Abstract
For pathogenic bacteria, the ability to sense and respond to environmental stresses encountered within the host is critically important, allowing them to adapt to changing conditions and express virulence genes appropriately. This review considers the diverse molecular mechanisms by which stress conditions are sensed by bacteria, how related signals are discriminated and how stress responses are integrated, highlighting recent studies in selected bacterial pathogens of clinical relevance.
“My senses get no rest-- but suffer from a constant strain.”
- Henry David Thoreau
Introduction
Pathogenic bacteria encounter a broad range of stress conditions within host microenvironments, including oxidative or nitrosative stress, acid pH, hypoxia, nutrient limitation and envelope damage (see accompanying review in this issue, Fang et al., 2016). In order to mount appropriate adaptive responses, these bacteria must be able to recognize and distinguish specific stresses. The exquisite ability of bacteria to discriminate stress conditions is illustrated by molecular oxygen (O2) and nitric oxide (NO·), whose molecular orbitals differ by a single unpaired electron. The subtle difference in the electronic configuration of the π antibonding molecular orbitals of O2 and NO· can be detected by dedicated sensors that elicit distinct responses tailored to the specific molecular signal. Alternatively, microorganisms may employ general stress responses that can be triggered by multiple environmental signals. For example, transcriptional, translational and post-translational regulation of the alternative sigma factor σS activates this general stress program in response to diverse stress signals. When multiple redundant or conflicting responses are received, bacteria must also integrate these signals and elicit the most appropriate response. This review discusses some of the best-understood molecular mechanisms that allow pathogenic bacteria to discriminate among stress signals, the signaling networks that transmit these signals, and the mechanisms by which multiple signals are integrated to regulate the expression of stress-resistance pathways and virulence genes.
Bacterial Sensing Motifs
Sensory molecules utilize a range of motifs to discriminate among the panoply of signals encountered by bacteria during their associations with hosts. These motifs include thiol groups in redox-active cysteines, ionizable groups in histidine, aspartic and glutamic acid residues, metal cofactors, protein domains and secondary structures in RNA molecules. Sensing motifs can be situated within transcription factors, sensor-kinases of two-component systems (TCS), alternative sigma factor regulatory cascade components, and non-coding or untranslated regions of RNA.
Thiols
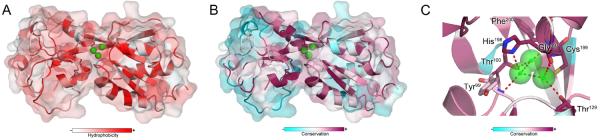
Cysteine residues participate in disulfide formation, electron donation, hydrolysis, metal binding, and redox catalysis and sensing. The redox state of cysteine thiol groups ranges from −2 to +6, making thiols some of the most versatile and widely utilized bacterial sensors of oxidative and nitrosative stress (Vazquez-Torres, 2012). Oxidizable thiols often occupy hydrophobic pockets and have low pKa values due to hydrogen-bonding with vicinal charged residues. Based on the mechanism of action of peroxidases, bacterial cysteine-based sensors of H2O2, such as the broadly conserved OxyR or OhrR homologs of Staphylococcus aureus, Pseudomonas aeruginosa and Xanthomonas campestris, are thought to rely on nucleophilic attack of the ionized thiolate group (-S−) to the peroxo bond (-O-O-) of biologically relevant inorganic and organic hydroperoxides (ROOH) (Vazquez-Torres, 2012). The sulfenic acid generated in this reaction tends to be a short-lived intermediate, giving rise to more stable disulfides, mixed disulfides or sulfonamides (-SN-). However, the recently determined structure of OxyR from P. aeruginosa (PaOxyR) has suggested an alternative mechanism for how thiols sense H2O2 (Jo et al., 2015) (Figure 1). The peroxidatic Cys199 in PaOxyR is located in a conserved hydrophobic pocket (Figure 1A,B). Three H2O molecules make contact with the backbone and side-chains of conserved residues, including Cys199 (Figure 1C). Incoming H2O2 replaces one of the H2O molecules bound in the proximity of peroxidatic Cys199. Nucleophilic attack of Cys199-SH to the proximal OA atom of H2O2 produces a sulfenic acid and an OBH− anion that, with the help of nearby H2O molecules, accepts the proton from the Cys199 thiol group. Highly conserved residues contact the three H2O molecules, suggesting that this mechanism is preserved among OxyR orthologs. The simultaneous deprotonation of the thiol, cleavage of the peroxo group and release of H2O might explain why the peroxidatic reaction of PaOxyR occurs with a rapid second order rate constant of 10−5 M−1 sec−1 (Aslund et al., 1999).
Figure 1. Sensing of H2O2 by OxyR.
The Pseudomonas aeruginosa OxyR structure suggests a role for H2O molecules in H2O2 sensing. (A) Peroxidatic Cys199 is in a hydrophobic pocket of OxyR. Hydrophobic and hydrophilic residues are shown in red and white, respectively. Panel B shows the structure of PaOxyR with the conserved residues forming the pocket where the three H2O molecules reside. Conserved amino acids (purple) were identified using the ConSurf server with input of 75 OxyR orthologs identified by PSI-BLAST in the SWISS-PROT protein sequence database (Celniker et al., 2013; Ashkenazy et al., 2010). (C) Three H2O molecules (green spheres) establish a network of hydrogen bonding with amino acid residues in the vicinity of Cys199. Red dashes show putative hydrogen-bonding of H2O with the backbone, and side-chains of conserved residues. The H2O molecule most proximal to Cys199 is replaced by H2O2. The two H2O molecules remaining in the pocket aid in the protonation of a leaving OH− group arising from peroxidatic cleavage of H2O2 by the Cys199-reduced thiol. Residues interacting with the three H2O molecules are highly conserved (purple) in OxyR, suggesting a common mechanism of H2O2 sensing among OxyR orthologs. PDB code 4Y0M was used for PyMol analysis.
In addition to directly sensing reactive species, thiols are scaffolds on which redox-active [Fe-S] clusters (Crack et al., 2013) and zinc fingers with dual structural and redox-sensing activities (Henard et al., 2014) are assembled. For example, the fumarate-nitrate reduction regulator FNR, which controls the response to anaerobic conditions in many bacterial enteropathogens, employs a redox-active [4Fe-4S] cluster coordinated by cysteine residues, while the regulator NsrR, which is a common sensor and regulator of the bacterial NO response, uses a [4Fe-4S] cluster coordinated by three cysteines and a glutamate (Crack et al. 2015, Tucker et al. 2008). Iron in [4Fe-4S] clusters directly interacts with O2, O2.−, or NO· (see below). Yet in other instances, metals such as zinc do not sense reactive species but rather modulate the reactivity of coordinating cysteines, as is the case for the four cysteine zinc-finger motif of the stringent response regulator DksA (Henard et al. 2015; Crawford et al, 2016). Oxidation or S-nitrosylation of DksA thiols triggers the release of zinc, formation of disulfide bonds, and the loss of α-helical structure. Together, these conformational changes in DksA cause the downregulation of translational machinery in response to oxidative and nitrosative stress.
Metal Centers
Bacterial regulators use the redox properties of iron to sense the diatomic molecules O2, O2·−, NO· and CO, as well as redox-cycling drugs. The metalloproteins PerR in Gram-positive bacteria, DosT in mycobacteria, and NsrR in eubacteria utilize mononuclear iron, heme groups, or [Fe-S] clusters as cofactors, respectively. The mechanisms of sensing oxidative or nitrosative stress differs amongst these metalloregulators. Iron in PerR acts as a Fenton catalyst that reduces H2O2. Hydroxyl radical produced in situ irreversibly oxidizes His37 to oxo-histidine, thereby derepressing the PerR regulon (Ji et al., 2015; Lee and Helmann, 2006). Ferrous iron in heme prosthetic groups of bacterial sensors directly binds to NO·, CO or O2 in penta- or hexa-coordinated geometries that promote signal transduction. [4Fe-4S] clusters coordinated by 4 cysteine residues react differently with NO·, O2, O2·−, or redox-cycling compounds. Reaction of NO· with [4Fe-4S] clusters, such as those found in FNR of Gram-negative bacteria and the WhiB transcription factors of mycobacteria and streptomycetes entails binding of 8 NO· molecules in a multiphasic reaction that produces a pair of dinuclear dinitrosyl iron complexes known as Roussin's red ester (Crack et al., 2011; Crack et al., 2013). [4Fe-4S] clusters can also serve as extremely sensitive O2 sensors. Oxidation of the [4Fe-4S] cluster in dimeric FNR by O2 proceeds through a transient [3Fe-4S] intermediate that yields inactive [2Fe-2S] monomeric protein (Crack et al., 2014). Spontaneous oxidation of [4Fe-4S] clusters by O2 may have contributed to the initial characterization of the NO· sensor NsrR as a [2Fe-2S] metalloprotein rather than a [4Fe-4S] protein (Crack et al. 2015). A [2Fe-2S] cluster is, nevertheless, present in SoxR, a transcriptional regulator in α, β, γ, and δ-proteobacteria that for many years was believed to function as an O2.− sensor, but is now considered a sensor of redox-cycling compounds such as viologens, quinones or phenazines (Gu and Imlay, 2011; Lee et al., 2015).
