Abstract
Anopheles gambiae densovirus (AgDNV) is a potential microbial agent for paratransgenesis and gene transduction in An. gambiae, the major vector of human malaria in sub-Saharan Africa. Understanding the interaction between AgDNV and An. gambiae is critical for using AgDNV in a basic and applied manner for Anopheles gene manipulation. Here, we tested the effects of mosquito age, sex, blood feeding status, and potential for horizontal transmission using an enhanced green fluorescent protein (EGFP) reporter AgDNV system. Neither mosquito age at infection nor feeding regime affected viral titers. Female mosquitoes were more permissive to viral infection than males. Despite low viral titers, infected males were able to venereally transmit virus to females during mating, where the virus was localized with the transferred sperm in the spermathecae. These findings will be useful for designing AgDNV-based strategies to manipulate Anopheles gambiae.
Keywords: Malaria, Paratransgenesis, Gene transduction, Sex-specific differences, Vector-borne disease, Disease control
Introduction
Mosquitoes in the genus Anopheles are the only arthropods capable of transmitting the Plasmodium parasites that cause human malaria, which infects over 200 million people and kills nearly one million people globally each year (Enayati & Hemingway, 2010). Anopheles gambiae is the most efficient human malaria vector in sub-Saharan Africa (Mendes et al., 2008). Paratransgenesis, the genetic manipulation of insect symbiotic microorganisms to inhibit pathogen replication in the hosts, has been proposed as a novel strategy to control mosquito-borne diseases such as malaria (Ren, Hoiczyk & Rasgon, 2008; Rasgon, 2011; Wang & Jacobs-Lorena, 2013). Previous studies demonstrated that paratransgenesis is able to dramatically diminish Plasmodium parasite density in An. gambiae (Fang et al., 2011; Wang et al., 2012). In addition to pathogen restriction in individual hosts, methods to spread transgenic symbionts into natural mosquito populations are critical for the application of this technology (Rasgon, 2008). Understanding basic interactions between the paratransgenic agent and its host arthropod is critical to develop successful paratransgenesis-based approaches for disease control.
Densonucleosis viruses (densoviruses (DNVs)) are non-enveloped, single-stranded parvoviruses that have been identified from many invertebrate taxa, including various mosquito species (Carlson, Suchman & Buchatsky, 2006). Their stability, narrow host range, and pathogenicity make DNVs attractive as bio-pesticides (Carlson, Suchman & Buchatsky, 2006; Suzuki et al., 2015). In mosquitoes, Aedes aegypti densovirus (AeDNV) has been intensively studied as a transducing vector and biocontrol agent against Aedes mosquitoes (Allen-Miura et al., 1999; Ledermann et al., 2004; Gu et al., 2010). We recently reported that An. gambiae DNV (AgDNV) can efficiently transduce exogenous genes in An. gambiae without serious pathogenic effects; DNV-infected mosquitoes had similar immature and adult lifespan compared to uninfected controls, and infection by the virus had minimal effects on host gene expression (Ren, Hoiczyk & Rasgon, 2008; Ren & Rasgon, 2010; Ren et al., 2014; Suzuki et al., 2014; Suzuki et al., 2015). These lack of viral-induced pathogenic effects make AgDNV a potentially useful as a viral paratransgenic agent for malaria control, as the agent itself is unlikely to be eliminated from the population due to negative selection. However, nothing is currently known about how basic components of mosquito biology (such as aging, feeding and mating) affect AgDNV infection dynamics in the mosquito, which could potentially affect disease control efforts. Here, we tested the effects of mosquito age, sex, blood feeding status, and potential for horizontal transmission to investigate the potential for these factors to help or hinder AgDNV spread in natural populations.
Methods
Insect cell culture and mosquito rearing
Uninfected An. gambiae Moss55 cells were cultured in Schneider’s medium (Sigma) supplemented with 10% fetal bovine serum (FBS) at 28 °C. An. gambiae mosquitoes (Keele strain) were reared at 28 °C and 80% relative humidity with 12:12 h light:dark photoperiod and offered 10% sugar at all times. Female adult mosquitoes were blood fed on commercially purchased expired human blood through artificial feeding system. Larvae were fed with tetramin fish food. During experiments, mosquitoes were reared on sugar except in cases where the effect of bloodfeeding was being specifically examined.
