Abstract
Objectives
Describe the relationship between genital hiatus (GH) and perineal body (PB) measurements with increasing pelvic organ prolapse stage in a large cohort of women referred to Urogynecology clinic for pelvic floor disorders.
Methods
Retrospective chart review of all new patients seen in an academic Urogynecology clinic between 1/2007 and 9/2011. Data were extracted from a standardized intake form. All patients underwent a Pelvic Organ Prolapse Quantification (POPQ) exam. Descriptive statistics compared the study population. Analysis of variance (ANOVA) was used to compare GH and PB measurements by prolapse stage. Fishers least significant differences was used for post hoc comparisons of means between prolapse stages. Pearson's correlations were used to evaluate the associations between GH and PB measurements and patient characteristics.
Results
1595 women with POPQ exams comprised the study population. The mean age was 55.3 ±14.8 years with a BMI 30.3 ± 7.6 kg/m2, most women were parous (90%), 40% were Hispanic, 33% had undergone prior hysterectomy for indications exclusive of pelvic organ prolapse. Woman with any prior prolapse repair were excluded, 6.5% had a prior incontinence procedure. PB measurements were slightly larger for Stage 2 pelvic organ prolapse (POP), but overall did not vary across other prolapse stages (all P >0.05). In contrast, GH measurements increased through stage 3 POP, GH measurements decreased for stage 4 POP.
Conclusions
Mean PB measurements did not demonstrate large changes over prolapse stage, while GH measurements increased through stage 3 POP. GH serves as an important marker for underlying pelvic muscle damage.
Keywords: Genial Hiatus, Perineal Body, Pelvic Organ Prolapse
Introduction
Measurements of the external genitalia, specifically the perineal body (PB) and the genital hiatus (GH), comprise part of the assessment of prolapse using the Pelvic Organ Prolapse Quantification examination (POPQ)1. A deficient perineal body is thought to contribute to prolapse as the pelvic organs do not have a shelf on which to lie. The perineal body represents level III support as defined by DeLancey2. Increasing GH measurements have been associated with levator ani muscle injury and pelvic organ prolapse on both clinical and ultrasound measurements3-6. A large or gaping GH is sometimes treated with perineorrhaphy to increase perineal body length and decrease genital hiatus size. Conversely, for a GH that is too small, some surgeons may shorten the PB in order to increase the GH7-8.
One prior study demonstrated an association between advanced prolapse stage and an increased GH4, however, that study did not include a description of PB measurements as prolapse advances. Despite the standardized assessment of GH and PB measurements as part of the POPQ evaluation for POP, the association between PB, POP, and pelvic anatomy are poorly understood, as are the interactions between GH and PB measurements.
We sought to describe the relationship between GH and PB measurements with increasing pelvic organ prolapse stage in a large cohort of women referred for subspecialty evaluation for a pelvic floor disorder. We hypothesized that GH measurements increase with increasing stage of POP while PB measurements decrease.
Materials and Methods
At the University of New Mexico, we obtained approval from the Human Research Review Committee (HRRC#: 10-511) to perform a retrospective cohort study consisting of all new patients seen in the Urogynecology clinic at our institution from January 2007 through September 2011. All women underwent a standardized history and physical examination which included a POPQ examination to assess prolapse stage. Our standardized history includes a form that collects the same information from all new patients included pelvic floor disorder symptoms, past medical history, past surgical history and indications, medications and social history. All new patients undergo a POPQ examination, this included assessment of GH and PB during strain as described by Bump et al1. All POPQ measurements were performed under the supervision of fellowship trained urogynecologists. Data were extracted from patient records. All subjects who had a completed medical and surgical history information as well as a POPQ examination were included in the study. Women with a prior prolapse repair were excluded. Patient characteristics were also collected.
