Abstract
Background
In ischemic mitral regurgitation (IMR), ring annuloplasty is associated with a significant rate of recurrent MR. Ring size is based on inter-trigonal distance without consideration of LV size. However, LV size is an important determinant of mitral valve (MV) leaflet tethering pre and post repair. We aimed to determine if LV–MV ring mismatch (mismatch of LV size relative to ring size) is associated with recurrent MR in IMR patients post restrictive ring annuloplasty.
Methods
Patients with moderate or severe IMR from the two Cardiothoracic Surgical Network IMR trials who received MV repair were examined at 1-year post-surgery. Baseline LV size was assessed by LV end-diastolic (LVEDd) and end-systolic (LVESd) dimensions. LV–MV ring mismatch was calculated as ratio of LV to ring size (LVEDd/ring size and LVESd/ring size).
Results
At one year post ring, 45 (21%) of 214 repair patients had ≥moderate MR. In univariable logistic regression analysis, larger LVESd (p=0.02) and LVESd/ring size (p=0.007) were associated with recurrent MR. In multivariable models adjusted for age, sex, baseline LVEF and severe IMR, only LVESd/ring size (odd ratio per 0.5 increase: 2.20; 95% CI 1.05–4.62; p=0.038) remained significantly associated with 1-year MR recurrence.
Conclusions
LV-MV ring size mismatch is associated with increased risk of MR recurrence. This finding may be helpful in guiding ring size to prevent recurrent MR in patients undergoing MV repair and in identification of patients who may benefit from MV repair with additional subvalvular intervention or mitral valve replacement rather than repair alone.
Clinical Trial Registration
http://clinicaltrials.gov; Unique Identifiers: NCT-00806988 and NCT-00807040.
Keywords: mitral regurgitation, mitral valve ring annuloplasty, mitral valve repair, recurrent mitral regurgitation, ischemic mitral regurgitation
Introduction
Ischemic mitral regurgitation (IMR) is a common complication of coronary artery disease that conveys adverse prognosis by at least doubling the risk of late death.1, 2 It occurs in approximately 25% of patients following myocardial infarction, and in up to 50% in patients with heart failure, left ventricle (LV) dysfunction, and cardiomyopathies.2–6 The principal mechanism underlying the development of IMR is related to tethering of the mitral valve (MV), resulting from ischemic LV remodelling and distortion.2, 7–10
Currently, MV repair or MV replacement with or without concomitant coronary artery bypass graft (CABG) is performed for the surgical management of IMR and it is unclear which patients do better with MV replacement versus repair.11 An important limitation of MV ring repair for IMR is the significant recurrence rates of MR following ring annuloplasty.12 The two recent randomized clinical trials from the Cardiothoracic Surgical Trials Network (CTSN) on moderate and severe IMR confirmed a significant recurrence rate of moderate or greater MR of 11% and 33% respectively.13, 14 Moreover, the 2-year results recently published for patients with severe IMR suggested a progressive pattern of recurrence of IMR post ring annuloplasty (59% at 2 years in the MV repair group).15
Several studies have suggested that posterior leaflet tethering post restrictive annuloplasty is the main underlying mechanism associated with MR recurrence following MV ring repair.16–19 Kron and others have shown that the primary mechanism of recurrent MR in the CTSN was from persistent MV leaflet tethering despite ring annuloplasty.17 MV annuloplasty using a downsized ring size according to inter-trigonal distance and/or anterior MV leaflet size, may exacerbate posterior leaflet tethering especially if the lateral wall of the LV remains displaced relative to the posterior edge of the mitral annulus.16–19 For the same implanted ring size, the larger the LV, the greater the tethering of the posterior leaflet due to an increased distance between papillary muscle and coaptation surface of the posterior leaflet (Figure 1). We hypothesized that persistence of leaflet tethering post restrictive annuloplasty for IMR is related to a mismatch between LV dimension and ring size. Our objective was to determine if LV–MV ring mismatch is associated with significant recurrent MR in patients post restrictive ring annuloplasty.
Figure 1.
Mechanisms of Recurrent MR Post Ring Annuloplasty. Top panels: preoperative echocardiography showing LV dimension, MV leaflets tethering and lack of coaptation (left), and associated degree of severity (right). Bottom panels: postoperative echocardiography showing LV dimension, posterior MV leaflet tethering and lack of coaptation following MV repair (left) and associated degree of MR severity (right). The dimension of LV remained the same pre and post-surgery, while anteroposterior dimension of the MV annulus was significantly decrease following MV repair: this mismatch resulted in a significant MR post MV repair.
Methods
Patient Population
The patient population in the present study comes from two randomized trials on IMR patients conducted by the CTSN.13, 14, 20, 21 As previously described, patients with coronary artery disease and moderate or greater IMR were randomized (1:1 ratio) to determine optimal surgical management: i) 301 patients with moderate IMR were randomized to CABG alone vs CABG + MV repair, and ii) 251 patients with severe IMR were randomized to CABG + MV repair vs CABG + MV replacement. These two trials were conducted in 26 and 22 centers, respectively, with a coordinating center, an independent adjudication committee, and a data and safety monitoring board that oversaw trial progress. Participating centers institutional review board approved the protocol and all patients signed a written informed consent.
Complete inclusion and exclusion criteria of the two randomized clinical trials have been previously reported.13, 14, 20, 21 In the present study, 214 patients who underwent MV repair from both moderate and severe CTSN IMR trials and who had complete transthoracic echocardiogram at 1-year post surgery were included. Optimal medical treatment was prescribed by treating cardiologist for all patients.