Production of recombinant AgDNV samples
Recombinant viruses were produced by co-transfecting the recombinant AgDNV plasmid pUTRAcGFP (Suzuki et al., 2014) and the helper plasmid pBAgα (Ren, Hoiczyk & Rasgon, 2008) at a ratio of 2:1 using Lipofectamine LTX reagent (Life Technologies) according to the manufacturer’s protocol in 70% confluent Moss55 cells. After three days of incubation, cells were harvested and suspended in phosphate buffered saline (PBS). The cell suspension was subjected to freezing-thawing three times and cell debris removed by centrifugation. The supernatant was used as the viral inoculum for experiments.
Densovirus DNA quantification
For quantifying DNV levels in inoculum, samples were subjected to TURBO DNase (Ambion) treatment to digest any residual plasmid DNAs prior to DNA extraction. For quantification of DNV in mosquitoes, total DNA was extracted from DNV-injected mosquitoes or tissues using DNEasy kits (Qiagen) according to the manufacturer’s protocol. Quantitative polymerase chain reaction (PCR) (qPCR) was performed (either absolute titers using a standard curve or normalizing to the mosquito S7 gene depending on the experiment) using Quantitect SYBR Green Kit (Qiagen) on a RotorGene system (Qiagen) with previously described primers (Suzuki et al., 2014).
AgDNV infection of An. gambiae mosquitoes
For mosquito infections, 20 adult mosquitoes per treatment were injected intrathoracically with virus as previously described at 106 and 107 viral genome equivalents per ml (vge/ml) as described in the text (Suzuki et al., 2014; Suzuki et al., 2015). For testing the effect of mosquito age on infection, mosquitoes were infected at 1 and 7 days post-emergence, and were held for an additional seven days before assaying them for virus. For testing the effect of mosquito feeding on infection, we controlled for the effect of mosquito age by infecting mosquitoes in all three treatments seven days post-emergence, and held them for an additional seven days before being assayed for infection. Mosquitoes in treatment 1 were maintained only on sugar for the entire time. Mosquitoes in treatment 2 were bloodfed three days post-injection (10 days post-emergence). Mosquitoes in treatment 3 were bloodfed one day prior to virus injection (six days post-emergence). For monitoring viral in vivo replication, mosquitoes were infected five days post-emergence and sampled three, seven and 12 days post-injection.
Microscopy analysis of EGFP expression
After microinjection, five individual mosquitoes/sex/treatment were randomly collected at the time points listed in the text and fluorescence observed using an Olympus BX-41 epifluorescent microscope. Images were processed using Picture Frame software (Olympus). After microscopy analysis, mosquitoes were stored at −80 °C until DNA was extracted for qPCR analysis.
Venereal transmission
Male and female mosquitoes were separated at the pupal stage and kept in different cages. The 5-day-old males were injected with vUTRAcGFP or media as described above. Three days post-injection, males were allowed to mate with uninfected females for seven days. After mating, females were collected, examined for EGFP by microscopy, and their spermathecae dissected into PBS. Viral levels in the spermathecae and carcasses were assayed by qPCR.
Statistical analysis
Experiments with paired treatments were analyzed by t-tests. Experiments with > 2 treatments were analyzed by ordinary one-way or two-way analysis of variance (ANOVA) as appropriate with Bonferroni’s correction for multiple tests. Prior to analysis, data were tested for adherence to normality using the Shapiro-Wilk normality test. Data that did not conform to normality assumptions were log transformed prior to analysis. All analyses were conducted using GraphPad Prism v.7.
