Abstract
Background
Obesity and asthma are an important public health problem in Saudi Arabia. An increasing body of data supports the hypothesis that obesity is a risk factor for asthma. Asthma appears to be associated with low bone mineral density (BMD) due to long-term use of corticosteroids. Studies recently showed that weight bearing exercise training can increase mineral bone density, reduce weight and improve metabolic control.
Objective
The present study aimed to measure the effects of treadmill walking exercises on bone mineral status and inflammatory cytokines in obese asthmatic patients treated with long term intake of corticosteroids.
Methods
Eighty obese asthmatic patients of both sexes, their age ranged from 41 to 53 years. Subjects were divided into two equal groups: training group (group A) received aerobic exercise training on treadmill for six months in addition to the medical treatment where, the control group (group B) received only the medical treatment.
Results
The results of this study indicated a significant increase in BMD of the lumbar spine & the radius, serum calcium and high density lipoprotein cholesterol (HDL-c) & significant reduction in parathyroid hormone, leptin, tumor necrosis factor- alpha(TNF-α), interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-6 (IL-6), low density lipoprotein cholesterol (LDL-c), triglycerides (TG) and body mass index (BMI) in group (A), while these changes were not significant in group (B).Also; there was a significant difference between both groups at the end of the study.
Conclusion
Treadmill walking exercise training is an effective treatment policy to improve bone mineral status and modulates inflammatory cytokines and blood lipids profile in obese asthmatic patients with long term intake of corticosteroids.
Keywords: Bone mineral density, bronchial asthma, exercises, inflammatory cytokines, inhaled corticosteroids, obesity
Introduction
Asthma and obesity are common disorders with a prevalence that has increased substantially over recent decades. Obesity is a growing worldwide problem that has reached epidemic proportions. The World Health Organization estimates that more than 600 million adults were obese in 20141. Asthma is also a major health problem, which is estimated to affect about 300 million persons worldwide, with the global prevalence of asthma ranging from 1% to 18% of the general population2 and is expected to affect an additional 100 million people by 20253,4.
Obesity may not be only associated with lung mechanics, such as airway closure during tidal breathing and reduced expiratory residual capacity5, but also with a high expression of certain inflammatory mediators, such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6) and leptin6,7. Also, asthma and obesity can be associated with chronic low-grade systemic inflammation involving acute phase proteins such as C-reactive protein (CRP) and proinflammatory cytokines such as IL-6, which may be driven by adipose tissue inflammation8–12.
According to data from the Healthcare Cost and Utilization Project, corticosteroids were the most common cause of drug-related complications, accounting for 10% of such complications and 141,000 hospital stays in the United States in 200413.Corticosteroids-induced osteoporosis results from suppression of bone formation while bone resorption is promoted14. Moderate or high doses of inhaled corticosteroids (ICS) are associated with increased risk of osteoporosis15. Long-term low-dose ICS use increases the rate of bone marrow density (BMD) loss in patients with chronic obstructive pulmonary disease16.
Corticosteroids have detrimental effects on function and survival of osteoblasts and osteocytes, and with the prolongation of osteoclast survival, induce metabolic bone disease17. Inhaled corticosteroids are widely accepted for their potent anti-inflammatory effects in the lungs and low systemic activity18. The use of ICS is the standard maintenance therapy in the management of asthma19.
Obesity is also associated with less likelihood of achieving well-controlled asthma and less-favorable response to current asthma therapy20,21 and Global Initiative for asthma (GINA) 2014 recommends weight loss for all obese asthmatics3. The present study aimed to measure the effects of treadmill walking exercises on bone mineral status and inflammatory cytokines in obese asthmatic patients treated with long term intake of corticosteroids.
Material and methods
Subjects
Eighty obese asthmatic patients of both sexes (40 males & 40 females), their ages ranged between 41–53 years and their body mass index ranged between 30–37 kg/m2 were enrolled in the present randomized controlled trial. The chronicity of the bronchial asthma was not less than 10 years. Participants had been taking bronchodilator therapy in addition to inhaled beclomethasone dipropionate or budesonide in doses ranging from 1000 to 1600 µg/day for at least six months preceding the start of the study. All participants were asked to read and sign an informed consent document prior to participation. Exclusion criteria included endocrinal, renal, liver, cardiac disorders, diabetes and other chest disease rather than bronchial asthma, fractures occurring within the six months preceding the start of the study, disorders of bone metabolism such as osteoporosis and pregnancy, lactation, inadequate contraceptive precautions, amenorrhea or a history of irregular menstrual cycles during the 12 months preceding the start of the study and treatment with any medication likely to influence bone metabolism.