Doppler Echocardiographic Data
All echocardiographic exams were reviewed and analysed by an independent and central Core Laboratory.
Mitral regurgitation assessment
The degree of MR was graded according to the current recommendations using an integrative approach and MR was graded as none/ trace, mild, moderate or severe.22, 23 Parameters used to grade MR included: i) mitral jet area as a percentage of left atrial area, ii) vena contracta (VC) width, and iii) effective regurgitant orifice area (EROA). As recommended, VC was measured on a zoomed parasternal view as the narrowest width of the proximal MR jet and EROA was calculated using the proximal isovolumetric surface area (PISA) method.22
Left ventricular parameters
End-diastolic and end-systolic LV internal dimension (i.e. LVEDd and LVESd, respectively) were measured in the parasternal long-axis view at the tip of the mitral valve leaflets.24 LV end-diastolic and end-systolic volumes, as well as LVEF, were determined with the modified biplane Simpson method. Basal aneurysm was defined as presence of thinning and localized LV dilation or distortion. Dyskinesis was the presence of outward displacement of the inferoposterior LV wall during systole.17
Mitral Valve Ring and LV-MV ring Mismatch
The protocol of the randomized trials mandated the use of complete rigid or semi-rigid annuloplasty ring.13, 14, 20, 21 Ring sizing was determined according to inter-trigonal distance, inter-commissural distance or anterior MV leaflets size, and the ring was downsized per protocol. Specific ring type, surgical technique and myocardial-preservation method were at the surgeon’s discretion.
The LV-MV ring mismatch was determined by the ratio of end-diastolic or end-systolic LV dimensions and implanted ring size, and LVEDd/ring size and LVESd/ring size were calculated.
Study End-Points
The primary end-point for this study was the 1-year post repair recurrence of MR defined by moderate or greater MR (i.e. ≥ moderate MR grade).
Statistical Analysis
Continuous data were expressed as mean ± SD and compared with Analysis of Variance (ANOVA) and Kruskal-Wallis tests when appropriate according to quartiles of LV-MV ring mismatch defined as LVESd/ring size, and with T-tests or Wilcoxon rank-sum tests when appropriate according to presence or absence of significant 1-year recurrent MR defined as MR grade ≥ moderate. Categorical data were expressed as percentage and compared with the Chi-square or Fisher’s exact tests when appropriate. Univariable and multivariable logistic regression analyses were performed to determine the predictive value of LV dimensions parameters and LV-MV ring mismatch variables regarding significant recurrence of MR (i.e. MR grade ≥ moderate) at 1-year post repair. Variables entered in the model were pre-specified and perceived clinical relevance only, and we thus included in the model: age, sex, baseline severity of MR and LVEF. Results were reported as odds ratios (OR) with 95% confidence intervals (95% CI). A p value < 0.05 was considered statistically significant. Statistical analyses were performed with SAS version 9.4 (SAS, Cary, NC).
Result
Patients Characteristics
Table shows the baseline characteristics of the 214 patients included in this study. Mean age was 66±10 years, 67% were men and mean body surface area (BSA) was 1.93±0.25 m2. Ninety percent of patients underwent CABG concomitantly to MV repair procedure (Table).
Table.
Baseline characteristics of the study population according to quartiles of LV-MV ring mismatch
| Total Cohort (N=214) |
LV-MV Ring Mismatch (i.e. LVESd/ring size) | |||||
|---|---|---|---|---|---|---|
| Quartile 1 LVESd/ring<1.45 |
Quartile 2 1.45≤LVESd/ring <1.62 |
Quartile 3 1.62≤LVESd/ring <1.81 |
Quartile 4 1.81≤LVESd/ring |
P-value | ||
| Clinical data | ||||||
| Age (years) | 66 ± 10 | 68 ± 9 | 68 ± 9 | 65 ± 11 | 63 ± 10 | 0.02 |
| Male -- no. (%) | 144 (67) | 31 (58) | 31 (58) | 37 (70) | 43 (81) | 0.04 |
| Height (cm) | 169 ± 11 | 167 ± 12 | 169 ± 10 | 170 ± 11 | 171 ± 11 | 0.34 |
| Weight (kg) | 80 ± 17 | 76 ± 19 | 80 ± 18 | 80 ± 16 | 81 ± 16 | 0.44 |