PDZ, PAS, GAF and HAMP Domains
Bacterial regulatory proteins use a variety of conserved domains to sense diverse signals (Galperin et al., 2001). The PDZ (Post synaptic density protein, Drosophila disc large tumor suppressor, Zonula occludens-1 protein) (Hizukuri et al., 2014) domain, consisting of five to six β-strands and two to three α-helices, typically recognizes a few amino acid residues at the C-termini of target proteins. The DegS and RseP proteases, which function in the regulatory cascade controlling the activation of the alternative sigma factor σE (see below), contain PDZ domains that regulate their proteolytic activity, thus controlling the subsequent expression of downstream genes in response to extracytoplasmic stress and the associated accumulation of unfolded outer membrane proteins (Walsh et al., 2003; Hasselblatt et al., 2007; Hizukuri et al., 2014). PAS (Per Arnt Sim) and GAF (cGMP-specific phophodiesterases, Adenylyl cyclases and FhlA) domains, with their characteristic α/β folds, sense signals as diverse as O2, metabolites and redox potential. Most bacterial PAS-domain-containing proteins are part of the TCS family of sensor-kinases (Taylor and Zhulin 1999). Both PAS and the structurally related GAF domains can incorporate different cofactors, likely contributing to their sensory specificity. Binding of a signaling molecule to particular amino acids or cofactors in PAS and GAF domains leads to a protein conformational change that converts the sensor-kinase to an active form. Heme groups of the O2 receptors FixL and DosT are ligated to PAS and GAF domains, respectively, whereas a PAS-like fold in PhoQ and EvgS responds to antimicrobial peptides, divalent cations, or acid pH (Cho et al., 2006; Johnson et al., 2014). PhoQ also responses to acid stress, and the mechanism has been characterized in detail. PhoQ is an integral membrane protein with a periplasmic sensing domain, two transmembrane segments, and HAMP (Histidine kinases, Adenyl cyclases, Methyl-accepting proteins, and Phosphatases), catalytic and DHp (Dimerization and Histidine phosphotransfer) cytoplasmic domains. Cations that bridge cytoplasmic-membrane phospholipids and an acidic patch at helices α4 and α5 in the periplasmic domain of dimeric PhoQ maintain the sensor-kinase in an inactive configuration. Acid pH disrupts the hydrogen bond network and electrostatic interactions between the PhoQ acidic patch, His157 imidazole group, and the bridging divalent cations (Prost et al., 2007). The resulting conformational changes activate PhoQ signaling. Acid pH can independently activate PhoQ through allosteric interactions with the adaptor protein SafA. Protonation of a histidine residue in the PAS domain in the periplasmic portion of the EvgS sensor kinase stimulates binding of the SafA adaptor protein to the PAS domain of PhoQ (Johnson et al., 2014). HAMP domains are usually involved in transduction of signals generated by upstream domains. However in the case of the osmosensor EnvZ, the cytoplasmic HAMP domain appears to sense osmolytes directly; increasing osmolarity induces EnvZ kinase activity though gains in α-helicity in the coiled-coil four-helix bundle (Wang et al., 2012). PhoQ and EnvZ, along with their cognate response-regulator partners PhoP and OmpR, play essential roles in the virulence of Salmonella and other pathogens (Fields et al., 1989; Miller et al., 1989; Dorman et al., 1989; Hicks et al., 2015).
RNA Regulators
Specific motifs in the 5'-UTR of certain mRNAs can act as thermometers to regulate gene expression in response to temperature (Righetti and Narberhaus, 2014). Stable hairpin structures that block access to the translational start site at low temperature become destabilized at higher temperatures. Cis-acting motifs in the 5'-UTR of mRNAs or in non-coding RNAs can also sense metals and small metabolite ligands. Such structures known as riboswitches regulate metabolism and virulence by altering mRNA secondary structure to block ribosome access or induce early transcription termination. Examples of riboswitches include the 5'-UTR of the mgtA mRNA that controls translation of a high affinity Salmonella enterica Mg2+ transporter according to Mg2+ availability (Cromie et al., 2006) and non-coding regulatory RNA molecules that control expression of propanediol and ethanolamine catabolism genes in Listeria monocytogenes and Enterococcus faecalis in response to adenosyl cobalamine (DebRoy et al., 2014; Mellin et al., 2014). Although mgtA is not essential for S. enterica virulence, perhaps due to the presence of other magnesium transporters (Moncrief and Maguire, 1999), the L. monocytogenes riboswitches appear to play an important role in coordinately regulating nutrient acquisition and intracellular virulence gene expression (Oliva et al., 2015).
The last decade has witnessed a growing appreciation for the importance of small (50–400 nucleotide) non-coding RNA molecules (sRNAs) in the regulation of gene expression (Vogel, 2009). S. enterica expresses 280 sRNAs during intracellular growth (Srikumar et al., 2015). Although much remains to be learned about their function, recent investigations have shown that the sRNA PinT regulates S. enterica virulence gene expression and host-cell JAK-STAT signaling (Westermann et al., 2016). Various sRNAs have been implicated in bacterial responses to nutrient deprivation, acid pH, oxidative stress, osmolar stress and hypoxia, as well as in the pathogenesis of such diverse organisms as Brucella abortus, Chlamydia trachomatis, Enterobacteriaceae, Francisella novicida, Legionella pneumophila, L. monocytogenes, P. aeruginosa, S. aureus and Streptococcus pyogenes (Padalon-Brauch et al., 2008; Vogel, 2009; Michaux et al., 2014; Oliva et al., 2015). Small RNAs typically downregulate gene expression, but examples of positive regulation have also been described (Frohlich and Vogel, 2009). Many sRNAs interact with proteins, in particular the RNA chaperone Hfq (Chao et al., 2012) and the carbon-storage regulator CsrA (Vakulskas et al., 2015). Hfq facilitates the interaction of sRNAs with their mRNA targets, and its central importance is illustrated by the many bacterial species in which an hfq mutation attenuates growth and virulence, including Francisella tularensis, L. pneumophila, L. monocytogenes, Neisseria gonorrhoeae and S. enterica (Oliva et al., 2015). In contrast, the CsrA protein binds to mRNAs, thereby influencing their translation, turnover and/or elongation, and is antagonized by the binding of specific sRNAs such as CsrB/CsrC (Vakulskas et al., 2015).
Signaling Mechanisms
Conformational changes that regulate protein function in response to stress can be induced by a variety of mechanisms, including modification of thiol groups, protonation of imidazole groups, binding of diatomic gases to heme cofactors, 1e− oxidation of [Fe-S] clusters, or the presence of misfolded proteins in the periplasm. This section examines examples of structural changes that mediate signal transduction in sensors of oxidative/nitrosative stress, two-component systems and alternative sigma factors.
Sensors of Oxidative or Nitrosative Stress
The H-NOX protein of Shewanella oneidensis (SoH-NOX) is an NO· sensor that stimulates biofilm formation by regulating the levels of cyclic diguanosine monophosphate. The structures of SoH-NOX in the hexa-coordinated intermediate and the NO·-active penta-coordinated heme have revealed the mechanism by which binding of NO· to heme Fe2+ transduces signaling (Figures 2A,B). In the inactive conformation, van der Waals interactions of Iso5 and Pro116 in the N- and C-terminal subdomains of SoH-NOX maintain the heme in a bent configuration. Binding of NO· to Mn2+-loaded SoH-NOX produces a hexa-coordinated intermediate in which the bonding distance between the metal and His103 is stretched. Rupture of the His103-metal covalent bond gives rise to a relaxed penta-coordinate NO·-Fe2+ species with its heme in planar geometry (Giardina et al., 2008; Herzik et al., 2014). Dissociation of the His103-coordinating Fe2+ acts as a spring that transmits potential energy stored in the porphyrin ring (Figure 2B), rotating the αF helix where His103 is located and driving structural changes in the proximal and distal subdomains associated with the active form of the protein. The mechanistic insights revealed in SoH-NOX are likely generalizable to orthologs in bacterial pathogens. Binding of NO· to the heme prosthetic group of L. pneumophila H-NOX regulates cyclic-di-GMP metabolism and biofilm formation (Carlson et al., 2010).