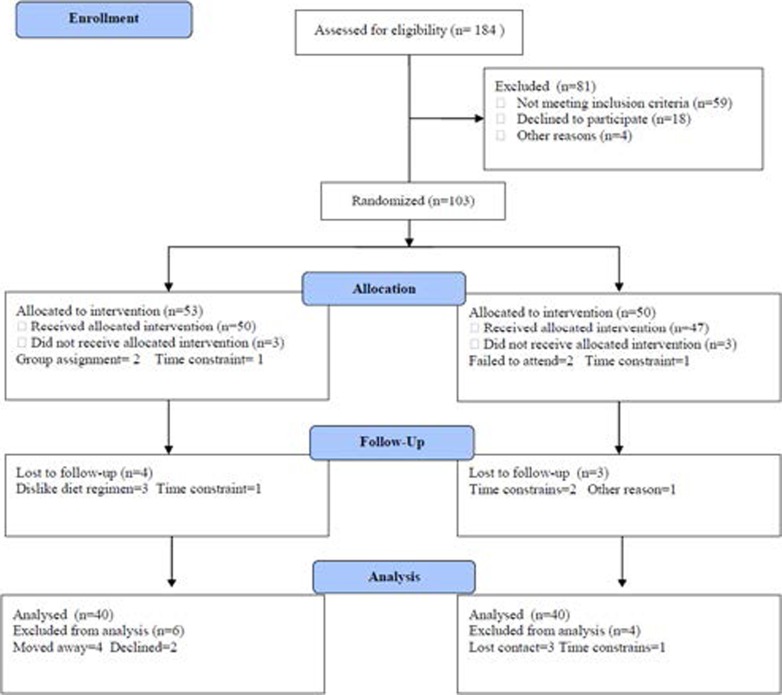
All the patients who participated in the study were nonsmokers and continued in their ordinary diet throughout the study. Following pre-training testing, a randomized block procedure was used to assign qualified participants into two equal groups; group (A) received treadmill walking exercise training .The second group (B) was asked not to participate in any structured exercise program for the duration of the study. The CONSORT diagram outlining the details of the screening, run-in and randomization phases of the study and reasons for participant exclusion can be found in figure (1). Informed consent was obtained from all participants. This study was approved by the Scientific Research Ethical Committee, Faculty of Applied Medical Sciences at King Abdulaziz University.
Figure (1).
Subjects screening and recruitment CONSORT diagram.
Measurements
• Bone mineral density measurements: Dual Energy X-RayAbsorptiometry ((DXA) GE Lunar Prodigy en-CORE software version 8.80, GE Medical Systems, Madison WI) was used to assess the BMD of lumbar spine (L2-L4) and the forearm (33% radius) sites assessed at baseline and after the eight month training period. Subjects removed all metal and plastic before being positioned on the DXA table.
• Chemical analysis: After fasting for 12 hours blood sample will be taken from each subject in clean plain tubes and for other diagnosis different types of anticoagulant would be used such as K2EDTA, Lithium heparin, and Na2 Citrate. All tubes would be centrifuged and plasma separated stored frozen at −20° and used for estimation of plasma tumor necrosis factor -alpha (TNF-α), interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-6 (IL-6), leptin and plasma lipid profile included Triglycerides, high density lipoprotein cholesterol (HDL-c) and low density lipoprotein cholesterol (LDL-c). However, parathyroid hormone (PTH) and serum calcium were measured in serum with an ELISA (Diagnostics Systems Laboratories, Inc., Webster, TX).
All measurements of bone mineral density (BMD) of lumbar spine & radius, serum calcium, parathyroid hormone, triglycerides, HDL-c, LDL-c, leptin, TNF-α, IL-2, IL-4, IL-6 and BMI were done before the starting of the study (pre-test) and after six months at the end of the study (post-test).
Procedures
Following the previous evaluation , all patients were divided randomly into the following groups:
• Group (A): Forty obese asthmatic patients of both sexes (23 males & 17 females), who received moderate intensity aerobic exercise training program. The training program consisted of treadmill exercise of progressive increasing intensity and frequency. The starting treadmill speed was 5 kilometer/hour and at 2 degrees angle of inclination. Then gradually increased at 2 minute intervals until it reached the initial intensity of 60% of the maximal heart rate. Then increased gradually until reaching 80% of the maximal heart rate by the end of the six months. The duration of each session was 30 minutes, including warming up for 5 minutes, 20 minutes for the active stage of training and finally 5 minutes for the cooling down. This training was repeated three times per week for six months.
• Group (B): Forty obese asthmatic patients of both sexes (21 males & 19 females) were asked to maintain their ordinary current life style with the usual medical therapy for six months.
