Abstract
A proportion of reproductive age women are affected by gynecologic malignancies. This patient population is faced with difficult decisions, related to their cancer care and treatment, as well as future childbearing potential. Therefore, it is important for gynecologists to be familiar with fertility sparing management options in patients with cervical, ovarian, and endometrial cancer. In addition to understanding the surgical approaches available, providers should be able to counsel patients regarding their eligibility for and the indications and limitations of fertility sparing therapy for gynecologic cancer, allowing for appropriate referrals. A comprehensive PUBMED literature search was conducted using the key words “fertility preservation,” “cervical cancer,” “endometrial cancer,” “ovarian cancer,” “borderline tumor of the ovary,” “germ cell tumor,” “obstetrical outcomes,” “chemotherapy,” and “radiation.” The following review summarizes fertility sparing options for patients with cervical, ovarian and endometrial cancer, with an emphasis on appropriate patient selection, oncologic, and obstetric outcomes.
Keywords: cervical cancer, endometrial cancer, fertility preservation, gynecologic malignancy, ovarian cancer
Traditionally, surgical management for the treatment of gynecologic cancer is viewed as a “sterilizing” procedure, given the common removal of the adnexa and uterus. Consequently, younger patients faced with this diagnoses are concerned about cure and fertility, particularly those that have not yet completed childbearing.
It is anticipated that there will be 1,529,560 new cancers diagnosed in 2010 with 569,000 deaths.1 Of these malignancies, 83,750 will affect the female genital tract, with an estimated 27,710 deaths. Fifteen to 21% of affected women will be less than 40 years of age at the time of diagnosis.2 This population of patients may have disease identified at an early stage and could potentially be cured, with fertility preservation being a priority at the time of disease diagnosis.3 Furthermore, we have seen a continuous trend in developed nations of delayed childbearing, which will result in an increase proportion of women diagnosed with a gynecologic cancer before their first pregnancy.4
Unfortunately, fertility-sparing options may not be offered to appropriate patients for various reasons, including lack of knowledge, unfamiliarity with the recommended surgical procedure, or concern over compromised cancer outcome. Alternately, patients facing a new cancer diagnosis may not be emotionally ready to discuss the complex risks and benefits surrounding this decision.
This review will describe the available evidence for fertility preservation in patients with cervical, ovarian, and endometrial cancer. Appropriate patient selection, surgical options, and related obstetric outcomes will be covered.
Cervical cancer
It is projected that there will be 12,200 new cases of cervical cancer diagnosed in the United States in 2010, with 4210 deaths.1 More than 1800 of these patients will be under the age of 40 years and potentially desire fertility preservation.5,6
The standard surgical treatment for patients with International Federation of Gynecology and Obstetrics (FIGO) stage I–IIA cervical cancer is radical hysterectomy. However, selected patients with early-stage squamous cell carcinoma of the cervix may be potential candidates for fertility preserving surgical interventions. Microinvasion (FIGO stage IA1), defined as less than 3 mm of stromal invasion, may be safely managed with cervical conization or large loop excision of the transformation zone (LLETZ).7 These patients have a 0.8% risk of lymph node metastasis in the absence of lymph vascular space invasion (LVSI).8 Diakomanolis et al9 also described the use of laser CO2 conization. Seventy-three women underwent laser CO2 conization with no recurrences after a mean follow-up of 54 months.9 Our groups recommended criteria for conservative management based on review of the literature include: (1) a negative endocervical curettage at completion of the procedure; (2) absence of LVSI (the risk of tumor recurrence increases from 3.2% to 9.7% with LVSI); and (3) a negative endocervical margin, given 10% risk of more extensive disease in individuals with positive margins at completion of biopsy.10,11 In patients who meet the above criteria, the risk of disease recurrence is less than 0.5%.
Unlike squamous cell lesions, adenocarcinoma is a glandular lesion and is considered multifocal, with up to 13% of patients having foci of disease separated by ≥2 mm of stromal mucosa.12 Furthermore, the complex architecture of endocervical glands, with invagination, branching, and tunnel formation makes determination of depth of invasion problematic.13 Bisseling et al14 performed a retrospective review of the treatment of cervical microinvasive adenocarcinoma, in which 16 patients with stage IA1 disease were managed with conization. Over an average follow-up period of 72 months there were no documented recurrences. In addition, McHale et al13 investigated survival and fertility outcomes in patients with adenocarcinoma in situ and those with microinvasive disease between 1985 and 1996. Twenty of 41 women with adenocarcinoma in situ underwent cervical conization. In the 5 patients with positive margins, 2 recurred and 1 developed invasive disease. Four of 20 women with stage IA lesions underwent cervical conization to preserve fertility, with no evidence of recurrence at 5 years follow-up. If fertility preserving options are used in patients with squamous lesions or adenocarcinoma, it is essential to have satisfactory margins free of disease.15
Patients who undergo a cervical cone biopsy or LLETZ for fertility preserving purposes should understand the potential attendant obstetric risk of preterm delivery. A metaanalysis published in 2006 by Kyrgiou et al,16 reported obstetric outcomes pooled from 27 evaluable studies. Cold knife cone was significantly associated with preterm delivery (relative risk [RR], 2.59; 95% confidence interval [CI], 1.80–3.72) and low birth-weight (RR, 2.53; 95% CI, 1.19–5.36). LLETZ was also significantly associated with preterm delivery and low birth-weight (RR, 1.70; 95% CI, 1.24–2.35 and RR, 1.82; 95% CI, 1.09–3.06, respectively). More recently, a large retrospective study was performed evaluating 241,701 women delivering singleton pregnancies. In this population, no increased risk of preterm delivery was seen in women who had undergone a LLETZ before the index pregnancy.17
Patients with greater than 3 mm of stromal invasion, defined as having FIGO stage IA2-IB1 disease, have a 7% risk of nodal metastasis, and definitive surgical treatment includes pelvic lymphadenectomy.18 For this group of patients, the fertility preserving option is a radical trachelectomy (RT), which includes resection of the entire cervix and surrounding parametria, and can be performed vaginally, abdominally, laparoscopically, and robotic assisted. First described by Dargent19 in 1987 in France, the vaginal radical trachelectomy (VRT) is preceded by a laparoscopic bilateral pelvic lymphadenectomy. Technically, the VRT is performed by dividing the uterus proximal to the cervical isthmus, and suturing the uterus to the vagina. Intraoperative frozen section should be used on both the endocervical margin and nodal tissue, with completion radical hysterectomy if tumor extends to within 5 mm of the margin.20,21 It is our recommendation that all patients offered this intervention satisfy 5 main criteria: (1) desiring preservation of fertility; (2) compliant with follow-up; (3) squamous cell carcinoma or adenocarcinoma with exclusion of undifferentiated and clear cell histologies; (4) FIGO stage IA1 with LVSI or stage IA2-IB1 lesion ≤2 cm; and (5) no evidence of pelvic lymph node metastasis. The overall complication rate for VRT of 2.5%, and the 4% recurrence and death rate are similar to those for traditional abdominal radical hysterectomy.6,22 The 2010 National Comprehensive Cancer Network (NCCN) Guidelines support cervical conization for the treatment of stage IA1 cervical cancer with negative margins, as well as RT plus pelvic lymph node dissection in patients desiring fertility preservation.23
In addition to the vaginal approach, both abdominal and robotic assisted RTs have been described. The abdominal approach, used in patients with distorted vaginal anatomy, larger lesions or in centers where the vaginal approach is not mastered has been described with favorable outcomes.24 Ungár et al25 performed the procedure on 30 patients with stage IA2-IB2 disease with no recurrences after a median follow-up of 47 months. Other authors support the use of the abdominal approach, reporting larger parametrial margins.24 The robotic assisted RT was recently reviewed by Ramirez et al.26 Four patients underwent successful robotic assisted RT, with no intraoperative complications and no disease recurrence, with a median follow-up of 105 days.26 The median operative time was 339.5 minutes, with a median console time of 282.5 minutes, which the authors report as similar to published data for vaginal and abdominal approaches. We recommend that the initial surveillance of patients after RT include Papanicolaou smear with high-risk human papilloma virus (HR HPV) testing every 3 months. As described by Feratovic et al,27 physicians should have an understanding that the alteration in anatomy postoperatively may result in glandular cells appearing in cytology specimens, with misdiagnosis of atypical glandular cells of undetermined significance.
