Abstract
An improved protocol for the formal elimination of propene from organic substrates is reported. This process entails the ozonolytic conversion of an alkene to a methoxy hydroperoxide which undergoes fragmentation mediated by copper and iron. The use of soluble Cu(BF4)2 and Fe(BF4)2 results in reproducible results up to a 100 gram scale.
Introduction
The scalable synthesis of simple building block molecules can be a bottleneck in the drug discovery process. The development of scalable procedures to supply adequate quantities of desired intermediates is one avenue to overcome this problem.
As part of a program directed towards the synthesis of neurologically active terpenoid compounds, we required considerable quantities of 6-methylcyclohexenone (2a). An analysis of the known preparations of this compound suggested the most convenient method used dihydrocarvone (1a) as a starting material.1 Schreiber and co-workers described that dihydrocarvone can be subjected to ozonolysis in methanol to yield an intermediate methoxy hydroperoxide species (3) (Scheme 1).2 Earlier synthetic and mechanistic work by Čeković and co-workers3 suggested that peroxide O–O bonds are cleaved in the presence of Fe(ii) to generate a highly reactive alkoxy radical that undergoes β-fragmentation to form the corresponding alkyl radical.4 In the presence of Cu(ii), an alkylcopper species is thought to be formed and undergoes a β-hydrogen elimination to generate an alkene.3 Hence, without the isolation of highly reactive intermediate 3, sequential treatment of this methoxy hydroperoxide species with Cu(OAc)2 and FeSO4 produced 6-methylcyclohexenone (2a) via the above described fragmentation reaction.2,4 Indeed, we found this process to sufficiently produce gram quantities of 6-methylcyclohexenone,5 albeit with variable efficiency. However, further increases in scale resulted in depreciations of yield.
Scheme 1.
Synthesis of (+)-6-methycylohexenone from (+)-dihydrocarvone.2
Results and discussion
Optimization of reaction conditions
To develop a more reliable procedure to produce the requisite quantities of the volatile 2a (100 gram batches), we examined the effect of alternative copper and iron sources for the conversion of 1b to the non-volatile 2b (Table 1). When using the standard heterogeneous conditions, 2b was obtained from 1b in 77% yield. We hypothesized that the variable yield was attributed to the heterogeneity of the reaction mixture. Thus, replacement of Cu(OAc)2 with a soluble and inexpensive salt, Cu(BF4)2 (45% aqueous),6 resulted in a comparable yield (75%) of the desired product (entry 2). A second modification was made to exchange FeSO4 with the soluble Fe(BF4)2 (45% aqueous), which resulted in 71% yield (entry 3). Although these results demonstrated a slight depreciation in efficiency, these conditions produced a fully homogeneous reaction solution that was expected to provide more reproducible results while using inexpensive copper6 and iron7 sources. Control experiments in line with earlier work illustrated that both Cu(ii) and Fe(ii) were necessary for an efficient transformation (entries 4 and 5). A brief survey of readily available Cu(ii) salts (Cu(OAc)2, Cu(TFA)2, CuSO4, Cu(acac)2, Cu(2-ethylhexanoate)2, etc.), indicated that variation of this parameter had little effect on yield. In contrast, use of alternative Fe(ii) salts led to greater variations in yield (e.g. Fe(acac)2 and Fe(C2O4) were less efficient).
Table 1.
β-Fragmentation optimization
 | |||
|---|---|---|---|
| Entry | Cu(ii) | Fe(ii) | % Yielda |
| 1 | Cu(OAc)2·H2O | FeSO4·7H2O | 77 |
| 2 | Cu(BF4)2 (aq.) | FeSO4·7H2O | 75 |
| 3 | Cu(BF4)2 (aq.) | Fe(BF4)2 (aq.) | 71 |
| 4 | None | Fe(BF4)2 (aq.) | 7 |
| 5 | Cu(BF4)2 (aq.) | None | 0 |
Yield determined by 1H-NMR analysis after aqueous work-up using 1,3,5-trimethoxybenzene as an internal standard.
Examination of substrate scope
After establishing optimized conditions for this formal propene elimination, we investigated the scope of these newly developed homogeneous reaction conditions (Table 2). Using the soluble Cu(BF4)2 and Fe(BF4)2 salts, (+)-6-methylcyclohexenone (2a) was prepared from (+)-dihydrocarvone (1a) in 41% yield, along with 17% of the separable β,γ-unsaturated ketone (not shown). These conditions provided 2a with an optical purity that was similar to that of the earlier reported conditions.8 However, if material of high optical purity is not required, we found that methanesulphonic acid treatment of the crude organic extracts quantitatively isomerized the alkene to the enone to provide a higher yield of the desired product (55%).