Statistical analysis
Mean values of the investigated parameters was compared by student paired “t” test. While, the unpaired” test was be used to compare between the two groups (P<0.05).
Results
Both study groups were considered homogeneous regarding the demographic and baseline variables (table 1).
Table (1).
Mean value of baseline and demographic data for participants in both groups.
| Mean ± SD | Significance | ||
| Group (A) | Group(B) | ||
| Age (year) | 47.21±6.52 | 46.36±5.13 | P >0.05 |
| Gender (male/female) | 23/17 | 21/19 | P >0.05 |
| Weight (kg) | 70.63±6.17 | 73.18±6.22 | P >0.05 |
| Height (cm) | 169.53±6.71 | 171.21±6.85 | P >0.05 |
| BMI (kg/m2) | 32.45± 3.21 | 33.17± 3.42 | P >0.05 |
| FVC (L) | 2.52 ± 0.82 | 2.31 ± 0.70 | P>0.05 |
| FEV1 (L) | 1.35 ± 0.65 | 1.28 ± 0.59 | P>0.05 |
| FEV1/FVC (%) | 48.77 ± 7.86 | 49.12 ± 8.23 | P>0.05 |
| MVV (L/minute) | 47.64 ± 9.14 | 44.51 ±10.12 | P>0.05 |
BMI = Body mass index; FVC = forced vital capacity; FEV1= forced expiratory volume in the first second; FEV1/FVC = Ratio between forced expiratory volume in the first second and forced vital capacity; MVV=Maximum voluntary ventilation.
The results of this study indicated a significant increase in BMD of the lumbar spine & the radius, serum calcium and HDL & significant reduction in parathyroid hormone, leptin, LDL, TG, TNF-α, IL-2, IL-4, IL-6 and BMI in group (A), while these changes were not significant in group (B) (Table 2 and 3).
Table (2).
Mean value and significance of serum calcium and parathyroid hormone, BMD of lumbar spine, BMD of radius, HDL-c, LDL-c, TG, BMI, Leptin, TNF-α, IL-2, IL-4 and IL-6 in group (A) before and at the end of the study.
| Mean ± SD | T-value | Significance | ||
| Before | After | |||
|
Serum Calcium(ng/dl) |
8.67 ±1.18 | 10.61± 1.17* | 6.41 | P <0.05 |
|
Parathyroid Hormone (ng/dl) |
14.48 ±2.22 | 12.26± 2.34* | 6.32 | P <0.05 |
|
BMD of lumbarspine (mg/cm) |
120.96± 9.17 | 150.32± 8.37* | 8.23 | P <0.05 |
|
BMD of radius(mg/cm) |
266.62± 12.37 | 311.36± 11.35* | 9.17 | P <0.05 |
| HDL-c (mg/dl) | 34.73 ± 5.61 | 37.92 ± 4.58* | 8.64 | P <0.05 |
| LDL-c (mg/dl | 133.71 ± 13.21 | 120.32 ± 11.45* | 8.91 | P <0.05 |
| TG (mg/dl) | 155.36 ± 12.64 | 127.73 ± 11.27* | 10.20 | P <0.05 |
| BMI (Kg/m2) | 35.15 4.63 | 4.4831.41* | 9.21 | P <0.05 |
| Leptin (Ng/ml) | 5.4340.32 | 4.4635.21* | 7.13 | P <0.05 |
| TNF-α (pg/mL) | 5.71± 1.76 | 4.22 ± 1.14* | 6.52 | P <0.05 |
| IL-2 (pg/mL) | 7.86 ± 2.05 | 4.42 ± 1.57* | 5.32 | P <0.05 |
| IL-4(pg/mL) | 5.32± 1.51 | 3.41± 1.26* | 5.40 | P <0.05 |
| IL-6 (pg/mL) | 8.76 ± 2.32 | 5.34 ± 1.85* | 6.19 | P <0.05 |
BMD: Bone marrow density; HDL-c: High density lipoprotein cholesterol; LDL-c: Low density lipoprotein cholesterol; TG: Triglyceride; BMI: Body Mass index; TNF- α = tumor necrosis factor - alpha; IL-2: Interleukin-2; IL-4: Interleukin-4; IL-6: Interleukin-6;
indicates a significant difference, P < 0.05.
Table (3).