| Body surface area (m2) | 1.93 ± 0.25 | 1.87 ± 0.28 | 1.93 ± 0.24 | 1.94 ± 0.22 | 1.95 ± 0.24 | 0.30 |
| Body mass index (kg/m2) | 28 ± 5 | 27 ± 6 | 28 ± 5 | 28 ± 6 | 28 ± 4 | 0.76 |
| Medical History and Presentation at Baseline -- no./total no. (%) |
||||||
| Atrial Fibrillation | 44/214 (21) | 9/53 (17) | 12/53 (23) | 11/53 (21) | 12/53 (23) | 0.88 |
| Diabetes | 91/214 (43) | 23/53 (43) | 28/53 (53) | 16/53 (30) | 22/53 (42) | 0.13 |
| Heart Failure | 121/214 (57) | 22/53 (42) | 32/53 (60) | 36/53 (68) | 31/53 (58) | 0.04 |
| Hypertension | 178/214 (83) | 45/53 (85) | 47/53 (89) | 41/53 (77) | 43/53 (81) | 0.44 |
| Myocardial Infarction | 157/214 (73) | 39/53 (74) | 35/53 (66) | 41/53 (77) | 41/53 (77) | 0.51 |
| Renal Insufficiency | 37/214 (17) | 9/53 (17) | 11/53 (21) | 5/53 (9) | 12/53 (23) | 0.29 |
| Stroke | 21/214 (10) | 4/53 (8) | 5/53 (9) | 7/53 (13) | 5/53 (9) | 0.80 |
| CABG | 17/209 (8) | 5/52 (10) | 4/52 (8) | 3/52 (6) | 5/51 (10) | 0.88 |
| ICD | 19/214 (9) | 1/53 (2) | 6/53 (11) | 4/53 (8) | 8/53 (15) | 0.08 |
| PCI | 50/214 (23) | 14/53 (26) | 9/53 (17) | 11/53 (21) | 16/53 (30) | 0.39 |
| NYHA Class 3 or 4 | 94/213 (44) | 16/53 (30) | 23/52 (44) | 25/53 (47) | 29/53 (55) | 0.08 |
| Concomitant Procedures – no. (%) | ||||||
| CABG | 192 (90) | 48 (91) | 47 (89) | 48 (91) | 47 (89) | 0.98 |
| Left Atrial Appendage | 17 (8) | 5 (9) | 4 (8) | 3 (6) | 5 (9) | 0.94 |
| MAZE | 21 (10) | 4 (8) | 4 (8) | 6 (11) | 7 (13) | 0.70 |
| Tricuspid valve repair | 10 (5) | 3 (6) | 2 (4) | 3 (6) | 2 (4) | 1.00 |
| Echocardiographic data | ||||||
| Severe mitral regurgitation -- no. (%) | 90 (42) | 18 (34) | 23 (43) | 20 (38) | 29 (55) | 0.15 |
| Vena Contracta (mm) | 6.2 ± 1.9 | 5.5 ± 1.7 | 6.3 ± 1.9 | 6.1 ± 1.6 | 7.0 ± 2.2 | <0.001 |
| Effective Regurgitant Orifice (cm2) | 0.29 ± 0.16 | 0.25 ± 0.11 | 0.31 ± 0.13 | 0.27 ± 0.11 | 0.34 ± 0.23 | 0.05 |
| LV ejection fraction (%) | 41 ± 11 | 50 ± 10 | 44 ± 11 | 37 ± 9 | 34 ± 8 | <0.001 |
| LVEDd (mm) | 56 ± 8 | 48 ± 5 | 53 ± 4 | 57 ± 5 | 64 ± 5 | <0.001 |
| LVESd (mm) | 46 ± 9 | 36 ± 5 | 43 ± 3 | 48 ± 4 | 56 ± 5 | <0.001 |
| LV end-diastolic volume (ml) | 185 ±61 | 143 ± 40 | 161 ± 39 | 191 ± 46 | 245 ± 59 | <0.001 |
| LV end-systolic volume (ml) | 112 ± 51 | 73 ± 29 | 92 ± 30 | 120 ± 30 | 164 ± 54 | <0.001 |
| LV – MV ring mismatch data | ||||||
| Ring Size | ||||||
| Mean size (mm) | 27.9 ± 2.0 | 28.4 ± 2.3 | 27.7 ± 1.7 | 27.6 ± 2.0 | 27.9 ± 1.8 | 0.46 |
| LVEDd/Ring Size | 2.00 ± 0.27 | 1.71 ± 0.18 | 1.90 ± 0.12 | 2.06 ± 0.11 | 2.31 ± 0.22 | <0.001 |
| LVESd/Ring Size | 1.64 ± 0.30 | 1.28 ± 0.15 | 1.54 ± 0.05 | 1.72 ± 0.05 | 2.03 ± 0.20 | <0.001 |
CABG: coronary artery bypass graft; ICD: implanted cardioverter defibrillator; LV: left ventricle; LVEDd: LV end-diastolic dimension; LVESd: LV end-systolic dimension; MR: mitral regurgitation; MV: mitral valve; NYHA: New York Heart Association; PCI: percutaneous coronary intervention. Values are means ± SD.
At baseline, IMR was severe in 42% of patients included in this study: mean VC was 6.2±1.9 mm and EROA was 0.29±0.16 cm2. Mean LVEDd and LVESd were 56±8 mm and 46±9 mm, respectively. LVEF was 41±11% (Table). The mean implanted ring size was 27.9±2.0 mm (Table). Mean LVEDd/ring size and LVESd/ring size were 2.00±0.27 and 1.64±0.30, respectively.
At baseline, 67 (32%) patients had evidence of basal aneurysm or dyskinesis and these patients also had larger LV (LVEDd: 59±8 vs 54±7 mm, p<0.0001; LVESd: 50±8 vs 44±8 mm, p<0.0001) as well as higher LV-MV ring mismatch (LVEDd/ring size: 2.13±0.27 vs 1.94±0.26 mm, p<0.0001; LVESd/ring size: 1.81±0.29 vs 1.57±0.28 mm, p<0.0001) than patients without basal aneurysm or dyskinesis.
The clinical characteristics of patients were similar across quartiles of LVESd/ring size, except for younger age (p=0.02) and higher prevalence of men (p=0.04) and heart failure (p=0.04) in the highest quartiles (Table). As expected, patients in the highest quartiles of LVESd/ring size had lower LVEF (p=0.05) and larger LV dimensions and volumes (all p>0.001) (Table), but ring size was similar across quartiles (p=0.46).