Table 2.
β-Fragmentation substrate scope
Isolated yield.
17% of the β,γ-enone was isolated.
Treated with MsOH during aqueous work-up.
2.0 equiv. of Cu(OAc)2·H2O & 1.2 equiv. of FeSO4·7H2O was employed.
Further examination of the substrate scope demonstrated that the Robinson annulation adducts9,10 1b and 1c were suitable substrates, providing the allylic alcohols 2b and 2c in 70% and 57% yield, respectively. While it was determined that (−)-isopulegol, 1d, could be transformed to the allylic alcohol 2d11 under the previously reported conditions using Cu(OAc)2 and FeSO4 in 43% yield, use of the tetrafluoroborate salts reported herein resulted in significantly lower yields of the desired product. Although the yield is modest for the degradation of isopulegol, it is noteworthy that the product 2d was previously prepared by a five-step sequence in 2% overall yield after a resolution,12 or from (+)-pulegone in five steps with a 25% overall yield.13
β-Fragmentation of the quaternary substituted isopropylidene substrate 1e proceeds to form 2e in 58% yield. Since substrate 1e was prepared from 2e by an organocuprate conjugate addition of an isopropylidene nucleophile to an enone, this transformation represents a method to temporarily mask enone functionality. Intermediate transformations could be conducted exploiting the differential reactivities of ketones and enones.
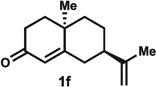
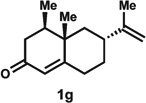
Enone 1f was also tolerated under the reaction conditions to form dienone 2f in a 61% yield.9 If air was excluded from the reaction, nootkatone (1g), a readily available diterpenoid natural product, yielded the kinetically favoured alkene product 2g in 58% yield. While 2g could be isolated by column chromatography with SiO2, it was observed that the double bond isomerized into the thermodynamically favoured position to form a conjugated dienone (not shown) when the purified compound was allowed to stand in CDCl3 at room temperature.
100 gram synthesis of 6-methylcyclohexenone
Following an examination of the substrate scope, we applied the use of Cu(BF4)2 and Fe(BF4)2 to the synthesis of 6-methylcyclohexenone (2a) on a 100 gram scale (Scheme 2). As a point of comparison, using the previously reported heterogeneous conditions gave the desired product 2a in 37% yield. In contrast, employing the newly developed homogeneous reaction conditions yielded 2a in 55% yield on the same scale. Furthermore, the aqueous work-up of the homogeneous reaction solution is more conveniently performed due to the increased solubility of the metal salts.
Scheme 2.
Scalable synthesis of 6-methylcyclohexenone.
Conclusions
This report described an improved procedure for the in situ generation and fragmentation of methoxy hydroperoxides to form alkenes by employing soluble Cu(ii) and Fe(ii) tetrafluoroborate salts. These homogeneous conditions have been employed for the scalable synthesis of an important building block chemical, 6-methylcyclohexenone, and may be useful for other applications.
While this work has focused on the derivatization of readily abundant isopropylidene-containing compounds, other uses for this chemistry could be envisioned. The conjugate addition of an organocopper isopropylidene nucleophile to an enone, followed by ozonolysis and copper- and iron-mediated fragmentation to reform an enone, as in 2e → 1e → 2e, formally represents a protection–deprotection sequence of the reactive enone functional group. This protection–deprotection sequence could be coupled productively with a variety of synthetic transformations.
Supplementary Material
Acknowledgments
Financial support for this work was provided by Yale University and the NSF (GFRP to A. W. S.). Dr. Brandon Mercado is gratefully acknowledged for X-ray analysis of compound 1c.
Footnotes
Electronic supplementary information (ESI) available. CCDC 1477346. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ob00941g
Notes and references
- 1.For known preparations of 6-methylcyclohexenone, see: Yanagita M, Inayama S, Kitagawa R. J. Org. Chem. 1956;21:612. Stork G, White WN. J. Am. Chem. Soc. 1956;78:4604. Reich HJ, Renga JM, Reich IL. J. Am. Chem. Soc. 1975;97:5434. Ito Y, Hirao T, Saegusa T. J. Org. Chem. 1978;43:1011. Cory RM, Chan DMT, Naguib YMA, Rastall MH, Renneboog RM. J. Org. Chem. 1980;45:1852. Chong B-Y, Ji Y-I, Oh S-S, Yang J-D, Baik W, Koo S. J. Org. Chem. 1997;62:9323. Mukaiyama T, Matsuo J, Kitagawa H. Chem. Lett. 2000:1250. Marques FA, Lenz CA, Simonelli F, Maia NS, Noronha Sales Maia BHL, Vellasco AP, Eberlin MN. J. Nat. Prod. 2004;67:1939. doi: 10.1021/np049771x.
