 0.27 |
| Total score | 3.63 (3.08–4.18) | 2.23 (2.12–2.34) | 0.002 | 4.00 (3.47–4.53) | 3.56 (3.37–3.75) | 0.28 |
n = 33 treatment centres (16 in EE and 17 in WE).
Figures represent mean HCI score per centre (reported) and per patient (actual) with 95% confidence interval (CI). Please note that for the WE reported RHZE score it was not possible to calculate a 95% CI as every centre answered positively.
Maximum HCI scores per indicator: routine DST: 1; RHZE: 2; cART ≤ 2 months: 2.
cART, combination antiretroviral therapy; DST, drug susceptibility testing; NA, not applicable; RZHE, rifampicin, isoniazid, pyrazinamide and ethambutol.
Table 3.
Distribution of survey-reported health care index (HCI) scores in Eastern (EE) and Western Europe (WE)
| Score | EE
|
WE
|
||
|---|---|---|---|---|
| Centres | Centres | |||
| n | % | n | % | |
| 5 | 5 | 31 | 9 | 53 |
| 4 | 2 | 13 | 0 | 0 |
| 3 | 8 | 50 | 7 | 41 |
| 2 | 0 | 0 | 1 | 6 |
| 1 | 1 | 6 | 0 | 0 |
| 0 | 0 | 0 | 0 | 0 |
Total HCI scores from survey data were comparable across the two regions (3.63 in EE and 4.00 in WE; P = 0.74), whereas a difference in HCI scores was noted between the regions when considering total HCI scores derived from clinically recorded data (2.23 in EE and 3.53 in WE; P < 0.0001). Reported HCI scores in EE were significantly higher compared with actual scores in EE (3.63 versus 2.23, respectively; P = 0.002) (Table 2), especially in relation to the indicators of anti-TB DST and RHZE initiation (Table 2). In WE, the reported versus actual HCI scores did not differ significantly (4.00 versus 3.56, respectively; P = 0.28) (Table 2).
The vast majority of centres in both regions had a total reported HCI score of 3 or above (93% in EE; 94% in WE; Table 3); however, when looking at actual clinical practice, the proportion of patients with an HCI score of 3 and above was considerably lower, particularly in EE (42% in EE; 75% in WE).
In EE, the actual HCI score for starting cART within the first 2 months (0.90; maximum score = 2) as well as a lack of DST (0.34; maximum score = 1) contributed significantly to a low total actual HCI score in EE (2.23; maximum score = 4) (Table 2). In WE, a lack of DST (0.62; maximum score = 1) specifically contributed to lowering the total actual HCI score (Table 2).
Discussion
This study found significant differences between EE and WE in relation to health care delivery for HIV/TB-coinfected patients, with a substantially less integrated approach in EE.
The availability of OST and anti-TB drugs, usage of DOT and preferred NRTI backbone also differed across regions, whereas strategies for TB diagnostics, anti-TB therapy and other issues related to cART were not reported to differ significantly between EE and WE.
Anti-TB treatment has traditionally been hospital-based in many countries of the EE region, but with the introduction of directly observed treatment short course (DOTS) there was a shift towards more out-patient care [14,15]. Current policies on collaborative HIV/TB service provision recommend the same facility and same visit for HIV and TB treatment and care [16]. The present study shows that only about half of all centres in EE offered treatment for TB and HIV at the same facility. Provision of HIV care, TB treatment and OST at different facilities, or even in different cities, is likely to result in suboptimal patient care with a negative impact on all aspects of the HIV/TB care cascade. A recent study from South Africa showed that co-location of TB/HIV care shortened the time to ART initiation [17] and a review from 2007 of several studies from across the world found that co-location of services for PWID infected with HIV and TB increased uptake of services and improved clinical outcome [18]. Several studies have demonstrated that early initiation of cART in HIV/TB-coinfected patients optimizes treatment outcome particularly in patients with advanced immunodeficiency [13,19,20]. Despite reporting of good access to cART, there was no consensus among respondents on the timing of cART initiation in relation to anti-TB treatment, a finding that may reflect diverging guidelines and calls for further investigation. Active substance abuse has been shown to negatively affect treatment adherence, and access to OST for PWID increases adherence to both HIV and TB treatment [18,21,22]. This study documents that OST is still poorly available in centres in EE. Increased cooperation across disease specialities (specifically drug dependence, TB and HIV programmes) should be a key health service priority in the EE region [9,16,18,23].