Recurrence of Mitral Regurgitation at 1 Year Post Surgery
Forty five patients (21%) had significant recurrent MR defined as moderate or greater MR 1-year post ring annuloplasty. Patients with recurrent MR had lower weight (p=0.049) and a trend for lower BSA and body mass index (both p=0.06; Online Table). Moreover, prevalence of atrial fibrillation (p=0.049), previous CABG (p=0.003) and percutaneous coronary intervention (p=0.03) were higher in patients with recurrent MR compared to those without significant MR at 1-year, whereas they had lower prevalence of diabetes (p=0.006) (Online Table). As expected, patients with moderate or severe recurrent MR at 1-year post surgery had more severe degree of IMR at baseline (71% vs 34% of severe IMR; p<0.0001; Online Table). LVEDd was similar between patients with and without recurrence of MR (57±7 vs 55±8 mm; p=0.14) whereas LVESd was significantly larger in recurrent MR patients (48±8 vs 45±9 mm; p=0.01) (Online Table). Ring size was similar between groups and only LVESd/ring size was significantly higher in patients with recurrent MR (1.75±0.29 vs 1.61±0.30; p=0.006).
In univariable logistic regression analyses, LVEDd and LVEDd/ring size were not significantly associated with recurence MR (all p>0.05; Figure 2). However, LVESd (OR per 10 mm increase: 1.62; 95% CI 1.09–2.40; p=0.02) and LVESd/ring size (OR per 0.5 increase: 2.15; 95% CI 1.23–3.76; p=0.007) reached significance to predict 1-year recurent MR post ring annuloplasty (Figure 2).
Figure 2.
Univariable and Multivariable Predictors of Recurrent MR. CI: confidence interval; LV: left ventricle; LVEDd: LV end-diastolic dimension; LVESd: LV end-systolic dimension; MR: mitral regurgitation; MV: mitral valve; OR: odds ratio. *: multivariable model is adjusted for age, sex, LV ejection fraction and severe MR at baseline.
After adjustment for age, sex, baseline severity of MR and LVEF, LVESd/ring size remained significantly associated with higher risk of recurent MR at 1-year post surgery (OR: 2.20; 95% CI 1.05–4.62; p=0.038) whereas there was only a trend for LVESd (OR: 1.65; 95% CI 0.94–2.90; p=0.079) (Figure 2). Further adjustment for atrial fibrilation or diabetes provided similar results: LVESd/ring size remained associated with higher risk of recurrence of MR (all p<0.05) whereas there was only a trend for LVESd (all p<0.10).
In order to account for body size effect, we performed two sub analyses confirming the persistant association between LV-MV ring mismatch and recurrence of MR at 1-year post repair: i) using LV dimensions indexed by BSA to calculate LV-MV ring mismatch ([LVESd/BSA]/ring size: adjusted OR=4.65; 95%CI 1.41–15.37; p=0.01), and ii) adjusting for BSA in addition to the variables included in the model (LVESd/ring size: adjusted OR=2.35; 95%CI 1.10–4.99; p=0.027).
There was a significant interaction bewteen LVESd/ring size and sex (p=0.02). The association between LVESd/ring size and recurrence of MR stratified by sex showed a similar direction for both males and females but only reached statistical significance for females.
Discussion
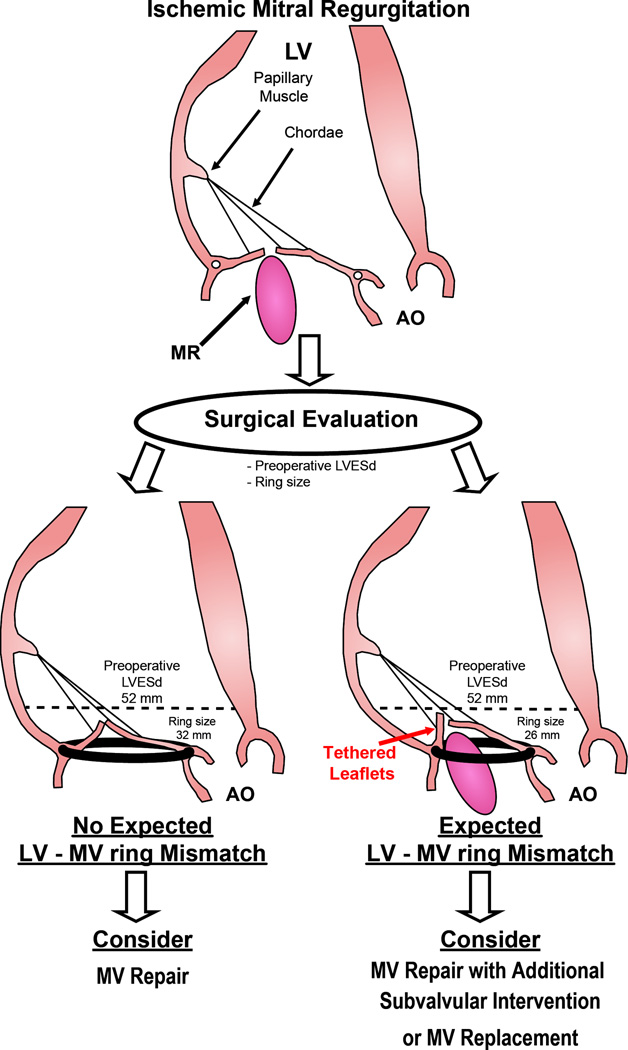
This study shows that LVESd/ring size ratio is an independent predictor of recurrent IMR following restrictive MV ring annuloplasty. LVESd/ring size ratio is a measure of degree of mismatch between LV size to mitral annular ring size. This study shows that an increase in LV-MV ring mismatch by 0.5 was associated with a more than 2-fold higher risk of developing recurrent MR at 1 year. These findings suggest that LV-MV ring mismatch could be useful in determining patients at high risk for recurrent MR following restrictive MV ring annuloplasty and thus guide surgical decision making to optimize benefit for IMR patients (Figure 3).