Data were analyzed using SAS v9.3 (Cary, NC) and descriptive statistics were performed on this study population. Stages of POP were derived from the POPQ examination. POP stage 0 and stage 1 were combined, as stage 0 had a small sample size compared to the other stages. Analysis of variance (ANOVA) was used for comparisons among means by prolapse stage. If there is an overall significant difference in means by ANOVA then Fisher's least significant differences method of post hoc comparison of these was performed to determine where the differences in means lies. Pearson's correlations were used to evaluate the associations between GH and PB measurements and patient characteristics. Correlation strengths were defined as “very strong” 0.8-1.00, “strong 0.60-.79”, “moderate” 0.40-5.9, “weak” 0.20-0.39, “very weak” 0.00-0.19.9
Results
A total of 1,595 women with recorded POPQ exams but without a history of prior prolapse repair comprised the study population were included in this study. 188 women were excluded for a history of prior prolapse repair surgery and 330 women were excluded for incomplete medical records The mean age and BMI were 55.3 ±14.8 years and 30.3 ± 7.6 kg/m2 respectively. Most women were parous (90%), 36.5% (n=581) were Hispanic, 33% (n=530) had undergone prior hysterectomy and 6.5% (n=104) had a prior incontinence procedure. The majority of subjects had stage 2 POP (50.7%), followed by stage 0,1 POP (19.8%), stage 3 POP (16.3%), and stage 4 POP (13.5%). Patient characteristics are listed in Table 1.
Table 1. Patient Characteristics.
| Patient Characteristics | Mean ± SD N (%) |
|---|---|
| Age (years) | 55.3 ± 14.8 |
| BMI (kg/m2) | 30.3 ± 7.6 |
| Nulliparous | 154 (9.7) |
| Prior hysterectomy | 530 (33.2) |
| Prior anti-incontinence surgery | 104 (6.5) |
| Hispanic | 581 (36.5) |
| Non-Hispanic White | 709 (44.5) |
| American Indian | 177 (11.0) |
| African American | 31 (2.0) |
| Other race | 95 (6.0) |
| Stage 0,1 POP | 315 (19.8) |
| Stage 2 | 809 (50.7) |
| Stage 3 | 255 (16.0) |
| Stage 4 POP | 216 (13.5) |
| GH | 3.6 ± 1.3 |
| PB | 3.3 ± 0.9 |
| BA | -0.5 ± 2.1 |
| BP | -1.1 ± 1.7 |
| C | -5.2 ± 3.9 |
| D | -5.7 ± 4.2 |
Mean PB and GH measurements were calculated for each POP stage. The overall mean PB measurement was 3.34 ± 0.9 cm and mean GH measurement was 3.59 ± 1.3. PB measurements were slightly larger for Stage 2 POP (p <0.01), otherwise did not vary across other prolapse stages. Although there was a statistically significant difference between Stage 2 POP (3.44cm) and all other stages (mean 3.23 cm) it was unlikely to be clinically significant. In contrast, GH measurements increased through stage 3 POP, until stage 4 POP where mean GH measurements were smaller (all p<0.01). (Table 2) Figure 1 demonstrates box plots for the mean GH and PB measurements for increasing stages of POP.
Table 2. PB and GH across POP Stages.
| Stage 0,1 N=314 |
Stage 2 N=807 |
Stage 3 N=254 |
Stage 4 N=216 |
Overall N=1595 |
p value | |
|---|---|---|---|---|---|---|
| PB mean ± SD cm (range) |
3.28 ±0.8a (1, 5) |
3.44 ± 0.9b (0, 6) |
3.22 ± 1.1a (1, 10) |
3.20 ± 0.9a (1, 9) |
3.34 ± 0.9 | <0.01 |
| GH mean ± SD cm (range) |
2.74 ±0.8a (1, 5) |
3.60 ± 1.0b (0, 9) |
4.83 ± 1.3c (2, 10) |
3.4 ± 1.6d (1, 10) |
3.59 ± 1.3 | <0.01 |
Fisher's least significant difference is indicated by superscripts a, b, c, d. Means with different letters are significantly different
Figure 1. Boxplot of PB and GH across pelvic organ prolapse stages.