Mean value and significance of serum calcium and parathyroid hormone, BMD of lumbar spine, BMD of radius, HDL-c, LDL-c, TG, BMI, Leptin, TNF-α, IL-2, IL-4 and IL-6 in group (B) before and at the end of the study.
| Mean ± SD | T-value | Significance | ||
| Before | After | |||
|
Serum Calcium(ng/dl) |
8.75± 1.23 | 8.96 ±1.19 | 0.63 | P >0.05 |
|
Parathyroid Hormone (ng/dl) |
14.26 ±2.18 | 14.72 ±2.11 | 0.58 | P >0.05 |
|
BMD of lumbar spine (mg/cm) |
122.16± 7.98 | 120.47± 7.87 | 1.85 | P >0.05 |
| BMD of radius(mg/cm) | 268.12± 10.21 | 265.96± 9.73 | 1.92 | P >0.05 |
| HDL-c (mg/dl) | 34.86 ± 5.28 | 34.12 ± 5.13 | 0.87 | P >0.05 |
| LDL-c (mg/dl | 132.94 ± 12.17 | 133.34 ± 12.05 |
0.68 | P >0.05 |
| TG (mg/dl) | 154.93 ± 11.88 | 155.15 ± 11.64 |
0.51 | P >0.05 |
| BMI (Kg/m2) | 34.78 4.21 | 34.88 4.12 | 0.45 | P >0.05 |
| Leptin (Ng/ml) | 39.98 ± 4.75 | 40.12 ± 3.64 |
0.51 | P >0.05 |
| TNF-α (pg/mL) | 5.66± 1.45 | 5.87 ± 1.33 | 0.63 | P >0.05 |
| IL-2 (pg/mL) | 7.72 ± 2.24 | 7.93 ± 2.06 | 0.42 | P >0.05 |
| IL-4(pg/mL) | 5.21± 1.63 | 5.35 ± 1.41 | 0.64 | P >0.05 |
| IL-6 (pg/mL) | 8.67 ± 2.01 | 8.82 ± 2.13 | 0.53 | P >0.05 |
BMD: Bone marrow density; HDL-c: High density lipoprotein cholesterol; LDL-c: Low density lipoprotein cholesterol; TG: Triglyceride ; BMI: Body Mass index; TNF- α = tumor necrosis factor - alpha; IL-2 : Interleukin-2; IL-4 : Interleukin-4; IL-6 : Interleukin-6.
Also; there was a significant difference between both groups at the end of the study (Table 4).
Table (4).
Mean value and significance of serum calcium and parathyroid hormone, BMD of lumbar spine, BMD of radius, HDL-c, LDL-c, TG, BMI, Leptin, TNF-α, IL-2, IL-4 and IL-6 in group (A) and group (B) at the end of the study.
| Mean ± SD | T-value | Significance | ||
| Group (A) | Group (B) | |||
|
Serum Calcium(ng/dl) |
0.6.1± 1.1.7* | 8.96 ± 1.19 |
6.87 | P <0.0.5 |
|
Parathyroid Hormone (ng/dl) |
12.26± 2.34* | 14.72 ±2.11 |
6.76 | P < 0.05 |
| HHDDL-c (mg/dl) | 37.92 ± 4.58* | 34.12 ± 5.13 |
8.68 | P < 0.05 |
|
BMD of lumbar spine (mg/cm) |
150.32± 8.37* | 120.47± 7.87 |
8.57 | P < 0.05 |
|
BMD of radius(mg/cm) |
311.3.6± 11.35* | 265.9.6± 9.73 | 7.24 | P < 0.05 |
| LDDL-c (mg/dl | 120.32 ± 11.45* | 133.34 ± 12.05 |
8.63 | P < 0.05 |
| TG (mg/dl) | 127.73 ± 11.2.7* | 155.15 ± 11.64 |
9.11 | P < 0.05 |
| BMI (Kg/m2) | 4.4831.41* | 34.88 4.12 | 9.42 | P < 0.05 |
| Leptin (Ng/ml) | 4.4635.21* | 40.12 ± 3.64 |
7.53 | P < 0.05 |
| TNF-α (pg/mL) | 4.22 ± 1.14* | 5.87 ± 1.33 |
6.65 | P < 0.05 |
| IL-2 (pg/mL) | 4.42 ± 1.57* | 7.93 ± 2.006 | 5.71 | P < 0.05 |
| IL-4(pg/mL) | 3.41± 1.26* | 5.35 ± 1.41 | 5.58 | P < 0.05 |
| IL-6(pg/mL) | 5.34 ± 1.85* | 8.82 ± 2.13 |
6.46 | P < 0.05 |
BMD: Bone marrow density; HDL-c: High density lipoprotein cholesterol; LDL-c: Low density lipoprotein cholesterol; TG: Triglyceride; BMI: Body Mass index; TNF-α = tumor necrosis factor - alpha; IL-2: Interleukin-2; IL-4: Interleukin-4; IL-6: Interleukin-6;
indicates a significant difference, P < 0.05.