Figure 3.
LV-MV Ring Mismatch Concept and Clinical Implication. Proposed algorithm for applying LV-MV ring mismatch concept to define patients at high risk for recurrence of MR post repair. LV-MV ring mismatch uses a simple and highly reproducible measurement that can be performed in vast majority of patients. This concept which can be readily determined in the operative room can provide a useful guide to determining the best surgical option to improve outcomes of IMR patients.
Mechanism of Recurrent MR Post MV Repair
Several experimental or clinical studies have shown persistent tethering of the MV leaflets following ring annuloplasty is one of the main mechanisms associated with recurrent MR in IMR patients (Figure 1).16, 18, 19 Indeed, these studies reported that the reduced mobility of the posterior leaflet following ring annuloplasty contributes to incomplete coaptation (Figure 1).18 The anterior MV annulus section is, at least in part, attached to the fibrous trigone and therefore relatively fixed, as opposed to the posterior MV annulus. Given the attachment of anterior MV annulus to the fibrous trigone, the posterior section of the MV annulus becomes most affected by MV ring annuloplasty which serves to reduce mitral annular area by reducing the anterior-posterior dimension. If the posterior lateral wall of the left ventricle remains displaced relative to the mitral annulus following ring insertion, this has the potential to exacerbate the tethering on the mitral leaflets with the posterior mitral leaflet more affected due to its smaller overall length (Figure 1). Exacerbation of tethering may be an important mechanistic basis for the positive association of increased LV to ring mismatch with recurrence of MR post ring annuloplasty. Experimental studies have demonstrated increased strains on the posterior annulus and leaflet following restrictive annuloplasty which further predisposes to leaflet immobility and tethering.18, 19, 25, 26
Echocardiography to Predict Recurrence MR Post MV Repair
Several studies have identified echocardiographic parameters of leaflet tethering or LV remodeling to predict risk of recurrent MR post restrictive annuloplasty in IMR patients: MV leaflets angles, tethering length, tenting area, LV dimensions, LV sphericity index have been previously reported to be associated with recurrence of MR post MV repair.16, 27–33 However, the majority of these were single center studies with retrospective analyses and had relatively small sample of IMR patients. More recently, Kron et al examined factors associated with recurrent MR in the CTSN severe IMR trial and showed that basal LV aneurysm or dyskinesis was associated with significant recurrent MR over a 2 year period following ring annuloplasty.17 Basal aneurysms result from ischemic LV remodeling and dilation and are incorporated within the LV dimension measure. Indeed, basal aneurysms were associated with larger LV dimensions as well as higher LV-MV ring mismatch. However, basal aneurysms can be variably interpreted and dependent on imaging technique. LV-MV ring mismatch is a simple, reproducible measure that can be applied widely in the clinical setting and is based on mechanistic principles which integrate both ischemic LV dilation and mitral leaflet tethering.
MR is a systolic phenomenon and MV leaflet tethering is best examined during end-systole as opposed to end-diastole to study the mechanistic impact. This likely explains the significant univariable association between end-systolic parameters (i.e. LVESd and LVESd/ring size) and recurrent MR, but not with end-diastolic parameters. The multivariable models confirmed an independent association between LVESd/ring size and recurrence of MR post repair (Figure 2).
The concept of LV-MV ring mismatch as calculated by the ratio between LV end-systolic dimension to ring size is a measure of the disassociation between the normal spatial relationship between LV and MV apparatus. MV repair with ring annuloplasty has the potential to disrupt the balance of this LV-MV geometry if the size of the newly reduced mitral annulus relative to the LV is not taken into account. As previously reported, tethering of the MV posterior leaflet can be exacerbated following restrictive ring annuloplasty especially if the papillary muscles remain laterally displaced relative to the mitral annulus/ring, thereby worsening the mismatch post MV repair between LV and MV size.16–19 Current understanding of the mechanisms associated with recurrence of MR post repair support the usefulness of the LV-MV ring mismatch concept presented in this paper.
Clinical Implications
Recurrence of MR following MV ring annuloplasty in IMR patients is recognized as the main adverse effect of the procedure.12–15 Indeed, although MV annuloplasty is perhaps less technically challenging, quicker and associated with lower short term complications than MV replacement, the high rate of recurrent MR post repair attenuates the potential benefit of MV annuloplasty. However, it is important to consider that a successful (without recurrent MR) MV repair was associated with a greater degree of LV reverse remodeling compared to MV replacement, highlighting the importance of selecting which patients will likely have a durable MV repair. In addition, patients in which there was successful MV surgery without recurrence of MR, had less heart failure events,15 suggesting a more favorable outcome with a durable MV surgery for IMR. Longer term follow up is needed to examine adverse effects of MV prosthesis versus ring and differences in LV remodeling between the subgroups. The identification of patients at higher risk for recurrent MR is important for clinical decision making in deciding the right operation for patients with IMR.
In our study, we show that LV-MV ring mismatch is potentially useful to define at risk patients for recurrence of MR post repair (Figure 3). If there is a large LV-MV ring mismatch then an alternative treatment strategy such as subvalvular intervention or MV replacement may be considered.12, 17, 34, 35 LV-MV ring mismatch uses a simple and highly reproducible measurement that can be performed in vast majority of patients. This concept which can be readily determined in the operative room can provide a useful guide to determining the best surgical option to improve outcomes of IMR patients.