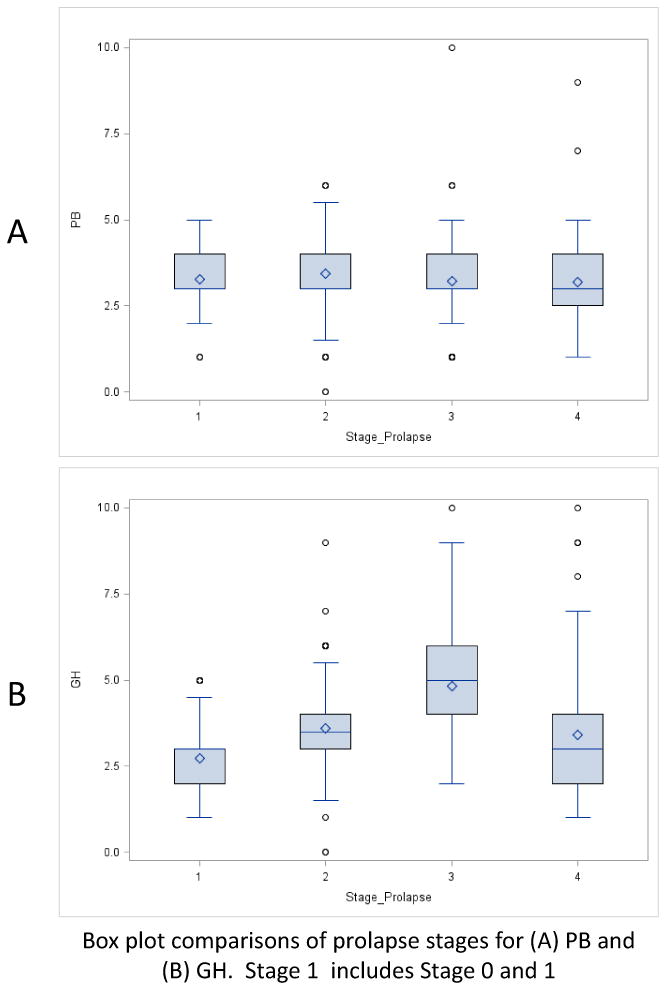
We performed a multivariate analysis of factors that may affect GH and PB measurements, including parity, hysterectomy status and prior anti-incontinence surgery. Both parity and hysterectomy status were significant and were then analyzed separately. When the data was separated into parous versus non-parous women, there were no significant finds for PB measurements comparing nulliparous versus parous women, except that in parous women PB followed the same pattern of the overall group, with PB significantly larger for stage 2 but unlikely to be clinically relevant. GH in the nulliparous group did not change significantly across prolapse stage, whereas GH for the parous groups followed the same pattern as the overall group. Interestingly, the mean GH was larger in the parous group for each prolapse stage. (Table 3) We then analyzed the data in relation to hysterectomy status. GH measurements were different between Stage 2 and 4, with women without a prior hysterectomy having larger GHs. However across prolapse stage the same pattern was seen as the overall group with GH increasing until Stage 4 in which a decrease was seen. Women in the Stage 0,1 group had significantly smaller PB measurements. PB findings across stages were mixed and are presented in Table 4. For stage 2 prolapse we evaluated the actual length, or most distal aspect of the prolapse (-1, 0, +1). The data followed the same pattern seen with increasing GH measurements with advancing prolapse. Most distal measurement -1, (GH 3.2 ±0.93), most distal measurement 0 (GH 3.8 ±0.90) and most distal measurement +1 (GH 4.0 ± 0.9) all p <0.01. There were no significant changes among PB measurements (3.4, 3.5, 3.4 respectively) p=0.08
Table 3. PB and GH with Nulliparous versus Parous across POP Stages.
| Parity Nulliparous = 154 Parous =1437 |
Stage 0,1 N=314 |
Stage 2 N=807 |
Stage 3 N=254 |
Stage 4 N=216 |
p value across POP stage | |
|---|---|---|---|---|---|---|
| PB mean ± SD cm (range) |
Nulliparous | 3.3 ±0.86 | 3.5 ±0.64 | 3.8 ±0.89 | 3.2 ±0.78 | 0.76 |
| Parous | 3.3a ±0.84 | 3.4b ±0.89 | 3.2b ±1.07 | 3.2b ±0.95 | ||
| p value between parity groups | 0.76 | 0.92 | 0.19 | 0.83 | ||
| GH mean ± SD cm (range) |
Nulliparous | 2.4 ±0.64 | 2.8 ±0.79 | 2.8 ±0.75 | 2.6 ±1.70 | <0.01 |
| Parous | 2.8b ±0.87 | 3.6b ±0.96 | 4.9c ±1.29 | 3.5b ±1.57 | ||
| p value between parity groups | <0.01 | <0.01 | <0.01 | 0.03 |
Fisher's least significant difference is indicated by superscripts a, b, c, d. Means with different letters are significantly different
Table 4. PB and GH with and without a hysterectomy across POP Stages.
