Abstract
Background
Occult hepatitis B infections are becoming a major global threat, but the available data on its prevalence in various parts of the world are often divergent.
Objective
This study aimed to detect occult hepatitis B virus in hepatitis B surface antigen-negative serum using anti-HBc as a marker of previous infection.
Patient and methods
A total of 1000 randomly selected hepatitis B surface antigen-negative sera from blood donors were tested for hepatitis B core antibody and hepatitis B surface antibody using an ELISA and nested polymerase chain reaction was done using primers specific to the surface gene (S-gene).
Results
Of the 1000 samples 55 (5.5%) were found to be reactive, of which 87.3% (48/55) were positive for hepatitis B surface antibody, indicating immunity as a result of previous infection however, that does not exclude active infection with escaped mutant HBV. Nested PCR results showed the presence of hepatitis B viral DNA in all the 55 samples that were positive for core protein, which is in agreement with the hepatitis B surface antibody result.
Conclusion
This study reveals the 5.5% prevalence of occult hepatitis B among Malaysian blood donors as well as the reliability of using hepatitis B core antibody in screening for occult hepatitis B infection in low endemic, low socioeconomic settings.
Keywords: Hepatitis B, surface antigen, core antibody, polymerase chain reaction, occult hepatitis B infection
Introduction
Hepatitis B surface antigen (HBsAg) is the main diagnostic marker for hepatitis B infection and for screening of donated blood. Escaped mutants might be as a result of post transcriptional effect of the mutation on HBsAg expression as described previously1,2. In addition, it is a known fact that surface antigen mutation reduced effectiveness of diagnostics and allowed for humoral immune escaped thereby reducing vaccination effectiveness3. While occult hepatitis B is characterised by undetectable levels of surface antigen, but detectable levels of viral DNA4. Occult hepatitis B infection is becoming a major global threat due to: (a) the effect on the health of children born to carrier mothers despite the presence of passive immunoglobulin at birth5–7; (b) immune escape of current vaccines8,9; and (c) spread through blood and blood products in post transfusion infection, organ donation, and sexual transmission10,11. There are several concerns surrounding occult hepatitis B infection in clinical settings, including reduced sensitivity to available diagnostic tests, lack of immunity following vaccination with non-mutant HBV variants (vaccine escaped mutant), and failure of passive immunization with HBV immunoglobulin G12. The failure of diagnostic assays to detect HBV poses a major risk to recipients of blood transfusions or organ donations13,14. Since hepatitis B surface mutants are stable and can be spread through either vertical or horizontal transmission15, both a highly sensitive molecular method and an affordable and reliable serological test are required for the diagnosis of occult hepatitis B infection. Therefore, this study aimed at detecting occult hepatitis B among hepatitis B surface antigen negative blood donors using anti-HBc as a marker and compared these results to detection of the hepatitis B S-gene by nested PCR.
Methodology
Sample collection
One thousand serum samples were randomly selected from a pool of hepatitis B surface antigen-negative sera from anonymized leftovers from initial blood testing of blood donors at the National Blood Centre in Kuala Lumpur, Malaysia. Systematic random sampling random selection of first sample systematic selection through a pre-determined interval N/n = nth term using online sampling software (https://www.randomizer.org/)
Anti-HBc testing
Hepatitis B core antibodies were tested for by using a commercially available ELISA kit (DRG International Inc. New Jersey, USA). Serum samples were diluted in wash solution (1:5). Then, 50 µl of the diluted serum was added to the micro wells, which are coated with purified recombinant hepatitis B core antigen according to the manufacturer's instructions. The TECAN Magellan ELISA reader version 6.4 was used to measure the absorbance at 450 nm and the results were interpreted based on the optical density (OD).
DNA extraction
HBV DNA was extracted from 200 µl of serum using a QIAampBlood mini kit (Qiagen, Hilden, Germany) according to the manufacturer's instruction. Briefly, 20 µl of protease was added to the serum in a 1.5 ml tube. Then, 200 µl of cell lysis solution (AL buffer) was added, vortexed, and incubated for 10 min at 56°C. The DNA was eluted using 50 µl of elution buffer and stored at −20°C until further analysis.