A comprehensive review of the literature regarding obstetric outcomes in patients undergoing RT is shown in Table 1.25,28–36 A total of 582 patients, represented in 10 studies, had 257 pregnancies with a 64% live birth rate. There were 23 recurrences and 12 deaths. Patients should understand that pregnancies after RT are complicated by preterm delivery and miscarriage, with first and second trimester loss rates as high as 19% and 9.5%, respectively.37 Thus, referral to a Maternal-Fetal Medicine specialist for consultation before surgery may be warranted in this patient population.
TABLE 1.
Oncologic and obstetric outcomes in patients with cervical cancer after radical trachelectomy
| Author | Patients | Pregnancies | Live births | Recurrences | Deaths |
|---|---|---|---|---|---|
| Shepherd et al28 | 123 | 55 | 28 | 5 | 4 |
| Dargent et al29 | 96 | 55 | 36 | 4 | 3 |
| Burnett et al30 | 21 | 3 | 2 | 0 | 0 |
| Bernardini et al31 | 80 | 22 | 18 | 7 | 4 |
| Plante et al32 | 72 | 50 | 36 | 2 | 1 |
| Schlaerth et al33 | 10 | 4 | 2 | 0 | 0 |
| Schneider et al34 | 36 | 7 | 4 | 1 | 0 |
| Boss et al35 | 19 | 2 | 2 | 0 | 0 |
| Ungár et al25 | 30 | 3 | 2 | 0 | 0 |
| Mathevet et al36 | 95 | 56 | 34 | 4 | 0 |
| Total | 582 | 257 (44%) | 164 (64%) | 23 (3.9%) | 12 (0.2%) |
Eskander. Fertility preservation in patients with gynecologic malignancies. Am J Obstet Gynecol 2011.
As an alternative approach to trachelectomy, neoadjuvant chemotherapy (NACT) has been used in patients with larger cervical lesions desiring to preserve their fertility, mostly in European centers. The largest such series, published by Maneo et al, described 21 patients with stage IB1 cervical cancer who were treated with NACT, followed by cold knife cone and pelvic lymph node dissection. All patients were treated with 3 cycles of cisplatin, paclitaxel, and ifosfamide. Twenty patients underwent cervical conization and pelvic lymphadenectomy. No relapses were noted after a median follow-up of 69 months.38
In those instances where patients require a radical hysterectomy for treatment of cervical cancer, lateral ovarian transposition (LOT) should be discussed. Chambers et al39 reported that 71% of patients maintained ovarian function after LOT and pelvic RT. The preservation of function correlated with the estimated scatter dose to the ovaries. The rate of ovarian failure was 11% with doses ≤300 cGy, compared with 50% if the estimated dose was >300 cGy.
Ovarian cancer
Ovarian cancer represents a spectrum of malignancies with varying prognosis and patterns of spread. In 2010, there will be a projected 21,880 new cases and 13,850 deaths.1 Although the majority of patients will present with advanced disease, low malignant potential tumors, FIGO stage I tumors, and germ cell malignancies are more common in women of reproductive age. It is estimated that as many as 3719 of these malignancies will affect women of childbearing potential, with disease-specific 5-year survival approaching 80% in this young patient population.40
Borderline tumors of the ovary are characterized by a lack of stromal invasion as well as serous, mucinous, or endometrioid histology. The median age at diagnosis is 45, with greater than 34% of patients being less than 40 years of age.41 Traditionally, these tumors are managed with total abdominal hysterectomy and bilateral salpingo-oophorectomy, given that 25% of borderline tumors are re-classified as invasive on final pathologic review.42 In those patients desiring fertility preservation, however, surgical management may be limited to unilateral salpingo-oophorectomy (USO) with complete surgical staging, provided that the tumor appears confined to 1 ovary.43 Ovarian cystectomy is not unreasonable; however, patients should be counseled regarding recurrence rates greater than 30%. If there is bilateral ovarian involvement and complete resection can be achieved, ovarian cystectomy is the treatment of choice.44 Complete surgical staging, including exploration of the entire abdominal cavity, peritoneal washings, infracolic omentectomy, and multiple peritoneal biopsies is important, as 20% of patients may have noninvasive as well as invasive metastatic implants.45 Table 2 illustrates obstetric outcomes in patients with borderline ovarian tumors undergoing fertility preserving surgery.46–55 A total of 10 studies were identified, with 626 total patients. There were 185 reported pregnancies and 107 live births. Despite 111 recurrences (18%), there was only 1 patient death. These data indicate that fertility preservation should be considered in young patients desiring future childbearing who are appropriately staged and in whom the primary tumor can be completely resected. When frozen section pathology is unclear, we advocate a 2-step approach, with conservative removal of the primary lesion at initial surgery, reserving the option for more comprehensive surgery at a later time when final pathologic evaluation shows invasive disease.
TABLE 2.