| Hysterectomy Status No hysterectomy N=1061 Hysterectomy N=530 |
Stage 0,1 N=314 |
Stage 2 N=807 |
Stage 3 N=254 |
Stage 4 N=216 |
p value across POP stage | |
|---|---|---|---|---|---|---|
| PB mean ± SD cm (range) |
No Hysterectomy | 3.2a,b ±0.85 | 3.4a ±0.91 | 3.2a,b ±1.11 | 3.1b ±0.98 | <0.01 |
| Prior Hysterectomy | 3.4a,b ±0.82 | 3.5a ±0.82 | 3.2c ±0.93 | 3.2b,c ±0.99 | <0.01 | |
| p value between hysterectomy groups | 0.04 | 0.27 | 0.67 | 0.62 | ||
| GH mean ± SD cm (range) |
No Hysterectomy | 2.7a ±0.83 | 3.6b ±0.95 | 4.9c ±1.34 | 4.0d ±2.10 | <0.01 |
| Prior Hysterrectomy | 2.7a ±0.89 | 3.5b ±1.00 | 4.8c ±1.23 | 3.2d ±1.34 | <0.01 | |
| p value between hysterectomy groups | 0.85 | 0.02 | 0.59 | 0.01 |
Fisher's least significant difference is indicated by superscripts a, b, c, d. Means with different letters are significantly different
Pearson correlation coefficients demonstrated a strong correlation between GH, Ba (r=0.61, p<0.01), moderate correlations for Bp (r=0.46, p<0.01), and C (r=0.42, p<0.01), and weak correlation between GH, age, BMI, parity, and D. PB demonstrated weak to very weak correlations only to all of the variables assessed.. All correlations are reported in Table 5.
Table 5. Correlations of Patient Characteristics and PB and GH Measurements.
| Patient Characteristic | PB r (p value) |
GH r (p value) |
|---|---|---|
| Age | -0.03 (0.24) | 0.04 (0.08) |
| BMI | 0.11 (<0.01) | 0.08 (<0.01) |
| Parity | -0.05 (0.05) | 0.27 (<0.01) |
| Ba | -0.09 (<0.01) | 0.61 (<0.01)* |
| C | -0.08 (<0.01) | 0.42 (<0.01)§ |
| Bp | -0.06 (0.03) | 0.46 (<0.01)§ |
| D | -0.05 (0.05) | 0.32 (<0.01) |
Strong correlation
Moderate correlation
Given the unexpected finding of GH measurements decreasing with stage 4 POP, the correlations were reassessed without stage 4 data, however, this did not result in any changes to the correlations.
Discussion
We found that as prolapse increases, GH measurements also increase until Stage 4 prolapse, where mean GH decreased. This is in contrast to PB measurements which exhibited little change with advancing prolapse. Our findings support that GH measurements vary with prolapse, but changes in GH are not associated with concurrent changes in PB measurements. It is unclear why mean GH measurements decreased in this cohort with very advanced prolapse; it may be that once the pelvic organs have completely protruded beyond the GH that the pressure on the genital hiatus is diminished, and thus, its size likewise decreases. Alternatively, it may be that the number of women with stage 4 prolapse in this study was small and our observation is spurious. Finally, measurement of the GH in women with Stage 4 prolapse can be challenging since the prolapsed organs often obscure the genital opening. We had hypothesized that the PB would decrease as GH increased with advancing stage of prolapse, but found little relationship between the two measures. This calls into question the common surgical practices of altering the perineal body in order to impact genital hiatus size.