The proportion of centres in the survey that reported offering HIV testing to all patients with TB was substantially higher than the most recent estimate of the proportion of patients with TB with a known HIV status in Europe (59%) [2]. This could represent inclusion bias towards centres that performed well on this measure or it could reflect obsequiousness bias; in addition, some patients with TB who are offered an HIV test may decline to be tested.
Regular TB screening of HIV-infected patients was markedly higher in EE; however, considering the scale of the TB epidemic in EE, the reported TB screening prevalence of 80% in EE may not be sufficient. However, the respondents may have interpreted ‘regular TB screening’ differently. WHO recommends that all HIV-infected patients should be screened for TB using a clinical symptom-based algorithm [16], but this algorithm was not specified in the survey and it is likely that the reported screening prevalence indicates actual tests (tuberculin skin testing, chest X-ray, interferon gamma release assay etc.) and not a clinical algorithm. In that case, the proportion TB-screened in EE should be considered high and the low TB screening prevalence found in WE (40%) may thus reflect the relatively low TB burden in these countries. Of additional concern is that TB rapid diagnostic tests were available in only half of all HIV clinics in EE. Furthermore, access to preferred second-line agents such as moxifloxacin, prothionamide or ethionamide, and cycloserine was rather limited considering the high prevalence of MDR TB in this region. Use of less potent fluoroquinolones and less active or less well tolerated second-line drugs is a major concern in terms of drug resistance amplification, especially for resistance to drugs such as linezolid and bedaquiline, to which most strains would be expected to be susceptible. Drug supply may well be substantially worse at treatment centres that did not participate in the survey, for example, in nonurban settings.
The main limitation of this study is that it is based on a self-reported survey, and ‘obsequiousness’ bias, where respondents alter their reply in the direction they perceive to be desired by the investigator, cannot be ruled out. The calculation of an HCI score and comparisons between reported and actual clinical practices sought to explore the magnitude of this particular bias, and it appeared that a discrepancy indeed was present between actual and reported practices in EE. Alternatively, the overall data reported in the survey were correct but observed practice may have differed because of temporary shortages of TB drugs, nonuptake of cART, or interruptions to the supply of laboratory reagents. The sizable proportion of patients with an observed HCI score below 3 in both regions, although larger in EE than in WE, is worrying, and will be the focus of planned analyses. Considering the discrepancy between reported and actual HCI scores, it is likely that the survey data in general underestimate the difference in actual health care provision between WE and EE. It should be noted that the HCI scores calculated based on the present survey did not correspond exactly to the criteria used in the original HCI score (Table 1) [13]. The health care index has been derived using step-wise regression in a study population very similar to the current one [13]. Other variables, such as CD4 cell count, were considered for inclusion in the score, but they were not found to significantly predict clinical outcome and therefore were not included in the final HCI equation. It should be noted that using the HCI score to indicate quality of treatment has its limitations [13]. The three parameters included in the score, although important, do not necessarily provide a comprehensive picture of clinical performance. Nonetheless, as the aim was not to evaluate clinical performance, but to identify differences in reported versus actual HCI scores, the impact of this limitation on our conclusions is likely to be small.
Additional limitations include the fact that the centres surveyed are, in general, larger hospitals and thus presumably not representative of the entire region, especially rural areas. Intra-country and intra-regional variations were observed, although the sample size did not allow us to investigate this further. Despite the small sample size, the treatment centres surveyed treat a relatively large share (7%) of the total number of new TB cases in the HIV-infected population in Europe, estimated to be 19 000 in 2013 [2].