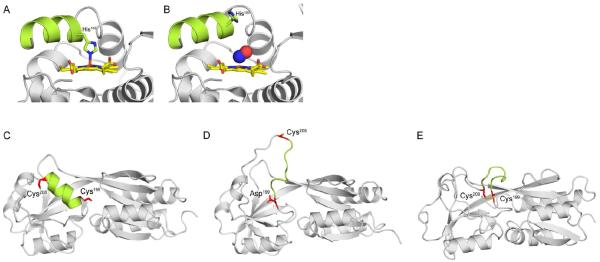
Figure 2. Mechanisms of signal transduction in response to NO· and H2O2.
(A) Reduced H-NOX from Shewanella oneidensis with the penta-coordinated heme (yellow) bound to His103. (B) Binding of NO· (blue and red spheres) to the H-NOX heme severs the bond between His103 and Fe2+. Free His103 rotates the αF helix (green), activating signal transduction. Recent structures of the H2O2 sensor OxyR from Pseudomonas aeruginosa have revealed the molecular mechanism of sensing and signal transduction in response to H2O2. (C) Peroxidatic Cys199 and Cys208 are 15–17Å apart in opposite ends of an α-helix (green). (D) Oxidation of Cys199 by H2O2 produces a sulfenic acid (-SOH) intermediate that is mimicked by a C199D mutation. Oxidation of Cys199 triggers new hydrogen-bonding interactions between the sulfenylated group and Arg201 and Phe200 (not shown). (E) The new hydrogen-bonding disorganizes the intervening α-helix, increasing the reactivity of Cys208 and facilitating formation of a disulfide. (C) and (D) show structures of reduced and OxyR C199D proteins from P. aeruginosa, whereas (E) shows oxidized OxyR from Escherichia coli. PDB codes 4U99, 4U9B, 4Y0M, 4XWS, and 1IBA were used to generate the images in panels (A–E), respectively.
Sensing of reactive oxygen species by OxyR contributes to bacterial pathogenesis (Fang et al., 2016), and OxyR has served as model system to study the molecular mechanisms by which this sensing is translated into regulatory outputs. Sulfenylation of the thiolate side chain of Cys199 is a key event in the formation of an intramolecular disulfide with Cys208. Disulfide bond formation stabilizes massive conformational changes in the OxyR tetramer (Zheng et al., 1998). As Cys199 and Cys208 are separated by 17Å by an α-helix in the E. coli protein (Figure 2C), a long-standing question in the field has been how these residues are rearranged to form a disulfide bond. The recent structure of a P. aeruginosa OxyR C199D variant, representing the Cys199 sulfenylated intermediate, has provided insights into the mechanism by which disulfide bond formation takes place (Jo et al., 2015). The Od1 atom of Asp199, representing the -OH group of sulfenylated Cys199, hydrogen-bonds with the amide and carbonyl backbone groups of Arg201 and Phe200. These new hydrogen bonds displace Phe200 in the intercysteine α-helix to form a new hydrophobic pocket in the protein. Consequently, the intercysteine α-helix is destabilized (Figure 2D), increasing both Cys208 reactivity and providing opportunities for interaction between Cys199-SOH and Cys208-SH groups to form an intramolecular disulfide (Figure 2E).
Two-Component Systems
As described above, the functional versatility of PAS sensor domains is remarkable. The PAS domains of ArcB and PhoQ are two interesting examples of this diversity. Reduction of cysteine thiols in the PAS domain of the ArcB sensor-kinase, which helps regulate carbon and electron flow in response to oxygen availability and the redox state of the membrane quinone pool, activates autophosphorylation and subsequent phosphorylation and activation of the ArcA response regulator. Conversely, oxidation of the thiols forms a disulfide bond with resulting inhibition of kinase activity (Malpica et al., 2004). In contrast, the PAS domain of the dimeric PhoQ sensor-kinase forms a flat surface that interacts with phospholipids of the outer leaflet of the cytoplasmic membrane via divalent cation bridges (Bader et al., 2005). Disruption of metal bridges between the PhoQ acidic surface and the negatively-charged membrane leads to a scissoring movement of the PhoQ sensor domain. The outward pulling of the PhoQ sensor domain triggers rotation and repositioning of the transmembrane four-helix bundle, an event that is transmitted downstream to the cytoplasmic HAMP domain (Molnar et al., 2014). As histidine kinases are regulated by the position of the catalytic domain rather than the accessibility of the phosphoacceptor histidine residue, conformational changes in the HAMP domain disrupt interactions of the catalytic domain with both HAMP and DHp domains, possibly adopting a kinase competent conformation (Matamouros et al., 2015). Acting as general base, an acidic and polar residue deprotonates the phosphoacceptor histidine in the DHp domain, a critical step that facilitates attack of ATP γ-P in the autophosphorylation reaction (Casino et al., 2014).
Alternative Sigma Factors
Sigma factors are components of RNA polymerase required for the initiation of transcription. In addition to the “housekeeping” sigma factors that control the expression of most genes, bacteria have alternative sigma factors responsible for the regulation of specific gene subsets under particular conditions. S. enterica possesses five alternative sigma factors, two of which (σE, σS) have been directly implicated in virulence (Fang et al., 2016).
σE is a so-called extracytoplasmic function (ECF) sigma factor because it responds to stress conditions affecting the cell envelope. A proteolytic cascade involving two PDZ domain-containing proteases allows activation of the σE regulon in response to the accumulation of misfolded periplasmic proteins (Walsh et al., 2003) or acidic pH (Muller et al., 2009). Although σE is essential for viability in E. coli, a σE-deficient S. enterica mutant is viable but avirulent, most likely due to increased susceptibility to a variety of stresses (Humphreys et al., 1999; Testerman et al., 2002; Crouch et al., 2005). σS coordinately regulates the expression of many genes during stationary phase and in response to diverse stresses. Although σS is required for S. enterica virulence, its contribution to virulence in other bacterial species varies (Dong and Schellhorn, 2010). The particular importance of σS in S. enterica may result from its regulation of the spv plasmid virulence genes that disrupt host-cell signaling and induce cell death (Fang et al., 1992; Guiney and Fierer, 2011). Interestingly, strains of the human-adapted S. enterica serovar Typhi frequently exhibit reduced σS expression (Robbe-Saule et al., 2003), perhaps because they lack spv plasmids, and because σS interferes with the expression of the S. enterica Typhi Vi polysaccharide capsule (Santander et al., 2007).
Other alternative sigma factors have also been found to contribute to bacterial virulence, including σN in P. aeruginosa (Totten et al., 1990), SigB in Gram-positive bacteria (Hecker et al., 2007), σN and σS in Borrelia burgdorferi (Fisher et al., 2005) and multiple sigma factors in M. tuberculosis (Kazmierczak et al., 2005).
Signal Discrimination
The challenges associated with specific stresses require specific adaptive responses. Iron is poorly able to discriminate among diverse reactive oxygen and nitrogen species. Nonetheless, iron in the context of a polypeptide chain can be remarkably specific at recognizing cognate signals. The following section discusses a few examples that illustrate the principles governing signal discrimination by regulatory proteins.
Discrimination of reactive species by redox-active sensors
Iron in metalloproteins and sulfur in redox-sensing cysteine residues are used by many regulatory proteins as sensors of diverse reactive oxygen and nitrogen species. However, some sensors are exquisitely selective for certain reactive molecules. For instance, heme prosthetic groups in PAS or GAF domains of E. coli FixL and M. tuberculosis DosS/DosT sensor kinases, respectively, preferentially bind to O2, whereas heme cofactors in H-NOX and DNR proteins from S. oneidensis and P. aeruginosa prefer NO· (Plate and Marletta, 2013). The geometry and allosteric interactions of the porphyrin ring with neighboring residues define the selectivity of heme groups for O2 or NO· (Figure 3). Hydrogen-bonding of O2-bound to a hexa-coordinated Fe2+ in a flat heme with arginine or tyrosine residues are some of the salient characteristics that make DosS, DosT, and FixL efficient O2 traps (Liebl et al., 2002; Vos et al., 2012). In contrast, NO· does not establish hydrogen bonding with dedicated sensors such as H-NOX and DNR, and the nitrosyl iron is penta-coordinated (Herzik et al., 2014; Plate and Marletta, 2013). Rapid (7 picoseconds) recombination of a gas ligand with Fe2+ further enhances the discriminatory abilities of dedicated sensors (Lobato et al., 2014).