Others have found an association between enlarged GH, increased prolapse, and levator ani muscle anatomy3-7, 10-13. Several of these studies were performed on a general gynecologic population.10-11 Regardless, this suggests that an enlarged GH may indicate underlying levator ani muscle damage. One group reported that a GH + PB of ≥ 8.5 cm could help identify women with levator avulsion.12 DeLancey et al found that the urogenital hiatus, which was determined by palpation of the hiatus and the use of a ruler, was increased in women with prolapse compared to women without POP. However, that study used the Baden Walker classification system for POP instead of the POPQ examination so PB measurements were not reported for any of the 28 women in that study.3 Further work by this group compared the presence of levator ani defects, as determined by MRI, in women with and without POP. They found that women with prolapse were more likely to have levator ani defects or injury noted on MRI than women without POP. They also noted that women with POP had larger GH measurements than those women without POP.6 Similar to the results presented here, Ghetti et al4 assessed severity of POP relative to levator hiatus size and function in a large cohort of women. They found prolapse severity was positively correlated with GH but not PB, however they did not provide any specific information on PB measurements. Interesting, the authors also found that GH increased with increased stage of POP until Stage 3 without additional change for stage 4 prolapse. Similar findings of increased GH with increased prolapse were recently reported by Lowder et al. They found a GH of >3.75 to be strongly associated with apical prolapse.13 In contrast, the information contained in this current work is on a larger cohort of women and found a decrease in GH measurements from Stage 3 to Stage 4 POP. In addition, we report detailed information on PB size with respect to increasing stages of POP, and demonstrate no clinically significant change in PB with advancing prolapse. Further sub-analysis revealed interesting information related to parity and hysterectomy status. Neither PB or GH changed significantly with increasing prolapse stage in nulliparous women. However, in parous women a similar pattern was observed as the overall group with increasing GH until stage 4 in which there was no difference compared to stage 2. Furthermore, women without prior hysterectomies had larger GH measurements for stage 2 and 4 prolapse compared to women with prior hysterectomies, although overall the pattern of GH across prolapse stage was the same in the overall group regardless of hysterectomy status. The findings of PB measurements with consideration of hysterectomy status were mixed. Women with stage 0, 1 prolapse and no prior hysterectomy had larger PB measurements compared to those with prior hysterectomy. Similar changes in PB size were noted across the prolapse stages irrespective or prior hysterectomy status, these changes were statistically significant but do seem to be clinically relevant. These findings demonstrate that parity and hysterectomy may contribute to pelvic floor muscle damage and pelvic organ prolapse.
The strengths of this study include the large number of subjects and the standardized method of collecting medical history and POPQ examinations. All POPQ values were collected by individuals well-trained in obtaining POPQ measurements. Limitations of this study include those inherent in its retrospective nature. As with all retrospectively designed studies, causation cannot be remarked upon. We also excluded women with a known history of prior surgery for POP but prior POP surgery was obtained by patient self-report and it is possible women may not have accurately remembered the details of their prior surgical history. However, the large numbers in this study should mitigate this effect. In addition, we assessed the effect of nulliparity versus parity on GH and PB measurements but did not collect data on those that had cesarean delivery and can therefore not comment on this effect either.
In conclusion, mean PB measurements did not demonstrate any clinically significant changes relative to prolapse stage, while GH measurements increased through stage 3 POP. GH appears to be marker for underlying pelvic muscle damage, however PB does not. Perineorrhaphy is often performed for what is felt to be deficient PB or to reduce the size of the GH, however, it is unclear if this is a helpful or necessary component to prolapse repair. These findings highlight an area of needed future study, including what the effect perineorrhaphy has on GH and PB and its effect on recurrence of POP and pelvic floor function.
Acknowledgments
Funding: Supported by a pilot grant from the Clinical and Translational Science Center at the University of New Mexico, National Center for Research Resources and the National Center for Advancing Translational Sciences through grant number UL1-RR031977.
Financial Disclaimers/Conflict of Interest: Gena C Dunivan, MD receives research support from Pelvalon, Inc. Rebecca G Rogers, MD is DSMB chair for the TRANSFORM trial sponsored by American Medical Systems and receives royalties from UpToDate and McGraw Hill.
Footnotes
Presentation Information: Poster presentation at the Society of Gynecologic Surgeons 41st Annual Scientific Meeting in Orlando, FL. March 22-25, 2015.