Nested Polymerase Chain Reaction (PCR)
Two sets of published primers were used to amplify the hepatitis B S-gene from the DNA extracted from the serum samples16 with the first round of PCR using the outer primers, which amplify a 916 bp segment which includes the S-gene, under the following conditions: 30 cycles of 94°C for 5 min, 94°C for 30 sec, 63.8°C for 30 sec, and 72°C for 60 sec, followed by a final extension at 72°C for 10 min. The second round of PCR was performed using the inner primers, which amplify the 656 bp S-gene amplicon of the surface antigen under the following conditions: 30 cycles of 94°C for 5 min, 94°C for 30 sec, 63.8°C for 30 sec, and 72°C for 60 sec, with a final extension at 72°C for 8 min. A 50 µl reaction mixture containing 1 µl of DNA sample, 25 µl PCR pre mixed solution (Promega, USA), 1.25 µl of forward and reverse primers(final concentration 0.5 µM), and 21.5 µl of nuclease free water. This reaction was used for both the first and second rounds of PCR amplification using a thermal cycler (Bio-Rad) as described previously16. Genomic hepatitis B DNA and no DNA template control were used as positive and negative controls, respectively. The PCR products were analysed by gel electrophoresis using 1.5% (w/v) agarose gel (Seakem LE, USA). PCR products were visualised with UV illuminator and the product size was determined by comparison to the DNA molecular mark er (GeneDireX). Sample bands corresponded to the size of the positive control bands. To ensure that the primers used in this study specifically amplified the S-gene of HBV, they were tested against a DNA sample from an HBV-positive serum that is positive for anti-Hbc, anti-HBe, and negative anti-HBs.. The absolute detection limit of the nested PCR was determined by a 10-fold dilution of the standard HBV-positive serum and the detection limit was found to 5 copies per µL.
Data analysis
The data from the findings was analyzed using SPSS statistic version 20 (Chicago, Illinois). The percentage of occult hepatitis B infection prevalence was then calculated.
Ethical approval
Ethical approval to carry out this study was obtained from the Institute for Medical Research (IMR) in Malaysia with reference No. NMRR-12-469-11762 and the Faculty of Medicine and Health Sciences Ethical Committee, Universiti Putra, Malaysia (Ref: UPM/.FPSK/100-9/2-MJKEtikaPen)..
Results
Of the 1000 samples that were screened, 5.5% (55/1000) were found to be anti-HBc-positive, all of which were also positive for HBV DNA. Additionally, 84.8% (848/1000) of the samples were anti-HBs-positive (Table 1).
Table 1.
Serological markers and molecular detection of HBV DNA.