Discussion
As asthma appears to be associated with low bone mineral density (BMD) due to long-term use of corticosteroids and studies recently showed that weight bearing exercise training can increase mineral bone density, reduce weight and improve metabolic control, so this study aimed to evaluate the efficacy of treadmill walking exercises on markers of bone metabolism and inflammatory cytokines in obese asthmatic patients with long term intake of corticosteroids. The results of this study indicated a significant increase in BMD of the lumbar spine & the radius, serum calcium and HDL-c & significant reduction in parathyroid hormone, leptin, TNF-α, IL-2, IL-4, IL-6, LDL-c, TG and BMI in group (A), while these changes were not significant in group (B) which means that treadmill walking exercise training is an effective treatment policy to improve bone mineral status and modulates inflammatory cytokines and blood lipids profile in obese asthmatic patients with long term intake of corticosteroids , these results agreed with many previous studies in this area.
Lester etal. stated that training interventions of longer duration (6–36 months) have consistently reported positive bone mineral density increases, whereas those of shorter durations (< 6 months) have failed to show similar adaptations22. Also, Nordstrom etal. reported that athletes who participate in weight-bearing activities have higher BMD than sedentary controls23. While, Bonaiuti et al. stated that all prescribed exercise programs, including aerobic exercise, resistance exercises or walking are effective at 1 year or more in slowing loss of bone marrow density. Fast walking is recommended as the best prevention and treatment strategy for osteoporosis in postmenopausal women as it is most similar to activities of daily living and may produce the greatest compliance24. However, Hind et al. reported that regular weight-bearing exercise sessions, two to three times weekly, over a period of at least 6 months in pre- or early pubertal children with cystic fibrosis improves BMD25. While, Remes et al. said that physical activity is an important factor in attaining peak bone mass26. Also, Lin et al. stated that weight-bearing exercise had a greater positive effect on BMD than the non-weight-bearing exercise27. Moreover, Douchi et al. confirmed that lumbar spine BMD increased significantly in individual studies of strength training with/without endurance exercise training28. Finally, weight bearing exercise is universally recognized as a major and effective prophylaxis against osteoporosis, to firstly stimulate bone accretion during growth; secondly, to stimulate bone accretion once bone loss has occurred and thirdly, to prevent bone loss25.
The possible mechanism by which exercise maintains the skeletal integrity are: changes in the biochemical structure of the blood by altering the level of its component which has a role in the integrity of normal skeletal and mechanical load of the exercise which can modify and increase bone mass. The rise in serum calcium is mainly due to the effects of exercise induced acidosis. So, when the parathyroid hormone falls the excess hydrogen ions reversibly displace calcium ions from imidazole groups of the albumin molecule, thus causing the serum calcium to rise29. The increase in bone mineral density after exercise may be due to increase in serum calcium associated with decreased parathyroid hormone following exercise training30.
Concerning the inflammatory cytokines, the mean values of leptin, TNF-α, IL-2, IL-4 and IL-6 were significantly decreased after aerobic exercise training. These results are in line with many previous studies, Dekker et al. stated that a 12-week exercise intervention resulted in a significant decrease in circulating IL-6 in subjects with type 2 diabetes mellitus who underwent an exercise program without weight loss31. Also, Mikkelsen et al. proved that life-long endurance exercise was associated with a lower level of the inflammatory markers CRP and IL-6 in elderly subjects32. While, Sugawara et al. concluded that the levels of elevated inflammatory cytokines decreased significantly after intervention with an anti-inflammatory nutrition combined with the low-intensity exercise in stable elderly COPD patients33. In addition, there is evidence of lowered IL-6 and TNF-α after prolonged exercise in obese women34 and decreased TNF-α after 12 weeks of aerobic exercise in patients with heart disease 35. Moreover, in obese postmenopausal women with type 2 diabetes, 14 weeks of aerobic exercise decreased CRP by 15% and marginally decreased IL-6 (p=0.07)36. Likewise, 12 week of exercise reduced IL-18 levels by 17.5% in patients with metabolic syndrome37.
The exact mechanisms by which physical activity may reduce inflammation are not entirely understood, there is some data pointing to factors that may contribute to an effect of repeated bouts of muscle contraction leading to improvements in inflammatory status over time38. Exercise training-induced improvements in inflammatory status may also result from the modulation of intracellular signaling pathways and cellular function that are mediated by nitric oxide (NO) and ROS39. However, physical activity may lower leptin concentrations not only due to the decreased body fat mass, but potentially through an increase in leptin sensitivity40.