Oncologic and obstetric outcomes in patients with borderline ovarian tumors undergoing fertility preserving surgery
| Author | Patients | Pregnancies | Live births | Recurrences | Deaths |
|---|---|---|---|---|---|
| Zanetta et al46 | 189 | 44 | N/A | 35 | 0 |
| Lim-Tan et al47 | 35 | 8 | 6 | 6 | 0 |
| Morice et al48 | 44 | 17 | 10 | 9 | 0 |
| Boran et al49 | 62 | 13 | 10 | 4 | 0 |
| Fauvet et al50 | 162 | 30 | 18 | 27 | 0 |
| Donnez et al51 | 16 | 12 | 12 | 3 | 0 |
| Seracchiolo et al52 | 19 | 6 | 6 | 1 | 0 |
| Camatte et al53 | 17 | 8 | 8 | 9 | 0 |
| Morris et al55 | 43 | 25 | 16 | 14 | 1 |
| Gotlieb et al54 | 39 | 22 | 21 | 3 | 0 |
| Total | 626 | 185 (30%) | 107 (58%) | 111 (18%) | 1 (0.2%) |
N/A, not available.
Eskander. Fertility preservation in patients with gynecologic malignancies. Am J Obstet Gynecol 2011.
Malignant germ cell tumors account for 5% of ovarian malignancies and unlike other types of ovarian cancer, fertility preservation is the standard of care. The median age of affected patients is 19 years, with the majority of patients having stage I disease. The recommended management of young patients with suspected malignant germ cell tumors of the ovary includes: (1) intact removal of the tumor; (2) sparing of the fallopian tube if not adherent to the tumor; (3) procurement of cytologic washings or harvesting of ascites fluid; (4) examination and palpation of the omentum with removal of suspicious areas; and (5) examination and palpation of the ileac and aortocaval nodes with biopsy of abnormal areas.56 In addition, 90–95% of malignant germ cell tumors of the ovary are curable with the use of postoperative systemic chemotherapy.57 Gershenson58 described 40 patients treated with surgery and multiagent chemotherapy for malignant germ cell tumors of the ovary. The median age at onset of therapy was 15 years. All 28 patients treated with vincristine, doxorubicin, and cyclophosphamide (VAC) chemotherapy resumed regular menstrual function, with only 3 patients having persistent menstrual dysfunction. Of 16 patients attempting pregnancy, 11 delivered 22 healthy infants. Table 3 illustrates obstetric outcomes pooled from 7 articles describing patients with ovarian germ cell tumors.59–64 A total of 515 patients were evaluated, with 185 pregnancies and 148 live births. Amenorrhea rates after completion of fertility sparing surgery and chemotherapy were less than 3%. Nine percent of patients experienced recurrence with a death rate of 3%.
TABLE 3.
Oncologic and obstetric outcomes in patients with malignant germ cell tumors treated conservatively
| Author | Patients | Pregnancies | Live births | Recurrences | Deaths |
|---|---|---|---|---|---|
| Gershenson58 | 40 | 22 | 22 | 3 | 2 |
| Kanazawa et al59 | 21 | 11 | 9 | 1 | 1 |
| Low et al60 | 74 | 19 | 14 | 7 | 2 |
| Gershenson et al63 | 71 | 37 | 30 | 10 | 4 |
| Zanetta et al61 | 138 | 41 | 28 | 16 | 3 |
| Perrin et al57 | 45 | 8 | 7 | 4 | 2 |
| Tangir et al62 | 64 | 47 | 38 | 5 | 3 |
| Total | 453 | 185 (41%) | 148 (80%) | 46 (10%) | 17 (3.8%) |
Eskander. Fertility preservation in patients with gynecologic malignancies. Am J Obstet Gynecol 2011.
The conservative management of invasive epithelial ovarian cancers is uncommon, and the literature describing patient and obstetric outcomes is sparse. Traditionally, management of invasive epithelial ovarian cancer, which accounts for 80% of ovarian malignancies, includes total abdominal hysterectomy, bilateral salpingo-oophorectomy, omentectomy, peritoneal cytology, and biopsies as well as pelvic and paraaortic lymph node dissection. This is followed by adjuvant chemotherapy in all cases aside from completely staged, FIGO IA grade 1 and IB grade 1 lesions. However, in patients with well-differentiated, encapsulated, unilateral lesions without adhesions or ascites, fertility preserving surgery in the form of a unilateral salpingo-oophorectomy and complete staging, with preservation of the uterus and contralateral ovary may be considered.20,43 In patients desiring to preserve fertility, it is recommended that biopsy of a normal appearing contralateral ovary be avoided as this can result in mechanical infertility. If the contralateral ovary appears grossly normal, the risk of occult malignancy is less than 3%. There have been 328 cases of fertility conserving surgery reported in the literature, with 119 pregnancies and a 96% live birth rate (Table 4).65–72
TABLE 4.
Oncologic and obstetric outcomes in patient with invasive epithelial ovarian cancer treated with fertility sparing surgery
| Author | Patients | Pregnancies | Live births | Recurrences | Deaths |
|---|---|---|---|---|---|
| Colombo et al65 | 56 | 25 | 16 | 3 | 2 |
| Zanetta et al66 | 84 | 33 | 22 | 5 | 3 |
| Duska et al67 | 6 | 2 | 2 | 1 | 1 |
| Morice et al68 | 34 | 10 | 7 | 10 | 4 |
| Schilder et al69 | 52 | 17 | 26 | 5 | 2 |
| Park et al70 | 62 | 22 | 22 | 11 | 6 |
| Raspagliesi et al71 | 10 | 3 | 3 | 0 | 0 |
| Colombo et al72 | 24 | 7 | 6 | 7 | 2 |
| Total | 328 | 119 (36%) | 104 (87%) | 42 (13%) | 20 (6%) |
Eskander. Fertility preservation in patients with gynecologic malignancies. Am J Obstet Gynecol 2011.
A proportion of patients with early-stage epithelial ovarian cancer may require adjuvant chemotherapy. Patients menstruating before treatment have a higher rate of amenorrhea and oligomenorrhea when treated with alkylating agents, with a depression in follicular maturation and primordial follicle development.73 The return of menses and ovulation after treatment appear to be a function of age, related to the number of oocytes that can be recruited after chemotherapy. Bines et al74 investigated 2500 patients receiving multiple cycles of alkylating agents, including cyclophosphamide and 5-fluorouracil, with 40% of patients ≤40 years of age developing amenorrhea, in comparison to 76% of patients 41 years and older. Investigation into the use of ovarian suppression during chemotherapy has shown promising preliminary results. Recchia et al75 studied 100 women receiving concurrent goserilin therapy with adjuvant chemotherapy. With a median follow-up of over 6 years 67% of patients recovered normal menses, including 100% of women less than 40 years of age.
Alternative options, including embryo, oocyte and ovarian tissue cryopreservation have been explored. Greatest success has been achieved with oocyte harvesting, followed by in vitro fertilization and embryo cryopreservation. For patients who are not in a position to create embryos, or who lack a sperm donor, cryopreservation of unfertilized oocytes or ovarian tissue may be discussed. Unfortunately, success rates with these methods is limited, although recent advances in vitrification and modifications in freezing solutions have improved the live birth rates.76 Finally, a discussion regarding surrogacy and adoption may be appropriate for those patients unable to, or electing not to attempt pregnancy.
