Abstract
This study tested the hypothesis that deletion of angiotensin type 1a receptors (AT1a) from the paraventricular nucleus of hypothalamus (PVN) attenuates anxiety-like behavior, hypothalamic-pituitary-adrenal (HPA) axis activity, and cardiovascular reactivity. We used the Cre/LoxP system to generate male mice with AT1a specifically deleted from the PVN. Deletion of the AT1a from the PVN reduced anxiety-like behavior as indicated by increased time spent in the open arms of the elevated plus maze. In contrast, PVN AT1a deletion had no effect on HPA axis activation subsequent to an acute restraint challenge but did reduce hypothalamic mRNA expression for corticotropin-releasing hormone (CRH). To determine whether PVN AT1a deletion inhibits cardiovascular reactivity, we measured systolic blood pressure, heart rate, and heart rate variability (HRV) using telemetry and found that PVN AT1a deletion attenuated restraint-induced elevations in systolic blood pressure and elicited changes in HRV indicative of reduced sympathetic nervous activity. Consistent with the decreased HRV, PVN AT1a deletion also decreased adrenal weight, suggestive of decreased adrenal sympathetic outflow. Interestingly, the altered stress responsivity of mice with AT1a deleted from the PVN was associated with decreased hypothalamic microglia and proinflammatory cytokine expression. Collectively, these results suggest that deletion of AT1a from the PVN attenuates anxiety, CRH gene transcription, and cardiovascular reactivity and reduced brain inflammation may contribute to these effects.
Keywords: corticosterone, corticotropin-releasing hormone, neuroinflammation, stress, anxiety
anxiety is the most common neuropsychiatric illness with nearly 30% of the adult population in the US reporting a lifetime prevalence of some type of anxiety disorder (35). In addition to excessive uncontrollable anxiety, patients with anxiety disorders frequently present with impairment of the hypothalamic-pituitary-adrenal (HPA) axis (reviewed by Ref. 20) and overactivation of the sympatho-cardiovascular system (57). While the etiology of anxiety disorders are not fully understood, research from several groups has implicated the renin-angiotensin system (RAS) in the underlying pathophysiology (36, 48, 59).
Angiotensin II (ANG II) is the effector peptide of the RAS and exerts the majority of its physiological effects via activation of angiotensin type 1 receptor in humans or its homolog, angiotensin type 1a receptors (AT1a), in rodents. Preclinical studies utilizing laboratory rodents have found that activation of AT1a in the brain promotes stress-response and anxiety-like behavior and exposure to chronic stress augments the expression of AT1a within the paraventricular nucleus of hypothalamus (PVN) (2, 42). The PVN contains neuronal phenotypes critically involved in the regulation of the HPA axis and sympathetic nervous system activity. Specifically, the PVN contains neurosecretory neurons synthesizing corticotropin-releasing hormone (CRH) that project to the median eminence and initiate activation of the HPA axis. Additionally, the PVN contains neurons with projections to the brain stem and spinal cord. These projections influence sympathetic nervous activity, and therefore, these PVN neurons are deemed “preautonomic.” Interestingly, icv administration of ANG II increases CRH mRNA in the PVN (3, 12), and losartan delivered with the same route of administration suppresses the increased CRH mRNA and elevated plasma catecholamines that are observed subsequent to immobilization stress (33). Together, these results suggest stress exposure promotes AT1a activation within the PVN to regulate the HPA axis and sympathetic nervous system; however, the long-term physiological and behavioral consequences of ablating AT1a signaling specifically in the PVN has not been evaluated.
In addition to the control of endocrine axes and autonomic function, ANG II and AT1a have been implicated in the neuroinflammation that is associated with several brain-based diseases (59, 69). In this regard, accumulating evidence from several studies has identified a link between inflammation and neuropsychiatric illness (27, 49, 60). Patients with anxiety disorders have increased inflammation (70), and administration of inflammatory endotoxins elicits HPA axis activation and feelings of anxiety in human subjects (55). Studies using laboratory rodents indicate that administration of exogenous proinflammatory cytokines promotes HPA axis activation (19), sympatho-cardiovascular excitation (61, 64), and anxiety-like behavior (56), effects that are attenuated by delivery of anti-inflammatory agents (24, 34, 56, 74). Interestingly, nondiscrete central administration of an angiotensin receptor antagonist ameliorates neuroinflammation (5) and reduces anxiety-like behavior in rodents (59). Whether abrogation of AT1a signaling specifically in the PVN attenuates local neuroinflammation, which in turn is predictive of altered stress responsiveness, is unknown.
To study the physiological and behavioral consequences of chronic inhibition of hypothalamic AT1a, we utilized the Cre-LoxP system to generate mice that have AT1a selectively deleted from the PVN (PVN AT1a KO mice). Specifically, mice expressing Cre recombinase driven by Sim1 (Sim1-Cre) were bred to mice with the gene coding for AT1a flanked by LoxP sites (AT1a flox/flox). Sim1 is a transcription factor that is mainly expressed in the PVN (46), and therefore, Sim1 can be used to drive the expression of Cre that deletes the floxed AT1a gene specifically from the PVN. Here, we use PVN AT1a KO mice and their littermate AT1a flox/flox controls to test the hypothesis that long-term deletion of AT1a from the PVN attenuates stress-induced HPA axis activation, cardiovascular reactivity, and anxiety-like behavior.
MATERIALS AND METHODS
Animals.
The details about the generation, validation, and characterization of the PVN AT1a KO mice have been described previously (14). In brief, PVN AT1a KO mice were male mice and homologous for floxed AT1a (AT1a flox/flox) and expressing Sim1-Cre. It was previously determined that Sim1-Cre on its own did not affect stress responsiveness (23), and consequently, littermate male mice that were homologous for floxed AT1a (AT1a flox/flox mice) but did not express Sim1-Cre were used as controls. All mice were at least 8 wk old at the initiation of the studies, individually housed, and given ad libitum access to pelleted rodent chow and water. All procedures were approved by the University of Florida Institutional Animal Care and Use Committee.
Dual in situ hybridization and immunohistochemistry.
To confirm that the PVN AT1a KO mice indeed have AT1a specifically deleted from the PVN and to further investigate the phenotype of cells where the deletion of AT1a occurs in the PVN, we performed dual RNAscope in situ hybridization (ISH; Advanced Cell Diagnostics, Hayward, CA) for AT1a as well as immunohistochemistry (IHC) for HuC/D (neuronal specific marker) and Iba-1 (microglia marker) using brain sections from AT1a flox/flox mice (n = 3) and PVN AT1a KO mice (n = 3). Additionally, we evaluated the expression of AT1a mRNA in brain regions previously reported to coexpress Sim1 and/or AT1a. These regions included the PVN, subfornical organ (SFO), basomedial amygdala (BMA), central nucleus of amygdala (CeA), and the lateral hypothalamus (LH) (4, 25). General procedures for tissue preparation and RNAscope ISH were previously described (15, 62, 71). Briefly, mice were transcardially perfused with 4% RNase-free paraformaldehyde (PFA) after euthanasia by an overdose of sodium pentobarbital. Brains were quickly extracted, submerged in RNase-free 4% PFA for 4 h, and then submerged in RNase-free 30% sucrose at 4°C for 2 days. Brains were then stored at −80°C until being sectioned. Brain sectioning was conducted in a Leica CM3050S cryostat in RNase-free conditions. Brains were sectioned coronally at 20 μm, rinsed twice in RNase-free PBS, and then mounted onto Superfrost Plus Gold slides. Mounted brain sections were air-dried for ∼20 min at room temperature and then were stored at −80°C. On the day we conducted RNAscope ISH, brain sections were air-dried for ∼30 min at room temperature, incubated in pretreat 4 (Advanced Cell Diagnostics) for 20 min, and hybridized with specific probes following procedures described in the RNAscope Multiplex Fluorescent Kit User Manual PART 2 (Advanced Cell Diagnostics). The probes used for this study were: 1) DapB, negative control, 2) Ubc, positive control, 3) AT1a. Following the protocol for ISH, brain sections underwent IHC for HuC/D and Iba-1. Briefly, sections were rinsed four times in 50 mM potassium PBS (KPBS) and placed in a blocking solution (50 mM KPBS with 2% normal donkey serum and 0.2% Triton X-100) for 1 h at room temperature. Subsequently, sections were incubated in blocking solution containing rabbit polyclonal anti-Iba-1 (1:500; Wako Chemicals, Richmond, VA) and mouse monoclonal anti-HuC/D (1:500; Life Technologies, Eugene, OR) for overnight at 4°C. Sections were then brought to room temperature, rinsed, and incubated for 2 h in blocking solution containing Alexa-Fluor 488 donkey anti-rabbit (1:500; Jackson ImmunoResearch, West Grove, PA) and Cy3 donkey anti-mouse (1:500, Jackson ImmunoResearch). After another series of rinses, slides were coverslipped.