| HBsAg | Anti-HBs | Ant-HBc | Nucleic Acid Amplification Test |
Polymerase Chain Reaction (PCR) |
|
| Positive | 0 | 848 | 55 | 0 | 55 |
| Negative | 1000 | 152 | 945 | 1000 | 945 |
| Total | 1000 | 1000 | 1000 | 1000 | 1000 |
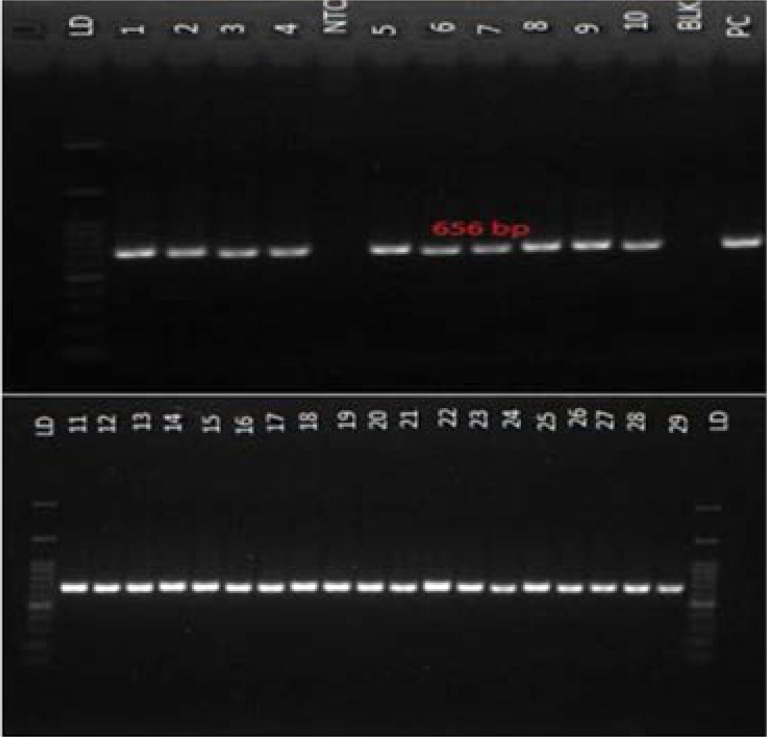
The results of the nested PCR show the presence of a 656 bp fragment, indicating a positive result with the no template and blank showing no band as such excluding chances of contaminant from the PCR reagents. Positive control also showed a specific band that is similar in size with the positive samples confirming its positivity (Figure 1).
Figure 1.
Positive nested PCR result from hepatitis B surface-negative, core-positive serum samples of healthy volunteers. A 656 bp DNA product was amplified using primers specific for hepatitis B S-gene. All lanes show a PCR product of the expected size. LD: 100 bp molecular weight marker; PC: Positive control; and NTC: Non-template control (NTC). 160×161mm (72 × 72 DPI)
Of the 5.5% (55/1000) of the samples that were positive for occult hepatitis B, 87.3% (48/55) were also hepatitis B surface antibody-positive, indicating immunity as a result of previous infection17, while 12.7% (7/55) were negative for the presence of the antibody. However, none of these samples were hepatitis B surface antigen-positive.
Discussion
Hepatitis B is of global health importance with about 350 million chronic carriers worldwide, constituting a major global threat. According to World Health Organization (WHO), Western Pacific region accounts for about 50% of the world's chronic hepatitis B infection18. Likewise, in Malaysia the prevalence of HBV among children decreased to 0.4% from 3.0% prior to introduction universal infant hepatitis B vaccination19. However, the prevalence in general population ranges from 1.5 to 9.8% but reported to be lower (0.4%) among repeated blood donors20. In this study HBV DNA was detected in HBsAg-negative serum using nested PCR, which is in line with previous studies that demonstrated the same result in peripheral blood mononuclear cells, serum, and liver samples21–24. Nested PCR approach improves the sensitivity of the PCR assay by avoiding artefacts and non-specific binding associated with the conventional PCR method as shown in this study In serum, HBV DNA levels are less than 104 copies/mL25, which is considerably lower than those that are HBsAg-positive22,23. The use of NAT in detecting HBV has been a debatable issue, since the viral load in occult infection is usually very low, thereby reducing the effectiveness of NAT testing as shown in this study where NAT and HBsAg negative sera of blood donors were found to be HBV DNA positive by nested PCR (Table 1). Therefore, there is a need for a more sensitive HBsAg system that is capable of detecting a very low HBsAg level and S gene mutants in order to reduce the risk of transfusion and organ transplant associated hepatitis B infection.