Endometrial Cancer
Endometrial cancer is the most common gynecologic malignancy, with a projected 43,470 new cases in 2010 and 7950 deaths.1 Eight to 14% of affected patients will be of childbearing age, highlighting the importance of fertility preservation in this population. Standard therapy for endometrial cancer includes total hysterectomy and bilateral salpingo-oophorectomy with or without pelvic and paraaortic lymph node dissection, depending on risk factors and apparent cancer stage.
Fertility preserving options in endometrial cancer are currently limited to hormonal methods. Thus, successful treatment is dependent on hormone receptor expression on cancer cells.77 Response rates range from 26% to 89% in estrogen and progesterone receptor positive tumors, and are as low as 8–17% in those that are receptor negative.20,78 It is our recommendation that patients offered hormonal treatment satisfy the following criteria: (1) grade 1 well-differentiated tumor; (2) absence of LVSI on adequate curettage specimen; (3) no evidence of myometrial invasion on magnetic resonance imaging; (4) no evidence of metastatic disease on computed tomography (CT) imaging; (5) no evidence of a suspicious adnexal mass on CT or pelvic ultrasound imaging, as up to 29% of premenopausal women diagnosed with endometrial cancer may have a concurrent ovarian malignancy; and (6) strong and diffuse expression of progesterone receptors on immunohistochemistry staining of the endometrial biopsy or curettage specimen.79
It is important to ensure that patients desiring to proceed with hormonal management are extensively counseled regarding potential risks. Clinicians should understand that there is no scientifically proven optimal progestin. Previous regimens have included megestrol acetate, medroxyprogesterone acetate, and the progesterone releasing intrauterine device. In addition, the dose to be administered and duration of therapy are unclear. Current convention is to treat with megestrol acetate 160 mg daily with repeat endometrial sampling in 3 months to determine whether there is disease regression, persistence, or progression. Ramirez et al80 reviewed 81 patients in 27 articles, with grade 1 endometrial adenocarcinoma managed hormonally. Sixty-two patients (76%) responded to treatment, with a median time to response of 12 weeks. Of those, 15 patients (24%) recurred, and 6 had residual adenocarcinoma identified at the time of hysterectomy. The median time to recurrence was 19 months, and 19 patients never responded.80 In our practice, patients desiring fertility preservation, who meet previously described criteria, are managed with megestrol acetate 160 mg daily or medroxyprogesterone acetate 600 mg daily for 3 months. Repeat sampling of the endometrium is then performed by curettage. If persistence or progression is identified, recommendation to proceed with hysterectomy is made. In cases where regression occurs, continued hormonal therapy for an additional 6–9 months is acceptable. At completion of treatment, in the absence of relapse, the patient is encouraged to pursue pregnancy, with close follow-up after delivery. As endometrial cancer is linked to obesity, polycystic ovarian syndrome, and anovulation, many women with the diagnosis may have primary or secondary infertility, and require assisted reproductive technologies. Thus, concurrent referral to reproductive endocrinology may be warranted.81,82 The safety of the hormonal changes of pregnancy and medications used in assisted reproductive technology are unclear.
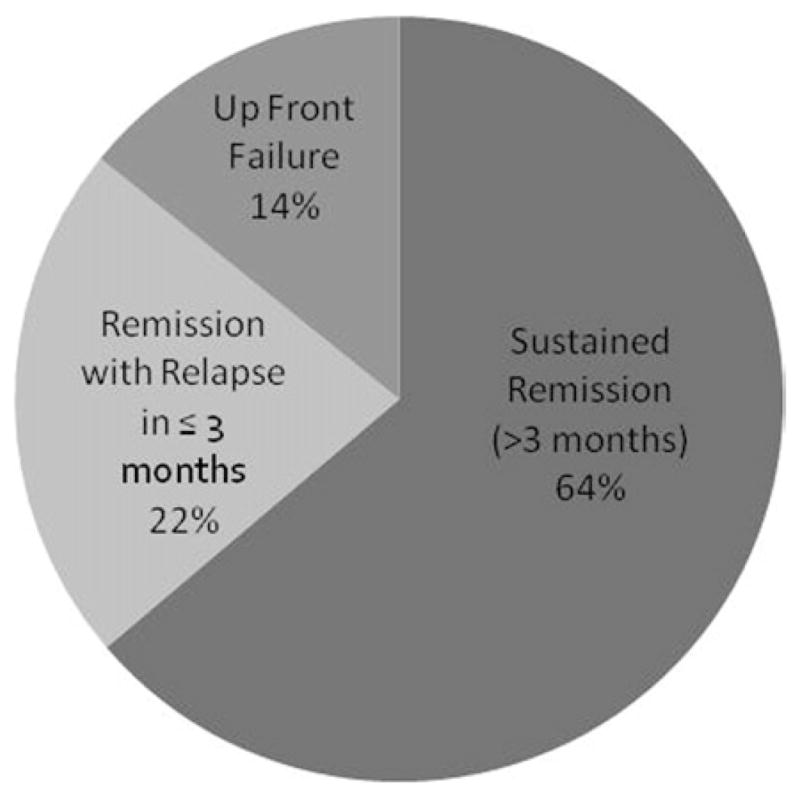
Table 5 summarizes 11 studies detailing patient outcomes and live births in women with endometrial cancer treated conservatively with hormone therapy. Despite a large number of pooled papers, the total number of patients remains low. Of all women attempting to conceive, there was a 47% live birth rate. Remission was seen in 81% of patients, with relapse occurring in 28% of that cohort. A total of 18% of patients failed up front hormonal therapy and required hysterectomy (Figure).77,83–92
TABLE 5.
Regression, relapse and obstetric outcomes in women with endometrial cancer treated conservatively with progestin therapy
| Author | Patients | Regression | Relapse | Live births | Progesterone |
|---|---|---|---|---|---|
| Randall and Kurman83 | 12 | 9 | 1 | 6 | Megestrol or MPA |
| Duska et al84 | 12 | 10 | 1 | 5 | MPA |
| Imai et al85 | 14 | 8 | 3 | 3 | MPA |
| Kaku et al86 | 12 | 9 | 2 | 1 | MPA |
| Wang et al87 | 9 | 8 | 4 | 3 | Megestrol |
| Niwa et al88 | 12 | 12 | 8 | 5 | MPA |
| Lowe et al89 | 2 | 2 | 0 | 8 | Megestrol |
| Sardi et al90 | 4 | 3 | 0 | 3 | MPA |
| Yang et al91 | 6 | 4 | 2 | 2 | Megestrol |
| Farhi et al77 | 4 | 3 | 1 | 2 | Progestin |
| Gotlieb et al92 | 13 | 13 | 6 | 9 | Megestrol |
| Total | 100 | 81 (81%) | 28 (28%) | 47 (47%) |
MPA, medroxyprogesterone acetate.