Image analysis.
Fluorescent images were captured with the same optimized exposure time under which fluorescence was abundantly detected in sections hybridized with the Ubc (positive control) but was undetectable in sections hybridized with DapB (negative control). The integrated density of AT1a was counted using ImageJ. The frequency of colocalizations of AT1a mRNA with either HuC/D-positive cells or Iba-1-positive cells was counted manually. It was considered a colocalization when at least two AT1a mRNA molecules were detected within a cell.
Testing anxiety-like behavior using elevated plus maze.
Anxiety-like behavior was tested in the elevated plus maze (EPM) between 0800 and 1200 with mice (AT1a flox/flox mice, n = 36; AT1a KO mice, n = 35) that had not previously been exposed to stress. The EPM consisted of two opposing open arms (31 × 6 cm) and two opposing closed arms (31 × 6 cm) elevated 41 cm from the floor. Mice were placed in the center of the EPM and then were allowed to explore the maze freely for 5 min. To minimize the effect of olfactory cues, the maze was cleaned with 30% ethanol after each test. Testing sessions were recorded by a ceiling-mounted camera connected to a personal computer running TopScan software (CleverSys, Reston, VA).
Restraint induced HPA activation.
AT1a flox/flox mice (n = 18) and PVN AT1a KO mice (n = 17) underwent a 30 min restraint challenge to evaluate the influence of AT1a expressed within the PVN on systemic corticosterone (CORT) subsequent to an acute psychogenic stressor. Mice were restrained in clear plastic ventilated tubes, and tail vein blood samples (30 μl) were collected immediately after the onset of restraint to reflect basal CORT levels. Thirty minutes after the onset of restraint, blood samples were again collected, and mice were released from the restrainers. Recovery blood samples were collected at 60 and 120 min after the onset of restraint. Blood samples were centrifuged at 6,500 rpm for 15 min at 4°C. Plasma was extracted and stored at −80°C until undergoing radioimmunoassay for CORT with an I125 kit (MP Biomedicals, Orangeburg, NY) as previously described (37–39).
Assessment of the cardiovascular response to stress.
AT1a flox/flox mice (n = 4) and PVN AT1a KO mice (n = 4) were used to assess the effect of deleting AT1a from the PVN on cardiovascular reactivity to psychogenic stress. Cardiovascular activity was recorded using PhysioTel PA-C10 Pressure Transmitter (Data Sciences International, New Brighton, MN). To implant the transmitter, we anesthetized mice with isoflurane and made an incision (∼1 cm) on the midline of the ventral neck, which allowed the exposure of the left carotid artery. Subsequently, a 1 cm long segment of the carotid artery was isolated from the vagus nerve with two sutures (size: 6-0); the cranial end of the segment was permanently ligated, and the caudal end of the segment was temporally occluded. Next, the carotid artery was punctured with a 25-gauge bent needle, and the catheter of the transmitter was slid into the artery lumen and then sutured to the artery. Subsequently, the transmitter body was implanted subcutaneously on the left flank, and the incision on the neck was sutured. After the surgery, mice were allowed to recover for 1 wk. On the day of the experiment, mice underwent a 30 min restraint challenge in their home cages, and cardiovascular parameters were continuously recorded. Cardiovascular data were analyzed with Dataquest A.R.T. Analysis version 4.31 (Data Sciences International). For analysis of heart rate variability (HRV), blood pressure waveforms were sampled at 1,000 Hz and segmented at 10 s. Interbeat intervals (IBIs) were determined by detecting max dP/dt point. The beat-by-beat time series of blood pressure data were then interpolated at a frequency of 50 Hz. The power spectral density of IBI data was analyzed by periodogram with a periodogram resolution of 128. The frequency bands for HRV were defined as: low frequency, 0.4–1.5 Hz; high frequency, 1.5–4 Hz. The same parameters have been used in several previous studies (18, 72). The HRV was calculated as the LF-to-HF ratio. Cardiovascular data collected at 10 s were condensed into 10 min bins. Condensed data were then averaged for genotypes to generate curves representing the systolic blood pressure, heart rate, and HRV of AT1a flox/flox mice and PVN AT1a KO mice.
RNA isolation and cDNA synthesis.
AT1a flox/flox mice (n = 9) and AT1a KO mice (n = 8) were euthanized immediately after they were taken out of the housing room. Brains were extracted and flash-frozen in dry ice-cooled 2-methylbutane. Hypothalami were dissected from flash-frozen brains in a cryostat as described previously (14). The procedure area and instruments were cleaned with RNaseZap (Ambion, Foster City, CA) to minimize RNA degradation. Collected tissues were lysed in Buffer RLT (Qiagen, Valencia, CA), and RNAs were isolated using an RNeasy Mini Kit (Qiagen). DNase treatment was performed (Qiagen) to minimize genomic DNA contamination of the RNA samples. cDNAs were subsequently synthesized from RNA using iScript (Bio-Rad Laboratories, Hercules, CA).
Semiquantitative real-time PCR.
Diluted (1:5) cDNA samples, mixed with TaqMan Gene Expression Master Mix and TaqMan Probes (Applied Biosystems, Foster City, CA), were analyzed for gene expression on the StepOne Real-Time PCR system (Applied Biosystems). All samples were run in duplicate. Genes of interest included corticotrophin-releasing hormone (CRH; Mm1293920), arginine vasopressin (AVP; Mm00437761), oxytocin (OXT; Mm01329577), tumor necrosis factors alpha (TNF-α; Mm00443260), interleukin 6 (IL-6; Mm00446190), interleukin 1 beta (IL-1β; Mm00434228), and cluster of differentiation molecule 11b (CD11b; Mm00434455). Ribosomal protein L32 (Mm02528467) was used as a housekeeping gene. To quantify the real-time PCR results, the expression of the gene of interest in each genotype was first normalized to the expression of L32. Subsequently, the normalized expression level was plotted as the percentage of the AT1a flox/flox group.
Statistical analysis.
All data were analyzed and graphed using Prism 5 (GraphPad Software, La Jolla, CA) and are presented as means and SE. Statistical significance was determined by the appropriate analysis of variance or a one-tailed Student's t-test.
RESULTS
Coexpression of Sim1-cre and AT1a flox/flox selectively decreases the expression of AT1a in neurons located within the PVN.