Limitations
There are several limitations that need to be considered. Due to the relative small sample size, and number of patients with recurrent MR, the findings of this study need to be interpreted as hypothesis generating. Moreover, a significant association between LVESd and recurrence of MR could not be excluded and further studies need to be performed to address this point. However, our findings supporting an independent association between LV-MV ring mismatch and recurrence of MR is supported by mechanistic standpoint and validate in the present study.
Our analyses showed an interaction between LV-MV ring mismatch and sex. We cannot rule out a differential impact of LV-MV ring mismatch based on sex. The mechanism for this interaction is unclear and was not a pre-specified sub analyses.
Different MV tethering patterns (asymmetric vs symmetric tethering) were not analyzed in the context of this study and deserve additional investigation. Moreover, the findings of this study are not applicable to the patients with Type 1 lesion.
Conclusions
LV-MV ring size mismatch is associated with increased risk of MR recurrence. This finding may be helpful in guiding ring size to prevent recurrent MR in patients undergoing MV repair and in identification of patients who may benefit from MV repair with additional subvalvular intervention or mitral valve replacement rather than repair.
Supplementary Material
Clinical Perspective.
What is new?
In this analysis of the two randomized clinical trials focused on the surgical treatment of ischemic mitral regurgitation, we demonstrated the importance of mismatch between left ventricular size relative to mitral valve ring size, quantitated as LVESd/ring size ratio, as an independent predictor of recurrent ischemic mitral regurgitation following restrictive ring annuloplasty.
The mechanistic basis behind this association between left ventricular to mitral valve ring mismatch and the recurrence of mitral regurgitation post ring annuloplasty relates to the persistence of mitral leaflets tethering.
What are the clinical implications?
The main adverse effect of the restrictive ring annuloplasty in patients with ischemic mitral regurgitation is the high rate of recurrence of mitral regurgitation post-surgery: up to 30% at one year and 60% at 2 years.
The findings of this study supports the usefulness of the left ventricular to mitral valve mismatch to determine patients who may benefit from additional subvalvular interventions to the restrictive ring annuloplasty or mitral valve replacement rather than mitral valve repair alone.
Acknowledgments
Funding Sources: Supported by a cooperative agreement (U01 HL088942) funded by the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute of Neurological Disorders and Stroke of the NIH and the Canadian Institutes of Health Research (CIHR). Supported in part by NIH/NHLBI R01 HL092101 (JH) and R01 HL109506 (RAL). RC is supported by a post-doctoral fellowship grant from CIHR.
Footnotes
Conflict of Interest and Disclosures: GA has served as consultant for Abbott Vascular, Edwards and Atricure. DA has served as consultant for Celldon Corp. PG has served as consultant for Abbott Vascular, Tendyne and Bracco Diagnostics; has received research funding from Abbott Vascular, Edwards Lifesciences, Medtronic, Boston Scientific and Tendyne; has Echo Core Laboratory contracts from Valtech Cardio, Tendyne. MM sits at the Steering Committees of trials for Edwards Lifesciences and Abbott Vascular (uncompensated); has received travel expenses paid for committee meetings. The other authors have reported no relationships relevant to the contents of this paper to disclose.
References
- 1.Bursi F, Enriquez-Sarano M, Nkomo VT, Jacobsen SJ, Weston SA, Meverden RA, Roger VL. Heart failure and death after myocardial infarction in the community: the emerging role of mitral regurgitation. Circulation. 2005;111:295–301. doi: 10.1161/01.CIR.0000151097.30779.04. [DOI] [PubMed] [Google Scholar]
- 2.Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic mitral regurgitation. Long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103:1759–1764. doi: 10.1161/01.cir.103.13.1759. [DOI] [PubMed] [Google Scholar]
- 3.Lamas GA, Mitchell GF, Flaker GC, Smith SC, Jr, Gersh BJ, Basta L, Moye L, Braunwald E, Pfeffer MA. Clinical significance of mitral regurgitation after acute myocardial infarction. Survival and Ventricular Enlargement Investigators. Circulation. 1997;96:827–833. doi: 10.1161/01.cir.96.3.827. [DOI] [PubMed] [Google Scholar]
- 4.Koelling TM, Aaronson KD, Cody RJ, Bach DS, Armstrong WF. Prognostic significance of mitral regurgitation and tricuspid regurgitation in patients with left ventricular systolic dysfunction. Am Heart J. 2002;144:524–529. doi: 10.1067/mhj.2002.123575. [DOI] [PubMed] [Google Scholar]