Eskander. Fertility preservation in patients with gynecologic malignancies. Am J Obstet Gynecol 2011.
FIGURE. Remission, relapse and up front failure rates in patients with endometrial adenocarcinoma treated hormonally.
Eskander. Fertility preservation in patients with gynecologic malignancies. Am J Obstet Gynecol 2011.
Conclusion
In summary, cervical, ovarian, and endometrial cancer affect a proportion of women for whom fertility preservation is a priority. It is important to understand the options, limitations, and eligibility criteria as they apply to this patient population. At times, fertility preserving options may not reflect the standard treatment, and in these instances, patients will be forced to weigh the risks and benefits associated with each treatment option. Ultimately, careful oncologic, genetic, reproductive, and psychologic counseling is needed before offering young cancer patients a nonstandard therapy. Thus, a multidisciplinary approach, including gynecologic oncology, maternal fetal medicine, as well as reproductive endocrinology, is recommended to maximize patient understanding and fertility potential.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Makar A, Tropé C. Fertility preservation in gynecologic cancer. Acta Obstet Gynecol Scand. 2001;80:794–802. doi: 10.1034/j.1600-0412.2001.080009794.x. [DOI] [PubMed] [Google Scholar]
- 3.Wright J, Shah M, Mathew L, et al. Fertility preservation in young women with epithelial ovarian cancer. Cancer. 2009;115:4118–26. doi: 10.1002/cncr.24461. [DOI] [PubMed] [Google Scholar]
- 4.Martin J, Hamilton B, Sutton P, Ventura S, Menacker F, Kirmeyer S. Births: final data for 2004. Natl Vital Stat Rep. 2006;55:1–101. [PubMed] [Google Scholar]
- 5.Wang S, Sherman M, Hildesheim A, Lacey JJ, Devesa S. Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the United States for 1976–2000. Cancer. 2004;100:1035–44. doi: 10.1002/cncr.20064. [DOI] [PubMed] [Google Scholar]
- 6.Gien L, Covens A. Fertility-sparing options for early stage cervical cancer. Gynecol Oncol. 2010;117:350–7. doi: 10.1016/j.ygyno.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 7.Stehman F, Rose P, Greer B, et al. Innovations in the treatment of invasive cervical cancer. Cancer. 2003;98(Suppl):2052–63. doi: 10.1002/cncr.11676. [DOI] [PubMed] [Google Scholar]
- 8.Benedetti-Panici P, Maneschi F, D’Andrea G, et al. Early cervical carcinoma: the natural history of lymph node involvement redefined on the basis of thorough parametrectomy and giant section study. Cancer. 2000;88:2267–74. [PubMed] [Google Scholar]
- 9.Diakomanolis E, Haidopoulos D, Rodolakis A, et al. Laser CO(2) conization: a safe mode of treating conservatively microinvasive carcinoma of the uterine cervix. Eur J Obstet Gynecol Reprod Biol. 2004;113:229–33. doi: 10.1016/j.ejogrb.2003.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Buckley S, Tritz D, Van Le L, et al. Lymph node metastases and prognosis in patients with stage IA2 cervical cancer. Gynecol Oncol. 1996;63:4–9. doi: 10.1006/gyno.1996.0268. [DOI] [PubMed] [Google Scholar]
- 11.Roman L, Felix J, Muderspach L, et al. Influence of quantity of lymph-vascular space invasion on the risk of nodal metastases in women with early-stage squamous cancer of the cervix. Gynecol Oncol. 1998;68:220–5. doi: 10.1006/gyno.1998.4943. [DOI] [PubMed] [Google Scholar]
- 12.Ostör A, Duncan A, Quinn M, Rome R. Adenocarcinoma in situ of the uterine cervix: an experience with 100 cases. Gynecol Oncol. 2000;79:207–10. doi: 10.1006/gyno.2000.5957. [DOI] [PubMed] [Google Scholar]
- 13.McHale M, Le T, Burger R, Gu M, Rutgers J, Monk B. Fertility sparing treatment for in situ and early invasive adenocarcinoma of the cervix. Obstet Gynecol. 2001;98(Pt 1):726–31. doi: 10.1016/s0029-7844(01)01544-7. [DOI] [PubMed] [Google Scholar]
- 14.Bisseling K, Bekkers R, Rome R, Quinn M. Treatment of microinvasive adenocarcinoma of the uterine cervix: a retrospective study and review of the literature. Gynecol Oncol. 2007;107:424–30. doi: 10.1016/j.ygyno.2007.07.062. [DOI] [PubMed] [Google Scholar]
- 15.Salani R, Puri I, Bristow R. Adenocarcinoma in situ of the uterine cervix: a metaanalysis of 1278 patients evaluating the predictive value of conization margin status. Am J Obstet Gynecol. 2009;200:182, e1–5. doi: 10.1016/j.ajog.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet. 2006;367:489–98. doi: 10.1016/S0140-6736(06)68181-6. [DOI] [PubMed] [Google Scholar]
- 17.Werner C, Lo J, Heffernan T, Griffith W, McIntire D, Leveno K. Loop electrosurgical excision procedure and risk of preterm birth. Obstet Gynecol. 2010;115:605–8. doi: 10.1097/AOG.0b013e3181d068a3. [DOI] [PubMed] [Google Scholar]
- 18.Covens A, Rosen B, Murphy J, et al. How important is removal of the parametrium at surgery for carcinoma of the cervix? Gynecol Oncol. 2002;84:145–9. doi: 10.1006/gyno.2001.6493. [DOI] [PubMed] [Google Scholar]
- 19.Dargent D, Mathevet P. Schauta’s vaginal hysterectomy combined with laparoscopic lymphadenectomy. Baillieres Clin Obstet Gynaecol. 1995;9:691–705. doi: 10.1016/s0950-3552(05)80392-x. [DOI] [PubMed] [Google Scholar]
- 20.Tewari K, Di Saia P. Ovulatory failure, fertility preservation and reproductive strategies in the setting of gynecologic and non-gynecologic malignancies. Eur J Gynaecol Oncol. 2006;27:449–61. [PubMed] [Google Scholar]
- 21.Tanguay C, Plante M, Renaud M, Roy M, Têtu B. Vaginal radical trachelectomy in the treatment of cervical cancer: the role of frozen section. Int J Gynecol Pathol. 2004;23:170–5. doi: 10.1097/00004347-200404000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Marchiole P, Benchaib M, Buenerd A, Lazlo E, Dargent D, Mathevet P. Oncological safety of laparoscopic-assisted vaginal radical trachelectomy (LARVT or Dargent’s operation): a comparative study with laparoscopic-assisted vaginal radical hysterectomy (LARVH) Gynecol Oncol. 2007;106:132–41. doi: 10.1016/j.ygyno.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 23.National Comprehensive Cancer Network (NCCN) [Accessed Dec. 12, 2010];NCCN Guidelines Version 1.2011 Cervical Cancer. Available at: www.nccn.org.
