Abstract
Background
Malignant germ cell tumors of the ovary are relatively rare, making up less than 10% of all ovarian cancers. However, although they represent only a small fraction of ovarian cancers overall, they frequently affect adolescent women of reproductive age, making fertility sparing treatment of paramount importance. Malignant germ cell tumors are subdivided into dysgerminoma and non-dygerminomatous tumors. The most common types of nondysgerminomatous tumors are yolk sac and immature teratoma. Mixed germ cell tumors with embryonal carcinoma, nongestational choriocarcinoma, and polyembryoma are less common. Embryonal carcinomas, though rare, are one of the most malignant cancers arising in the ovary.
Case
A 19-year-old female with abdominal pain and massive ascites was found to have a malignant mixed ovarian germ cell tumor with a large embryonal component which was treated via surgical resection and chemotherapy.
Conclusions
Malignant germ cell tumors frequently affect adolescent women of reproductive age. Management of these tumors requires consideration of fertility sparing surgical techniques and chemotherapy management. Using these techniques, the vast majority of patients will maintain their ovarian function and the ability to bear children after their recovery.
Keywords: Mixed ovarian germ cell tumor, Embryonal carcinoma of the ovary, Fertility sparing surgery, BEP adjuvant chemotherapy
Introduction
Germ cell tumors of the ovary are relatively uncommon, making up less than 10% of ovarian cancers overall.1 Only 2% to 3% of germ cell tumors are malignant.2 These tumors originate from the primitive germ cell and then gradually differentiate to mimic the developmental tissues of embryonic origin (ectoderm, mesoderm, endoderm), and the extraembryonic tissues (yolk sac and trophoblast). Germ cell tumors that originate in the ovary have homologous counterparts in the testes.2 Pure embryonal carcinomas are extremely rare, more commonly occurring as a component of a mixed germ cell tumor. They are composed of primitive embryonal cells.
Most of these lesions are diagnosed by finding a palpable abdominal mass, often associated with pain. At times the pain is exacerbated by capsular rupture or torsion of the neoplasm, which classically results in hemoperitoneum. Because these tumors occur in a predominantly reproductive age group, they are commonly diagnosed in pregnancy.3 Many germ cell tumors produce serum markers that are specific and sensitive enough to be used clinically to follow disease progression, but initial diagnosis is largely based on imaging via ultrasound or computed tomography (CT). For early stage disease the aim is to minimize toxicity without compromising efficacy of treatment.4 Fertility-sparing surgery is feasible in most patients and at least 80% of those who receive postoperative chemotherapy will retain reproductive function.5 The prognosis for malignant ovarian germ cell tumors has improved vastly over the last 30 years, mainly due to modern combination chemotherapy.4 Today cure rates approach 100% for those with early-stage disease and at least 75% for patients with advanced-stage disease.
In this report, we present the case of a malignant mixed ovarian germ cell tumor with a large embryonal carcinoma component.
Case Report
A 19-year-old nulliparous female of Korean descent presented to our clinic with a history of intermittent abdominal pain and increasing abdominal girth which had slowly been worsening over two months. Though initial workup was begun in the outpatient setting, these symptoms soon prompted an emergency room visit for intractable abdominal pain, nausea, vomiting, shortness of breath, and tense abdominal distension. The patient also complained of significant weight gain over the same time period in conjunction with what she described as “abdominal swelling.” Her last menstrual period was 9 weeks prior to her presentation. The patient denied any history of sexual activity. She had no significant past medical or surgical history. On exam, she was found to have decreased breath sounds in the bilateral lung bases, tense ascites, diffuse tenderness to palpation of the abdomen, and distant bowel sounds.