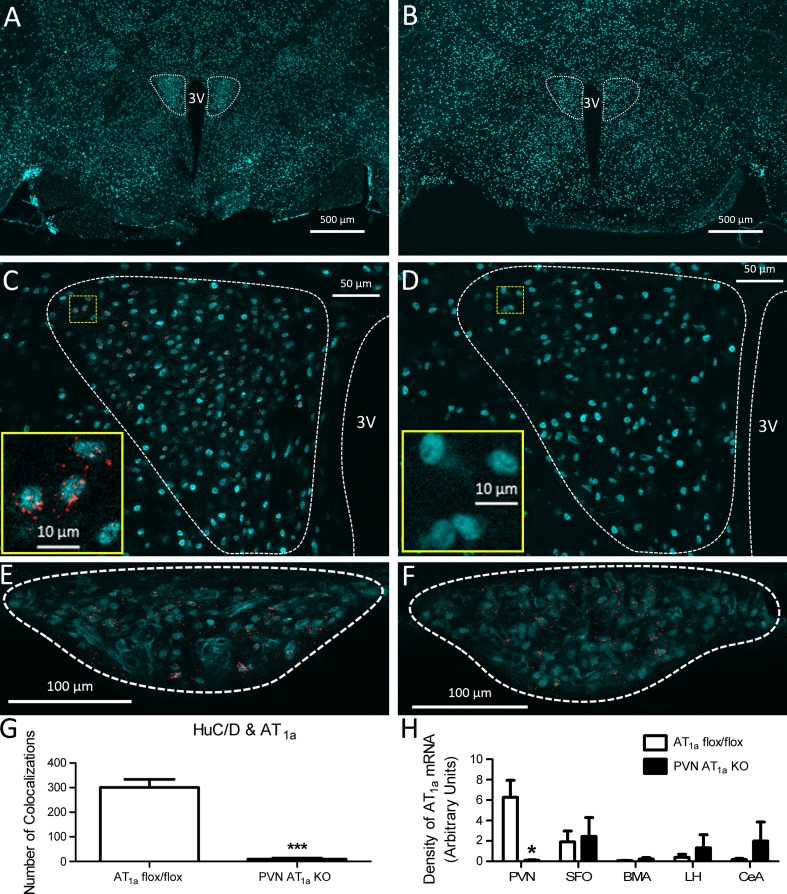
We found that AT1a mRNA was predominantly localized to cells that were positive for HuC/D (Fig. 1C), indicating that within the PVN, AT1a are expressed on neurons. The PVN AT1a KO mice had significantly fewer HuC/D-positive cells that expressed AT1a mRNA (t(4) = 8.90, P < 0.0001) in the PVN compared with that of AT1a flox/flox mice (Fig. 1G), demonstrating effective Sim1-Cre-mediated deletion of AT1a from PVN neurons. Moreover, relative to AT1a flox/flox mice, PVN AT1a KO mice had reduced density of AT1a mRNA within the PVN (t(4) = 3.70, P < 0.05, Fig. 1H). Importantly, this effect was specific to the PVN because expression of AT1a mRNA in the SFO, BMA, CeA, and LH (Fig. 1, E, F, and H) was similar between PVN AT1a KO mice and AT1a flox/flox controls.
Fig. 1.
Coexpression of Sim1Cre and AT1a flox/flox decreases the expression of angiotensin type 1a receptor (AT1a) mRNA in neurons within the paraventricular nucleus of hypothalamus (PVN). ×2.5 images of a coronal sections through the PVN (dotted outline) of an AT1a flox/flox mouse (A) and a PVN AT1a KO mouse (B). C: ×20 image showing AT1a mRNA (depicted as punctate red dots) frequently colocalize with HuC/D (cyan, marker for neurons) in the PVN (enclosed with white dashed line) of an AT1a flox/flox mouse. Inset, high magnification image of the region enclosed with yellow dashed line in C. D: ×20 image showing that AT1a mRNA is not detected in the PVN of a PVN AT1a KO mouse (cyan indicates HuC/D). Inset, high magnification image of the region enclosed with yellow dashed line in D. ×20 image showing that AT1a mRNA (depicted as punctate red dots) frequently colocalize with HuC/D (cyan) in the SFO (enclosed with white dashed line) of an AT1a flox/flox mouse (E) and a PVN AT1a KO mouse (F). G: compared with AT1a flox/flox mice, PVN AT1a KO mice had significantly decreased number of HuC/D-positive cells (neurons) that express AT1a mRNA. H: quantification of the density of AT1a mRNA signal in different brain nuclei of AT1a flox/flox mice and PVN AT1a KO mice. SFO, subfornical organ; BMA, basomedial amygdala; LH, lateral hypothalamus; CeA, central nucleus of amygdala. Bars represent means and SE. *P < 0.05, ***P < 0.001.
Deletion of AT1a from the PVN reduces anxiety-like behavior.
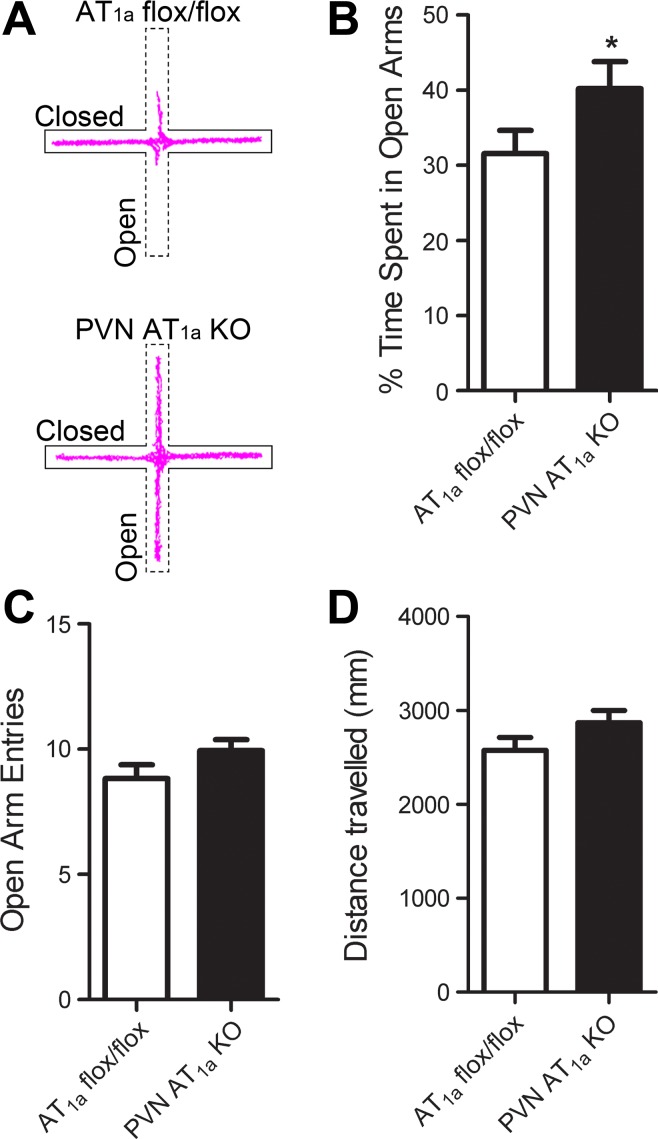
To determine whether deletion of AT1a from the PVN decreases anxiety-like behavior, we tested PVN AT1a KO mice and AT1a flox/flox mice in the EPM. The PVN AT1a KO mice spent more time in the open arms (t(68) = 1.82, P < 0.05, Fig. 2, A and B) but had similar open arm entries and travelled similar distance relative to AT1a flox/flox mice (Fig. 2, C and D).
Fig. 2.
Deletion of AT1a from the PVN reduces anxiety-like behavior. A: representative locator traces of an AT1a flox/flox mouse (top) and a PVN AT1a KO mouse (bottom) in the elevated plus maze (EPM). B: compared with AT1a flox/flox mice, PVN AT1a KO mice spent more time in the open arms of EPM but had similar open arm entries (C) and travelled similar distances (D). Bars represent means and SE. *P < 0.05.
Deletion of AT1a from the PVN reduces the expression of CRH mRNA in the hypothalamus but does not affect the activation of the HPA axis.
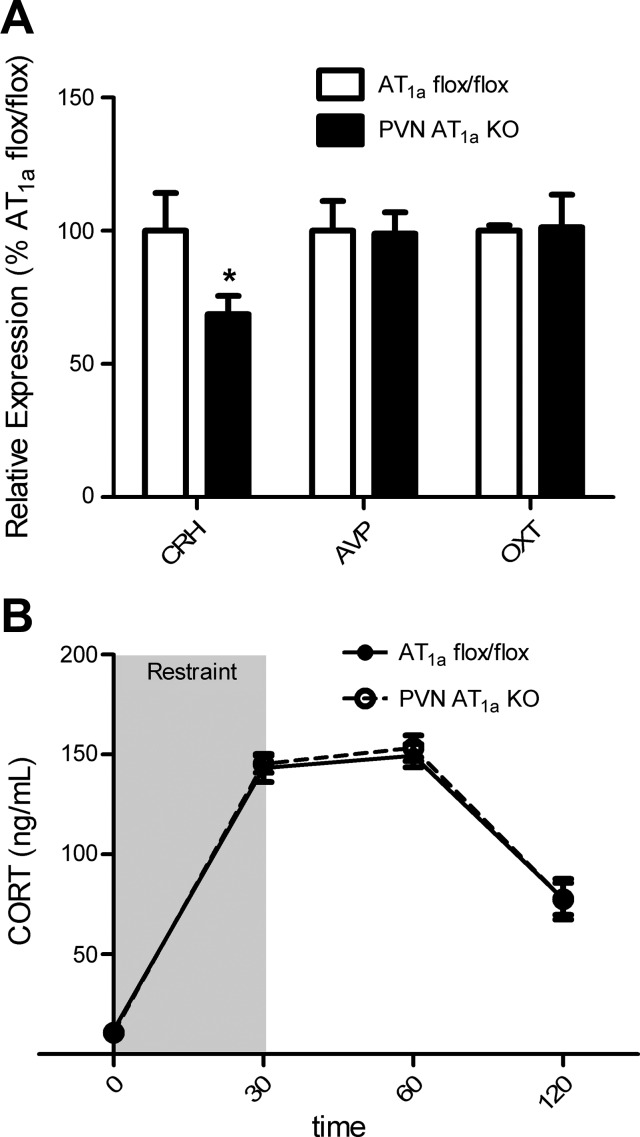
Deletion of AT1a from the PVN significantly attenuated (t(12) = 1.98, P < 0.05) CRH mRNA expression in the hypothalamus but did not affect the mRNA expression of AVP and OXT (Fig. 3A). Despite this difference, deletion of AT1a from the PVN had no effect on plasma CORT in response to a 30 min restraint challenge (Fig. 3B).