Authorship Contribution: G Dunivan: Protocol/project development, Data collection, Data Analysis, Manuscript writing
K Lyons: Data Analysis, Manuscript writing
P Jeppson: Protocol/project development, Manuscript editing
C Ninivaggio: Protocol/project development, Manuscript editing
Y Komesu: Protocol/project development, Manuscript editing
F Alba: Protocol/project development, Manuscript editing
R Rogers: Protocol/project development, Manuscript editing
Contributor Information
Gena C Dunivan, Division of Urogynecology, Department of OBGYN, University of New MexicoAlbuquerque, NM
Katherine E Lyons, Department of OBGYN, University of New Mexico, Albuquerque, NM.
Peter C Jeppson, Division of Urogynecology, Department of OBGYN, University of New Mexico, Albuquerque, NM.
Cara S Ninivaggio, Division of Urogynecology, Department of OBGYN, University of New Mexico, Albuquerque, NM.
Yuko M Komesu, Division of Urogynecology, Department of OBGYN, University of New Mexico, Albuquerque, NM.
Frances M Alba, Division of Urology, Department of Surgery, University of New Mexico, Albuquerque, NM.
Rebecca G Rogers, Division of Urogynecology, Department of OBGYN, University of New Mexico, Albuquerque, NM
References
- 1.Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10–7. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 2.DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166:1717–24. doi: 10.1016/0002-9378(92)91562-o. [DOI] [PubMed] [Google Scholar]
- 3.DeLancey JO, Hurd WW. Size of the urogenital hiatus in the levator ani muscles in normal women and women with pelvic organ prolapse. Obstet Gynecol. 1998;91(3):364–8. doi: 10.1016/s0029-7844(97)00682-0. [DOI] [PubMed] [Google Scholar]
- 4.Ghetti C, Gregory W, Edwards S, et al. Severity of pelvic organ prolapse associated with measurements of pelvic floor function. Int Urogynecol J. 2005;16:432–436. doi: 10.1007/s00192-004-1274-1. [DOI] [PubMed] [Google Scholar]
- 5.Dietz HP, Simpson JM. Levator trauma is associated with pelvic organ prolapse. BJOG. 2008;115:979–984. doi: 10.1111/j.1471-0528.2008.01751.x. [DOI] [PubMed] [Google Scholar]
- 6.DeLancey JO, Morgan DM, Fenner DE, et al. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol. 2007;109:295–302. doi: 10.1097/01.AOG.0000250901.57095.ba. [DOI] [PubMed] [Google Scholar]
- 7.Kanter G, Jeppson P, McGuire B, et al. Perineorrhaphy: commonly performed yet poorly understood. A survey of surgeons. Int Urogynecol J. 2015;26(12):1797–1801. doi: 10.1007/s00192-015-2762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haylen BT, Younis M, Naidoo S, et al. Perineorrhaphy quantitative assessment (Pe-QA) Int Urogynecol J. 2015 Apr;26(4):539–44. doi: 10.1007/s00192-014-2528-1. [DOI] [PubMed] [Google Scholar]
- 9.Evens J. Straightforward Statistics for the Behavioral Sciences. Belmont, CA: Brooks/Cole Publishing Company; 1996. [Google Scholar]
- 10.Kahn M, Breitkopf C, Valley M, et al. Pelvic Organ Support Study (POSST) and bowel symptoms; Straining at stool is associated with perineal and anterior vaginal descent in a general gynecologic population. Am J Obstet Gynecol. 2005;192:1516–22. doi: 10.1016/j.ajog.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 11.Swift S, Woodman P, O'Boyle A, et al. Pelvic Organ Support Study(POSST): The distribution, clinical definition, and epidemiologic condition of pelvic organ support defects. Am J Obstet Gynecol. 2005;192:795–806. doi: 10.1016/j.ajog.2004.10.602. [DOI] [PubMed] [Google Scholar]
- 12.Volloyhaug I, Wong V, Shek K, et al. Does levator avulsion cause distension of the genital hiatus and perineal body? Int Urogynecol J. 2013;24:1161–1165. doi: 10.1007/s00192-012-1993-7. [DOI] [PubMed] [Google Scholar]
- 13.Lowder J, Oliphant S, Shepherd J, et al. Genital hiatus size is associated with and predictive of apical vaginal support loss. Am J Obstet Gynecol. 2016 Jun;214(6):718.e1–8. doi: 10.1016/j.ajog.2015.12.027. [DOI] [PubMed] [Google Scholar]