Conclusions and study implications
This study documents significant differences in the organization and delivery of health care for HIV/TB within Europe, depicting a more fragmented health care system in EE with likely adverse consequences for adherence. The availability of anti-TB drugs with activity against MDR and XDR TB as well as OST remains limited in EE, where the need for them is highest. Findings from this study emphasize the need for an increased focus on patient-centred care and integration across specialities. In line with recent publications and recommendations [2,9,24–26], this should include offering all patients with TB an HIV test, regularly screening HIV-positive patients for TB, making OST and rapid TB diagnostics available in HIV/TB treatment centres, and finally ensuring that adequate anti-TB treatment and cART are widely available and initiated in a timely fashion. Linking regional differences in the organization and availability of HIV/TB health care described here to treatment outcomes is crucial for future care and warrants further analyses. We aim to address these questions in the on-going TB:HIV study (http://www.chip.dk/TBHIV).
Acknowledgments
Sources of funding: EuroCOORD EU 7th framework programme; The Danish Council for Independent Research (DFF); Research Council, Copenhagen University Hospital, Rigshospitalet.
Appendix
TB:HIV study group: Argentina: M. H. Losso, J. J. Toibaro, D. Palmero, B. J. Bartoletti, E. Warley, O. Gear, O. G. Messina, M. Michans, H. Laplume, D. David, C. Marson, F. P. Scapelatto, D. Dalessandro, S. Lupo, G. Costilla Campero, M. Herbst, C. Remondegui, and C. Elias; Belarus: I. Karpov, A. Vassilenko, E. Skrahina, A. Skrahin, A. Zalutskya, O. Kondratenko, V. Mitsura, V. Bondarenko, O. Suetnov and D. Paduto; Belgium: S. Dewit, M. C. Payen, C. Noscoi, K. Kabeya. Chile: M. Wolff and C. Cortes; Denmark: N. Obel, G. Kronborg and O. Kirk; Estonia: V. Iljna and T. Kummik; France: M. Bryand and F. Dabis; Georgia: N. Bolokadze, N. Lanchava, N. Bablishvili, L. Goginashvili, L. Mikiashvili and K. Mshvidobadze; Italy: E. Girardi, A. Di Biagio, A. Matteelli, A. Apostoli, S. Barzoni, G. Lapadula, M. Purgatorio and S. Carbonara; Latvia: B. Rozentale, I. Zeltina and I. Janushkevich; Lithuania: S. Caplinskas, Z. Kancauskienne and I. Caplinskienne; Mexico: B. Crabtree, A. Pina, J. S. Madero, J. Mosqueda and J. A. Villanueva; Poland: A. Grzeszczuk, M. Bura, A. Garlicki, M. Inglot, B. Knysz, J. Kozlowska, J. Loster, E. Mularska, R. Podlasin, M. Thompson and A. Wiercinska-Drapalo; Romania: D. Duiculescu and S. Tetradov; Russia: A. Rakhmanova, A. Yakovlev, A. Panteleev, A. Turkalova, Y. Vlasova, T. Trotimora, E. Borodulina, L. Chumanova and Y. Mashkova; Spain: J. M. Miro, J. A. Caylá, A. Moreno, J. P. Millet, A. Orcau, L. Fina, L. del Baño, L. L. Roldán, A. Romero, J. A. Martínez, C. Manzardo, J. González, G. Tudo, H. Knobel, F. Sanchez, M. Salvadó, A. Curran, M. T. Tórtola, D. Pozamczer, M. Saumoy, F. Alcaide, X. Martínez-Lacasa, E. Cuchí, M. A. Sambeat, V. Pomar, P. Coll, P. Miralles, S. Moreno, J. A. Iribarren, M. Ibarguren and T. Aldamiz; Switzerland: H. Furrer, M. Rickenbach and M. Sagette; UK: F. A. Post, R. F. Miller, A. Chapman, D. Dockrell, E. Wilkins, G. Cooke, J. Ainsworth, D. Macallen, J. Dhar, N. Vora, S. Mullaney and S. Kegg; Ukraine: G. Kyselyova.