- 2.(a) Schreiber SL. J. Am. Chem. Soc. 1980;102:6163. [Google Scholar]; (b) Schreiber SL, Liew W. J. Am. Chem. Soc. 1985;107:2980. [Google Scholar]
- 3.For seminal work and mechanistic studies of FeSO4/Cu(OAc)2 mediated decomposition of hydroperoxides, see: Čeković Ž, Green MM. J. Am. Chem. Soc. 1974;96:3000. Čeković Ž, Dimitrijević L, Djokić G, Srnić T. Tetrahedron. 1979;35:2021. Čeković Ž, Cvetković M. Tetrahedron Lett. 1982;23:3791.
- 4.For examples of Fe(ii) mediated decomposition of peroxides followed by subsequent β-fragmentation, see: Canonica L, Danieli B, Lesma G, Palmisano G. Helv. Chim. Acta. 1987;70:701. Paquette LA, Reagan J, Schreiber SL, Teleha CA. J. Am. Chem. Soc. 1989;111:2331. McCullough KJ, Motomura Y, Masuyama A, Nojima M. Chem. Commun. 1998:1173. Nonami Y, Baran J, Sosnicki J, Mayr H, Masuyama A, Nojima M. J. Org. Chem. 1999;64:4060. Masuyama A, Sugawara T, Nojima M, McCullough KJ. Tetrahedron. 2003;59:353. Gui J, Wang D, Tian W. Angew. Chem., Int. Ed. 2011;50:7093. doi: 10.1002/anie.201101893.
- 5.For examples of total syntheses that use 6-methylcyclohexenone generated from ozonolysis and Cu/Fe mediated β-fragmentation of dihydrocarvone, see: Solladié G, Hutt J. J. Org. Chem. 1987;52:3560. Monti H, Audran G, Monti J, Leandri G. J. Org. Chem. 1996;61:6021. Ohba M, Iizuka K, Ishibashi H, Fujii T. Tetrahedron. 1997;53:16977. Aubin Y, Audran G, Monti H. Tetrahedron Lett. 2006;47:3669. Jiang C, Bhattacharyya A, Sha C. Org. Lett. 2007;9:3241. doi: 10.1021/ol071124k. Fazakerley NJ, Helm MD, Procter DJ. Chem. – Eur. J. 2013;19:6718. doi: 10.1002/chem.201300968. Zhou B, Ren J, Liu X, Zhu H. Tetrahedron. 2013;69:1189. Muchalski H, Xu L, Porter NA. Org. Biomol. Chem. 2015;13:1249. doi: 10.1039/c4ob02377c.
- 6.45% aqueous Cu(BF4)2 was purchased from TCI America for $20 per mol.
- 7.45% aqueous Fe(BF4)2 was purchased from Strem Chemicals, Inc. for $119 per mol.
- 8.White JD, Grether UM, Lee C. Org. Synth. 2005;82:108. [Google Scholar]
- 9.Jansen BJ, Kreuger JA, De Groot A. Tetrahedron. 1989;45:1447. [Google Scholar]
- 10.Nussbaumer C. Helv. Chim. Acta. 1990;73:1621. [Google Scholar]
- 11.For syntheses using allylic alcohol 2d, see: Shimizu M, Kamikubo T, Ogasawara K. Synlett. 1998:655. Zhao H, Donnelly AC, Kusuma BR, Brandt GEL, Brown D, Rajewski RA, Vielhauer G, Holzbeierlein J, Cohen MS, Blagg BSJ. J. Med. Chem. 2011;54:3839. doi: 10.1021/jm200148p.
- 12.For a five-step synthesis of 2d involving a resolution, see: Goering HL, Blanchard JP. J. Am. Chem. Soc. 1954;76:5405. Goering H, Schmidt WW, Singleton VD., Jr J. Org. Chem. 1979;44:2282.
- 13.For a five-step synthesis of 2d starting from pulegone, see: Oppolzer W, Petrzilka M. Helv. Chim. Acta. 1978;61:2755. Thurkauf A, Tius MA. J. Chem. Soc., Chem. Commun. 1989:1593.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.