Our finding revealed 5.5% prevalence of anti-HBc among healthy blood donors, which is higher than what was reported in Italy26, Egypt27, Germany, UK28 and USA29, but less than what was reported in Korea30 and Greece31. This study revealed a 100% prevalence of occult hepatitis in hepatitis B core antibody-positive healthy individuals. This is similar to the frequency reported in China32. Additionally, other studies have reported finding occult hepatitis B virus more frequently in individuals with anti-HBc-positive serology than in those with anti-HBc-negative serology33,34. On the other hand, a prevalence of 33.3% was reported in North East China32, as compared to 0.11% in Taiwan35. The risk transmission depends on presence of anti-HBs and viral dose; viral load: copies/ml x vol. of plasma, the higher the viral load, and the higher the chance of transmission. Anti-HBs; in donor or passive also influence the rate of transmission. Immune status of the recipient is also an important determinant of its transmission36. In a look back data from Japan and Hong Kong: transmission of HBV from donors with OHBI was approximately 3%36. In Malaysia NAT has been implemented in the National Blood Centre since 5th November 2007 and was used to test the donated blood from others catchment hospitals such as Klang, Kajang, Kuala Kubu Baru, Termerloh, Bentong, Raub, Kuala Lipis and Seremban. There was sufficient evidence to support that the effectiveness of using NAT blood screening test to detect HBV infection in donated blood. Hence, reducing the risk of transfusion associated HBV infection. However, it is not cost-effective to implement it in the low endemic setting as such anti-HBc can be considered in such setting.
The risk of transmitting occult hepatitis B infection dependents on presence of copies of HBV DNA in the plasma and the volume of the plasma transfused therefore, the higher the viral load in the transfused blood the higher the chance of infectivity37. The presence of high anti-HBs in the donors blood also influence the rate of infectivity38. Immune status of the recipient and donors also play an important role in determining infectivity36. Recent infectivity data indicate transmission rate 3.8% 38, but the rate is higher in unvaccinated recipients of occult hepatitis B blood or blood products. Blood products from donors with occult hepatitis B carry a high risk of HBV transmission by transfusion. This may justify safety measures such as anti-HBc and HBV nucleic acid test screening depending on epidemiology. Anti-HBc may be used in < 2% to 4% prevalence, while, HBV-NAT in high endemic areas37. Occult hepatitis B infections have significant clinical importance since they can become reactivated when the immune system is suppressed and can be transmitted through blood or blood product transfusion, organ transplant, and sexual intercourse. It may also enhance the progression of liver fibrosis and, subsequently, hepatocellular carcinoma.
Conclusion
This study revealed a 5.5 % prevalence of occult hepatitis in asymptomatic, healthy blood donors. We found that 100% of samples that were positive for hepatitis B core antibodies were also positive for HBV DNA, thus indicating the reliability of hepatitis B core antibody in screening for occult hepatitis B infection, especially in low socioeconomic, low endemic settings. Therefore, complimenting screening methods like anti-HBc and NAT based on epidemiology may be of important benefit in decreasing the risk associated with post transfusion HBV infection as such anti-HBc may be used in < 2% to 4% prevalence, while, HBV-NAT in high endemic areas. The detection of hepatitis B viral DNA in both the so-called “immune as a result of natural infection” and the isolated anti-core group indicates the need for further study and reclassification.
Acknowledgments
The authors are grateful to Sanofi Aventis-Malaysia and the Faculty of Medicine and Health Sciences, Universiti Putra, Malaysia for their financial support of this research.
Authors' contributions
SA Hudu and Z Sekawi conceived the idea for this project. R Hassan and SA Hudu collected serum sample for this study. SA Hudu and M I Saeed performed the experiments. SA Hudu wrote the first draft of the manuscript, which was substantially revised by Z Sekawi, YA Malik, NM Taib, NS Harma, MI Saeed, AS Alshrari and R Hassan. All authors read and approved the final manuscript.
Competing interests
We declare no competing interests.