TB:HIV Steering Committee: M. Bruyand, J. Caylá, D. Duiculesku, H. Furrer, E. Girardi, M. H. Losso, J. D. Lundgren, R. F. Miller, J. M. Miro, N. Obel, A. Panteleev (Co-Chair), F. A. Post (Co-Chair), A. Skrahin and J. J. Toibaro.
Statistical centre: L. Shepherd, A. Schultze and A. Mocroft.
Coordinating centre: A. M. Werlinrud Efsen, M. Mansfeld, B. Aagaard, B. R. Nielsen, A. H. Fisher, R. S. Brandt, D. Raben, D. Podlekareva and O. Kirk.
Footnotes
Conflicts of interest
None of the authors has a conflict of interest to declare.
References
- 1.UNAIDS. UNAIDS Report on the Global AIDS Epidemic. Geneva: UNAIDS; 2013. [accessed 02 December 2013]. Available at http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf. [Google Scholar]
- 2.World Health Organization. Global Tuberculosis Report. Geneva: World Health Organization; 2014. [accessed 02 December 2014]. Available at http://www.who.int/tb/publications/global_report/gtbr14_main_text.pdf?ua=1. [Google Scholar]
- 3.Podlekareva DN, Mocroft A, Post FA, et al. HIV/TB Study Writing Group. Mortality from HIV and TB coinfections is higher in Eastern Europe than in Western Europe and Argentina. AIDS. 2009;18:2485–2495. doi: 10.1097/QAD.0b013e3283326879. [DOI] [PubMed] [Google Scholar]
- 4.Dehovitz J, Uuskula A, El-Bassel N. The HIV epidemic in Eastern Europe and Central Asia. Curr HIV/AIDS Rep. 2014;11:168–176. doi: 10.1007/s11904-014-0202-3. [DOI] [PubMed] [Google Scholar]
- 5.Dara M, Dadu A, Kremer K, Zaleskis R, Kluge HH. Epidemiology of tuberculosis in WHO European Region and public health response. Eur Spine J. 2013;4:549–555. doi: 10.1007/s00586-012-2339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dooley KE, Kim PS, Williams SD, Hafner R. TB and HIV therapeutics: pharzmacology research priorities. AIDS Res Treat. 2012;2012:874083. doi: 10.1155/2012/874083. Epub 2012 Jul 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skrahina A, Hurevich H, Zalutskaya A, et al. Multidrug-resistant tuberculosis in Belarus: the size of the problem and associated risk factors. Bull World Health Organ. 2013;91:36–45. doi: 10.2471/BLT.12.104588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Multidrug and Extensively Drug-Resistant TB (M/XDR-TB): 2010 Global Report on Surveillance and Response. Geneva: World Health Organization; 2010. [accessed 12 January 2014]. Available at http://whqlibdoc.who.int/publications/2010/9789241599191_eng.pdf. [Google Scholar]
- 9.World Health Organization. The global plan to stop tuberculosis 2006–2015. Stop TB partnership. Geneva: World Health Organization; 2006. [accessed 12 January 2014]. Available at http://www.stoptb.org/assets/documents/global/plan/GlobalPlanFinal.pdf. [Google Scholar]
- 10.Floyd K, Hutubessy R, Samyshkin Y, et al. Health-systems efficiency in the Russian Federation: tuberculosis control. Bull World Health Organ. 2006;84:43–51. doi: 10.2471/blt.04.018705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimitrova B, Balabanova D, Atun R, Drobniewski F, Levicheva V, Coker R. Health service providers’ perceptions of barriers to tuberculosis care in Russia. Health Policy Plan. 2006;21:265–274. doi: 10.1093/heapol/czl014. [DOI] [PubMed] [Google Scholar]
- 12.Lazarus JV, Olsen M, Ditiu L, Matic S. Tuberculosis-HIV co-infection: policy and epidemiology in 25 countries in the WHO European region. HIV Med. 2008;9:406–414. doi: 10.1111/j.1468-1293.2008.00567.x. [DOI] [PubMed] [Google Scholar]