Labs included a hemoglobin of 15.9, hematocrit of 48.8, and platelets of 346. Liver function tests including bilirubin, alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase were all within normal limits. Her β-human chorionic gonadotropin (β-hCG) was 77. CA-125 tumor marker, CA 19-9, and CEA were all within normal limits. However, α-fetoprotein (AFP) was elevated at 100 as was her lactate dehydrogenase (LDH) at 269. Computed tomography demonstrated small bilateral pleural effusions, massive generalized ascites, and a large pelvic mass measuring 11 × 11 cm, attached to the left adnexa, most likely arising from the left ovary. The mass was predominantly solid and was noted to be hypervascular, with blood supply arising from the left gonadal vessels. There was no intraperitoneal studding. See Fig. 1. Transabdominal ultrasound demonstrated a mixed cystic and solid midline pelvic mass measuring 11.2 × 8.8 × 10.7 cm, the solid component of which measured 7.7 × 6.8 × 7.7 cm and demonstrated internal flow. The origin of the mass could not be ascertained, but was felt to be likely from the left ovary because a separate, normal left ovary could not be visualized. The right ovary appeared normal, measuring 3.1 × 2 × 2.2 cm. See Fig. 2. Both the CT and ultrasound noted massive ascites.
Fig 1.
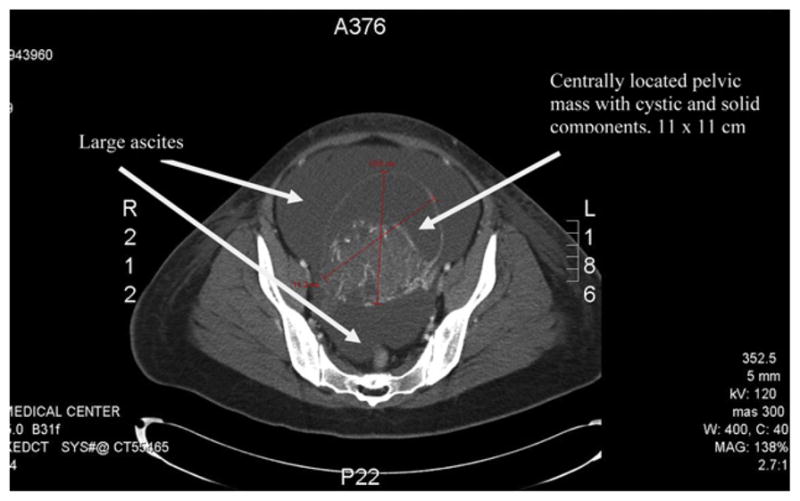
CT of abdomen and pelvis demonstrating a large pelvic mass attached to the left adnexa, most likely arising from the left ovary, also significant ascites.
Fig 2.
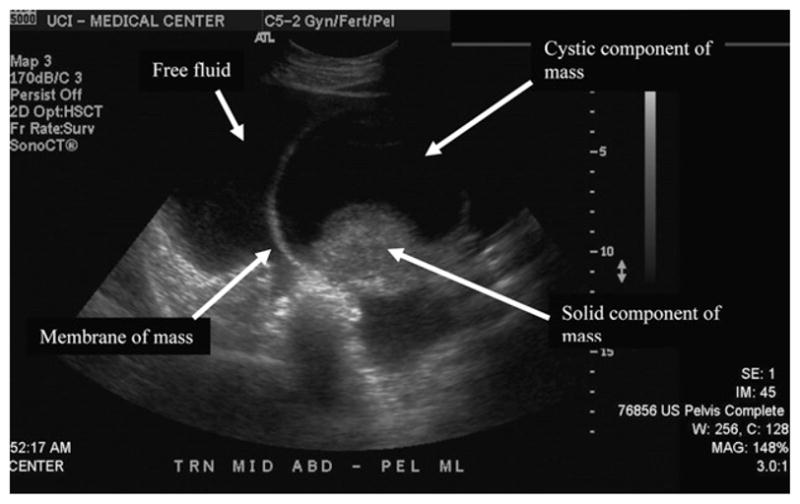
Transabdominal ultrasound demonstrating mixed cystic and solid midline pelvic mass with significant free fluid in the pelvis.