Results
Mosquito age and AgDNV replication
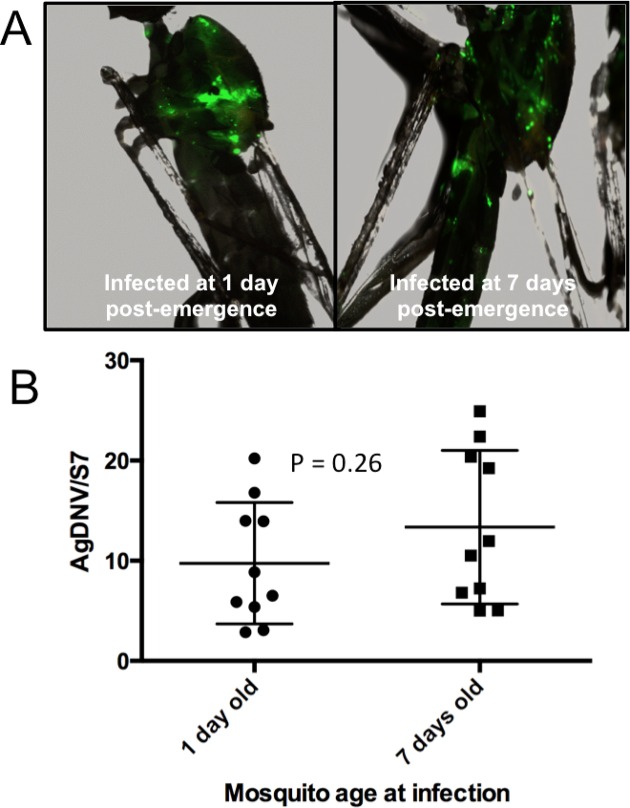
Adult female mosquitoes (one or seven days post emergence) were intrathoracically inoculated with 107 viral genome equivalents (vge) of EGFP-transducing AgDNV (vUTRAcGFP) (Suzuki et al., 2014). Seven days post infection, mosquitoes were collected and examine for EGFP expression by fluorescent microscope. Mosquitoes infected at one and seven days post emergence exhibited qualitatively similar levels of EGFP expression (Fig. 1A), and quantitatively similar levels of viral genomes by qPCR (T = 1.17, df = 18, P = 0.26) (Fig. 1B).
Figure 1. Effect of mosquito age at infection on AgDNV replication.
Mosquitoes were infected at either one or seven days post-emergence. (A) EGFP expression. (B) Quantitation of AgDNV by qPCR. Infection levels between treatments are not statistically different (T-test).
Mosquito blood feeding and AgDNV replication
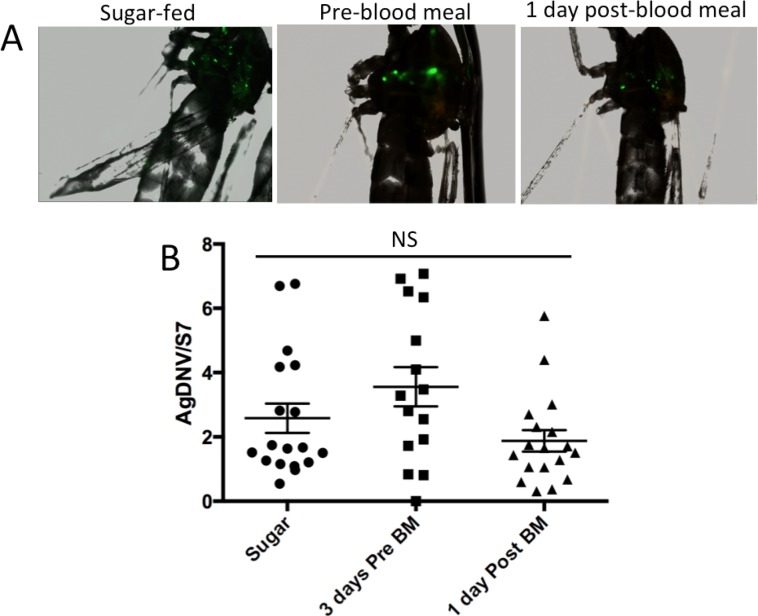
Adult females were injected with 106 vUTRAcGFP at three days before blood feeding, one day after blood feeding, or were only sugar-fed. Seven days post infection, mosquitoes were collected and examine for EGFP expression by fluorescent microscope. All three treatments exhibited qualitatively similar levels of EGFP expression (Fig. 2A). We then confirmed fluorescence results by qPCR to measure viral titers. Data were log transformed and analyzed by one-way ANOVA. Infection titers between treatments were not statistically different (F2, 48 = 0.5136, P = 0.6).
Figure 2. Effect of mosquito feeding on AgDNV replication.
Mosquitoes were either sugar-fed, infected three days pre-blood meal, or infected one day post blood-meal. (A) EGFP expression. (B) Quantitation of AgDNV by qPCR. Infection levels between treatments are not statistically different (ANOVA).