Figure 3. Discrimination of O2 and NO· by H-NOX proteins.

Dedicated sensors of reactive species discriminate closely related diatomic gases. H-NOX proteins from Caldoanaerobacter subterraneus (A) and Shewanella oneidensis (B) can discriminate between O2 and NO·, respectively. The heme (yellow) of C. subterraneus H-NOX is hexa-coordinated with O2, the porphyrin ring, and the imidazole group of His102. O2 maintains hydrogen-bonding interactions (red dashes) with the hydroxyl group of Tyr140. In contrast, the heme of NO·-dedicated sensors such as S. oneidensis H-NOX is penta-coordinated. Panel (B) shows the NO· molecule (red and blue spheres) in the proximal face of the porphyrin ring. The short side-chain of Cys141 does not allow hydrogen-bonding after NO· binding at the distal face of the porphyrin ring (not shown). PDB codes 1U55 and 4U9B were used for the analysis of C. subterraneus H-NOX and S. oneidensis H-NOX, respectively.
Thiol-based regulators such as OxyR can respond to either reactive oxygen or reactive nitrogen species (Seth et al., 2012). However, kinetics suggest that OxyR proteins are better at sensing H2O2 than other reactive species. The structure of PaOxyR C199D indicates that a complex hydrogen-bonding network of H2O2 with backbone amide and carbonyl groups with the predicted hydroxyl group of a Cys199-SOH intermediate helps the OxyR triggering thiol to preferentially sense H2O2 (Jo et al., 2015). The H2O2 molecule also establishes hydrogen-bonding with two H2O molecules trapped by nearby Thr100, Thr129, His198 and Asp199 residues. Preferential sensing of alkylhydroperoxides by OhrR provides another example of the importance that the local protein environment plays in signal discrimination. A patch of hydrophobic residues adjacent to the sensory Cys15 in OhrR from Bacillus subtilis accommodates the aliphatic chain of organic hydroperoxides (Hong et al., 2005).
Solvent-exposed [Fe-S] clusters in regulatory proteins are remarkably selective for O2, O2.−, NO· or redox-active drugs. The redox potential of the cluster, accessibility of redox moieties, and cluster reactivity control signal discrimination in these metalloproteins. The [2Fe-2S] cluster in Streptomyces coelicolor SoxR is more stringent in its interactions with redox-active drugs than its closely-related orthologs from E. coli or P. aeruginosa. Amide groups from two branched-chain amino acids on one side of the cluster in S. coelicolor SoxR increase the electropositive environment of the cluster, making the solvent-exposed iron more resistant to oxidation (Lee et al., 2015). Accordingly, an O2-resistant [4Fe-4S]2+-containing FNR paralog of Pseudomonas putida has a positively charged arginine residue in place of serine found in the O2-sensitive FNR protein of E. coli (Ibrahim et al., 2015), and an L28H substitution increases the stability of the E. coli FNR [4Fe-4S]2+ cluster in the presence of O2 (Bates et al., 2000). In addition to affecting electropositivity by decreasing the accessibility of cysteine residues that coordinate one of the irons in the [Fe-S] cluster, bulky side groups of branched-chain amino acids slow both the O2-dependent 1e− oxidation of [Fe-4S]2+ to the intermediate [3Fe-4S]+ and the spontaneous rearrangement to the [2Fe-2S]2+ cluster (Jervis et al., 2009).
Discrimination of ions by metal sensors
All organisms must maintain homeostatic concentrations of metal ions within the cell. Many metalloproteins of central metabolism, DNA synthesis and repair can interchangeably utilize different divalent metals in vitro, but metal usage in vivo is quite narrow. The Irving-Williams series defines the affinity, in decreasing order, of Cu2+, Zn2+, Co2+, Fe2+, Mg2+ and Mn2+ for organic molecules. By keeping the effective cytoplasmic concentration of Cu2+ and Zn2+ at around 10−21 M and 10−15 M, respectively, weaker binding sites remain available to divalent cations in the Irving-Williams series that are less competitive (Waldron and Robinson, 2009). Metal availability is an important determinant of the preferential binding of Fe2+ to the B. subtilis Fur protein, which regulates transcription in response to iron levels (Ma et al., 2012). However, metal availability does not explain the preference of the repressor MntR (regulator of manganese transport) for Mn2+ over Fe2+, because the intracytoplasmic concentration of these metals is similar, and Fe2+ occupies a higher position in the ranked order of the Irving-Williams series. Rather, allostery explains the preference of MntR for Mn2+ (McGuire et al., 2013). Two Mn2+ cations are hepta-coordinated and penta-coordinated at two adjacent metal binding sites designated A- and C in MntR, whereas Fe2+, Zn2+ and Co2+ bind with less optimal tetrahedral or pentahedral geometries that prevent the formation of the binuclear cluster. Moreover, binding of Mn2+ to the A site orients the side chains of two bridging glutamates to participate in both A and C site coordination. The correct geometry of binding at the second metal site by the cognate ion imparts proper allosteric regulation needed for the binding of the MntR and Fur repressors to operators of cognate genes (Ma et al., 2012; McGuire et al., 2013).
Discrimination of signals that activate sigma factors and two-component systems
Alternative sigma factors and TCS regulate virulence programs in response to cognate signals. Specific signal transduction and recognition of cognate inputs are important for the fidelity of the adaptive responses regulated by these signaling pathways. Highly specific protein-protein interactions, allostery and adaptor proteins allow alternative sigma factors and TCS to activate adaptive responses that are appropriate to the relevant challenges. Specific activation of extracytoplasmic sigma factors such as σE in enterobacteria or σK in mycobacteria is controlled with a high degree of precision provided by sequential proteolysis of the cognate anti-sigma factor by intracytoplasmic membrane S1P (site one) and S2P (site two) proteases. Tandem PDZ domains in the S2P protease RseP act as a filter to prevent cleavage of large periplasmic domains (Hizukuri et al., 2014). Unfolded outer membrane proteins activate proteolysis of the RseA periplasmic domain by the S1P RseS. Truncated RseA can then interact with the PDZ domains of RseP. The β-hairpin-like structure of RseP adds further specificity by directing the extended conformation of the transmembrane segment 3 of RseA to the proteolytic active site (Akiyama et al., 2015). Reaction kinetics and cleavage site sequence also contribute to S2P protease specificity (Langosch et al., 2015).
Adaptor proteins further refine signaling fidelity. The mycobacterial Rip1 protease can degrade anti-sigma factors of σM, σK and σL. Adaptor proteins may help Rip1 achieve specificity in signaling, as suggested by selective tethering of the Rip1 PDZ domain to the anti-sigma factor σM, but not to the anti-sigma factors σK and σL, by the adaptor Ppr1 (Schneider et al., 2013). Allosteric interactions between the adaptor SafA and the PhoQ periplasmic sensor domain specifically link EvgS/EvgA and PhoQ/PhoP signaling in response to acid pH (Eguchi et al., 2012). The adaptor IraP also provides specificity in σS-dependent PhoP signaling (Battesti et al., 2013).
Signal discrimination is well illustrated in the cognate interactions between sensor kinases and response regulators. Response regulators harbor a loop, connecting helices α1 and α2, that sits at the interface of the sensor kinase interaction, and four nonconserved residues in this loop determine the specificity of the interaction with cognate histidine kinases (Casino et al., 2009). Mutations in three or four of these interfacial residues are sufficient to switch partner specificity (Capra et al., 2010). A saturating combinatorial library within the four key residues of PhoQ showed the unexpected existence of 1,659 functional variants, even though only 13 residue combinations have been reported in all PhoQ orthologs examined (Podgornaia and Laub, 2015). Thus, despite a high degree of potential degeneracy in the protein-protein interface, the connectivity of functional variants and pressures to maintain identity among closely-related proteins likely constrain the evolution of TCS partners.
Regulatory Networks and Signal Integration
The genetic networks governing stress responses are often highly complex. The complexity of regulatory circuitry determines the performance characteristics of the network and allows multiple regulatory inputs. The presence of autoregulatory and feedforward loops influences the responsiveness, cell-to-cell variation and temporal dynamics of gene expression within a network (Alon, 2007; Salazar and Laub, 2015).