- 24.Einstein M, Park K, Sonoda Y, et al. Radical vaginal versus abdominal trachelectomy for stage IB1 cervical cancer: a comparison of surgical and pathologic outcomes. Gynecol Oncol. 2009;112:73–7. doi: 10.1016/j.ygyno.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ungár L, Pálfalvi L, Hogg R, et al. Abdominal radical trachelectomy: a fertility-preserving option for women with early cervical cancer. BJOG. 2005;112:366–9. doi: 10.1111/j.1471-0528.2004.00421.x. [DOI] [PubMed] [Google Scholar]
- 26.Ramirez P, Schmeler K, Malpica A, Soliman P. Safety and feasibility of robotic radical trachelectomy in patients with early-stage cervical cancer. Gynecol Oncol. 2010;116:512–5. doi: 10.1016/j.ygyno.2009.10.063. [DOI] [PubMed] [Google Scholar]
- 27.Feratovic R, Lewin S, Sonoda Y, et al. Cytologic findings after fertility-sparing radical trachelectomy. Cancer. 2008;114:1–6. doi: 10.1002/cncr.23256. [DOI] [PubMed] [Google Scholar]
- 28.Shepherd J, Spencer C, Herod J, Ind T. Radical vaginal trachelectomy as a fertility-sparing procedure in women with early-stage cervical cancer-cumulative pregnancy rate in a series of 123 women. BJOG. 2006;113:719–24. doi: 10.1111/j.1471-0528.2006.00936.x. [DOI] [PubMed] [Google Scholar]
- 29.Dargent D, Franzosi F, Ansquer Y, Martin X, Mathevet P, Adeline P. Extended trachelectomy relapse: plea for patient involvement in the medical decision. Bull Cancer. 2002;89:1027–30. [PubMed] [Google Scholar]
- 30.Burnett A, Roman L, O’Meara A, Morrow C. Radical vaginal trachelectomy and pelvic lymphadenectomy for preservation of fertility in early cervical carcinoma. Gynecol Oncol. 2003;88:419–23. doi: 10.1016/s0090-8258(02)00142-7. [DOI] [PubMed] [Google Scholar]
- 31.Bernardini M, Barrett J, Seaward G, Covens A. Pregnancy outcomes in patients after radical trachelectomy. Am J Obstet Gynecol. 2003;189:1378–82. doi: 10.1067/s0002-9378(03)00776-2. [DOI] [PubMed] [Google Scholar]
- 32.Plante M, Renaud M, François H, Roy M. Vaginal radical trachelectomy: an oncologically safe fertility-preserving surgery: an updated series of 72 cases and review of the literature. Gynecol Oncol. 2004;94:614–23. doi: 10.1016/j.ygyno.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 33.Schlaerth J, Spirtos N, Schlaerth A. Radical trachelectomy and pelvic lymphadenectomy with uterine preservation in the treatment of cervical cancer. Am J Obstet Gynecol. 2003;188:29–34. doi: 10.1067/mob.2003.124. [DOI] [PubMed] [Google Scholar]
- 34.Schneider A, Possover M, Köhler C. New concepts for staging and therapy of cervix cancer by endoscopic surgery. Zentralbl Gynakol. 2001;123:250–4. doi: 10.1055/s-2001-14787. [DOI] [PubMed] [Google Scholar]
- 35.Boss E, van Golde R, Beerendonk C, Massuger L. Pregnancy after radical trachelectomy: a real option? Gynecol Oncol. 2005;99(Suppl 1):S152–6. doi: 10.1016/j.ygyno.2005.07.071. [DOI] [PubMed] [Google Scholar]
- 36.Mathevet P, Laszlo de Kaszon E, Dargent D. Fertility preservation in early cervical cancer. Gynecol Obstet Fertil. 2003;31:706–12. doi: 10.1016/s1297-9589(03)00200-5. [DOI] [PubMed] [Google Scholar]
- 37.Jolley J, Battista L, Wing D. Management of pregnancy after radical trachelectomy: case reports and systematic review of the literature. Am J Perinatol. 2007;24:531–9. doi: 10.1055/s-2007-986680. [DOI] [PubMed] [Google Scholar]
- 38.Maneo A, Chiari S, Bonazzi C, Mangioni C. Neoadjuvant chemotherapy and conservative surgery for stage IB1 cervical cancer. Gynecol Oncol. 2008;111:438–43. doi: 10.1016/j.ygyno.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 39.Chambers SK, Chambers JT, Kier R, Peschel RE. Sequelae of lateral ovarian transposition in irradiated cervical cancer patients. Int J Radiat Oncol Biol Phys. 1991;20:1305–8. doi: 10.1016/0360-3016(91)90242-v. [DOI] [PubMed] [Google Scholar]
- 40.Chan J, Urban R, Cheung M, et al. Ovarian cancer in younger vs older women: a population-based analysis. Br J Cancer. 2006;95:1314–20. doi: 10.1038/sj.bjc.6603457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skírnisdóttir I, Garmo H, Wilander E, Holmberg L. Borderline ovarian tumors in Sweden 1960–2005: trends in incidence and age at diagnosis compared to ovarian cancer. Int J Cancer. 2008;123:1897–901. doi: 10.1002/ijc.23724. [DOI] [PubMed] [Google Scholar]
- 42.Wingo S, Knowles L, Carrick K, Miller D, Schorge J. Retrospective cohort study of surgical staging for ovarian low malignant potential tumors. Am J Obstet Gynecol. 2006;194:e20–2. doi: 10.1016/j.ajog.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 43.National Comprehensive Cancer Network (NCCN) [Accessed Dec. 12, 2010];NCCN Guidelines Version 2.2011 epithelial ovarian cancer/fallopian tube cancer/primary peritoneal cancer. Available at: www.nccn.org.