An. gambiae females are more permissive to AgDNV than males
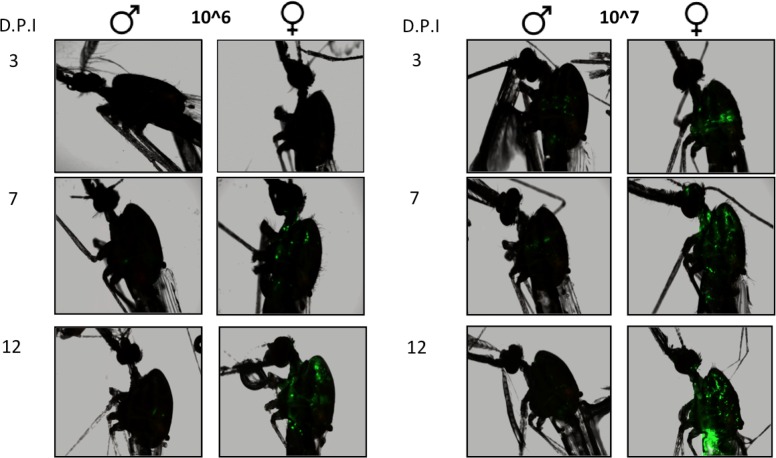
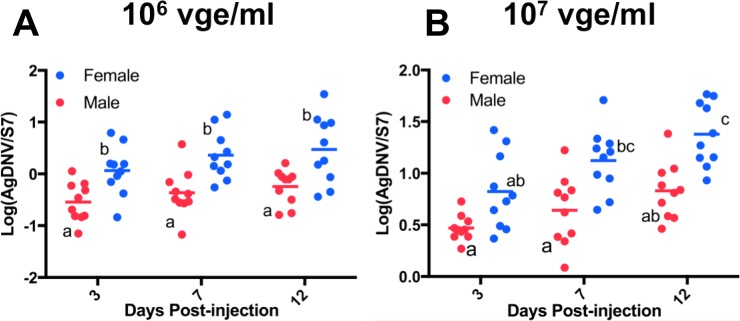
The 5-day-old female and male mosquitoes were injected with 106 or 107 vUTRAcGFP and assayed at three, seven and 12 days post-injection to monitor EGFP expression. Female mosquitoes showed an increase in EGFP fluorescence in a dose- and time-dependent manner (Fig. 3). Fluorescence results were independently confirmed by qPCR (Fig. 4). Data were log transformed and analyzed by ordinary 2-way ANOVA. At 106 vge/ml, at all time points females exhibited greater viral titers compared to males. (F1, 54 = 31.73, P < 0.0001). There was a trend toward increased viral titer in females over time, but this was not significant (F2, 54 = 2.897, P = 0.06). At 107 vge/ml, viral titers varied significantly by both sex (F1, 54 = 36.34, P < 0.0001) and time (F2, 54 = 11.86, P < 0.0001) (Fig. 4). The interaction terms were not significant.
Figure 3. Effect of sex, time, and viral dose on AgDNV replication, assessed by EGFP fluorescence.
At all time points and dosages, females exhibit greater EGFP expression compared to males. D.P.I., days post-injection.
Figure 4. Effect of sex, time, and viral dose on AgDNV replication, assessed by qPCR.
At all time points and dosages, females exhibit greater AgDNV titers compared to males. Data were analyzed by ANOVA. Treatments with different lowercase letters denote statistical significance (P < 0.05).
AgDNV can be venereally transmitted from males to females
Although females were more permissive to AgDNV infection than males, males did get infected. We therefore investigated the possibility that males could transfer AgDNV to females during mating. Male mosquitoes were injected with 107 vUTRAcGFP or media as a control. Three days-post injection, males were mated to uninfected females. Mosquitoes were allowed to mate for seven days, after which females were collected to assay for virus. EGFP was not observed in females mated to AgDNV-infected males (data not shown). Spermathecae of mated females were dissected and the spermathecae and carcasses assayed for virus by qPCR. Data were analyzed by ordinary one-way ANOVA. Viral titers were significantly higher in spermethecae of females mated to infected males (F3, 8 = 34.04, P < 0.0001) compared to the spermethecae of females mated to uninfected males, or the carcasses of females mated to either male type (Fig. 5).
Figure 5. AgDNV can be venereally transmitted from males to females, and is present in the spermathecae.