Transcriptional regulators can control gene expression by directly interacting with RNA polymerase to enhance the initiation of transcription (activation), by impeding transcription (repression), or by antagonizing the actions of intrinsic transcriptional silencers, such as the nucleoid-associated protein H-NS (counter-silencing) (Navarre et al., 2007). Transcriptional activation requires the precise localization of activator binding in relation to the −35 box of the promoter, whereas the architectural requirements of counter-silencing are more flexible (Cox et al., 2007; Will et al., 2014; Will et al., 2015). For this reason, genes acquired by horizontal transfer tend to be incorporated into existing regulatory networks by counter-silencing rather than by the de novo evolution of direct activation circuits (Navarre et al., 2006; Will et al., 2014). This suggests that counter-silencing has played a major role in facilitating bacterial evolution (Will et al., 2015). The S. enterica PhoP regulon, which is critically important for virulence (Miller et al., 1989; Fields et al., 1989), provides an instructive example of a network that includes both ancestral genes regulated by direct activation and horizontally-acquired genes regulated by counter-silencing, and these two classes can be distinguished by their promoter architecture (Will et al., 2014).
The convergence of multiple inputs in a regulatory network permits the integration of multiple signals in the control of common pathways of stress resistance or virulence gene expression (Fang, 2005; Yoon et al., 2009; Shen and Fang, 2012). For example, multiple levels of transcriptional, translational and post-translational control of the alternative sigma factor σS confer responsiveness of this general stress pathway to diverse environmental signals including nutritional availability, temperature, osmolarity and pH (Klauck et al., 2007). The versatility of counter-silencing is illustrated by the complex network controlling expression of the genes carried by Salmonella Pathogenicity Islands 1 and 4 (SPI-1 and SPI-4, respectively), which are required for bacterial adherence, invasion of host cells and the induction of pyroptosis in macrophages (Collazo and Galan, 1997; Fink and Cookson, 2007; Gerlach et al., 2007). Expression of SPI-1 and SPI-4 genes is controlled by multiple transcriptional regulators that promote gene expression by countering silencing by H-NS (Schechter et al., 1999; Olekhnovich and Kadner, 2007; Main-Hester et al., 2008). Despite overlapping actions and binding sites, the regulators have different phenotypes and patterns of regulation (Schechter et al., 1999; Lucas and Lee, 2001; Fabrega and Vila, 2013). Overexpression of the SPI-1 master regulator HilA, or either of the AraC family regulators HilC or HilD alone, is able to promote SPI-1 gene expression (Bajaj et al., 1995; Schechter and Lee, 2001), but only the combination of HilA and HilD allows SPI-4 gene expression (Main-Hester et al., 2008). Thus, as each individual regulator responds to distinctive conditions, the complex interaction of multiple counter-silencers can allow the activation of virulence genes either in response to different environmental signals (SPI-1) or only when a specific combination of environmental conditions is present (SPI-4).
Concluding Remarks
Bacterial pathogens exhibit a remarkable ability to sense and respond to the varied environmental conditions encountered during interaction with their hosts. Intricate sensing mechanisms involving thiols, metal centers, specialized protein domains and riboswitches are being increasingly defined. Allostery, specific protein-protein interactions, and adaptor proteins allow bacterial sensing motifs to discriminate among similar stress signals that require distinct adaptive responses. On the other hand, the plasticity of bacterial regulatory networks confers both versatility and efficiency, as multiple signals can be integrated to control the expression of common stress response pathways. The innumerable variations created from common regulatory motifs provide fascinating insights into the evolution of bacterial stress responses and virulence.
ACKNOWLEDGMENTS
The authors are grateful for support from the National Institutes of Health and Veterans Administration to FCF (AI44486, AI101084, AI118962), ERF (AI112101), TT (T32 GM 873015) and AVT (AI54959, BX02073).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akiyama K, Mizuno S, Hizukuri Y, Mori H, Nogi T, Akiyama Y. Roles of the membrane-reentrant beta-hairpin-like loop of RseP protease in selective substrate cleavage. Elife. 2015;4 doi: 10.7554/eLife.08928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon U. Network motifs: theory and experimental approaches. Nat Rev Genet. 2007;8:450–461. doi: 10.1038/nrg2102. [DOI] [PubMed] [Google Scholar]
- Ashkenazy H, Erez E, Martz E, Pupko T, Ben-Tal N. ConSurf 2010: calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res. 2010;38:W529–W533. doi: 10.1093/nar/gkq399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslund F, Zheng M, Beckwith J, Storz G. Regulation of the OxyR transcription factor by hydrogen peroxide and the cellular thiol-disulfide status. Proc Natl Acad Sci U S A. 1999;96:6161–6165. doi: 10.1073/pnas.96.11.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader MW, Sanowar S, Daley ME, Schneider AR, Cho U, Xu W, Klevit RE, Le Moual H, Miller SI. Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell. 2005;122:461–472. doi: 10.1016/j.cell.2005.05.030. [DOI] [PubMed] [Google Scholar]
- Bajaj V, Hwang C, Lee CA. HilA is a novel OmpR/ToxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol Microbiol. 1995;18:715–727. doi: 10.1111/j.1365-2958.1995.mmi_18040715.x. [DOI] [PubMed] [Google Scholar]
- Bates DM, Popescu CV, Khoroshilova N, Vogt K, Beinert H, Munck E, Kiley PJ. Substitution of leucine 28 with histidine in the Escherichia coli transcription factor FNR results in increased stability of the [4Fe-4S](2+) cluster to oxygen. J Biol Chem. 2000;275:6234–6240. doi: 10.1074/jbc.275.9.6234. [DOI] [PubMed] [Google Scholar]
- Battesti A, Hoskins JR, Tong S, Milanesio P, Mann JM, Kravats A, Tsegaye YM, Bougdour A, Wickner S, Gottesman S. Anti-adaptors provide multiple modes for regulation of the RssB adaptor protein. Genes Dev. 2013;27:2722–2735. doi: 10.1101/gad.229617.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capra EJ, Perchuk BS, Lubin EA, Ashenberg O, Skerker JM, Laub MT. Systematic dissection and trajectory-scanning mutagenesis of the molecular interface that ensures specificity of two-component signaling pathways. PLoS Genet. 2010;6:e1001220. doi: 10.1371/journal.pgen.1001220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson HK, Vance RE, Marletta MA. H-NOX regulation of c-di-GMP metabolism and biofilm formation in Legionella pneumophila. Mol Microbiol. 2010;77:930–942. doi: 10.1111/j.1365-2958.2010.07259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casino P, Miguel-Romero L, Marina A. Visualizing autophosphorylation in histidine kinases. Nat Commun. 2014;5:3258. doi: 10.1038/ncomms4258. [DOI] [PubMed] [Google Scholar]
- Casino P, Rubio V, Marina A. Structural insight into partner specificity and phosphoryl transfer in two-component signal transduction. Cell. 2009;139:325–336. doi: 10.1016/j.cell.2009.08.032. [DOI] [PubMed] [Google Scholar]
- Celniker G, Nimrod G, Ashkenazy H, Glaser F, Martz E, Mayrose I, Pupko T, Ben-Tal N. ConSurf: using evolutionary data to raise testable hypotheses about protein function. Isr. J. Chem. 2013;53:199–206. [Google Scholar]
- Chao Y, Papenfort K, Reinhardt R, Sharma CM, Vogel J. An atlas of Hfq-bound transcripts reveals 3' UTRs as a genomic reservoir of regulatory small RNAs. EMBO J. 2012;31:4005–4019. doi: 10.1038/emboj.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri RR, Morgan E, Peters SE, Pleasance SJ, Hudson DL, Davies HM, Wang J, van Diemen PM, Buckley AM, Bowen AJ, Pullinger GD, Turner DJ, Langridge GC, Turner AK, Parkhill J, Charles IG, Maskell DJ, Stevens MP. Comprehensive assignment of roles for Salmonella typhimurium genes in intestinal colonization of food-producing animals. PLoS Genet. 2013;9:e1003456. doi: 10.1371/journal.pgen.1003456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho US, Bader MW, Amaya MF, Daley ME, Klevit RE, Miller SI, Xu W. Metal bridges between the PhoQ sensor domain and the membrane regulate transmembrane signaling. J Mol Biol. 2006;356:1193–1206. doi: 10.1016/j.jmb.2005.12.032. [DOI] [PubMed] [Google Scholar]