Fig. 3.
Deletion of AT1a from the PVN reduces CRH mRNA expression in the hypothalamus but does not affect the corticosterone (CORT) response to restraint. A: compared with AT1a flox/flox mice, PVN AT1a KO mice had decreased mRNA expression for corticotropin-releasing hormone (CRH) but had similar mRNA expression for arginine vasopressin (AVP) and oxytocin (OXT) in the hypothalamus. B: AT1a flox/flox mice and AT1a KO mice had similar CORT responses to 30 min restraint. Bars represent means and SE. *P < 0.05.
Deletion of AT1a from the PVN attenuates cardiovascular reactivity to stress.
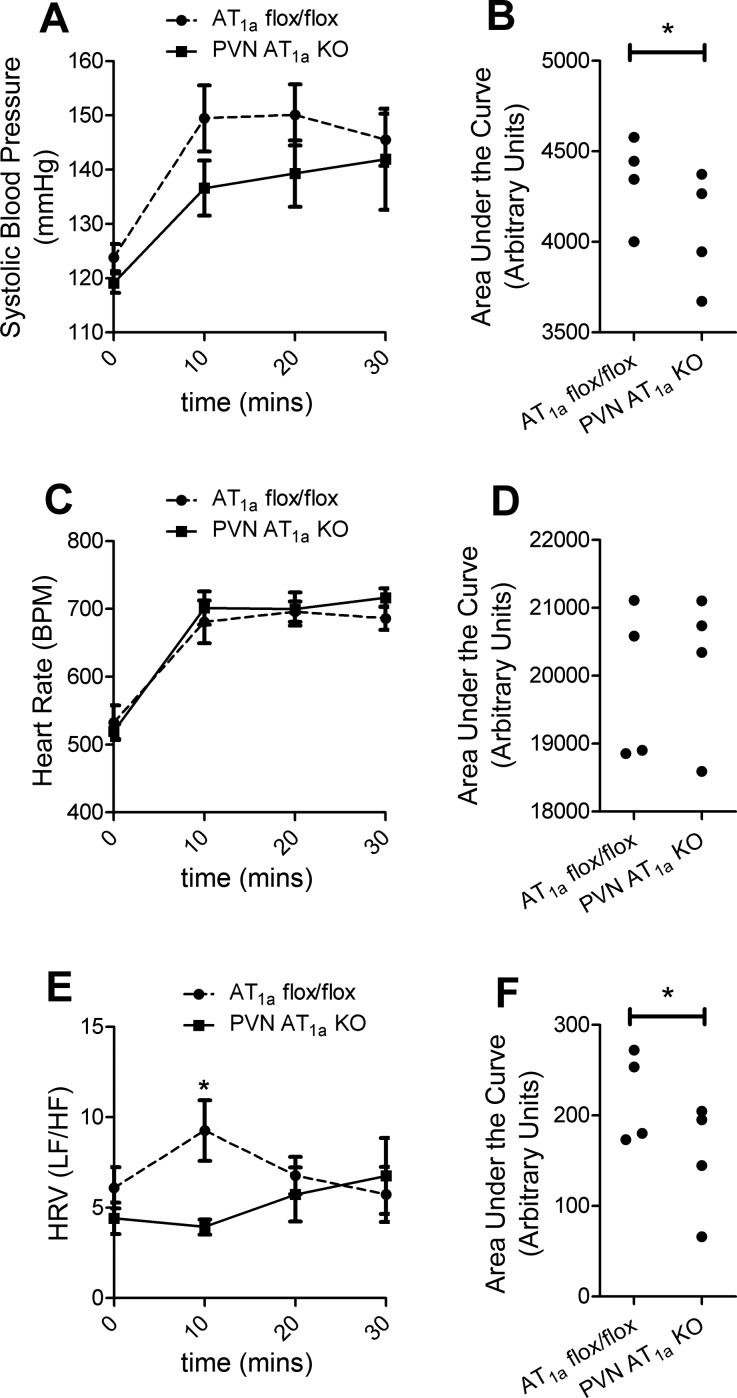
We measured systolic blood pressure, heart rate, and HRV in PVN AT1a KO and AT1a flox/flox mice with telemetry to test whether deletion of AT1a from the PVN affects cardiovascular function basally and subsequent to restraint stress. Deletion of AT1a from the PVN did not affect basal systolic blood pressure (Fig. 4A) but significantly attenuated (t(3) = 2.37, P < 0.05) the integrated pressor response during restraint (Fig. 4B). However, the heart rate response to restraint was not affected by deletion of AT1a from the PVN (Fig. 4, C and D). We then calculated HRV as the LF-to-HF ratio with increases indicative of elevated sympathetic nervous system activity and parasympathetic withdrawal. Deletion of AT1a in PVN had no effect on basal HRV; however, 10 min after the onset of restraint, HRV was significantly decreased in PVN AT1a KO mice relative to AT1a flox/flox controls (t(3) = 3.32, P < 0.05, Fig. 4E), an effect that was also present with the integrated response (t(3) = 2.625, P < 0.05, Fig. 4F).
Fig. 4.
Deletion of AT1a from the PVN attenuates cardiovascular reactivity to stress. Compared with AT1a flox/flox mice, PVN AT1a KO mice had decreased pressor responses (A, B) but had similar heart rate responses to restraint (C, D). Additionally, relative to AT1a flox/flox mice, PVN AT1a KO mice also had altered heart rate variability (HRV) 10 min after restraint (E) that was also evident in the integrated HRV response (F). Bars represent means and SE. *P < 0.05.
In addition, deletion of AT1a from the PVN significantly decreased adrenal gland weight relative to body weight (AT1a flox/flox mice, 0.473 ± 0.0217%; PVN AT1a KO mice, 0.416 ± 0.0251%; t(33) = 1.72; P < 0.05).
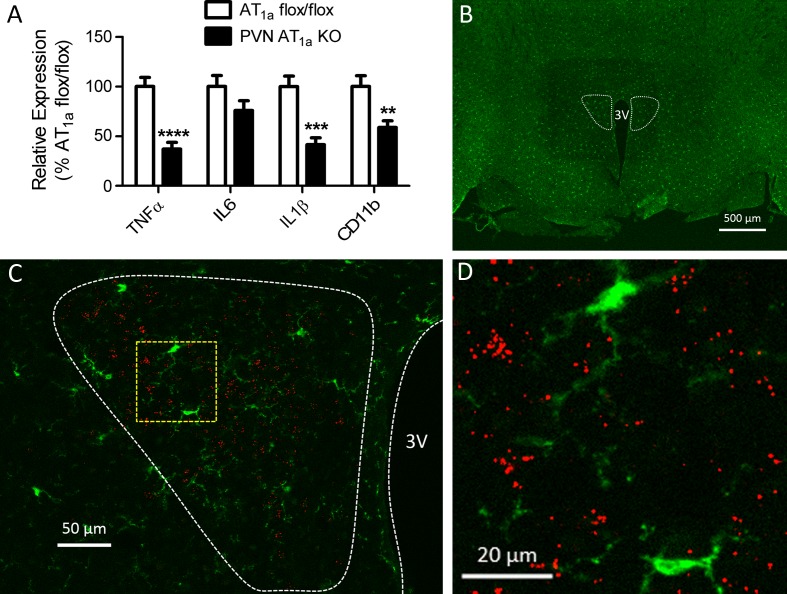
Deletion of AT1a from the PVN attenuates hypothalamic expression of proinflammatory cytokines and decreases activation of microglia.