- 5.Flynn M, Curtin R, Nowicki ER, Rajeswaran J, Flamm SD, Blackstone EH, Mihaljevic T. Regional wall motion abnormalities and scarring in severe functional ischemic mitral regurgitation: A pilot cardiovascular magnetic resonance imaging study. J Thorac Cardiovasc Surg. 2009;137:1063–1070. doi: 10.1016/j.jtcvs.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 6.Schroder JN, Williams ML, Hata JA, Muhlbaier LH, Swaminathan M, Mathew JP, Glower DD, O'Connor CM, Smith PK, Milano CA. Impact of mitral valve regurgitation evaluated by intraoperative transesophageal echocardiography on long-term outcomes after coronary artery bypass grafting. Circulation. 2005;112:I293–I298. doi: 10.1161/CIRCULATIONAHA.104.523472. [DOI] [PubMed] [Google Scholar]
- 7.Kono T, Sabbah HN, Rosman H. Left ventricular shape is the primary determinant of functional mitral regurgitation in heart failure. J Am Coll Cardiol. 1992;20:1594–1598. doi: 10.1016/0735-1097(92)90455-v. [DOI] [PubMed] [Google Scholar]
- 8.Hueb AC, Jatene FB, Moreira LF, Pomerantzeff PM, Kallas E, de Oliveira SA. Ventricular remodeling and mitral valve modifications in dilated cardiomyopathy: new insights from anatomic study. J Thorac Cardiovasc Surg. 2002;124:1216–1224. doi: 10.1067/mtc.2002.125342. [DOI] [PubMed] [Google Scholar]
- 9.Levine RA, Schwammenthal E. Ischemic mitral regurgitation on the threshold of a solution: from paradoxes to unifying concepts. Circulation. 2005;112:745–758. doi: 10.1161/CIRCULATIONAHA.104.486720. [DOI] [PubMed] [Google Scholar]
- 10.Kumanohoso T, Otsuji Y, Yoshifuku S, Matsukida K, Koriyama C, Kisanuki A, Minagoe S, Levine RA, Tei C. Mechanism of higher incidence of ischemic mitral regurgitation in patients with inferior myocardial infarction: quantitative analysis of left ventricular and mitral valve geometry in 103 patients with prior myocardial infarction. J Thorac Cardiovasc Surg. 2003;125:135–143. doi: 10.1067/mtc.2003.78. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM, III, Thomas JD, Members AATF. 2014 AHA/ACC Guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American heart association task force on practice guidelines. Circulation. 2014;129:e521–e643. doi: 10.1161/CIR.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 12.Magne J, Senechal M, Dumesnil JG, Pibarot P. Ischemic Mitral Regurgitation : a Complex Multifaceted Disease. Cardiology. 2009;112:244–259. doi: 10.1159/000151693. [DOI] [PubMed] [Google Scholar]
- 13.Acker MA, Parides MK, Perrault LP, Moskowitz AJ, Gelijns AC, Voisine P, Smith PK, Hung JW, Blackstone EH, Puskas JD, Argenziano M, Gammie JS, Mack M, Ascheim DD, Bagiella E, Moquete EG, Ferguson TB, Horvath KA, Geller NL, Miller MA, Woo YJ, D'Alessandro DA, Ailawadi G, Dagenais F, Gardner TJ, O'Gara PT, Michler RE, Kron IL. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med. 2014;370:23–32. doi: 10.1056/NEJMoa1312808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith PK, Puskas JD, Ascheim DD, Voisine P, Gelijns AC, Moskowitz AJ, Hung JW, Parides MK, Ailawadi G, Perrault LP, Acker MA, Argenziano M, Thourani V, Gammie JS, Miller MA, Page P, Overbey JR, Bagiella E, Dagenais F, Blackstone EH, Kron IL, Goldstein DJ, Rose EA, Moquete EG, Jeffries N, Gardner TJ, O'Gara PT, Alexander JH, Michler RE. Surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med. 2014;371:2178–2188. doi: 10.1056/NEJMoa1410490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein D, Moskowitz AJ, Gelijns AC, Ailawadi G, Parides MK, Perrault LP, Hung JW, Voisine P, Dagenais F, Gillinov AM, Thourani V, Argenziano M, Gammie JS, Mack M, Demers P, Atluri P, Rose EA, O'Sullivan K, Williams DL, Bagiella E, Michler RE, Weisel RD, Miller MA, Geller NL, Taddei-Peters WC, Smith PK, Moquete E, Overbey JR, Kron IL, O'Gara PT, Acker MA. Two-Year Outcomes of Surgical Treatment of Severe Ischemic Mitral Regurgitation. N Engl J Med. 2016;374:344–353. doi: 10.1056/NEJMoa1512913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hung J, Papakostas L, Tahta SA, Hardy BG, Bollen BA, Duran CM, Levine RA. Mechanism of recurrent ischemic mitral regurgitation after annuloplasty: continued LV remodeling as a moving target. Circulation. 2004;110:II85–II90. doi: 10.1161/01.CIR.0000138192.65015.45. [DOI] [PubMed] [Google Scholar]
- 17.Kron IL, Hung J, Overbey JR, Bouchard D, Gelijns AC, Moskowitz AJ, Voisine P, O'Gara PT, Argenziano M, Michler RE, Gillinov M, Puskas JD, Gammie JS, Mack MJ, Smith PK, Sai-Sudhakar C, Gardner TJ, Ailawadi G, Zeng X, O'Sullivan K, Parides MK, Swayze R, Thourani V, Rose EA, Perrault LP, Acker MA. Predicting recurrent mitral regurgitation after mitral valve repair for severe ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2015;149:752.e1–761.e1. doi: 10.1016/j.jtcvs.2014.10.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green GR, Dagum P, Glasson JR, Nistal JF, Daughters GT, Ingels NB, Jr, Miller DC. Restricted posterior leaflet motion after mitral ring annuloplasty. Ann Thorac Surg. 1999;68:2100–2106. doi: 10.1016/s0003-4975(99)01175-3. [DOI] [PubMed] [Google Scholar]