- Collazo CM, Galan JE. The invasion-associated type-III protein secretion system in Salmonella--a review. Gene. 1997;192:51–59. doi: 10.1016/s0378-1119(96)00825-6. [DOI] [PubMed] [Google Scholar]
- Cox RS, Surette MG, Elowitz MB. Programming gene expression with combinatorial promoters. Mol Syst Biol. 2007;3:145. doi: 10.1038/msb4100187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crack JC, Green J, Thomson AJ, Le Brun NE. Iron-sulfur clusters as biological sensors: the chemistry of reactions with molecular oxygen and nitric oxide. Acc Chem Res. 2014;47:3196–3205. doi: 10.1021/ar5002507. [DOI] [PubMed] [Google Scholar]
- Crack JC, Smith LJ, Stapleton MR, Peck J, Watmough NJ, Buttner MJ, Buxton RS, Green J, Oganesyan VS, Thomson AJ, Le Brun NE. Mechanistic insight into the nitrosylation of the [4Fe-4S] cluster of WhiB-like proteins. J Am Chem Soc. 2011;133:1112–1121. doi: 10.1021/ja109581t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crack JC, Munnoch J, Dodd EL, Knowles F, Al Bassam MM, Kamali S, Holland AA, Cramer SP, Hamilton CJ, Johnson MK, Thomson AJ, Hutchings MI, Le Brun NE. NsrR from Streptomyces coelicoler is a nitric oxide-sensing [4Fe-4S] cluster protein with a specialized regulatory function. J Biol Chem. 2015;290:12689–12704. doi: 10.1074/jbc.M115.643072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crack JC, Stapleton MR, Green J, Thomson AJ, Le Brun NE. Mechanism of [4Fe-4S](Cys)4 cluster nitrosylation is conserved among NO-responsive regulators. J Biol Chem. 2013;288:11492–11502. doi: 10.1074/jbc.M112.439901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford MA, Tapscott T, Fitzsimmons LF, Liu L, Reyes AM, Libby SJ, Trujillo M, Fang FC, Radi R, Vàzquez-Torres A. Redox-active sensing by bacterial DksA transcription factors is determined by cysteine and zinc content. MBio. 2016;7:e02161–15. doi: 10.1128/mBio.02161-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromie MJ, Shi Y, Latifi T, Groisman EA. An RNA sensor for intracellular Mg(2+) Cell. 2006;125:71–84. doi: 10.1016/j.cell.2006.01.043. [DOI] [PubMed] [Google Scholar]
- Crouch ML, Becker LA, Bang IS, Tanabe H, Ouellette AJ, Fang FC. The alternative sigma factor sigma is required for resistance of Salmonella enterica serovar Typhimurium to anti-microbial peptides. Mol Microbiol. 2005;56:789–799. doi: 10.1111/j.1365-2958.2005.04578.x. [DOI] [PubMed] [Google Scholar]
- DebRoy S, Gebbie M, Ramesh A, Goodson JR, Cruz MR, van Hoof A, Winkler WC, Garsin DA. Riboswitches. A riboswitch-containing sRNA controls gene expression by sequestration of a response regulator. Science. 2014;345:937–940. doi: 10.1126/science.1255091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong T, Schellhorn HE. Role of RpoS in virulence of pathogens. Infect Immun. 2010;78:887–897. doi: 10.1128/IAI.00882-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman CJ, Chatfield S, Higgins CF, Hayward C, Dougan G. Characterization of porin and ompR mutants of a virulent strain of Salmonella typhimurium: ompR mutants are attenuated in vivo. Infect Immun. 1989;57:2136–2140. doi: 10.1128/iai.57.7.2136-2140.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubbs JM, Mongkolsuk S. Peroxide-sensing transcriptional regulators in bacteria. J Bacteriol. 2012;194:5495–5503. doi: 10.1128/JB.00304-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi Y, Ishii E, Yamane M, Utsumi R. The connector SafA interacts with the multi-sensing domain of PhoQ in Escherichia coli. Mol Microbiol. 2012;85:299–313. doi: 10.1111/j.1365-2958.2012.08114.x. [DOI] [PubMed] [Google Scholar]
- Fabrega A, Vila J. Salmonella enterica serovar Typhimurium skills to succeed in the host: virulence and regulation. Clin Microbiol Rev. 2013;26:308–341. doi: 10.1128/CMR.00066-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang FC. Sigma cascades in prokaryotic regulatory networks. Proc Natl Acad Sci U S A. 2005;102:4933–4934. doi: 10.1073/pnas.0501417102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang FC, Frawley ER, Tapscott T, Vazquez-Torres A. Stresses and stress responses in bacterial-host interactions. Cell Host Microbe. 2016;x:xxx–xxx. doi: 10.1016/j.chom.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang FC, Libby SJ, Buchmeier NA, Loewen PC, Switala J, Harwood J, Guiney DG. The alternative sigma factor KatF (RpoS) regulates Salmonella virulence. Proc Natl Acad Sci U S A. 1992;89:11978–11982. doi: 10.1073/pnas.89.24.11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields PI, Groisman EA, Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science. 1989;243:1059–1062. doi: 10.1126/science.2646710. [DOI] [PubMed] [Google Scholar]
- Fink SL, Cookson BT. Pyroptosis and host cell death responses during Salmonella infection. Cell Microbiol. 2007;9:2562–2570. doi: 10.1111/j.1462-5822.2007.01036.x. [DOI] [PubMed] [Google Scholar]
- Fisher MA, Grimm D, Henion AK, Elias AF, Stewart PE, Rosa PA, Gherardini FC. Borrelia burgdorferi sigma54 is required for mammalian infection and vector transmission but not for tick colonization. Proc Natl Acad Sci U S A. 2005;102:5162–5167. doi: 10.1073/pnas.0408536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JW, Spector MP. How Salmonella survive against the odds. Annu Rev Microbiol. 1995;49:145–174. doi: 10.1146/annurev.mi.49.100195.001045. [DOI] [PubMed] [Google Scholar]
- Frohlich KS, Vogel J. Activation of gene expression by small RNA. Curr Opin Microbiol. 2009;12:674–682. doi: 10.1016/j.mib.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Fuangthong M, Helmann JD. The OhrR repressor senses organic hydroperoxides by reversible formation of a cysteine-sulfenic acid derivative. Proc Natl Acad Sci U S A. 2002;99:6690–6695. doi: 10.1073/pnas.102483199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galperin MY, Nikolskaya AN, Koonin EV. Novel domains of the prokaryotic two-component signal transduction systems. FEMS Microbiol Lett. 2001;203:11–21. doi: 10.1111/j.1574-6968.2001.tb10814.x. [DOI] [PubMed] [Google Scholar]
- Gerlach RG, Jackel D, Geymeier N, Hensel M. Salmonella pathogenicity island 4-mediated adhesion is coregulated with invasion genes in Salmonella enterica. Infect Immun. 2007;75:4697–4709. doi: 10.1128/IAI.00228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardina G, Rinaldo S, Johnson KA, Di Matteo A, Brunori M, Cutruzzola F. NO sensing in Pseudomonas aeruginosa: structure of the transcriptional regulator DNR. J Mol Biol. 2008;378:1002–1015. doi: 10.1016/j.jmb.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Gu M, Imlay JA. The SoxRS response of Escherichia coli is directly activated by redox-cycling drugs rather than by superoxide. Mol Microbiol. 2011;79:1136–1150. doi: 10.1111/j.1365-2958.2010.07520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiney DG, Fierer J. The role of the spv genes in Salmonella pathogenesis. Front Microbiol. 2011;2:129. doi: 10.3389/fmicb.2011.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselblatt H, Kurzbauer R, Wilken C, Krojer T, Sawa J, Kurt J, Kirk R, Hasenbein S, Ehrmann M, Clausen T. Regulation of the sigmaE stress response by DegS: how the PDZ domain keeps the protease inactive in the resting state and allows integration of different OMP-derived stress signals upon folding stress. Genes Dev. 2007;21:2659–2670. doi: 10.1101/gad.445307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker M, Pane-Farre J, Volker U. SigB-dependent general stress response in Bacillus subtilis and related gram-positive bacteria. Annu Rev Microbiol. 2007;61:215–236. doi: 10.1146/annurev.micro.61.080706.093445. [DOI] [PubMed] [Google Scholar]