References
- 1.Hass M, Hannoun C, Kalinina T, Sommer G, Manegold C, Günther S. Functional analysis of hepatitis B virus reactivating in hepatitis B surface antigen-negative individuals. Hepatology. 2005;42(1):93–103. doi: 10.1002/hep.20748/full. PubMed. [DOI] [PubMed] [Google Scholar]
- 2.Baumert TF, Köck J, Blum HE. A novel target of hepatitis B virus mutations: Splicing of surface RNA. Hepatology. 2005;42(1):21–23. doi: 10.1002/hep.20791/full. PubMed. [DOI] [PubMed] [Google Scholar]
- 3.Weber B. Genetic variability of the S gene of hepatitis B virus: clinical and diagnostic impact. Journal of clinical virology. 2005;32(2):102–112. doi: 10.1016/j.jcv.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto K, Horikita M, Tsuda F, Itoh K, Akahane Y, Yotsumoto S, et al. Naturally occurring escape mutants of hepatitis B virus with various mutations in the S gene in carriers seropositive for antibody to hepatitis B surface antigen. Journal of Virology. 1994;68(4):2671–2676. doi: 10.1016/0270-9139(95)90546-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hudu S, Sekawi Z, Taib N, Malik Y, Harmal N. P24: Occult hepatitis B infection among vaccinated cohort. Journal of Viral Hepatitis. 2013;20(s3):30–30. doi: 10.1111/jvh.12166_23. [DOI] [Google Scholar]
- 6.Pan CQ, Duan ZP, Bhamidimarri KR, Zou HB, Liang XF, Li J, et al. An algorithm for risk assessment and intervention of mother to child transmission of hepatitis B virus. Clinical Gastroenterology and Hepatology. 2012;10(5):4529. doi: 10.1016/j.cgh.2011.10.041. [DOI] [PubMed] [Google Scholar]
- 7.Foaud H, Maklad S, Mahmoud F, El-Karaksy H. Occult hepatitis B virus infection in children born to HBsAg-positive mothers after neonatal passive-active immunoprophylaxis. Infection. 2015:1–8. doi: 10.1007/s15010-015-0733-6. [DOI] [PubMed] [Google Scholar]
- 8.Simon B, Kundi M, Puchhammer E. Analysis of Mutations in the S Gene of Hepatitis B Virus Strains in Patients with Chronic Infection by Online Bioinformatics Tools. Journal of Clinical Microbiology. 2013;51(1):163–168. doi: 10.1128/JCM.01630-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alter HJ. To have B or not to have B: Vaccine and the potential eradication of hepatitis B. Journal of Hepatology. 2012;57(4):715. doi: 10.1016/j.jhep.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 10.O'Brien SF, Zou S, Laperche S, Brant LJ, Seed CR, Kleinman SH. Surveillance of Transfusion-Transmissible Infections: Comparison of Systems in Five Developed Countries. Transfusion Medicine Reviews. 2012;26(1):38–57. doi: 10.1016/j.tmrv.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdullah SM. The effect of repeated blood donations on the iron status of male Saudi blood donors. Blood Transfusion. 2011;9(2):167. doi: 10.2450/2010.0040-10. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Chaar MC, Daniel Crowther R, Allain Anthony, Pierre Jean. Impact of hepatitis B virus surface protein mutations on the diagnosis of occult hepatitis B virus infection. Hepatology. 2010;52(5):1600–1610. doi: 10.1002/hep.23886. PubMed. [DOI] [PubMed] [Google Scholar]
- 13.Levicnik-Stezinar S. Hepatitis B surface antigen escape mutant in a first time blood donor potentially missed by a routine screening assay. Clinical laboratory. 2004;50(1–2):49–51. doi: 10.1111/j.1365-3148.2010.01007.x. [DOI] [PubMed] [Google Scholar]