Regarding blood lipid profile, our results proved that aerobic exercise training resulted in significant increase in HDL-c and significant reduction in LDL-c and TG , these results are in line with many previous studies as Bounds and colleagues stated that exercise training resulted in an increase in HDL-c (10.7%) and a concomitant fall in triglyceride (-25%) and total cholesterol (−3.5%)41. Where, exercise-induced increases in HDL-c and decreases in triglyceride are similar in hyper-and normo-cholesterolemic men and may be mediated, at least in part, by an increase in lipoprotein lipase activity42. However, aerobic exercise is efficacious for increasing HDL-C and decreasing TC, LDL-C, and TG in women43.
The current study has important strengths and limitations. The major strength is the supervised nature of the study. Supervising physical activity removes the need to question compliance or to rely on activity questionnaires. Further, all exercise sessions were supervised and adherence to activities was essentially 100%. Moreover, the study was randomized; hence, we can extrapolate adherence to the general population. In the other hand, the major limitation is the small sample size in both groups may limit the possibility of generalization of the findings in the present study. Finally, within the limit of this study, treadmill walking exercise training is an effective treatment policy to modulate bone mineral status, inflammatory cytokines and blood lipids profile in obese patients with bronchial asthma with long term intake of corticosteroids. Further researches are needed to explore the impact of exercise training upon quality of life and other biochemical parameters among patients with bronchial asthma.
Conclusion
Treadmill walking exercise training is an effective treatment policy to modulate bone mineral status, inflammatory cytokines and blood lipids profile in obese patients with bronchial asthma with long term intake of corticosteroids.
Acknowledgment
This project was funded by the Deanship of Scientific Research (DSR), KingAbdulaziz University, Jeddah, under grant no. (45/142/1432). The authors, therefore, acknowledge with thanks DSR technical and financial support.
References
- 1.World Health Organization (WHO), author Obesity and overweight Fact Sheet No. 311. 2015. [December 20, 2015]. Available at: http://www.who.int/mediacentre/factsheets/fs311/en/
- 2.Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–178. doi: 10.1183/09031936.00138707. PubMed. [DOI] [PubMed] [Google Scholar]
- 3.Global Initiative for Asthma (GINA), author Global Strategy for Asthma Management and Prevention. 2014. [February, 2015]. In http://www.ginasthma.org/
- 4.Global Initiative fos ashtma (GINA), author Global Burden of asthma. 2014. [February, 2015]. In http://www.ginasthma.org/
- 5.Lessard A, Turcotte H, Cormier Y, Boulet LP. Obesity and asthma: a specific phenotype? Chest. 2008;134:317–323. doi: 10.1378/chest.07-2959. PubMed. [DOI] [PubMed] [Google Scholar]
- 6.Gibson PG. Obesity and asthma. Ann Am Thorac Soc. 2013;10(Suppl):S138–S142. doi: 10.1513/AnnalsATS.201302-038AW. [DOI] [PubMed] [Google Scholar]
- 7.Sutherland TJ, Cowan JO, Young S, Goulding A, Grant AM. The association between obesity and asthma: interactions between systemic and airway inflammation. Am J Respir Crit Care Med. 2008;178:469–475. doi: 10.1164/rccm.200802-301OC. [DOI] [PubMed] [Google Scholar]
- 8.Jensen ME, Gibson PG, Collins CE, Wood LG. Airway and systemic inflammation in obese children with asthma. Eur Respir J. 2013;42:1012–1019. doi: 10.1183/09031936.00124912. [DOI] [PubMed] [Google Scholar]
- 9.Leiria L, Martins M, Saad M. Obesity and asthma: beyond TH2 inflammation. Metab Clin Exp. 2014;64:172–181. doi: 10.1016/j.metabol.2014.10.002. PubMed. [DOI] [PubMed] [Google Scholar]
- 10.Periyalil H, Gibson P, Wood L. Immunometabolism in obese asthmatics: are we there yet? Nutrients. 2013;5:3506–3530. doi: 10.3390/nu5093506. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wood LG, Garg ML, Gibson PG. A high-fat challenge increases airway inflammation and impairs bronchodilator recovery in asthma. J Allergy Clin Immunol. 2011;127:1133–1140. doi: 10.1016/j.jaci.2011.01.036. PubMed. [DOI] [PubMed] [Google Scholar]
- 12.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Sci New York NY. 1993;259:87–91. doi: 10.1126/science.7678183. PubMed. [DOI] [PubMed] [Google Scholar]
- 13.Elixhauser A, Owens P. for the Agency of Healthcare Research and Quality. Adverse drug events in U.S. hospitals, 2004. Healthcare Cost and Utilization Project. Statistical Brief. 2007;29:1–12. [Google Scholar]
- 14.Dore RK. How to prevent glucocorticoid-induced osteoporosis. Cleve Clin J Med. 2010;77:529–536. doi: 10.3949/ccjm.77a.10003. PubMed. [DOI] [PubMed] [Google Scholar]
- 15.Avenell A, Gillespie WJ, Gillespie LD. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database Syst Rev. 2009;2:CD000227. doi: 10.1002/14651858.CD000227.pub3. [DOI] [PubMed] [Google Scholar]
- 16.Mathioudakis AG, Amanetopoulou SG, Gialmanidis IP. Impact of long-term treatment with low-dose inhaled corticosteroids on the bone mineral density of chronic obstructive pulmonary disease patients: aggravating or beneficial? Respirology. 2013;18:147–153. doi: 10.1111/j.1440-1843.2012.02265.x. PubMed. [DOI] [PubMed] [Google Scholar]
- 17.Chee C, Sellahewa L, Pappachan JM. Inhaled corticosteroids and bone health. Open Respir Med J. 2014;8:85–92. doi: 10.2174/1874306401408010085. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoloff SW, Kelly HW. Updates on the use of inhaled corticosteroids in asthma. Curr Opin Allergy Clin Immunol. 2011;11:337–344. doi: 10.1097/ACI.0b013e328348a813. [DOI] [PubMed] [Google Scholar]
- 19.Global Strategy for asthma management and prevention. [2 September 2014]. http://www.ginasthma.org/local/uploads/files/GINA_Report_2014_Aug12.pdf.