Deletion of AT1a from the PVN significantly decreased hypothalamic expression of TNF-α (t(15) = 5.40, P < 0.0001), IL-1β (t(15) = 4.521, P < 0.001), and CD11b (t(15) = 3.149, P < 0.01) (Fig. 5A). To determine whether it is possible that ANG II acts on AT1a expressed on microglia to promote their activation and cytokine expression, we performed RNAscope ISH for AT1a mRNA with IHC for Iba-1 (microglia marker) on PVN sections obtained from AT1a flox/flox mice. As depicted in Fig. 5, B–D, AT1a mRNA was not colocalized with Iba-1 in the PVN.
Fig. 5.
AT1a mRNA is not expressed in microglia, but deletion of AT1a from the PVN decreases indexes of microglia infiltration and attenuates expression of proinflammatory cytokines in the hypothalamus. A: relative to AT1a flox/flox mice, PVN AT1a KO mice had decreased hypothalamic expression of TNF-α, IL-1β, and CD11b (marker for microglia). B: ×2.5 images of a coronal section through the PVN (dotted outline) of an AT1a flox/flox mouse. C: ×20 representative image showing that AT1a mRNA (depicted as punctate red dots) does not colocalize with Iba-1 (green, marker for microglia) in the PVN (enclosed within white dashed line) of AT1a flox/flox mice. D: high magnification image of the region enclosed with yellow dashed line in C. Bars represent means and SE. **P < 0.01, ***P < 0.001, ****P < 0.0001.
DISCUSSION
This study used genetically modified mice to test the hypothesis that chronic deletion of AT1a from the PVN attenuates endocrine, cardiovascular, and behavioral responses to psychogenic stress. Compared with AT1a flox/flox control mice, PVN AT1a KO mice had reduced CRH mRNA expression in the hypothalamus, lowered adrenal weight, diminished cardiovascular responses to restraint stress, and decreased anxiety-like behavior but similar plasma CORT. The altered physiological and behavioral phenotypes of PVN AT1a KO mice were accompanied by decreased indexes of hypothalamic inflammation and microglial infiltration. Collectively, these results suggest that deletion of AT1a from the PVN attenuates anxiety-like behavior and cardiovascular reactivity in response to psychogenic stressors, effects that may be the result of decreased hypothalamic inflammation and CRH expression.
We have previously confirmed that breeding Sim1-Cre mice to AT1a flox/flox mice significantly decreases ANG II binding to AT1a in the PVN without affecting binding in the SFO, supraoptic nucleus, median preoptic nucleus, and periventricular nucleus (14). The same study determined that AT1a expression was slightly, but significantly, decreased in the renal cortex, but this effect did not alter renal function (14). In the present study, Sim1-Cre also deleted AT1a from the PVN, but we conducted additional analyses to determine the cellular phenotype in which this deletion occurs. The colocalization of AT1a mRNA with HuC/D was significantly decreased in PVN AT1a KO mice, and AT1a mRNA and Iba-1 colocalizations were absent in AT1a flox/flox mice. These results indicate that Sim1-Cre-mediated deletion of AT1a predominantly occurs in neurons. Previous reports indicate that, in addition to the PVN, Sim1 is found in the LH and amygdala (4), and these brain nuclei express AT1a (25). Therefore, it is possible Sim1-Cre alters AT1a expression in the LH and amygdala; however, AT1a mRNAs in the LH, BMA, and CeA were similar between PVN AT1a KO mice and AT1a flox/flox mice. Collectively, these results indicate that Sim1-Cre deletes AT1a from PVN neurons, and consequently, any observed effects can be attributed to this specific deletion.
Central AT1a antagonism affects systemic RAS activity (44, 45), which in turn, affects renal handling of sodium, and therefore, it is possible that deleting AT1a from the PVN alters hydromineral balance. In this regard, we have previously found that under basal conditions, PVN AT1a KO and AT1a flox/flox mice have similar plasma renin activity, hematocrit, plasma protein concentration, plasma sodium concentration, and plasma glucose concentration (14). Additionally, under basal conditions, body weight and water and saline intakes are also similar (14). Collectively, these results suggest that the decreased anxiety-like behavior and inhibited stress-responsivity that we observed in PVN AT1a KO mice are not secondary to altered systemic RAS activity or impaired hydromineral balance but, rather, are the result of eliminating AT1a signaling from a brain nucleus that is known to coordinate the stress response.
The neuropeptide CRH is an established mediator of the stress response (16, 29, 65), and AT1a-expressing neurons within the PVN are implicated in its regulation. For example, studies conducted by Oldfield and colleagues (52) discovered that within the PVN, AT1a are expressed on parvocellular neurons with identified projections to the median eminence. Such neurons are known to produce CRH, and, indeed, mRNAs for CRH and AT1a colocalize in cells residing in the PVN (3). More recently, genetic reporting for AT1a was used in conjunction with IHC to demonstrate that AT1a-expressing neurons in the PVN colocalize with CRH immunoreactivity (31). In regard to functionality, acute injection or chronic infusion of exogenous ANG II into the brain increases CRH mRNA in the PVN (3, 12), indicating that AT1a stimulation in the PVN may regulate CRH expression. Our study tested the hypothesis that endogenous ANG II activates AT1a expressed on CRH neurons in the PVN to influence CRH synthesis. Relative to controls, hypothalamic CRH mRNA was decreased by deletion of AT1a from the PVN, but mRNAs for vasopressin and oxytocin were unaffected, indicating that under basal conditions, activation of AT1a in the PVN specifically upregulates hypothalamic CRH mRNA expression.
A subset of parvocellular PVN neurons initiates activation of the HPA axis by releasing CRH from axons terminating in the median eminence. This release of CRH stimulates the synthesis of proopiomelanocortin in the pituitary gland, which is processed into ACTH. ACTH is released into the blood from the anterior pituitary and causes the adrenal gland to secrete CORT into the systemic circulation. Given that eliminating AT1a in the PVN attenuated hypothalamic CRH mRNA, we hypothesized that stress-induced activation of the HPA axis would be blunted in PVN AT1a KO mice relative to controls. Contrary to this hypothesis, PVN AT1a KO mice and AT1a flox/flox control mice mounted similar CORT responses to acute restraint stress. Although unexpected, these results are consistent with earlier reports demonstrating that icv delivery of the AT1a antagonist, losartan, had no effect on ACTH and CORT but attenuated the increased CRH mRNA expression that occurs in the PVN subsequent to acute immobilization stress (33). Taken together, these results suggest that AT1a expressed on PVN neurons regulates hypothalamic CRH gene transcription but does not contribute to stress-induced activation of the HPA axis. Another implication of these results is that AT1a may be expressed on CRH neurons in the PVN that send their axons to brain regions other than the median eminence to affect cardiovascular and/or behavioral responses to stress.
In addition to sending axons to the median eminence, CRH neurons within the PVN project to hind-brain nuclei such as the nucleus of the solitary tract (NTS) (58). The NTS is heavily involved in the regulation of sympathetic nervous activity and cardiovascular function and expresses receptors for CRH (58, 68). Central administration of CRH significantly increases plasma catecholamines and blood pressure by activating CRH receptors residing in the brain (51). We hypothesized that the decreased CRH gene transcription that we observed in the PVN AT1a KO mice may attenuate sympathetic drive during restraint stress, resulting in reduced cardiovascular reactivity. Relative to AT1a flox/flox controls, PVN AT1a KO mice had decreased adrenal weight, blunted systolic blood pressure and heart rate variability indicative of reduced cardiac sympathetic outflow in response to acute restraint stress. These results are consistent with other studies demonstrating that microinjection of the AT1a antagonist, losartan, into the PVN decreased the pressor response to acute restraint stress (10), but microinjection of ANG II into the PVN augments renal sympathetic nerve activity by activating AT1a (75). Collectively, these results suggest that AT1a expressed on CRH neurons in the PVN can be inhibited to alleviate the increased sympathetic nervous activity that accompanies psychogenic stress exposure.