- 14.Thakur V, Kazim SN, Guptan RC, Hasnain SE, Bartholomeusz A, Malhotra V, et al. Transmission of G145R mutant of HBV to an unrelated contact. Journal of Medical Virology. 2005;76(1):40–46. doi: 10.1002/jmv.20321. [DOI] [PubMed] [Google Scholar]
- 15.Hunt CM, McGill JM, Allen MI, Condreay LD. Clinical relevance of hepatitis B viral mutations. Hepatology. 2000;31(5):1037–1044. doi: 10.1053/he.2000.6709. PubMed. [DOI] [PubMed] [Google Scholar]
- 16.Hudu S, Harmal N, Saeed M, Alshrari A, Malik Y, Niazlin M, et al. Naturallyoccurring hepatitis B virus surface antigen mutant variants in Malaysian blood donors and vaccinees. European Journal of Clinical Microbiology & Infectious Diseases. 2015:1–11. doi: 10.1007/s10096-015-2358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee H, Kiang P, Watanabe P, Halon P, Shi L, Church D. Hepatitis B virus infection and immunizations among asian american college students: infection, exposure, and immunity rates. Journal of American college health: J of ACH. 2013;61(2):67. doi: 10.1080/07448481.2012.753891. [DOI] [PubMed] [Google Scholar]
- 18.Clements CJ, Baoping Y, Crouch A, Hipgrave D, Mansoor O, Nelson CB, et al. Progress in the control of hepatitis B infection in the Western Pacific Region. Vaccine. 2006;24(12):1975–1982. doi: 10.1016/j.vaccine.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 19.Ng KP, Saw TL, Baki A, Rozainah K, Pang KW, Ramanathan M. Impact of the Expanded Program of Immunization against hepatitis B infection in school children in Malaysia. Med Microbiol Immunol. 2005;194(3):163–168. doi: 10.1007/s00430-004-0231-4. [DOI] [PubMed] [Google Scholar]
- 20.Yousuf R, Rapiaah M, Ahmed S, Rosline H, Salam A, Selamah S, et al. Trends in Hepatitis B Virus Infection among Blood Donors in Kelantan, Malaysia: a Retrospective Study. Southeast Asian J Trop Med Public Health. 2007;36(6):1070–1074. doi: 10.1.1.564.5646. [PubMed] [Google Scholar]
- 21.Kaneko S, Miller RH, Feinstone SM, Unoura M, Kobayashi K, Hattori N, et al. Detection of serum hepatitis B virus DNA in patients with chronic hepatitis using the polymerase chain reaction assay. Proceedings of the National Academy of Sciences. 1989;86(1):312–316. doi: 10.1073/pnas.86.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cabrerizo M, Bartolomé J, Caramelo C, Barril G, Carreño V. Molecular analysis of hepatitis B virus DNA in serum and peripheral blood mononuclear cells from hepatitis B surface antigen-negative cases. Hepatology. 2000;32(1):116–123. doi: 10.1053/jhep.2000.8541. [DOI] [PubMed] [Google Scholar]
- 23.Cacciola I, Pollicino T, Squadrito G, Cerenzia G, Villari D, de Franchis R, et al. Quantification of intrahepatic hepatitis B virus (HBV) DNA in patients with chronic HBV infection. Hepatology. 2000;31(2):507–512. doi: 10.1002/hep.510310235. PubMed. [DOI] [PubMed] [Google Scholar]
- 24.Marusawa H, Uemoto S, Hijikata M, Ueda Y, Tanaka K, Shimotohno K, et al. Latent hepatitis B virus infection in healthy individuals with antibodies to hepatitis B core antigen. Hepatology. 2000;31(2):488–495. doi: 10.1002/hep.510310232. PubMed. [DOI] [PubMed] [Google Scholar]
- 25.Carman WF, Van Deursen FJ, Mimms LT, Hardie D, Coppola R, Decker R, et al. The prevalence of surface antigen variants of hepatitis B virus in Papua New Guinea, South Africa, and Sardinia. Hepatology. 1997;26(6):1658–1666. doi: 10.1002/hep.510260640. PubMed. [DOI] [PubMed] [Google Scholar]
- 26.Manzini P, Girotto M, Borsotti R, Giachino O, Guaschino R, Lanteri M, et al. Italian blood donors with anti-HBc and occult hepatitis B virus infection. Haematologica. 2007;92(12):1664–1670. doi: 10.3324/haematol.11224. PubMed. [DOI] [PubMed] [Google Scholar]