- 13.Podlekareva DN, Grint D, Post FA, et al. for the HIV-TB Study Group. Health care index score and risk of death following tuberculosis diagnosis in HIV-positive patients. Int J Tuberc Lung Dis. 2013;17:198–206. doi: 10.5588/ijtld.12.0224. [DOI] [PubMed] [Google Scholar]
- 14.Keshavjee S, Gelmanova IY, Pasechnikov AD, et al. Treating multidrug-resistant tuberculosis in Tomsk, Russia: developing programs that address the linkage between poverty and disease. Ann N Y Acad Sci. 2008;1136:1–11. doi: 10.1196/annals.1425.009. [DOI] [PubMed] [Google Scholar]
- 15.Perelman MI. Tuberculosis in Russia. Int J Tuberc Lung Dis. 2000;4:1097–1103. [PubMed] [Google Scholar]
- 16.World Health Organization. WHO Policy on Collaborative TB/HIV Activities: Guidelines for National Programmes and Other Stakeholders. Geneva: World Health Organization; 2012. [accessed 12 January 2014]. Available at http://whqlibdoc.who.int/publications/2012/9789241503006_eng.pdf. [PubMed] [Google Scholar]
- 17.Kerschberger B1, Hilderbrand K, Boulle AM, et al. The effect of complete integration of HIV and TB services on time to initiation of antiretroviral therapy: a before-after study. PLoS ONE. 2012;7(10):e46988. doi: 10.1371/journal.pone.0046988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sylla L, Bruce RD, Kamarulzaman A, Altice FL. Integration and co-location of HIV/AIDS, tuberculosis and drug treatment services. Int J Drug Policy. 2007;18:306–312. doi: 10.1016/j.drugpo.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Havlir DV, Kendall MA, Ive P, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. NEJM. 2011;365:1482–1491. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanc F, Sok T, Laureillard D, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. NEJM. 2011;365:1471–1481. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spire B, Lucas GM, Carrieri MP. Adherence to HIV treatment among IDUs and the role of opioid substitution treatment (OST) Int J Drug Policy. 2007;18:262–270. doi: 10.1016/j.drugpo.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Grenfell P, Baptista Leite R, Garfein R, de Lussigny S, Platt L, Rhodes T. Tuberculosis, injecting drug use and integrated HIV-TB care: a review of the literature. Drug Alcohol Depend. 2013;129:180–209. doi: 10.1016/j.drugalcdep.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Marco A, Caylà JA, Serra M, et al. Predictors of adherence to tuberculosis treatment in a supervised therapy programme for prisoners before and after release. Study Group of Adherence to Tuberculosis Treatment of Prisoners. Eur Respir J. 1998;12:967–971. doi: 10.1183/09031936.98.12040967. [DOI] [PubMed] [Google Scholar]
- 24.Lazarus JV, Hoekstra M, Raben D, Delpech V, Coenen T, Lundgren JD HIV in Europe Initiative Steering Committee. The case for indicator condition-guided HIV screening. HIV Med. 2013;14:445–448. doi: 10.1111/hiv.12022. [DOI] [PubMed] [Google Scholar]
- 25.Lessem E, Keshavjee S. Russia: drug-resistant TB can be contained. Nature. 2014;506:295. doi: 10.1038/506295c. [DOI] [PubMed] [Google Scholar]
- 26.Lönnroth K, Castro KG, Chakaya JM. Tuberculosis control and elimination in 2010–50: cure, care, and social development. Lancet. 2010;375:1814–1829. doi: 10.1016/S0140-6736(10)60483-7. [DOI] [PubMed] [Google Scholar]