- 20.Camargo CA, Jr, Sutherland ER, Bailey W, et al. Effect of increased body mass index on asthma risk, impairment and response to asthma controller therapy in African Americans. Curr Med Res Opin. 2010;26:1629–1635. doi: 10.1185/03007995.2010.483113. PubMed. [DOI] [PubMed] [Google Scholar]
- 21.Forno E, Lescher R, Strunk R. Decreased response to inhaled steroids in overweight and obese asthmatic children. J Allergy Clin Immunol. 2011;127:741–749. doi: 10.1016/j.jaci.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lester M, Urso M, Evans R, Pierce J, Spiering B, Maresh C, Hatfield D, Kraemer W, Nindl B. Influence of exercise mode and osteogenic index on bone biomarker responses during short-term physical training. Bone. 2009;45(4):768–776. doi: 10.1016/j.bone.2009.06.001. PubMed. [DOI] [PubMed] [Google Scholar]
- 23.Nordström A, Olsson T, Nordström P. Sustained benefits from previous physical activity on bone mineral density in males. J Clin Endocrinol Metab. 2006;91(7):2600–2604. doi: 10.1210/jc.2006-0151. [DOI] [PubMed] [Google Scholar]
- 24.Bonaiuti D, Shea B, Iovine R, Negrini S, Robinson V, Kemper HC, Wells G, Tugwell P, Cranney A. Exercise for preventing and treating osteoporosis in postmenopausal women (review). The Cochrane Database of Systematic Reviews. Cochrane Database Syst Rev. 2002;3:CD000333. doi: 10.1002/14651858.CD000333. [DOI] [PubMed] [Google Scholar]
- 25.Hind K, Truscott J, Conway S. Exercise during childhood and adolescence: A prophylaxis against cystic fibrosis- related low bone mineral density?: Exercise for bone health in children with cystic fibrosis. J Cyst Fibrosis. 2008;7(4):270–276. doi: 10.1016/j.jcf.2008.02.001. PubMed. [DOI] [PubMed] [Google Scholar]
- 26.Remes T, Väisänen SB, Mahonen A, Huuskonen J, Kröger H, Jurvelin JS, Penttilä IM, Rauramaa R. The association of bone metabolism with bone mineral density, serum sex hormone concentrations, and regular exercise in middle-aged men. Bone. 2004;35(2):439–447. doi: 10.1016/j.bone.2004.04.020. PubMed. [DOI] [PubMed] [Google Scholar]
- 27.Lin L, Lo M, Yao W, Hung C. The effects of different weight-bearing exercise training on bone mineral density and bone metabolism in young men. Journal of Science and Medicine in Sport. 2005;12(Supplement 2):e123–e124. [Google Scholar]
- 28.Douchi T, Yamamoto S, Oki T, Maruta K, Kuwahata R, Yamasaki H, Nagata Y. The effects of physical exercise on body fat distribution and bone mineral density in postmenopausal women. Maturitas. 2000;35(1):25–30. doi: 10.1016/s0378-5122(00)00094-3. PubMed. [DOI] [PubMed] [Google Scholar]
- 29.Ljunghall S, Joborn H, Benson L, Fellström B, Wide L, Akerstrom G. Effects of physical exercise on serum calcium and parathyroid hormone. Eur J Clin Invest. 1984;14(6):469–473. doi: 10.1111/j.1365-2362.1984.tb01215.x. [DOI] [PubMed] [Google Scholar]
- 30.Grimston S, Tanguay K, Gundberg C, Hanley D. The caliotropic hormone response to changes in serum calcium during exercise in female. J Clin Endocrinol Metab. 1993;76(4):867–872. doi: 10.1210/jcem.76.4.8473398. [DOI] [PubMed] [Google Scholar]
- 31.Dekker M, Lee S, Hudson R, Kilpatrick K, Graham T, Ross R. An exercise intervention without weight loss decreases circulating interleukin-6 in lean and obese men with and without type 2 diabetes mellitus. Metabolism. 2007;56(3):332–338. doi: 10.1016/j.metabol.2006.10.015. PubMed. [DOI] [PubMed] [Google Scholar]