Exploratory laparotomy demonstrated nine liters of dark, blood-tinged ascites and a 10-cm cystic and solid mass originating from the left ovary. The cyst appeared ruptured. There was also a 1 × 1 cm omental nodule. There was no peritoneal carcinomatosis and no lesions on the liver or subdiaphragmatic areas. The pelvic and periaortic lymph nodes were normal on palpation. The uterus and right adnexa appeared normal with no lesions. Left salpingo-oophorectomy was performed and frozen section was consistent with choriocarcinoma of the ovary. Peritoneal biopsies were obtained, as well as pelvic washings. Infra-colic omentectomy was performed to include the area of the omental nodule.
Final pathology revealed mixed germ cell tumor with predominantly grade 3 immature teratoma (80%), embryonal carcinoma (15%), and mature teratoma (5%). Ascitic fluid and peritoneal biopsies were negative for malignancy. The omental nodule was found to be a focal, noncaseating granuloma, negative for acid-fast bacilli. Given these findings, the patient was given adjuvant chemotherapy consisting of three cycles of bleomycin, etoposide, and cisplatin (BEP). After this treatment, the patient’s tumor markers, including β-hCG, LDH, and AFP, returned to within normal limits. There were no significant complications associated with her chemotherapy. The patient resumed her menses 6 months after her last dose of chemotherapy and has continued to have regular menses each month, with flow and duration similar to that prior to her treatment.
Discussion
While there are many different malignant germ cell histologies, their treatment is the same: fertility-sparing surgery, if feasible, and postoperative chemotherapy for the majority of patients. Though malignant germ cell tumors of the ovary make up only a small proportion of ovarian cancers, less than 10%, they frequently affect adolescent women of reproductive age, making fertility sparing treatment of paramount importance. Embryonal carcinomas are one of the most malignant cancers arising in the ovary. With an age-adjusted incidence of 0.014 per 100,000 woman-years,6 they represent only 4% of all malignant ovarian germ cell tumors.3 They are a relatively undifferentiated product of the primordial germ cell. Macroscopically they are large, generally unilateral, with a yellow-grey tissue on transection. Histologically, polygonal or ovoid primitive epithelial cells with occasional gland-like structures are seen. They may secrete both β-hCG and AFP, which may aid in diagnosis and monitoring of treatment.1 Mean age of presentation is 15 years. Asian and black ethnic groups are affected by malignant germ cell tumors three times as frequently as Caucasian women.3
Definitive diagnosis is made by histology at the time of surgical excision. Preoperatively, the diagnosis is strongly suggested by findings of an adnexal mass on exam or pelvic imaging and elevation of tumor specific serum markers.
The standard management of malignant ovarian germ cell tumors is complete surgical excision. Most are unilateral, allowing for conservative, fertility sparing surgical treatment, with the addition of careful staging. In a retrospective case series, Nishio et al demonstrated that the type of surgical procedure was not an important prognostic factor for patients with malignant germ cell tumors of the ovary at all clinical stages, indicating that conservative, fertility sparing surgery is appropriate as long as chemotherapeutic agents are employed.7,8 Even in patients with bulky metastatic disease, a normal appearing uterus and contralateral ovary may be preserved allowing for future fertility options if desired. Though patients with bulky disease in the abdomen, pelvis, and retroperitoneum should be surgically cytoreduced to optimal residual disease if at all possible.3