- 44.Rao G, Skinner E, Gehrig P, Duska L, Miller D, Schorge J. Fertility-sparing surgery for ovarian low malignant potential tumors. Gynecol Oncol. 2005;98:263–6. doi: 10.1016/j.ygyno.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 45.Cadron I, Leunen K, Van Gorp T, Amant F, Neven P, Vergote I. Management of borderline ovarian neoplasms. J Clin Oncol. 2007;25:2928–37. doi: 10.1200/JCO.2007.10.8076. [DOI] [PubMed] [Google Scholar]
- 46.Zanetta G, Meni A, Brancatelli G, et al. Comparison of methods for monitoring young women with stage I borderline ovarian tumor after conservative surgery. Minerva Ginecol. 2001;53(Suppl 1):10–1. [PubMed] [Google Scholar]
- 47.Lim-Tan S, Cajigas H, Scully R. Ovarian cystectomy for serous borderline tumors: a follow-up study of 35 cases. Obstet Gynecol. 1988;72:775–81. [PubMed] [Google Scholar]
- 48.Morice P, Camatte S, El Hassan J, Pautier P, Duvillard P, Castaigne D. Clinical outcomes and fertility after conservative treatment of ovarian borderline tumors. Fertil Steril. 2001;75:92–6. doi: 10.1016/s0015-0282(00)01633-2. [DOI] [PubMed] [Google Scholar]
- 49.Boran N, Cil A, Tulunay G, et al. Fertility and recurrence results of conservative surgery for borderline ovarian tumors. Gynecol Oncol. 2005;97:845–51. doi: 10.1016/j.ygyno.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 50.Fauvet R, Poncelet C, Boccara J, Descamps P, Fondrinier E, Daraï E. Fertility after conservative treatment for borderline ovarian tumors: a French multicenter study. Fertil Steril. 2005;83:284–90. doi: 10.1016/j.fertnstert.2004.10.009. quiz 525–6. [DOI] [PubMed] [Google Scholar]
- 51.Donnez J, Munschke A, Berliere M, et al. Safety of conservative management and fertility outcome in women with borderline tumors of the ovary. Fertil Steril. 2003;79:1216–21. doi: 10.1016/s0015-0282(03)00160-2. [DOI] [PubMed] [Google Scholar]
- 52.Seracchioli R, Venturoli S, Colombo F, Govoni F, Missiroli S, Bagnoli A. Fertility and tumor recurrence rate after conservative laparoscopic management of young women with early-stage borderline ovarian tumors. Fertil Steril. 2001;76:999–1004. doi: 10.1016/s0015-0282(01)02842-4. [DOI] [PubMed] [Google Scholar]
- 53.Camatte S, Morice P, Pautier P, Atallah D, Duvillard P, Castaigne D. Fertility results after conservative treatment of advanced stage serous borderline tumour of the ovary. BJOG. 2002;109:376–80. doi: 10.1111/j.1471-0528.2002.01359.x. [DOI] [PubMed] [Google Scholar]
- 54.Gotlieb W, Flikker S, Davidson B, Korach Y, Kopolovic J, Ben-Baruch G. Borderline tumors of the ovary: fertility treatment, conservative management, and pregnancy outcome. Cancer. 1998;82:141–6. [PubMed] [Google Scholar]
- 55.Morris R, Gershenson D, Silva E, Follen M, Morris M, Wharton J. Outcome and reproductive function after conservative surgery for borderline ovarian tumors. Obstet Gynecol. 2000;95:541–7. doi: 10.1016/s0029-7844(99)00619-5. [DOI] [PubMed] [Google Scholar]
- 56.Billmire D. Malignant germ cell tumors in childhood. Semin Pediatr Surg. 2006;15:30–6. doi: 10.1053/j.sempedsurg.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 57.Perrin L, Low J, Nicklin J, Ward B, Crandon A. Fertility and ovarian function after conservative surgery for germ cell tumours of the ovary. Aust N Z J Obstet Gynaecol. 1999;39:243–5. doi: 10.1111/j.1479-828x.1999.tb03382.x. [DOI] [PubMed] [Google Scholar]
- 58.Gershenson D. Menstrual and reproductive function after treatment with combination chemotherapy for malignant ovarian germ cell tumors. J Clin Oncol. 1988;6:270–5. doi: 10.1200/JCO.1988.6.2.270. [DOI] [PubMed] [Google Scholar]
- 59.Kanazawa K, Suzuki T, Sakumoto K. Treatment of malignant ovarian germ cell tumors with preservation of fertility: reproductive performance after persistent remission. Am J Clin Oncol. 2000;23:244–8. doi: 10.1097/00000421-200006000-00007. [DOI] [PubMed] [Google Scholar]
- 60.Low J, Perrin L, Crandon A, Hacker N. Conservative surgery to preserve ovarian function in patients with malignant ovarian germ cell tumors: a review of 74 cases. Cancer. 2000;89:391–8. [PubMed] [Google Scholar]
- 61.Zanetta G, Bonazzi C, Cantù M, et al. Survival and reproductive function after treatment of malignant germ cell ovarian tumors. J Clin Oncol. 2001;19:1015–20. doi: 10.1200/JCO.2001.19.4.1015. [DOI] [PubMed] [Google Scholar]
- 62.Tangir J, Zelterman D, Ma W, Schwartz P. Reproductive function after conservative surgery and chemotherapy for malignant germ cell tumors of the ovary. Obstet Gynecol. 2003;101:251–7. doi: 10.1016/s0029-7844(02)02508-5. [DOI] [PubMed] [Google Scholar]
- 63.Gershenson DM, Miller AM, Champion VL, et al. Reproductive and sexual function after platinum-based chemotherapy in long-term ovarian germ cell tumor survivors: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:2792–7. doi: 10.1200/JCO.2006.08.4590. [DOI] [PubMed] [Google Scholar]
- 64.al Ge Reproductive function after chemotherapy for malignant ovarian germ cell tumors. Abstract 1491. American Society of Clinical Oncology Annual Clinical Meeting. 2002 [Google Scholar]
- 65.Colombo N, Chiari S, Maggioni A, Bocciolone L, Torri V, Mangioni C. Controversial issues in the management of early epithelial ovarian cancer: conservative surgery and role of adjuvant therapy. Gynecol Oncol. 1994;55(Pt 2):S47–51. doi: 10.1006/gyno.1994.1341. [DOI] [PubMed] [Google Scholar]
- 66.Zanetta G, Chiari S, Rota S, et al. Conservative surgery for stage I ovarian carcinoma in women of childbearing age. Br J Obstet Gynaecol. 1997;104:1030–5. doi: 10.1111/j.1471-0528.1997.tb12062.x. [DOI] [PubMed] [Google Scholar]