- 19.Kuwahara E, Otsuji Y, Iguro Y, Ueno T, Zhu F, Mizukami N, Kubota K, Nakashiki K, Yuasa T, Yu B, Uemura T, Takasaki K, Miyata M, Hamasaki S, Kisanuki A, Levine RA, Sakata R, Tei C. Mechanism of recurrent/persistent ischemic/functional mitral regurgitation in the chronic phase after surgical annuloplasty: importance of augmented posterior leaflet tethering. Circulation. 2006;114:I529–I534. doi: 10.1161/CIRCULATIONAHA.105.000729. [DOI] [PubMed] [Google Scholar]
- 20.Perrault LP, Moskowitz AJ, Kron IL, Acker MA, Miller MA, Horvath KA, Thourani VH, Argenziano M, D'Alessandro DA, Blackstone EH, Moy CS, Mathew JP, Hung J, Gardner TJ, Parides MK. Optimal surgical management of severe ischemic mitral regurgitation: to repair or to replace? J Thorac Cardiovasc Surg. 2012;143:1396–1403. doi: 10.1016/j.jtcvs.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith PK, Michler RE, Woo YJ, Alexander JH, Puskas JD, Parides MK, Hahn RT, Williams JB, Dent JM, Ferguson TB, Jr, Moquete E, Rose EA, Page P, Jeffries NO, O'Gara PT, Ascheim DD. Design, rationale, and initiation of the Surgical Interventions for Moderate Ischemic Mitral Regurgitation Trial: a report from the Cardiothoracic Surgical Trials Network. J Thorac Cardiovasc Surg. 2012;143:111–117. 117.e1. doi: 10.1016/j.jtcvs.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 23.Grayburn PA, Carabello B, Hung J, Gillam LD, Liang D, Mack MJ, McCarthy PM, Miller DC, Trento A, Siegel RJ. Defining "severe" secondary mitral regurgitation: emphasizing an integrated approach. J Am Coll Cardiol. 2014;64:2792–2801. doi: 10.1016/j.jacc.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the american society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Cheng A, Nguyen TC, Malinowski M, Liang D, Daughters GT, Ingels NB, Jr, Miller DC. Effects of undersized mitral annuloplasty on regional transmural left ventricular wall strains and wall thickening mechanisms. Circulation. 2006;114:I600–I609. doi: 10.1161/CIRCULATIONAHA.105.001529. [DOI] [PubMed] [Google Scholar]
- 26.Bothe W, Rausch MK, Kvitting JP, Echtner DK, Walther M, Ingels NB, Jr, Kuhl E, Miller DC. How do annuloplasty rings affect mitral annular strains in the normal beating ovine heart? Circulation. 2012;126:S231–S238. doi: 10.1161/CIRCULATIONAHA.111.084046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magne J, Pibarot P, Dagenais F, Hachicha Z, Dumesnil JG, Sénéchal M. Preoperative posterior leaflet angle accurately predicts outcome after restrictive mitral valve annuloplasty for ischemic mitral regurgitation. Circulation. 2007;115:782–791. doi: 10.1161/CIRCULATIONAHA.106.649236. [DOI] [PubMed] [Google Scholar]
- 28.McGee EC, Gillinov AM, Blackstone EH, Rajeswaran J, Cohen G, Najam F, Shiota T, Sabik JF, Lytle BW, McCarthy PM, Cosgrove DM. Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2004;128:916–924. doi: 10.1016/j.jtcvs.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 29.Kongsaerepong V, Shiota M, Gillinov AM, Song JM, Fukuda S, McCarthy PM, Williams T, Savage R, Daimon M, Thomas JD, Shiota T. Echocardiographic predictors of successful versus unsuccessful mitral valve repair in ischemic mitral regurgitation. Am J Cardiol. 2006;98:504–508. doi: 10.1016/j.amjcard.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 30.Roshanali F, Mandegar MH, Yousefnia MA, Rayatzadeh H, Alaeddini F. A prospective study of predicting factors in ischemic mitral regurgitation recurrence after ring annuloplasty. Ann Thorac Surg. 2007;84:745–749. doi: 10.1016/j.athoracsur.2007.04.106. [DOI] [PubMed] [Google Scholar]
- 31.Gelsomino S, Lorusso R, De Cicco G, Capecchi I, Rostagno C, Caciolli S, Romagnoli S, Dabroi U, Stefano P, Gensini GF. Five-year echocardiographic results of combined undersized mitral ring annuloplasty and coronary artery bypass grafting for chronic ischaemic mitral regurgitation. Eur Heart J. 2007;29:231–240. doi: 10.1093/eurheartj/ehm468. [DOI] [PubMed] [Google Scholar]
- 32.Calafiore AM, Gallina S, Di Mauro M. Mitral valve procedure in dilated cardiomyopathy : repair or replacement? Ann Thorac Surg. 2001;71:1146–1153. doi: 10.1016/s0003-4975(00)02650-3. [DOI] [PubMed] [Google Scholar]
- 33.Ereminiene E, Vaskelyte J, Benetis R, Stoskute N. Ischemic mitral valve repair: predictive significance of restrictive left ventricular diastolic filling. Echocardiography. 2005;22:217–224. doi: 10.1111/j.0742-2822.2005.03108.x. [DOI] [PubMed] [Google Scholar]
- 34.De Bonis M, Maisano F, La Canna G, Alfieri O. Treatment and management of mitral regurgitation. Nat Rev Cardiol. 2012;9:133–146. doi: 10.1038/nrcardio.2011.169. [DOI] [PubMed] [Google Scholar]
- 35.Nappi F, Lusini M, Spadaccio C, Nenna A, Covino E, Acar C, Chello M. Papillary Muscle Approximation Versus Restrictive Annuloplasty Alone for Severe Ischemic Mitral Regurgitation. J Am Coll Cardiol. 2016;67:2334–2346. doi: 10.1016/j.jacc.2016.03.478. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.