Thorough surgical staging to confirm apparent FIGO stage IA, grade I immature teratomas or apparent FIGO stage IA dysgerminomas is crucial as it might identify women who can safely avoid postoperative chemotherapy.8 Patients in these categories have high cure rates with surgery alone.2 However there is a strong trend toward exploring the feasibility of surgery followed by close surveillance for a much broader group of patients. Due to the rarity of ovarian germ cell tumors, it would not be feasible to perform a randomized controlled trial comparing surveillance and adjuvant chemotherapy.9 A retrospective review of patients with stage IA malignant germ cell tumors followed closely (surveillance only) after surgery demonstrated a relapse rate of 36% for nondysgerminomas and 22% for dysgerminomas. All relapses occurred within 13 months of surgery. 91% of patients who experienced relapses were successfully salvaged with first relapse chemotherapy. One patient died of pulmonary embolus during relapse chemotherapy. Overall disease-specific survival was 93% after a median follow-up of 6 years.9 The authors concluded that surveillance of stage IA dysgerminomas and nondysgerminomatous tumors was safe and acceptable practice provided patients comply with the strict surveillance program and avoid pregnancy, which can mask elevation in tumor markers and therefore hinder surveillance.9
The evolution in the chemotherapeutic treatment of mixed ovarian germ cell tumors constitutes a major advance in clinical management.5 Prior to the advent of combination chemotherapy, non-dysgerminomatous germ cell tumors carried a dismal prognosis. Survival following surgery for stage I disease was in the region of 5–20%.10 In the 1970s combination therapy with VAC (vincristine, actinomycin-D, and cyclophosphamide) was introduced, which improved the cure rate to 86% for stage I disease, but the rate of long-term remission in metastatic disease remained less than 50% at the cost of significant side effects.11 With the adoption of the PVB (cisplatin, vinblastine, bleomycin) regimen cure rates again climbed to more than 90% in patients with stage I disease, but toxicity remained substantial.12 In the 1980s, when a novel etopo-side containing regimen, BEP (bleomycin, etoposide, cisplatin) proved efficacious and relatively nontoxic in male germ cell tumors,13 it was soon tried in ovarian germ cell tumors14 and quickly became the standard of care.
However, this improvement in survival was not without some cost. Toxicities of the BEP regimen include hair loss, fatigue, nausea, and myelosuppression. Cisplatin is also associated with nerve damage manifested as peripheral neuropathy or hearing loss. An uncommon but potentially fatal side effect is bleomycin-induced pulmonary fibrosis; therefore patients should have pulmonary function testing before treatment to document baseline function and allow for surveillance of function during therapy.4 Yet another major area of concern is secondary malignancy related to etoposide in the form of acute myelogenous leukemia, which appears to be related to cumulative dose effect.15 While each of these toxicities are well known risks, they are relatively uncommon and usually short-lived when they occur due to the short duration of treatment, the standard being only three cycles. Therefore, the benefit of treatment clearly outweighs the risks for the majority of patients.
It is well known that a proportion of women may experience premature ovarian failure following multiagent chemotherapy. Brewer et al reported on 26 patients treated with BEP for dysgerminoma, 71% of which resumed normal menstrual function, and six of whom conceived.16 In a study by the gynecologic oncology group, 3% of malignant germ cell tumor survivors who underwent fertility-sparing surgeryand platinum based chemotherapy experienced premature menopause,17 which was consistent with prior data.18 It is clear that while the risk of infertility following treatment of malignant germ cell tumors is always a concern, the vast majority of patients will maintain their ovarian function and the ability to bear children after their recovery.