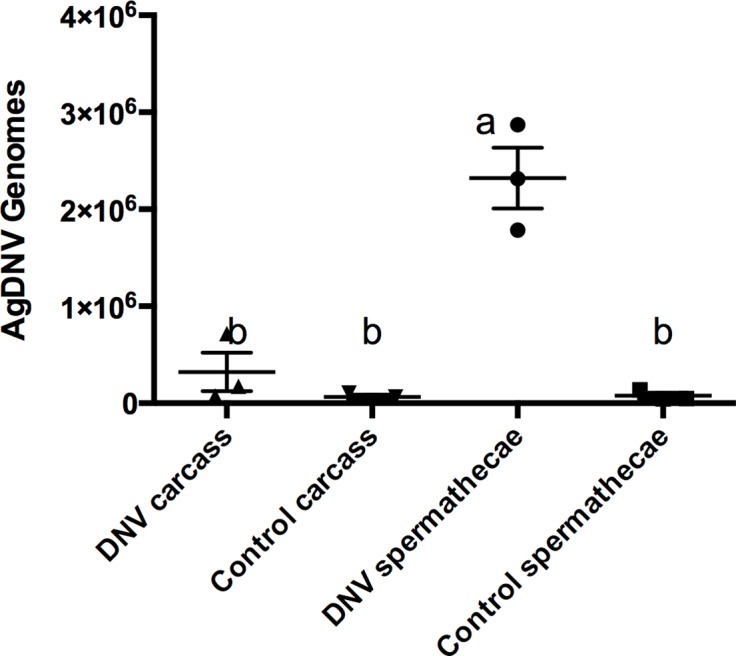
Data were analyzed by ANOVA.
Discussion
Paratransgenesis, the genetic manipulation of arthropod symbiotic microorganisms, is one potential strategy to control mosquito-borne diseases (Ren, Hoiczyk & Rasgon, 2008; Ren & Rasgon, 2010; Rasgon, 2011; Fang et al., 2011; Wang et al., 2012; Wang & Jacobs-Lorena, 2013). Interactions between mosquito hosts and their microbes need to be well-understood for successful design of paratransgenic control methods. Here, we investigated the effects of mosquito age, feeding status and sex on AgDNV infection.
Neither timing of infection after emergence or mosquito feeding significantly affected virus replication. However, female mosquitoes were more permissive to virus infection than males at multiple viral titers. We previously showed that the mosquito fat body is a main location of viral replication (Ren, Hoiczyk & Rasgon, 2008; Suzuki et al., 2014; Suzuki et al., 2015). Females are larger and have greater abundance of this tissue, and differences in infection levels may simply be due to differences in amounts of permissive tissue between sexes.
Although males were less permissive to virus infection than females, they were able to transmit the virus venereally to their mates. However, EGFP expression and qPCR indicated that transferred virus was restricted to the female spermatheca and did not disseminate in the female. This is similar to Ae. albopictus parvovirus (AaPV), which was venereally transmitted at a low rate without detectable dissemination (Barreau, Jousset & Bergoin, 1997). As AgDNV is transmitted vertically from mother to offspring (Ren, Hoiczyk & Rasgon, 2008), future studies should test the possibility that venereally transferred virus may be able to infect offspring during fertilization, as it may facilitate the spread of the virus into natural populations during a paratransgenic control release.
Supplemental Information
Acknowledgments
We thank Mrs. Rhiannon Barry for Insectary support. We thank Dr. Robert Tesh for his kind gift of the Mos55 cells.
Funding Statement
This research was funded by NIH grants R21AI088311, R21AI128918, R21AI111175, and R01AI116636 to JLR. TKB was partially supported by a Raman postdoctoral fellowship from the University Grant Commission, Government of India. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Tapan K. Barik conceived and designed the experiments, performed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Yasutsugu Suzuki conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Jason L. Rasgon conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Data Deposition
The following information was supplied regarding data availability:
All raw data are presented in the relevant figures.