- Henard CA, Tapscott T, Crawford MA, Husain M, Doulias PT, Porwollik S, Liu L, McClelland M, Ischiropoulos H, Vazquez-Torres A. The 4-cysteine zinc-finger motif of the RNA polymerase regulator DksA serves as a thiol switch for sensing oxidative and nitrosative stress. Mol Microbiol. 2014;91:790–804. doi: 10.1111/mmi.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzik MA, Jr., Jonnalagadda R, Kuriyan J, Marletta MA. Structural insights into the role of iron-histidine bond cleavage in nitric oxide-induced activation of H-NOX gas sensor proteins. Proc Natl Acad Sci U S A. 2014;111:E4156–E4164. doi: 10.1073/pnas.1416936111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks KG, Delbecq SP, Sancho-Vaello E, Blanc MP, Dove KK, Prost LR, Daley ME, Zeth K, Klevit RE, Miller SI. Acidic pH and divalent cation sensing by PhoQ are dispensable for systemic Salmonellae virulence. Elife. 2015;4:e06792. doi: 10.7554/eLife.06792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hizukuri Y, Oda T, Tabata S, Tamura-Kawakami K, Oi R, Sato M, Takagi J, Akiyama Y, Nogi T. A structure-based model of substrate discrimination by a noncanonical PDZ tandem in the intramembrane-cleaving protease RseP. Structure. 2014;22:326–336. doi: 10.1016/j.str.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Hong M, Fuangthong M, Helmann JD, Brennan RG. Structure of an OhrR-ohrA operator complex reveals the DNA binding mechanism of the MarR family. Mol Cell. 2005;20:131–141. doi: 10.1016/j.molcel.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Humphreys S, Stevenson A, Bacon A, Weinhardt AB, Roberts M. The alternative sigma factor, sigmaE, is critically important for the virulence of Salmonella typhimurium. Infect Immun. 1999;67:1560–1568. doi: 10.1128/iai.67.4.1560-1568.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim SA, Crack JC, Rolfe MD, Borrero-de Acuna JM, Thomson AJ, Le Brun NE, Schobert M, Stapleton MR, Green J. Three Pseudomonas putida FNR family proteins with different sensitivities to O2. J Biol Chem. 2015;290:16812–16823. doi: 10.1074/jbc.M115.654079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jervis AJ, Crack JC, White G, Artymiuk PJ, Cheesman MR, Thomson AJ, Le Brun NE, Green J. The O2 sensitivity of the transcription factor FNR is controlled by Ser24 modulating the kinetics of [4Fe-4S] to [2Fe-2S] conversion. Proc Natl Acad Sci U S A. 2009;106:4659–4664. doi: 10.1073/pnas.0804943106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji CJ, Kim JH, Won YB, Lee YE, Choi TW, Ju SY, Youn H, Helmann JD, Lee JW. Staphylococcus aureus PerR Is a hypersensitive hydrogen peroxide sensor using iron-mediated histidine oxidation. J Biol Chem. 2015;290:20374–20386. doi: 10.1074/jbc.M115.664961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo I, Chung IY, Bae HW, Kim JS, Song S, Cho YH, Ha NC. Structural details of the OxyR peroxide-sensing mechanism. Proc Natl Acad Sci U S A. 2015;112:6443–6448. doi: 10.1073/pnas.1424495112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD, Bell J, Clarke K, Chandler R, Pathak P, Xia Y, Marshall RL, Weinstock GM, Loman NJ, Winn PJ, Lund PA. Characterization of mutations in the PAS domain of the EvgS sensor kinase selected by laboratory evolution for acid resistance in Escherichia coli. Mol Microbiol. 2014;93:911–927. doi: 10.1111/mmi.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak MJ, Wiedmann M, Boor KJ. Alternative sigma factors and their roles in bacterial virulence. Microbiol Mol Biol Rev. 2005;69:527–543. doi: 10.1128/MMBR.69.4.527-543.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauck E, Typas A, Hengge R. The sigmaS subunit of RNA polymerase as a signal integrator and network master regulator in the general stress response in Escherichia coli. Sci Prog. 2007;90:103–127. doi: 10.3184/003685007X215922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langosch D, Scharnagl C, Steiner H, Lemberg MK. Understanding intramembrane proteolysis: from protein dynamics to reaction kinetics. Trends Biochem Sci. 2015;40:318–327. doi: 10.1016/j.tibs.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Lee JW, Helmann JD. The PerR transcription factor senses H2O2 by metal-catalysed histidine oxidation. Nature. 2006;440:363–367. doi: 10.1038/nature04537. [DOI] [PubMed] [Google Scholar]
- Lee KL, Singh AK, Heo L, Seok C, Roe JH. Factors affecting redox potential and differential sensitivity of SoxR to redox-active compounds. Mol Microbiol. 2015;97:808–821. doi: 10.1111/mmi.13068. [DOI] [PubMed] [Google Scholar]
- Liebl U, Bouzhir-Sima L, Negrerie M, Martin JL, Vos MH. Ultrafast ligand rebinding in the heme domain of the oxygen sensors FixL and Dos: general regulatory implications for heme-based sensors. Proc Natl Acad Sci U S A. 2002;99:12771–12776. doi: 10.1073/pnas.192311699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobato L, Bouzhir-Sima L, Yamashita T, Wilson MT, Vos MH, Liebl U. Dynamics of the heme-binding bacterial gas-sensing dissimilative nitrate respiration regulator (DNR) and activation barriers for ligand binding and escape. J Biol Chem. 2014;289:26514–26524. doi: 10.1074/jbc.M114.571398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas RL, Lee CA. Roles of hilC and hilD in regulation of hilA expression in Salmonella enterica serovar Typhimurium. J Bacteriol. 2001;183:2733–2745. doi: 10.1128/JB.183.9.2733-2745.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Faulkner MJ, Helmann JD. Origins of specificity and cross-talk in metal ion sensing by Bacillus subtilis Fur. Mol Microbiol. 2012;86:1144–1155. doi: 10.1111/mmi.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main-Hester KL, Colpitts KM, Thomas GA, Fang FC, Libby SJ. Coordinate regulation of Salmonella pathogenicity island 1 (SPI1) and SPI4 in Salmonella enterica serovar Typhimurium. Infect Immun. 2008;76:1024–1035. doi: 10.1128/IAI.01224-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpica R, Franco B, Rodriguez C, Kwon O, Georgellis D. Identification of a quinone-sensitive redox switch in the ArcB sensor kinase. Proc Natl Acad Sci USA. 2004;101:13318–13323. doi: 10.1073/pnas.0403064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matamouros S, Hager KR, Miller SI. HAMP Domain rotation and tilting movements associated with signal transduction in the PhoQ sensor kinase. MBio. 2015;6:e00616–e00615. doi: 10.1128/mBio.00616-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire AM, Cuthbert BJ, Ma Z, Grauer-Gray KD, Brunjes Brophy M, Spear KA, Soonsanga S, Kliegman JI, Griner SL, Helmann JD, Glasfeld A. Roles of the A and C sites in the manganese-specific activation of MntR. Biochemistry. 2013;52:701–713. doi: 10.1021/bi301550t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellin JR, Koutero M, Dar D, Nahori MA, Sorek R, Cossart P. Riboswitches. Sequestration of a two-component response regulator by a riboswitch-regulated noncoding RNA. Science. 2014;345:940–943. doi: 10.1126/science.1255083. [DOI] [PubMed] [Google Scholar]
- Michaux C, Verneuil N, Hartke A, Giard JC. Physiological roles of small RNA molecules. Microbiology. 2014;160:1007–1019. doi: 10.1099/mic.0.076208-0. [DOI] [PubMed] [Google Scholar]
- Miller SI, Kukral AM, Mekalanos JJ. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci U S A. 1989;86:5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar KS, Bonomi M, Pellarin R, Clinthorne GD, Gonzalez G, Goldberg SD, Goulian M, Sali A, DeGrado WF. Cys-scanning disulfide crosslinking and bayesian modeling probe the transmembrane signaling mechanism of the histidine kinase, PhoQ. Structure. 2014;22:1239–1251. doi: 10.1016/j.str.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncrief MB, Maguire ME. Magnesium transport in prokaryotes. J Biol Inorg Chem. 1999;4:523–527. doi: 10.1007/s007750050374. [DOI] [PubMed] [Google Scholar]
- Muller C, Bang IS, Velayudhan J, Karlinsey J, Papenfort K, Vogel J, Fang FC. Acid stress activation of the sigma(E) stress response in Salmonella enterica serovar Typhimurium. Mol Microbiol. 2009;71:1228–1238. doi: 10.1111/j.1365-2958.2009.06597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarre WW, McClelland M, Libby SJ, Fang FC. Silencing of xenogeneic DNA by H-NS-facilitation of lateral gene transfer in bacteria by a defense system that recognizes foreign DNA. Genes Dev. 2007;21:1456–1471. doi: 10.1101/gad.1543107. [DOI] [PubMed] [Google Scholar]