Most patients with mixed ovarian germ cell tumors are cured, but a small percentage develop recurrence usually within 24 months of primary diagnosis. Because of the rarity of relapse in this population, there is no standard approach with treatment strategies extrapolated from clinical experience with testicular cancer patients.5
References
- 1.Hogg R, Friedlander M. Management of embryonal carcinoma of the ovary. J Gynecol Oncol. 2002;7:234. [Google Scholar]
- 2.Katz VL, Lentz GM, Lobo RA, et al. Comprehensive Gynecology. 5. Philadelphia: Mosby Elsevier; 2007. Neoplastic diseases of the ovary; pp. 865–871. [Google Scholar]
- 3.DiSaia PJ, Creasman WT. Germ cell, stromal, and other ovarian tumors. In: Gaertner R, Simpson D, editors. Clinical Gynecologic Oncology. 7. Philadelphia: Mosby Elsevier; 2007. pp. 369–395. [Google Scholar]
- 4.Bailey J, Church D. Management of germ cell tumours of the ovary. Rev Gynaecol Pract. 2005;5:201. [Google Scholar]
- 5.Gershenson DM. Management of ovarian germ cell tumors. J Clin Oncol. 2007;25:2938. doi: 10.1200/JCO.2007.10.8738. [DOI] [PubMed] [Google Scholar]
- 6.Smith HO, Berwick M, Verschraegen C, et al. Incidence and survival rates for female malignant germ cell tumors. Obstet Gynecol. 2006;107:1075. doi: 10.1097/01.AOG.0000216004.22588.ce. [DOI] [PubMed] [Google Scholar]
- 7.Nishio S, Ushijima K, Fukui A, et al. Fertility-preserving treatment for patients with malignant germ cell tumors of the ovary. J Obstet Gynaecol Res. 2006;32:416. doi: 10.1111/j.1447-0756.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- 8.Tewari K, Cappuccini F, Disaia P, et al. Malignant germ cell tumors of the ovary. Obstet Gynecol. 2000;95:128. doi: 10.1016/s0029-7844(99)00470-6. [DOI] [PubMed] [Google Scholar]
- 9.Patterson DM, Murugaesu N, Holden L, et al. A review of the close surveillance policy for stage I female germ cell tumors of the ovary and other sites. Int J Gynecol Cancer. 2008;18:43. doi: 10.1111/j.1525-1438.2007.00969.x. [DOI] [PubMed] [Google Scholar]
- 10.Smith JP, Rutledge F, Sutow WW. Mixed gynecologic tumors in children. current approaches to treatment. Am J Obstet Gynecol. 1973;116:261. doi: 10.1016/0002-9378(73)91061-2. [DOI] [PubMed] [Google Scholar]
- 11.Gershenson DM, Copeland LJ, Kavanagh JJ, et al. Treatment of malignant nondysgerminomatous germ cell tumors of the ovary with vincristine, dactinomycin, and cyclophosphamide. Cancer. 1985;56:2756. doi: 10.1002/1097-0142(19851215)56:12<2756::aid-cncr2820561206>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 12.Vriesendorp R, Aalders JG, Sleijfer DT, et al. Treatment of malignant germ cell tumors of the ovary with cisplatin, vinblastine, and bleomycin (PVB) Cancer Treat Rep. 1984;68:779. [PubMed] [Google Scholar]
- 13.Williams SD, Birch R, Einhorn LH, et al. Treatment of disseminated germ-cell tumors with cisplatin, bleomycin, and either vinblastine or etoposide. N Engl J Med. 1987;316:1435. doi: 10.1056/NEJM198706043162302. [DOI] [PubMed] [Google Scholar]
- 14.Williams S, Blessing JA, Liao SY, et al. Adjuvant therapy of ovarian germ cell tumors with cisplatin, etoposide, and bleomycin: a trial of the Gynecologic Oncology Group. J Clin Oncol. 1994;12:701. doi: 10.1200/JCO.1994.12.4.701. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen-Bjergaard J, Daugaard G, Hansen SW, et al. Increased risk of myelodysplasia and leukemia after etoposide, cisplatin, and bleomycin for germ-cell tumours. Lancet. 1991;338:359. doi: 10.1016/0140-6736(91)90490-g. [DOI] [PubMed] [Google Scholar]
- 16.Brewer M, Gershenson DM, Herzog CE, et al. Outcome and reproductive function after chemotherapy for ovarian dysgerminoma. J Clin Oncol. 1999;17:2670. doi: 10.1200/JCO.1999.17.9.2670. [DOI] [PubMed] [Google Scholar]
- 17.Gershenson DM, Miller AN, Champion VL, et al. Reproductive and sexual function after platinum-based chemotherapy in long-term ovarian germ cell tumor survivors: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:2792. doi: 10.1200/JCO.2006.08.4590. [DOI] [PubMed] [Google Scholar]
- 18.Tangir J, Zelterman D, Ma W, et al. Reproductive function after conservative surgery and chemotherapy for malignant germ cell tumors of the ovary. Obstet Gynecol. 2003;101:251. doi: 10.1016/s0029-7844(02)02508-5. [DOI] [PubMed] [Google Scholar]