In addition to increasing plasma catecholamines and blood pressure, central administration of CRH also increases anxiety-like behavior (21, 41). Mice genetically engineered to overexpress CRH display increased anxiety-like behavior (66), and administration of a CRH type 1 receptor antagonist relieves anxiety-like behavior in rats (26, 28). Oral administration of candesartan, an AT1a antagonist permeable to the blood-brain-barrier, inhibits the expression of CRH in the hypothalamus (54) and decreases anxiety-like behavior in the EPM (59), suggesting that AT1a can be antagonized to suppress anxiety by altering the function of CRH neurons residing in the PVN. To the best of our knowledge, our study demonstrates for the first time that specific deletion of AT1a from PVN neurons decreases hypothalamic CRH mRNA expression as well as anxiety-like behavior as assessed in the EPM. These results conflict with those of a recent report demonstrating that selective deletion of AT1a from CRH cells had no effect on anxiety-like behavior in the EPM (31). While these discrepancies may be explained by differences in mouse strains, testing environment, and the degree of AT1a inhibition, it is clear that within the brain AT1a interacts with cells that produce CRH (3, 33), and there is strong preclinical and clinical evidence that preventing this interaction may influence the etiology of stress-related disorders (9, 14, 36, 59).
In addition to upregulating AT1a mRNA in the PVN (42), acute psychogenic stress also increases the concentration of ANG II in the brain and the periphery (73). Bloodborne ANG II can act on circumventricular organs, brain regions with an incomplete blood-brain barrier, to stimulate AT1a that regulate stress responsivity (22, 67) (38). In contrast, under nonpathological conditions the hypothalamus is protected by the blood-brain barrier, which prevents hydrophilic peptides, like ANG II, from accessing AT1a expressed in the PVN (6). Consequently, it is unlikely that the elevated hypothalamic ANG II that occurs subsequent to stress exposure has systemic origins, but rather, is synthesized in the brain. Consistent with this notion, microinjection of lisinopril, an ACE inhibitor that suppresses the synthesis of endogenous ANG II, into the PVN reduces the pressor response to acute restraint stress (10). These results suggest that stress exposure promotes the synthesis of brain-derived ANG II, which activates AT1a to modulate the physiological and behavioral responses to stress. Thus, the cardiovascular and behavioral effects observed in PVN AT1a KO mice are likely due to decreased binding of brain-derived ANG II to AT1a expressed on CRH neurons in the PVN.
In addition to decreasing CRH mRNA, deletion of AT1a from the PVN suppressed indexes of neuroinflammation and microglia infiltration. Clinical and preclinical studies have identified neuroinflammation as a potential contributor to the etiology of affective disorders (reviewed by Refs. 11, 30). For example, depressed patients present with higher concentrations of IL-1β in cerebrospinal fluid compared with healthy controls (43), and studies conducted in rodents found that exposure to psychological stressors significantly increased microglial infiltration and central expression of proinflammatory cytokines (7, 8, 63). Conversely, administration of exogenous proinflammatory cytokines mimics the anxiogenic and pressor responses observed during stress exposure (56, 64), and these effects are diminished by delivery of anti-inflammatory agents (32, 34, 50, 56, 64). Therefore, it is possible that the decreased neuroinflammation and microglia infiltration that occurred in PVN AT1a KO mice contribute to their dampened stress responsiveness.
Our neuroanatomical studies failed to observe colocalization of AT1a mRNA and the microglia marker, Iba-1, within the PVN of the AT1a flox/flox control mice. Although unexpected, this result is consistent with other studies demonstrating that, under basal conditions, AT1a mRNA is not present in microglia (47), and application of candesartan to primary microglia cultures does not affect microglia activation or the release of proinflammatory cytokines under basal conditions (5). These results then beg the question: How does deletion of AT1a from neurons suppress the activation of microglia as well as the expression of proinflammatory cytokines? In this regard, microglia reside in close proximity to AT1a-expressing neurons in the brain (13), and consequently, AT1a-expressing neurons and microglia are positioned to affect each other's function through paracrine signaling. Here, we propose that proinflammatory cytokines derived from AT1a-expressing neurons in the PVN may serve as this paracrine signal. Bath application of ANG II to neuronal cultures stimulate the release of TNF-α and IL-1β (1), but application of candesartan suppresses ANG II-induced release of these proinflammatory cytokines (5). Microglia express receptors for proinflammatory cytokines (17, 53), and stimulation of these receptors can trigger microglia to release their own proinflammatory cytokines (40). Thus, activation of AT1a expressed on neurons within the PVN may cause these neurons to release proinflammatory cytokines, which stimulate proinflammatory cytokine receptors expressed on microglia in close proximity, which in turn, elicits their release of proinflammatory cytokines. Deletion of AT1a from neurons within the PVN may prevent this “feed-forward” paracrine signaling, thereby inhibiting microglial infiltration and release of proinflammatory cytokines within the hypothalamus. Additional studies are required to evaluate the validity of this hypothesis.
The present study found that deletion of AT1a from the PVN attenuated CRH mRNA expression, blunted cardiovascular responses to psychogenic stress, and reduced anxiety-like behavior. Although AT1a mRNA was absent in microglia, deletion of AT1a from the PVN reduced hypothalamic indexes of microglia infiltration and proinflammatory cytokine expression, and these effects may contribute to the decreased cardiovascular reactivity and anxiolytic phenotype of PVN AT1a KO mice. Because AT1a are known to regulate CRH transcription and neuroinflammation, which in turn, are implicated in a variety of stress-related neuropsychiatric disorders, it is possible that augmented AT1a signaling within the central nervous system facilitates the onset of such disorders. Consequently, brain angiotensin receptor signaling may be a viable therapeutic target for the alleviation of stress-related diseases such as anxiety disorders.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-096830 (E. G. Krause), HL-122494 (E. G. Krause), HL-125805 (A. D. de Kloet), HL-116074 (A. D. de Kloet).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
L.W., A.D.d.K., and E.G.K. conception and design of research; L.W., H.H., J.A.S., A.D.d.K., and E.G.K. performed experiments; L.W., H.H., A.D.d.K., and E.G.K. analyzed data; L.W., A.D.d.K., and E.G.K. interpreted results of experiments; L.W., A.D.d.K., and E.G.K. prepared figures; L.W., A.D.d.K., and E.G.K. drafted manuscript; L.W., H.H., A.D.d.K., and E.G.K. edited and revised manuscript; L.W., H.H., J.A.S., and A.D.d.K. approved final version of manuscript.