- 67.Duska L, Chang Y, Flynn C, et al. Epithelial ovarian carcinoma in the reproductive age group. Cancer. 1999;85:2623–9. doi: 10.1002/(sici)1097-0142(19990615)85:12<2623::aid-cncr19>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 68.Morice P, Wicart-Poque F, Rey A, et al. Results of conservative treatment in epithelial ovarian carcinoma. Cancer. 2001;92:2412–8. doi: 10.1002/1097-0142(20011101)92:9<2412::aid-cncr1590>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 69.Schilder J, Thompson A, DePriest P, et al. Outcome of reproductive age women with stage IA or IC invasive epithelial ovarian cancer treated with fertility-sparing therapy. Gynecol Oncol. 2002;87:1–7. doi: 10.1006/gyno.2002.6805. [DOI] [PubMed] [Google Scholar]
- 70.Park J, Kim D, Suh D, et al. Outcomes of fertility-sparing surgery for invasive epithelial ovarian cancer: oncologic safety and reproductive outcomes. Gynecol Oncol. 2008;110:345–53. doi: 10.1016/j.ygyno.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 71.Raspagliesi F, Fontanelli R, Paladini D, di Re E. Conservative surgery in high-risk epithelial ovarian carcinoma. J Am Coll Surg. 1997;185:457–60. doi: 10.1016/s1072-7515(97)00066-5. [DOI] [PubMed] [Google Scholar]
- 72.Colombo N, Parma G, Lapresa M, Maggi F, Piantanida P, Maggioni A. Role of conservative surgery in ovarian cancer: the European experience. Int J Gynecol Cancer. 2005;15(Suppl 3):206–11. doi: 10.1111/j.1525-1438.2005.00428.x. [DOI] [PubMed] [Google Scholar]
- 73.Minton SE, Munster PN. Chemotherapy-induced amenorrhea and fertility in women undergoing adjuvant treatment for breast cancer. Cancer Control. 2002;9:466–72. doi: 10.1177/107327480200900603. [DOI] [PubMed] [Google Scholar]
- 74.Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14:1718–29. doi: 10.1200/JCO.1996.14.5.1718. [DOI] [PubMed] [Google Scholar]
- 75.Recchia F, Saggio G, Amiconi G, et al. Gonadotropin-releasing hormone analogues added to adjuvant chemotherapy protect ovarian function and improve clinical outcomes in young women with early breast carcinoma. Cancer. 2006;106:514–23. doi: 10.1002/cncr.21646. [DOI] [PubMed] [Google Scholar]
- 76.Hulvat MC, Jeruss JS. Maintaining fertility in young women with breast cancer. Curr Treat Options Oncol. 2009;10:308–17. doi: 10.1007/s11864-010-0116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Farhi D, Nosanchuk J, Silverberg S. Endometrial adenocarcinoma in women under 25 years of age. Obstet Gynecol. 1986;68:741–5. [PubMed] [Google Scholar]
- 78.Decruze S, Green J. Hormone therapy in advanced and recurrent endometrial cancer: a systematic review. Int J Gynecol Cancer. 2007;17:964–78. doi: 10.1111/j.1525-1438.2007.00897.x. [DOI] [PubMed] [Google Scholar]
- 79.Zivanovic O, Carter J, Kauff N, Barakat R. A review of the challenges faced in the conservative treatment of young women with endometrial carcinoma and risk of ovarian cancer. Gynecol Oncol. 2009;115:504–9. doi: 10.1016/j.ygyno.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 80.Ramirez P, Frumovitz M, Bodurka D, Sun C, Levenback C. Hormonal therapy for the management of grade 1 endometrial adenocarcinoma: a literature review. Gynecol Oncol. 2004;95:133–8. doi: 10.1016/j.ygyno.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 81.Yarali H, Bozdag G, Aksu T, Ayhan A. A successful pregnancy after intracytoplasmic sperm injection and embryo transfer in a patient with endometrial cancer who was treated conservatively. Fertil Steril. 2004;81:214–6. doi: 10.1016/j.fertnstert.2003.05.031. [DOI] [PubMed] [Google Scholar]
- 82.Pinto A, Gopal M, Herzog T, Pfeifer J, Williams D. Successful in vitro fertilization pregnancy after conservative management of endometrial cancer. Fertil Steril. 2001;76:826–9. doi: 10.1016/s0015-0282(01)01983-5. [DOI] [PubMed] [Google Scholar]
- 83.Randall T, Kurman R. Progestin treatment of atypical hyperplasia and well-differentiated carcinoma of the endometrium in women under age 40. Obstet Gynecol. 1997;90:434–40. doi: 10.1016/s0029-7844(97)00297-4. [DOI] [PubMed] [Google Scholar]
- 84.Duska L, Garrett A, Rueda B, Haas J, Chang Y, Fuller A. Endometrial cancer in women 40 years old or younger. Gynecol Oncol. 2001;83:388–93. doi: 10.1006/gyno.2001.6434. [DOI] [PubMed] [Google Scholar]
- 85.Imai M, Jobo T, Sato R, Kawaguchi M, Kuramoto H. Medroxyprogesterone acetate therapy for patients with adenocarcinoma of the endometrium who wish to preserve the uterus-usefulness and limitations. Eur J Gynaecol Oncol. 2001;22:217–20. [PubMed] [Google Scholar]
- 86.Kaku T, Yoshikawa H, Tsuda H, et al. Conservative therapy for adenocarcinoma and atypical endometrial hyperplasia of the endometrium in young women: central pathologic review and treatment outcome. Cancer Lett. 2001;167:39–48. doi: 10.1016/s0304-3835(01)00462-1. [DOI] [PubMed] [Google Scholar]
- 87.Wang CB, Wang CJ, Huang HJ, et al. Fertility-preserving treatment in young patients with endometrial adenocarcinoma. Cancer. 2002;94:2192–8. doi: 10.1002/cncr.10435. [DOI] [PubMed] [Google Scholar]
- 88.Niwa K, Tagami K, Lian Z, Onogi K, Mori H, Tamaya T. Outcome of fertility-preserving treatment in young women with endometrial carcinomas. BJOG. 2005;112:317–20. doi: 10.1111/j.1471-0528.2004.00398.x. [DOI] [PubMed] [Google Scholar]
- 89.Lowe M, Cooper B, Sood A, Davis W, Syrop C, Sorosky J. Implementation of assisted reproductive technologies following conservative management of FIGO grade I endometrial adenocarcinoma and/or complex hyperplasia with atypia. Gynecol Oncol. 2003;91:569–72. doi: 10.1016/j.ygyno.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 90.Sardi J, Anchezar Henry J, Paniceres G, Gomez Rueda N, Vighi S. Primary hormonal treatment for early endometrial carcinoma. Eur J Gynaecol Oncol. 1998;19:565–8. [PubMed] [Google Scholar]
- 91.Yang Y, Wu C, Chen C, Chang C, Wang K. Reevaluating the safety of fertility-sparing hormonal therapy for early endometrial cancer. Gynecol Oncol. 2005;99:287–93. doi: 10.1016/j.ygyno.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 92.Gotlieb W, Beiner M, Shalmon B, et al. Outcome of fertility-sparing treatment with progestins in young patients with endometrial cancer. Obstet Gynecol. 2003;102:718–25. doi: 10.1016/s0029-7844(03)00667-7. [DOI] [PubMed] [Google Scholar]