- Navarre WW, Porwollik S, Wang Y, McClelland M, Rosen H, Libby SJ, Fang FC. Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science. 2006;313:236–238. doi: 10.1126/science.1128794. [DOI] [PubMed] [Google Scholar]
- Olekhnovich IN, Kadner RJ. Role of nucleoid-associated proteins Hha and H-NS in expression of Salmonella enterica activators HilD, HilC, and RtsA required for cell invasion. J Bacteriol. 2007;189:6882–6890. doi: 10.1128/JB.00905-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva G, Sahr T, Buchrieser C. Small RNAs, 5' UTR elements and RNA-binding proteins in intracellular bacteria: impact on metabolism and virulence. FEMS Microbiol Rev. 2015;39:331–349. doi: 10.1093/femsre/fuv022. [DOI] [PubMed] [Google Scholar]
- Padalon-Brauch G, Hershberg R, Elgrably-Weiss M, Baruch K, Rosenshine I, Margalit H, Altuvia S. Small RNAs encoded within genetic islands of Salmonella typhimurium show host-induced expression and role in virulence. Nucleic Acids Res. 2008;36:1913–1927. doi: 10.1093/nar/gkn050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate L, Marletta MA. Nitric oxide-sensing H-NOX proteins govern bacterial communal behavior. Trends Biochem Sci. 2013;38:566–575. doi: 10.1016/j.tibs.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podgornaia AI, Laub MT. Protein evolution. Pervasive degeneracy and epistasis in a protein-protein interface. Science. 2015;347:673–677. doi: 10.1126/science.1257360. [DOI] [PubMed] [Google Scholar]
- Prost LR, Daley ME, Le Sage V, Bader MW, Le Moual H, Klevit RE, Miller SI. Activation of the bacterial sensor kinase PhoQ by acidic pH. Mol Cell. 2007;26:165–174. doi: 10.1016/j.molcel.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Righetti F, Narberhaus F. How to find RNA thermometers. Front Cell Infect Microbiol. 2014;4:132. doi: 10.3389/fcimb.2014.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe-Saule V, Algorta G, Rouilhac I, Norel F. Characterization of the RpoS status of clinical isolates of Salmonella enterica. Appl Environ Microbiol. 2003;69:4352–4358. doi: 10.1128/AEM.69.8.4352-4358.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runkel S, Wells HC, Rowley G. Living with stress: A lesson from the enteric pathogen Salmonella enterica. Adv Appl Microbiol. 2013;83:87–144. doi: 10.1016/B978-0-12-407678-5.00003-9. [DOI] [PubMed] [Google Scholar]
- Salazar ME, Laub MT. Temporal and evolutionary dynamics of two-component signaling pathways. Curr Opin Microbiol. 2015;24:7–14. doi: 10.1016/j.mib.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santander J, Wanda SY, Nickerson CA, Curtiss R. Role of RpoS in fine-tuning the synthesis of Vi capsular polysaccharide in Salmonella enterica serotype Typhi. Infect Immun. 2007;75:1382–1392. doi: 10.1128/IAI.00888-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter LM, Damrauer SM, Lee CA. Two AraC/XylS family members can independently counteract the effect of repressing sequences upstream of the hilA promoter. Mol Microbiol. 1999;32:629–642. doi: 10.1046/j.1365-2958.1999.01381.x. [DOI] [PubMed] [Google Scholar]
- Schechter LM, Lee CA. AraC/XylS family members, HilC and HilD, directly bind and derepress the Salmonella typhimurium hilA promoter. Mol Microbiol. 2001;40:1289–1299. doi: 10.1046/j.1365-2958.2001.02462.x. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Reddy SP, E HY, Evans HW, Glickman MS. Site-2 protease substrate specificity and coupling in trans by a PDZ-substrate adapter protein. Proc Natl Acad Sci U S A. 2013;110:19543–19548. doi: 10.1073/pnas.1305934110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth D, Hausladen A, Wang YJ, Stamler JS. Endogenous protein S-nitrosylation in E. coli: regulation by OxyR. Science. 2012;336:470–473. doi: 10.1126/science.1215643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Fang FC. Integrated stress responses in Salmonella. Int J Food Microbiol. 2012;152:75–81. doi: 10.1016/j.ijfoodmicro.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikumar S, Kröger C, Hébrard M, Colgan A, Owen SV, Sivasankaran SK, Cameron AD, Hokamp K, Hinton JC. RNA-seq brings new insights to the intra-macrophage transcriptome of Salmonella Typhimurium. PLoS Pathog. 2015;11:e1005262. doi: 10.1371/journal.ppat.1005262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testerman TL, Vazquez-Torres A, Xu Y, Jones-Carson J, Libby SJ, Fang FC. The alternative sigma factor sigmaE controls antioxidant defences required for Salmonella virulence and stationary-phase survival. Mol Microbiol. 2002;43:771–782. doi: 10.1046/j.1365-2958.2002.02787.x. [DOI] [PubMed] [Google Scholar]
- Totten PA, Lara JC, Lory S. The rpoN gene product of Pseudomonas aeruginosa is required for expression of diverse genes, including the flagellin gene. J Bacteriol. 1990;172:389–396. doi: 10.1128/jb.172.1.389-396.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker NP, Hicks MG, Clarke TA, Crack JC, Chandra G, Le Brun NE, Dixon R, Hutchings MI. The transcriptional repressor protein NsrR senses nitric oxide directly via a [2Fe-2S] cluster. PLoS One. 2008;3:e3623. doi: 10.1371/journal.pone.0003623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakulskas CA, Potts AH, Babitzke P, Ahmer BM, Romeo T. Regulation of bacterial virulence by Csr (Rsm) systems. Microbiol Mol Biol Rev. 2015;79:193–224. doi: 10.1128/MMBR.00052-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Torres A. Redox active thiol sensors of oxidative and nitrosative stress. Antioxid Redox Signal. 2012;17:1201–1214. doi: 10.1089/ars.2012.4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J. A rough guide to the non-coding RNA world of Salmonella. Mol Microbiol. 2009;71:1–11. doi: 10.1111/j.1365-2958.2008.06505.x. [DOI] [PubMed] [Google Scholar]
- Vos MH, Bouzhir-Sima L, Lambry JC, Luo H, Eaton-Rye JJ, Ioanoviciu A, Ortiz de Montellano PR, Liebl U. Ultrafast ligand dynamics in the heme-based GAF sensor domains of the histidine kinases DosS and DosT from Mycobacterium tuberculosis. Biochemistry. 2012;51:159–166. doi: 10.1021/bi201467c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron KJ, Robinson NJ. How do bacterial cells ensure that metalloproteins get the correct metal? Nat Rev Microbiol. 2009;7:25–35. doi: 10.1038/nrmicro2057. [DOI] [PubMed] [Google Scholar]
- Walsh NP, Alba BM, Bose B, Gross CA, Sauer RT. OMP peptide signals initiate the envelope-stress response by activating DegS protease via relief of inhibition mediated by its PDZ domain. Cell. 2003;113:61–71. doi: 10.1016/s0092-8674(03)00203-4. [DOI] [PubMed] [Google Scholar]
- Wang LC, Morgan LK, Godakumbura P, Kenney LJ, Anand GS. The inner membrane histidine kinase EnvZ senses osmolality via helix-coil transitions in the cytoplasm. EMBO J. 2012;31:2648–2659. doi: 10.1038/emboj.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann AJ, Förstner KU, Amman F, Barquist L, Chao Y, Schulte LN, Müller L, Reinhardt R, Stadler PF, Vogel J. Dual RNA-seq unveils noncoding RNA functions in host-pathogen interactions. Nature. 2016;529:496–501. doi: 10.1038/nature16547. [DOI] [PubMed] [Google Scholar]
- Will WR, Bale DH, Reid PJ, Libby SJ, Fang FC. Evolutionary expansion of a regulatory network by counter-silencing. Nat Commun. 2014;5:5270. doi: 10.1038/ncomms6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will WR, Navarre WW, Fang FC. Integrated circuits: how transcriptional silencing and counter-silencing facilitate bacterial evolution. Curr Opin Microbiol. 2015;23:8–13. doi: 10.1016/j.mib.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H, McDermott JE, Porwollik S, McClelland M, Heffron F. Coordinated regulation of virulence during systemic infection of Salmonella enterica serovar Typhimurium. PLoS Pathog. 2009;5:e1000306. doi: 10.1371/journal.ppat.1000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Aslund F, Storz G. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science. 1998;279:1718–1721. doi: 10.1126/science.279.5357.1718. [DOI] [PubMed] [Google Scholar]