REFERENCES
- 1.Agarwal D, Dange RB, Raizada MK, Francis J. Angiotensin II causes imbalance between pro- and anti-inflammatory cytokines by modulating GSK-3beta in neuronal culture. Br J Pharmacol 169: 860–874, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguilera G, Kiss A, Luo X. Increased expression of type 1 angiotensin II receptors in the hypothalamic paraventricular nucleus following stress and glucocorticoid administration. J Neuroendocrinol 7: 775–783, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Aguilera G, Young WS, Kiss A, Bathia A. Direct regulation of hypothalamic corticotropin-releasing-hormone neurons by angiotensin II. Neuroendocrinology 61: 437–444, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123: 493–505, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Benicky J, Sanchez-Lemus E, Honda M, Pang T, Orecna M, Wang J, Leng Y, Chuang DM, Saavedra JM. Angiotensin II AT1 receptor blockade ameliorates brain inflammation. Neuropsychopharmacology 36: 857–870, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biancardi VC, Son SJ, Ahmadi S, Filosa JA, Stern JE. Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood-brain barrier. Hypertension 63: 572–579, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blandino P Jr, Barnum CJ, Deak T. The involvement of norepinephrine and microglia in hypothalamic and splenic IL-1beta responses to stress. J Neuroimmunol 173: 87–95, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Blandino P Jr, Barnum CJ, Solomon LG, Larish Y, Lankow BS, Deak T. Gene expression changes in the hypothalamus provide evidence for regionally-selective changes in IL-1 and microglial markers after acute stress. Brain Behav Immun 23: 958–968, 2009. [DOI] [PubMed] [Google Scholar]
- 9.Braszko JJ, Karwowska-Polecka W, Halicka D, Gard PR. Captopril and enalapril improve cognition and depressed mood in hypertensive patients. J Basic Clin Physiol Pharmacol 14: 323–343, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Busnardo C, Tavares RF, Correa FM. Angiotensinergic neurotransmission in the paraventricular nucleus of the hypothalamus modulates the pressor response to acute restraint stress in rats. Neuroscience 270: 12–19, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Dantzer R. Cytokine, sickness behavior, depression. Neurol Clin 24: 441–460, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Kloet AD, Krause EG, Scott KA, Foster MT, Herman JP, Sakai RR, Seeley RJ, Woods SC. Central angiotensin II has catabolic action at white and brown adipose tissue. Am J Physiol Endocrinol Metab 301: E1081–E1091, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Kloet AD, Liu M, Rodriguez V, Krause EG, Sumners C. Role of neurons and glia in the CNS actions of the renin-angiotensin system in cardiovascular control. Am J Physiol Regul Integr Comp Physiol 309: R444–R458, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Kloet AD, Pati D, Wang L, Hiller H, Sumners C, Frazier CJ, Seeley RJ, Herman JP, Woods SC, Krause EG. Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus protect against diet-induced obesity. J Neurosci 33: 4825–4833, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Kloet AD, Wang L, Ludin JA, Smith JA, Pioquinto DJ, Hiller H, Steckelings UM, Scheuer DA, Sumners C, Krause EG. Reporter mouse strain provides a novel look at angiotensin type-2 receptor distribution in the central nervous system. Brain Struct Funct 221: 891–912, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Kloet ER. Hormones, brain and stress. Endocr Regul 37: 51–68, 2003. [PubMed] [Google Scholar]
- 17.Dopp JM, Mackenzie-Graham A, Otero GC, Merrill JE. Differential expression, cytokine modulation, and specific functions of type-1 and type-2 tumor necrosis factor receptors in rat glia. J Neuroimmunol 75: 104–112, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Duan SZ, Christe M, Milstone DS, Mortensen RM. Go but not Gi2 or Gi3 is required for muscarinic regulation of heart rate and heart rate variability in mice. Biochem Biophys Res Commun 357: 139–143, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunn AJ. Cytokine activation of the HPA axis. Ann NY Acad Sci 917: 608–617, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Faravelli C, Lo Sauro C, Lelli L, Pietrini F, Lazzeretti L, Godini L, Benni L, Fioravanti G, Talamba GA, Castellini G, Ricca V. The role of life events and HPA axis in anxiety disorders: a review. Curr Pharmaceut Design 18: 5663–5674, 2012. [DOI] [PubMed] [Google Scholar]
- 21.File SE, Johnston AL, Baldwin HA. Anxiolytic and anxiogenic drugs - changes in behavior and endocrine responses. Stress Med 4: 221–230, 1988. [Google Scholar]
- 22.Fry M, Ferguson AV. The sensory circumventricular organs: brain targets for circulating signals controlling ingestive behavior. Physiol Behav 91: 413–423, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Fyffe SL, Neul JL, Samaco RC, Chao HT, Ben-Shachar S, Moretti P, McGill BE, Goulding EH, Sullivan E, Tecott LH, Zoghbi HY. Deletion of Mecp2 in Sim1-expressing neurons reveals a critical role for MeCP2 in feeding behavior, aggression, and the response to stress. Neuron 59: 947–958, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gadek-Michalska A, Tadeusz J, Rachwalska P, Spyrka J, Bugajski J. Brain nitric oxide synthases in the interleukin-1beta-induced activation of hypothalamic-pituitary-adrenal axis. Pharmacol Rep 64: 1455–1465, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez AD, Wang G, Waters EM, Gonzales KL, Speth RC, Van Kempen TA, Marques-Lopes J, Young CN, Butler SD, Davisson RL, Iadecola C, Pickel VM, Pierce JP, Milner TA. Distribution of angiotensin type 1a receptor-containing cells in the brains of bacterial artificial chromosome transgenic mice. Neuroscience 226: 489–509, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gutman DA, Owens MJ, Skelton KH, Thrivikraman KV, Nemeroff CB. The corticotropin-releasing factor1 receptor antagonist R121919 attenuates the behavioral and endocrine responses to stress. J Pharmacol Exp Therapeut 304: 874–880, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Haarman BC, Riemersma-Van der Lek RF, de Groot JC, Ruhe HG, Klein HC, Zandstra TE, Burger H, Schoevers RA, de Vries EF, Drexhage HA, Nolen WA, Doorduin J. Neuroinflammation in bipolar disorder - A [(11)C]-(R)-PK11195 positron emission tomography study. Brain Behav Immun 40: 219–225, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Heinrichs SC, De Souza EB, Schulteis G, Lapsansky JL, Grigoriadis DE. Brain penetrance, receptor occupancy and antistress in vivo efficacy of a small molecule corticotropin releasing factor type I receptor selective antagonist. Neuropsychopharmacology 27: 194–202, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Herman JP, Cullinan WE. Neurocircuitry of stress: Central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci 20: 78–84, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Hou RH, Baldwin DS. A neuroimmunological perspective on anxiety disorders. Hum Psychopharm Clin 27: 6–14, 2012. [DOI] [PubMed] [Google Scholar]
- 31.Hurt RC, Garrett JC, Keifer OP Jr, Linares A, Couling L, Speth RC, Ressler KJ, Marvar PJ. Angiotensin type 1a receptors on corticotropin-releasing factor neurons contribute to the expression of conditioned fear. Genes Brain Behav 14: 526–533, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jarrett B, Godbout J, McKim D, Sheridan J. Minocycline attenuates stress-induced changes in leukocyte activation and anxiety-like behavior. Brain Behav Immun 40, Suppl: e23–e24, 2014. [Google Scholar]
- 33.Jezova D, Ochedalski T, Kiss A, Aguilera G. Brain angiotensin II modulates sympathoadrenal and hypothalamic pituitary adrenocortical activation during stress. J Neuroendocrinol 10: 67–72, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Karson A, Demirtas T, Bayramgurler D, Balci F, Utkan T. Chronic administration of infliximab (TNF-alpha inhibitor) decreases depression and anxiety-like behaviour in rat model of chronic mild stress. Basic Clin Pharmacol Toxicol 112: 335–340, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62: 617–627, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khoury NM, Marvar PJ, Gillespie CF, Wingo A, Schwartz A, Bradley B, Kramer M, Ressler KJ. The renin-angiotensin pathway in posttraumatic stress disorder: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are associated with fewer traumatic stress symptoms. J Clin Psychiatry 73: 849–855, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krause EG, de Kloet AD, Flak JN, Smeltzer MD, Solomon MB, Evanson NK, Woods SC, Sakai RR, Herman JP. Hydration state controls stress responsiveness and social behavior. J Neurosci 31: 5470–5476, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krause EG, de Kloet AD, Scott KA, Flak JN, Jones K, Smeltzer MD, Ulrich-Lai YM, Woods SC, Wilson SP, Reagan LP, Herman JP, Sakai RR. Blood-borne angiotensin II acts in the brain to influence behavioral and endocrine responses to psychogenic stress. J Neurosci 31: 15009–15015, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krause EG, Melhorn SJ, Davis JF, Scott KA, Ma LY, de Kloet AD, Benoit SC, Woods SC, Sakai RR. Angiotensin type 1 receptors in the subfornical organ mediate the drinking and hypothalamic-pituitary-adrenal response to systemic isoproterenol. Endocrinology 149: 6416–6424, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuno R, Wang JY, Kawanokuchi J, Takeuchi H, Mizuno T, Suzumura A. Autocrine activation of microglia by tumor necrosis factor-alpha. J Neuroimmunol 162: 89–96, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Kupferschmidt DA, Newman AE, Boonstra R, Erb S. Antagonism of cannabinoid 1 receptors reverses the anxiety-like behavior induced by central injections of corticotropin-releasing factor and cocaine withdrawal. Neuroscience 204: 125–133, 2012. [DOI] [PubMed] [Google Scholar]