- 32.Mikkelsen U, Couppé C, Karlsen A, Grosset J, Schjerling P, Mackey A, Klausen H, Magnusson S, Kjær M. Lifelong endurance exercise in humans: circulating levels of inflammatory markers and leg muscle size. Mech Ageing Dev. 2013;134(11–12):531–540. doi: 10.1016/j.mad.2013.11.004. PubMed. [DOI] [PubMed] [Google Scholar]
- 33.Sugawara K, Takahashi H, Kashiwagura T, Yamada K, Yanagida S, Homma M, Dairiki K, Sasaki H, Kawagoshi A, Satake M, Shioya T. Effect of anti-inflammatory supplementation with whey peptide and exercise therapy in patients with COPD. Respiratory Medicine. 2012;106:1526e15340. doi: 10.1016/j.rmed.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 34.You T, Berman D, Ryan A, Nicklas B. Effects of hypocaloric diet and exercise training on inflammation andadipocyte lipolysis in obese postmenopausal women. J Clin Endocrinol Metab. 2004;89(4):1739–1746. doi: 10.1210/jc.2003-031310. [DOI] [PubMed] [Google Scholar]
- 35.Larsen A, Aukrust P, Aarsland T, Dickstein K. Effect of aerobic exercise training on plasma levels of tumor necrosis factor alpha in patients with heart failure. Am J Cardiol. 2001;88(7):805–808. doi: 10.1016/s0002-9149(01)01859-8. PubMed. [DOI] [PubMed] [Google Scholar]
- 36.Giannopoulou I, Fernhall B, Carhart R, Weinstock R, Baynard T, Figueroa A, Kanaley J. Effects of diet and/or exercise on the adipocytokine and inflammatory cytokine levels of postmenopausal women with type 2 diabetes. Metabolism. 2005;54:866–875. doi: 10.1016/j.metabol.2005.01.033. PubMed. [DOI] [PubMed] [Google Scholar]
- 37.Troseid M, Lappegard KT, Mollnes T, Arnesen H, Seljeflot I. The effect of exercise on serum levels of in- terleukin-18 and components of the metabolic syndrome. Metab Syndr Relat Disord. 2009;7(6):579–584. doi: 10.1089/met.2009.0003. [DOI] [PubMed] [Google Scholar]
- 38.Beavers K, Brinkley T, Nicklas B. Effect of exercise training on chronic inflammation. Clin Chim Acta. 2010;411(11–12):785–793. doi: 10.1016/j.cca.2010.02.069. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheele C, Nielsen S, Pedersen B. ROS and myokines promote muscle adaptation to exercise. Trends Endocrinol Metab. 2009;20:95–99. doi: 10.1016/j.tem.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Mendez-Sanchez N, Gonzalez V, King-Martinez A, Sanchez H, Uribe M. Plasma leptin and the cholesterol saturation of bile are correlated in obese women after weight loss. J Nutr. 2002;132(8):2195–2198. doi: 10.1093/jn/132.8.2195. PubMed. [DOI] [PubMed] [Google Scholar]
- 41.Bounds R, Grandjean P, O'Brien B, Inman C, Crouse S. Diet and short term plasma lipoprotein lipid changes after exercise in trained men. Int J Sport Nutur Exerc Metab. 2000;10(2):114–127. doi: 10.1123/ijsnem.10.2.114. PubMed. [DOI] [PubMed] [Google Scholar]
- 42.Grandjean P, Crouse S, Rohack J. Influence of cholesterol status on blood lipid and lipoprotein enzyme responses to aerobic exercise. J Appl Physiol. 2000;89(2):472–480. doi: 10.1152/jappl.2000.89.2.472. PubMed. [DOI] [PubMed] [Google Scholar]
- 43.Kelley G, Kelley K, Tran Z. Aerobic exercise and lipids and lipoproteins in women: a meta-analysis of randomized controlled trials. J Women Health. 2004;3(10):1148–1164. doi: 10.1089/jwh.2004.13.1148. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]