- 27.Badrawy H, Bakry R. Anti-HBc and HBV-DNA detection in blood donors negative for hepatitis B virus surface antigen. American Journal of Molecular Biology. 2013;3:62–66. doi: 10.4236/ajmb.2013.31008. [DOI] [Google Scholar]
- 28.Soldan K, Davison K, Dow B. Estimates of the frequency of HBV, HCV, and HIV infectious donations entering the blood Supply in the United Kindom, 1996 to 2003. Euro Surveill. 2005;10(2):17–19. doi: 10.1111/j.1537-2995.2008.01718.x. PubMed. [DOI] [PubMed] [Google Scholar]
- 29.Kleinman SH, Kuhns MC, Todd DS, Glynn SA, McNamara A, DiMarco A. Frequency of HBV DNA detection in US blood donors testing positive for the presence of anti-HBc: implications for transfusion transmission and donor screening. Transfusion. 2003;43(6):696–704. doi: 10.1046/j.1537-2995.2003.00391.x. [DOI] [PubMed] [Google Scholar]
- 30.Seo DH, Whang DH, Song EY, Kim HS, Park Q. Prevalence of antibodies to hepatitis B core antigen and occult hepatitis B virus infections in Korean blood donors. Transfusion. 2011;51(8):1840–1846. doi: 10.1111/j.1537-2995.2010.03056.x. PubMed. [DOI] [PubMed] [Google Scholar]
- 31.Zervou EK, Dalekos GN, Boumba DS, Tsianos EV. Value of anti-HBc screening of blood donors for prevention of HBV infection: results of a 3-year prospective study in Northwestern Greece. Transfusion. 2001;41(5):652–658. doi: 10.1046/j.1537-2995.2001.41050652.x. PubMed. [DOI] [PubMed] [Google Scholar]
- 32.Fang Y, Shang Q-L, Liu J-Y, Li D, Xu W-Z, Teng X, et al. Prevalence of occult hepatitis B virus infection among hepatopathy patients and healthy people in China. Journal of Infection. 2009;58(5):383–388. doi: 10.1016/j.jinf.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45(2):507–539. doi: 10.1002/hep.21513. PubMed. [DOI] [PubMed] [Google Scholar]
- 34.Bréchot C, Thiers V, Kremsdorf D, Nalpas B, Pol S, Paterlini-Bréchot P. Persistent hepatitis B virus infection in subjects without hepatitis B surface antigen: clinically significant or purely “occult”? Hepatology. 2001;34(1):194–203. doi: 10.1053/jhep.2001.25172. PubMed. [DOI] [PubMed] [Google Scholar]
- 35.Li L, Chen PJ, Chen MH, Chak KF, Lin KS, Tsai SJL. A pilot study for screening blood donors in Taiwan by nucleic acid amplification technology: detecting occult hepatitis B virus infections and closing the serologic window period for hepatitis C virus. Transfusion. 2008;48(6):1198–1206. doi: 10.1111/j.1537-2995.2008.01672.x. PubMed. [DOI] [PubMed] [Google Scholar]
- 36.Yuen M-F, Wong DK-H, Lee C-K, Tanaka Y, Allain J-P, Fung J, et al. Transmissibility of hepatitis B virus (HBV) infection through blood transfusion from blood-donors with occult HBV infection. Clinical Infectious Diseases. 2011;52(5):624–632. doi: 10.1093/cid/ciq247. [DOI] [PubMed] [Google Scholar]
- 37.Allain JP, Mihaljevic I, Gonzalez-Fraile MI, Gubbe K, Holm-Harritshøj L, Garcia JM, et al. Infectivity of blood products from donors with occult hepatitis B virus infection. Transfusion. 2013;53(7):1405–1415. doi: 10.1111/trf.12096. [DOI] [PubMed] [Google Scholar]
- 38.Seed C, Kiely P. A method for estimating the residual risk of transfusion-transmitted HBV infection associated with occult hepatitis B virus infection in a donor population without universal anti-HBc screening. Vox sanguinis. 2013;105(4):290–298. doi: 10.1111/vox.12060. [DOI] [PubMed] [Google Scholar]