References
- Allen-Miura et al. (1999).Allen-Miura TM, Afanasiev BN, Olson KE, Beaty BJ, Carlson JO. Packaging of AeDNV-GFP transducing virus by expression of densovirus structural proteins from a sindbis virus expression system. Virology. 1999;257(1):54–61. doi: 10.1006/viro.1999.9622. [DOI] [PubMed] [Google Scholar]
- Barreau, Jousset & Bergoin (1997).Barreau C, Jousset FX, Bergoin M. Venereal and vertical transmission of the Aedes albopictus parvovirus in Aedes aegypti mosquitoes. American Journal of Tropical Medicine and Hygiene. 1997;57(2):126–131. doi: 10.4269/ajtmh.1997.57.126. [DOI] [PubMed] [Google Scholar]
- Carlson, Suchman & Buchatsky (2006).Carlson J, Suchman E, Buchatsky L. Densoviruses for control and genetic manipulation of mosquitoes. Advances in Virus Research. 2006;68:361–392. doi: 10.1016/S0065-3527(06)68010-X. [DOI] [PubMed] [Google Scholar]
- Enayati & Hemingway (2010).Enayati A, Hemingway J. Malaria management: past, present, and future. Annual Review of Entomology. 2010;55(1):569–591. doi: 10.1146/annurev-ento-112408-085423. [DOI] [PubMed] [Google Scholar]
- Fang et al. (2011).Fang W, Vega-Rodríguez J, Ghosh AK, Jacobs-Lorena M, Kang A, St Leger RJ. Development of transgenic fungi that kill human malaria parasites in mosquitoes. Science. 2011;331(6020):1074–1077. doi: 10.1126/science.1199115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu et al. (2010).Gu J-B, Dong Y-Q, Peng H-J, Chen X-G. A recombinant AeDNA containing the insect-specific toxin, BmK IT1, displayed an increasing pathogenicity on Aedes albopictus. American Journal of Tropical Medicine and Hygiene. 2010;83(3):614–623. doi: 10.4269/ajtmh.2010.10-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledermann et al. (2004).Ledermann JP, Suchman EL, Black WC, IV, Carlson JO. Infection and pathogenicity of the mosquito densoviruses AeDNV, HeDNV, and APeDNV in Aedes aegypti mosquitoes (Diptera: Culicidae) Journal of Economic Entomology. 2004;97(6):1828–1835. doi: 10.1093/jee/97.6.1828. [DOI] [PubMed] [Google Scholar]
- Mendes et al. (2008).Mendes AM, Schlegelmilch T, Cohuet A, Awono-Ambene P, De Iorio M, Fontenille D, Morlais I, Christophides GK, Kafatos FC, Vlachou D. Conserved mosquito/parasite interactions affect development of Plasmodium falciparum in Africa. PLoS Pathogens. 2008;4(5):e1000069. doi: 10.1371/journal.ppat.1000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon (2008).Rasgon JL. Using predictive models to optimize Wolbachia-based strategies for vector-borne disease control. Advances in Experimental Medicine and Biology. 2008;627:114–125. doi: 10.1007/978-0-387-78225-6_10. [DOI] [PubMed] [Google Scholar]
- Rasgon (2011).Rasgon JL. Using infections to fight infections: paratransgenic fungi can block malaria transmission in mosquitoes. Future Microbiology. 2011;6(8):851–853. doi: 10.2217/fmb.11.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, Hoiczyk & Rasgon (2008).Ren X, Hoiczyk E, Rasgon JL. Viral paratransgenesis in the malaria vector Anopheles gambiae. PLoS Pathogens. 2008;4(8):e1000135. doi: 10.1371/journal.ppat.1000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren et al. (2014).Ren X, Hughes GL, Niu G, Suzuki Y, Rasgon JL. Anopheles gambiae densovirus (AgDNV) has negligible effects on adult survival and transcriptome of its mosquito host. PeerJ. 2014;2:e584. doi: 10.7717/peerj.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren & Rasgon (2010).Ren X, Rasgon JL. Potential for the Anopheles gambiae densonucleosis virus to act as an “evolution-proof” biopesticide. Journal of Virology. 2010;84(15):7726–7729. doi: 10.1128/JVI.00631-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki et al. (2015).Suzuki Y, Barik TK, Johnson RM, Rasgon JL. In vitro and in vivo host range of Anopheles gambiae densovirus (AgDNV) Scientific Reports. 2015;5:12701. doi: 10.1038/srep12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki et al. (2014).Suzuki Y, Niu G, Hughes GL, Rasgon JL. A viral over-expression system for the major malaria mosquito Anopheles gambiae. Scientific Reports. 2014;4:5127. doi: 10.1038/srep05127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2012).Wang S, Ghosh AK, Bongio N, Stebbings KA, Lampe DJ, Jacobs-Lorena M. Fighting malaria with engineered symbiotic bacteria from vector mosquitoes. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(31):12734–12739. doi: 10.1073/pnas.1204158109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang & Jacobs-Lorena (2013).Wang S, Jacobs-Lorena M. Genetic approaches to interfere with malaria transmission by vector mosquitoes. Trends in Biotechnology. 2013;31(3):185–193. doi: 10.1016/j.tibtech.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.