- 42.Leong DS, Terron JA, Falcon-Neri A, Armando I, Ito T, Johren O, Tonelli LH, Hoe KL, Saavedra JM. Restraint stress modulates brain, pituitary and adrenal expression of angiotensin II AT(1A), AT(1B) and AT(2) receptors. Neuroendocrinology 75: 227–240, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Levine J, Barak Y, Chengappa KN, Rapoport A, Rebey M, Barak V. Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology 40: 171–176, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Mckinley MJ, Evered M, Mathai M, Coghlan JP. Effects of central losartan on plasma-renin and centrally mediated natriuresis. Kidney Int 46: 1479–1482, 1994. [DOI] [PubMed] [Google Scholar]
- 45.McKinley MJ, McBurnie MI, Mathai ML. Neural mechanisms subserving central angiotensinergic influences on plasma renin in sheep. Hypertension 37: 1375–1381, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Michaud JL, Rosenquist T, May NR, Fan CM. Development of neuroendocrine lineages requires the bHLH-PAS transcription factor SIM1. Genes Dev 12: 3264–3275, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miyoshi M, Miyano K, Moriyama N, Taniguchi M, Watanabe T. Angiotensin type 1 receptor antagonist inhibits lipopolysaccharide-induced stimulation of rat microglial cells by suppressing nuclear factor kappaB and activator protein-1 activation. Eur J Neurosci 27: 343–351, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Morrison FG, Ressler KJ. From the neurobiology of extinction to improved clinical treatments. Depress Anxiety 31: 279–290, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Najjar S, Pearlman DM, Hirsch S, Friedman K, Strange J, Reidy J, Khoukaz M, Ferrell RB, Devinsky O, Najjar A, Zagzag D. Brain biopsy findings link major depressive disorder to neuroinflammation, oxidative stress, and neurovascular dysfunction: a case report. Biol Psychiatry 75: e23–26, 2014. [DOI] [PubMed] [Google Scholar]
- 50.Neigh GN, Karelina K, Glasper ER, Bowers SLK, Zhang N, Popovich PG, DeVries AC. Anxiety after cardiac arrest/cardiopulmonary resuscitation exacerbated by stress and prevented by minocycline. Stroke 40: 3601–3607, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nijsen MJ, Croiset G, Stam R, Bruijnzeel A, Diamant M, de Wied D, Wiegant VM. The role of the CRH type 1 receptor in autonomic responses to corticotropin- releasing hormone in the rat. Neuropsychopharmacology 22: 388–399, 2000. [DOI] [PubMed] [Google Scholar]
- 52.Oldfield BJ, Davern PJ, Giles ME, Allen AM, Badoer E, McKinley MJ. Efferent neural projections of angiotensin receptor (AT1) expressing neurones in the hypothalamic paraventricular nucleus of the rat. J Neuroendocrinol 13: 139–146, 2001. [DOI] [PubMed] [Google Scholar]
- 53.Pinteaux E, Parker LC, Rothwell NJ, Luheshi GN. Expression of interleukin-1 receptors and their role in interleukin-1 actions in murine microglial cells. J Neurochem 83: 754–763, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Raasch W, Wittmershaus C, Dendorfer A, Voges I, Pahlke F, Dodt C, Dominiak P, Johren O. Angiotensin II inhibition reduces stress sensitivity of hypothalamo-pituitary-adrenal axis in spontaneously hypertensive rats. Endocrinology 147: 3539–3546, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry 58: 445–452, 2001. [DOI] [PubMed] [Google Scholar]
- 56.Rossi S, Sacchetti L, Napolitano F, De Chiara V, Motta C, Studer V, Musella A, Barbieri F, Bari M, Bernardi G, Maccarrone M, Usiello A, Centonze D. Interleukin-1beta causes anxiety by interacting with the endocannabinoid system. J Neurosci 32: 13896–13905, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roth WT, Doberenz S, Dietel A, Conrad A, Mueller A, Wollburg E, Meuret AE, Barr Taylor C, Kim S. Sympathetic activation in broadly defined generalized anxiety disorder. J Psychiatr Res 42: 205–212, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruyle BC, Callanan GF, Haney MM, Coldren KM, Heesch CM, Hasser EM. Localization of corticotropin releasing factor receptor 2 (CRFR2) in the nucleus tractus solitarii (nTS). FASEB J 30: 755–7., 2016. [Google Scholar]
- 59.Saavedra JM, Ando H, Armando I, Baiardi G, Bregonzio C, Juorio A, Macova M. Anti-stress and anti-anxiety effects of centrally acting angiotensin II AT1 receptor antagonists. Regul Pept 128: 227–238, 2005. [DOI] [PubMed] [Google Scholar]
- 60.Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, Suridjan I, Kennedy JL, Rekkas PV, Houle S, Meyer JH. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 72: 268–275, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shi Z, Gan XB, Fan ZD, Zhang F, Zhou YB, Gao XY, De W, Zhu GQ. Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxf) 203: 289–297, 2011. [DOI] [PubMed] [Google Scholar]
- 62.Smith JA, Wang L, Hiller H, Taylor CT, de Kloet AD, Krause EG. Acute hypernatremia promotes anxiolysis and attenuates stress-induced activation of the hypothalamic-pituitary-adrenal axis in male mice. Physiol Behav 136: 91–96, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sugama S, Fujita M, Hashimoto M, Conti B. Stress induced morphological microglial activation in the rodent brain: involvement of interleukin-18. Neuroscience 146: 1388–1399, 2007. [DOI] [PubMed] [Google Scholar]
- 64.Takahashi H, Nishimura M, Sakamoto M, Ikegaki I, Nakanishi T, Yoshimura M. Effects of interleukin-1 beta on blood pressure, sympathetic nerve activity, and pituitary endocrine functions in anesthetized rats. Am J Hypertens 5: 224–229, 1992. [DOI] [PubMed] [Google Scholar]
- 65.Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JM, Stalla GK, Blanquet V, Steckler T, Holsboer F, Wurst W. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet 19: 162–166, 1998. [DOI] [PubMed] [Google Scholar]
- 66.van Gaalen MM, Stenzel-Poore MP, Holsboer F, Steckler T. Effects of transgenic overproduction of CRH on anxiety-like behaviour. Eur J Neurosci 15: 2007–2015, 2002. [DOI] [PubMed] [Google Scholar]
- 67.van Houten M, Schiffrin EL, Mann JF, Posner BI, Boucher R. Radioautographic localization of specific binding sites for blood-borne angiotensin II in the rat brain. Brain Res 186: 480–485, 1980. [DOI] [PubMed] [Google Scholar]
- 68.Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 428: 191–212, 2000. [DOI] [PubMed] [Google Scholar]
- 69.Villapol S, Yaszemski AK, Logan TT, Sanchez-Lemus E, Saavedra JM, Symes AJ. Candesartan, an angiotensin II AT(1)-receptor blocker and PPAR-gamma agonist, reduces lesion volume and improves motor and memory function after traumatic brain injury in mice. Neuropsychopharmacology 37: 2817–2829, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vogelzangs N, Beekman AT, de Jonge P, Penninx BW. Anxiety disorders and inflammation in a large adult cohort. Transl Psychiatry 3: e249, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang L, de Kloet AD, Pati D, Hiller H, Smith JA, Pioquinto DJ, Ludin JA, Oh SP, Katovich MJ, Frazier CJ, Raizada MK, Krause EG. Increasing brain angiotensin converting enzyme 2 activity decreases anxiety-like behavior in male mice by activating central Mas receptors. Neuropharmacology 105: 114–123, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Williams TD, Chambers JB, Gagnon SP, Roberts LM, Henderson RP, Overton JM. Cardiovascular and metabolic responses to fasting and thermoneutrality in Ay mice. Physiol Behav 78: 615–623, 2003. [DOI] [PubMed] [Google Scholar]
- 73.Yang G, Xi ZX, Wan Y, Wang H, Bi G. Changes in circulating and tissue angiotensin II during acute and chronic stress. Biol Signals 2: 166–172, 1993. [DOI] [PubMed] [Google Scholar]
- 74.Yoshida S, Takeuchi T, Kotani T, Yamamoto N, Hata K, Nagai K, Shoda T, Takai S, Makino S, Hanafusa T. Infliximab, a TNF-alpha inhibitor, reduces 24-h ambulatory blood pressure in rheumatoid arthritis patients. J Hum Hypertens 28: 165–169, 2014. [DOI] [PubMed] [Google Scholar]
- 75.Zhu GQ, Patel KP, Zucker IH, Wang W. Microinjection of ANG II into paraventricular nucleus enhances cardiac sympathetic afferent reflex in rats. Am J Physiol Heart Circ Physiol 282: H2039–H2045, 2002. [DOI] [PubMed] [Google Scholar]